Simulation and Economic Analysis of the Biotechnological Potential of Biomass Production from a Microalgal Consortium
Abstract
1. Introduction
2. Results and Discussions
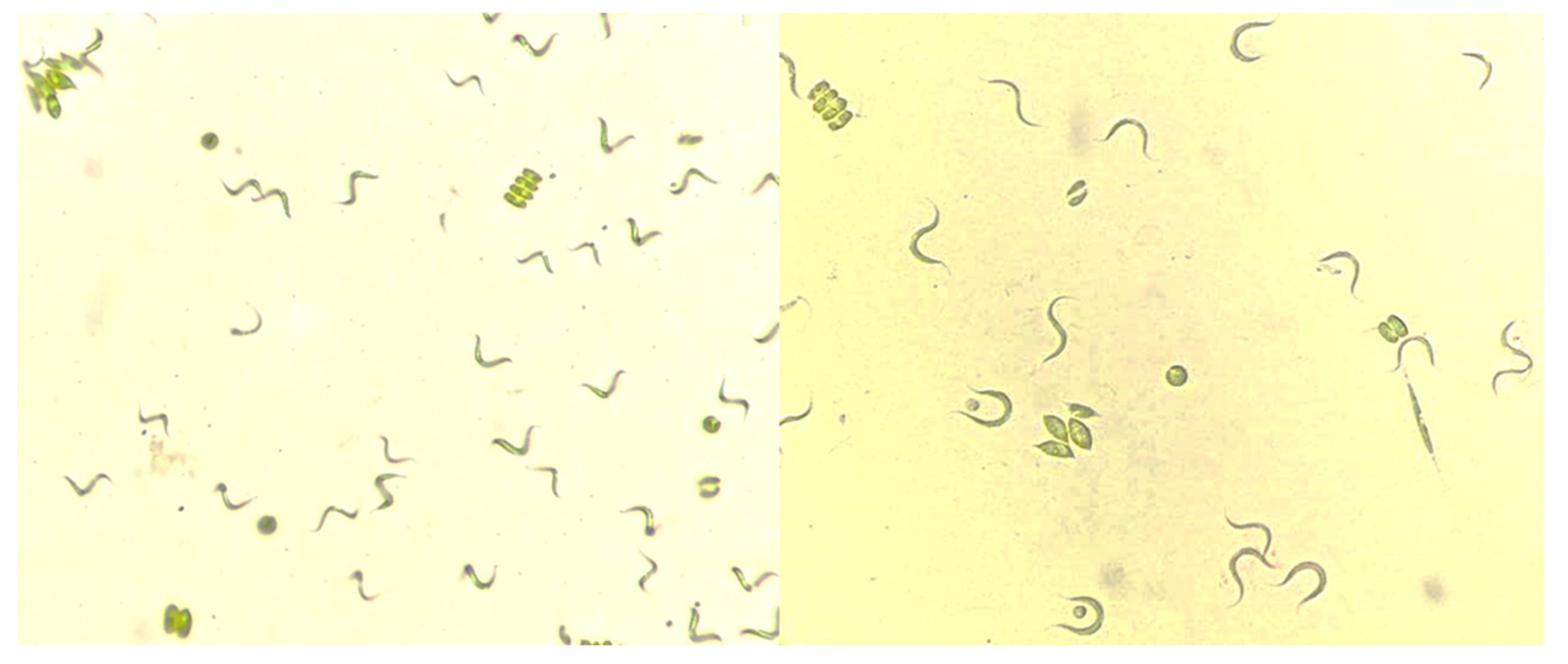
2.1. Experimental Biomass and Chlorophyll Production
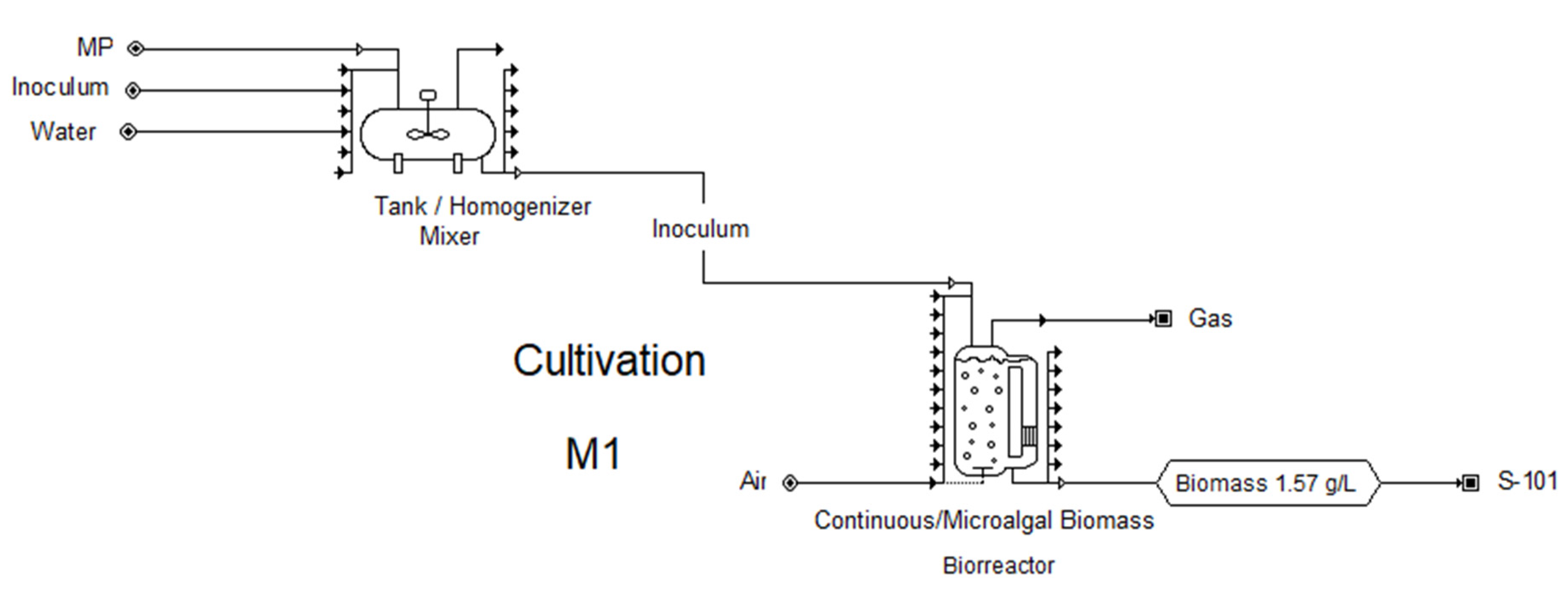
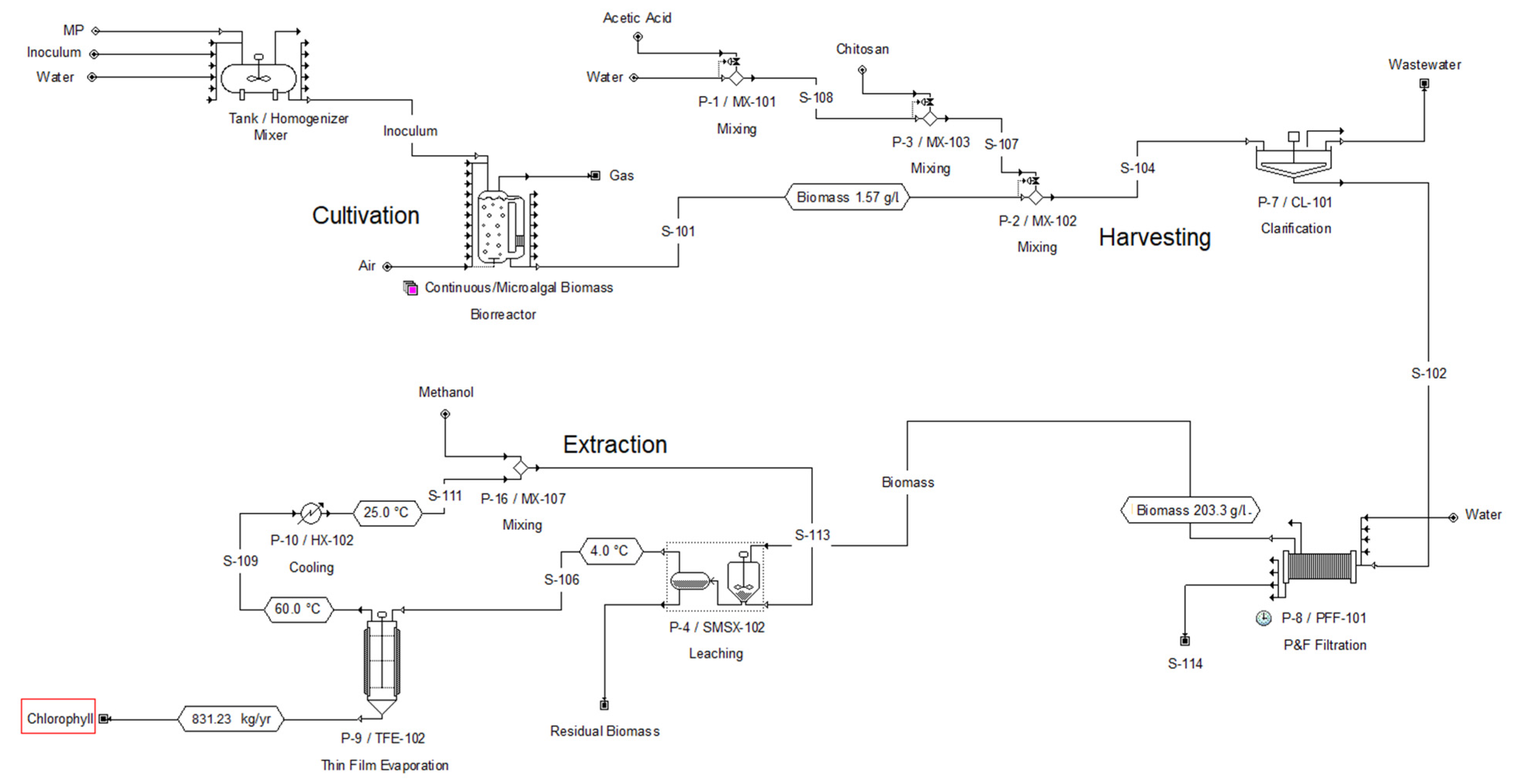
2.2. Results of the Techno-Economic Evaluation for the Production of Biomass from a Microalgal Consortium
2.3. Results of the Techno-Economic Evaluation of the Production of Chlorophyll from a Microalgal Consortium
3. Materials and Methods
3.1. Study Material
3.2. Determination of the Cell Growth
3.3. Determination of the Chlorophyll
3.4. Microalgae Biomass Production
3.5. Simulation of Microalgal Biomass Production and Techno-Economic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vázquez-Romero, B.; Perales, J.A.; Vree, J.H.; Böpple, H.; Steinrücken, P.; Barbosa, M.J.; Kleinegris, D.M.M.; Ruiz, J. Techno-economic analysis of microalgae production for aquafeed in Norway. Algal Res. 2022, 64, 102679. [Google Scholar] [CrossRef]
- Kholssi, R.; Ramos, P.V.; Marks, E.A.N.; Montero, O.; Rad, C. Biotechnological uses of microalgae: A review on the state of the art and challenges for the circular economy. Biocatal. Agric. Biotechnol. 2021, 36, 102114. [Google Scholar] [CrossRef]
- Balaji Prasath, B.; Elsawah, A.M.; Liyuan, Z.; Poon, K. Modeling and optimization of the effect of abiotic stressors on the productivity of the biomass, chlorophyll and lutein in microalgae Chlorella pyrenoidosa. J. Agric. Food Res. 2021, 5, 100163. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Nascimento, T.C.; Pinheiro, P.N.; Rosso, V.V.; Menezes, C.R.; Jacob-Lopes, E.; Zepka, L.Q. Insights on the intestinal absorption of chlorophyll series from microalgae. Food Res. Int. 2021, 140, 110031. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, P.M.; Torres-Acosta, M.; Collén, P.N.; Abreu, M.H. Extraction of chlorophyll from wild and farmed Ulva spp. Using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Sun, J.; Yang, L.; Xiao, S.; Chu, H.; Jiang, S.; Yu, S.; Zhou, X.; Zhang, Y. A promising microalgal wastewater cyclic cultivation technology: Dynamic simulations, economic viability, and environmental suitability. Water Res. 2022, 217, 118411. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- AlMahri, M.A.; Jung, K.; Alshehhi, M.; Bastidas-Oyanedel, J.R.; Schmidt, J.E. Techno-economic Assessment of Microalgae Biorefinery as a Source of Proteins, Pigments, and Fatty acids: A Case Study for the United Arab Emirates. In Biorefinery; Springer: Cham, Switzerland, 2019; pp. 679–693. [Google Scholar] [CrossRef]
- Elegbede, I.; Muritala, I.K.; Shuuluka, D.; Saheed, M.; Elegbeleye, O.W.; Li-Hammed, M.; Jinad, M. Algae Bioenergy. In Encyclopedia of Sustainable Management; Springer: Cham, Switzerland, 2023; pp. 1–7. [Google Scholar] [CrossRef]
- Castro, J.S.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 2020, 724, 138138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.; Sydney, E.B.; Assú-Tessari, L.F.; Soccol, C.R. Culture media for mass production of microalgae. In Biomass, Biofuels, Biochemicals: Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–50. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y.; Bashan, Y.; Bashan, Y. Microalgal heterotrophic and mixotrophic culturing for bio-refining: From metabolic routes to techno-economics. Algal Biorefin. 2015, 2, 61–131. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae cultivation and metabolites production: A comprehensive review. Biofuels Bioprod. Biorefin. 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Moon, M.; Kim, C.W.; Park, W.K.; Yoo, G.; Choi, Y.E.; Yang, J.W. Mixotrophic growth with acetate or volatile fatty acids maximizes growth and lipid production in Chlamydomonas reinhardtii. Algal Res. 2013, 2, 352–357. [Google Scholar] [CrossRef]
- Montero, E.; Olguín, E.J.; Philippis, R.; Reverchon, F. Mixotrophic cultivation of Chlorococcum sp. under non-controlled conditions using a digestate from pig manure within a biorefinery. J. Appl. Phycol. 2018, 30, 2847–2857. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Zhu, S.; Huo, S.; Feng, P. Developing designer microalgal consortia: A suitable approach to sustainable wastewater treatment. Microalgae Biotechnol. Dev. Biofuel Wastewater Treat. 2019, 2, 569–598. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, M.A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef]
- Paggi Matos, A. The impact of microalgae in food science and technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometryc review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406; [Google Scholar] [CrossRef]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef]
- Ahirwar, A.; Das, S.; Das, S.; Yang, Y.H.; Bhatia, S.K.; Vinayak, V.; Ghangrekar, M.M. Photosynthetic microbial fuel cell for bioenergy and valuable production: A review of circular bio-economy approach. Algal Res. 2023, 70, 102973. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engieneering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef]
- Sathinathan, P.; Parab, H.M.; Yusoff, R.; Ibrahim, S.; Vello, V.; Ngoh, G.C. Photobioreactor desing and parameters essential for algal cultivation using industrial wastewater: A review. Renew. Sustain. Energy Rev. 2023, 173, 113096. [Google Scholar] [CrossRef]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Nuñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerema, A.G.; Morales-Vargas, A.T.; Rodríguez-Nuñez, J.R. Production and physicochemical characterization of chitosan for the harvesting of wild microalgae consortia. Biotechnol. Rep. 2020, 28, e00554. [Google Scholar] [CrossRef] [PubMed]
- Valdovinos-García, E.M.; Petriz-Prieto, M.A.; Olán-Acosta, M.d.l.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Bravo-Sánchez, M.G. Production of Microalgal Biomass in Photobioreactors as Feedstock for Bioenergy and Other Uses: A Techno-Economic Study of Harvesting Stage. Appl. Sci. 2021, 11, 4386. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Bravo-Sánchez, M.G.; Olán-Acosta, M.d.l.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Petriz-Prieto, M.A. Technoeconomic Evaluation of Microalgae Oil Production: Efect of Cell Disruption Method. Fermentation 2022, 8, 301. [Google Scholar] [CrossRef]
- Bhatt, A.; Khanchandani, M.; Rana, M.S.; Prajapati, S.K. Techno-economic analysis of microalgae cultivation for commercial sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 370, 133456. [Google Scholar] [CrossRef]
- Liang, F.; Wen, X.; Geng, Y.; Ouyang, Z.; Luo, L.; Li, Y. Growth rate and biomass productivity of Chlorella as affected by culture depth and cell density in an open circular photobioreactor. J. Microbiol. Biotechnol. 2013, 23, 539–544. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2023. [Google Scholar] [CrossRef]
- Romero-García, J.M.; González-López, C.V.; Brindley, C.; Fernández-Sevilla, J.M.; Acién-Fernández, F.G. Simulation and Techno-Economical Evaluation of a Microalgal Biofertilizer Production Process. Biology 2022, 11, 1359. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Barajas-Fernández, J.; Olán-Acosta, M.; Petriz-Prieto, M.A.; Guzmán-López, A.; Bravo-Sánchez, M.G. Techno-Economic Study of CO2 Capture of a Thermoelectric Plant Using Microalgae (Chlorella vulgaris) for Production of Feedstock for Bioenergy. Energies 2020, 13, 413. [Google Scholar] [CrossRef]
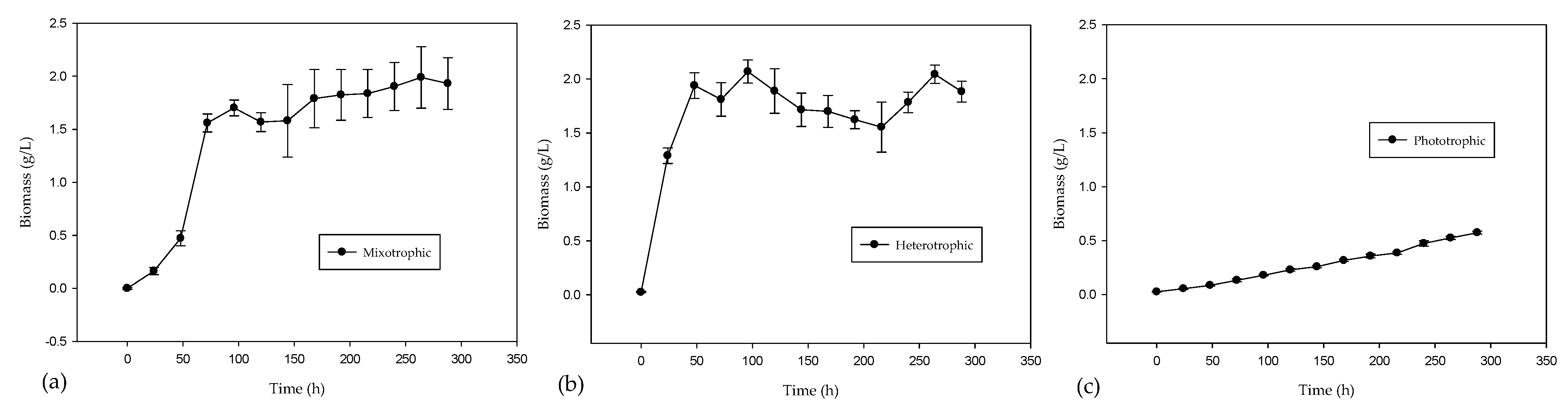
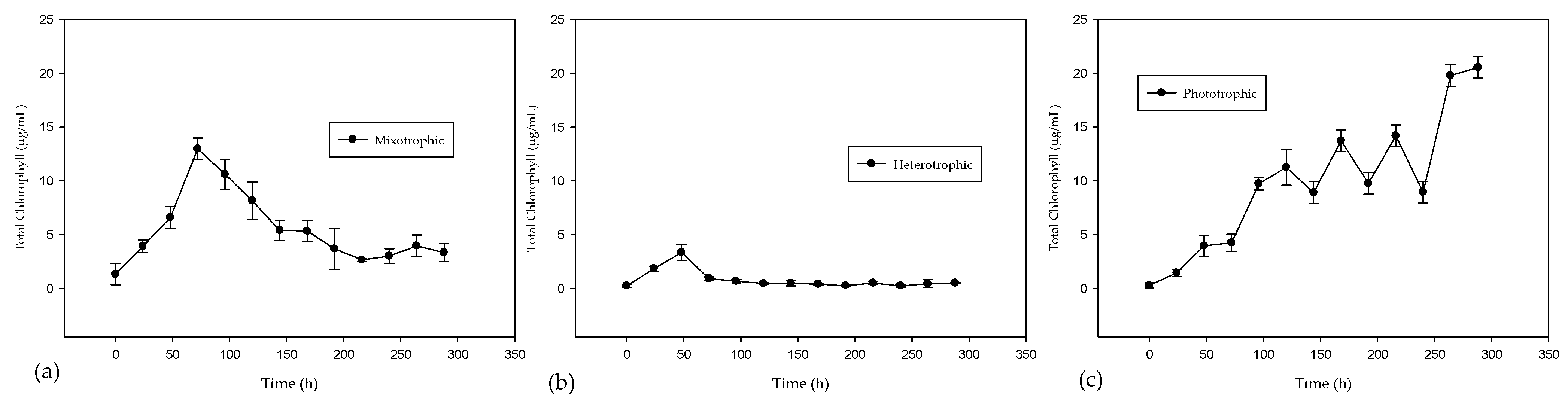



| Case | Biomass (g/L) | Chlorophyll (µg/L) |
|---|---|---|
| F1 | 0.04 | 4240 |
| F2 | 0.56 | 20,540 |
| H1 | 1.83 | 930 |
| H2 | 1.81 | 520 |
| M1 | 1.57 | 12,970 |
| M2 | 1.85 | 3330 |
| Equipment | Description | Units | Cost (USD) |
|---|---|---|---|
| Photobioreactor | Air lift fermenter (300 L) | 2176 | 4,352,000 |
| Homogenizer | Horizontal tank (10,000 L) | 1 | 1000 |
| Clarifier | CL-101 | 1 | 33,000 |
| Plate and frame filter | PFF-101 | 1 | 72,000 |
| Solid mixer–settler extractor | SMSX-102 | 1 | 11,000 |
| Evaporator | TFE-102 | 1 | 60,000 |
| Heat exchanger | HX-102 | 1 | 9000 |
| Reagents | per Liter | Cost (USD/ton) |
|---|---|---|
| KH2PO4 | 175 mg | 1650 |
| CaCl2·2H2O | 25 mg | 120 |
| MgSO4·7H2O | 75 mg | 95 |
| NaNO3 | 250 mg | 50 |
| K2HPO4 | 75 mg | 300 |
| NaCl | 25 mg | 78 |
| H3BO3 | 11.42 mg | 300 |
| Microelements Stock Solution | ||
| ZnSO4·7H2O | 8.82 g | 2000 |
| MnCl2·4H2O | 1.44 g | 500 |
| MoO3 | 0.71 g | 40,000 |
| CuSO4·5H2O | 1.57 g | 13,000 |
| Co(NO3)2·6H2O | 0.49 g | 9000 |
| Solution 1 | ||
| Na2EDTA | 50 g | 1500 |
| KOH | 3.1 g | 1300 |
| Solution 2 | ||
| FeSO4 | 4.98 g | 150 |
| H2SO4 (Conc.) | 1 mL | 330 |
| Case | Type of Crop | Residence Time (h) |
|---|---|---|
| F1 | Phototrophic | 72 |
| F2 | Phototrophic | 288 |
| H1 | Heterotrophic | 72 |
| H2 | Heterotrophic | 288 |
| M1 | Mixotrophic | 72 |
| M2 | Mixotrophic | 288 |
| Kind of Service | Price | Unity |
|---|---|---|
| Raw materials | ||
| Acid acetic | 730 | USD/ton |
| Chitosan | 224 | USD/ton |
| Water | 0.26 | USD/m3 |
| Services | ||
| Std power | 0.1 | USD/kWh |
| Steam | 12 | USD/ton |
| Chilled water | 0.4 | USD/ton |
| Glycol | 0.35 | USD/ton |
| Labor | ||
| Operator | 0.37 | USD/h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Capetillo, C.A.; Castillo-Baltazar, O.S.; Petriz-Prieto, M.A.; Guzmán-López, A.; Valdovinos-García, E.M.; Bravo-Sánchez, M.G. Simulation and Economic Analysis of the Biotechnological Potential of Biomass Production from a Microalgal Consortium. Mar. Drugs 2023, 21, 321. https://doi.org/10.3390/md21060321
Cabrera-Capetillo CA, Castillo-Baltazar OS, Petriz-Prieto MA, Guzmán-López A, Valdovinos-García EM, Bravo-Sánchez MG. Simulation and Economic Analysis of the Biotechnological Potential of Biomass Production from a Microalgal Consortium. Marine Drugs. 2023; 21(6):321. https://doi.org/10.3390/md21060321
Chicago/Turabian StyleCabrera-Capetillo, Christian Ariel, Omar Surisadai Castillo-Baltazar, Moisés Abraham Petriz-Prieto, Adriana Guzmán-López, Esveidi Montserrat Valdovinos-García, and Micael Gerardo Bravo-Sánchez. 2023. "Simulation and Economic Analysis of the Biotechnological Potential of Biomass Production from a Microalgal Consortium" Marine Drugs 21, no. 6: 321. https://doi.org/10.3390/md21060321
APA StyleCabrera-Capetillo, C. A., Castillo-Baltazar, O. S., Petriz-Prieto, M. A., Guzmán-López, A., Valdovinos-García, E. M., & Bravo-Sánchez, M. G. (2023). Simulation and Economic Analysis of the Biotechnological Potential of Biomass Production from a Microalgal Consortium. Marine Drugs, 21(6), 321. https://doi.org/10.3390/md21060321







