Photoprotective and Anti-Aging Properties of the Apical Frond Extracts from the Mediterranean Seaweed Ericaria amentacea
Abstract
1. Introduction
2. Results and Discussion
2.1. TPC and TFC in E. amentacea Extracts
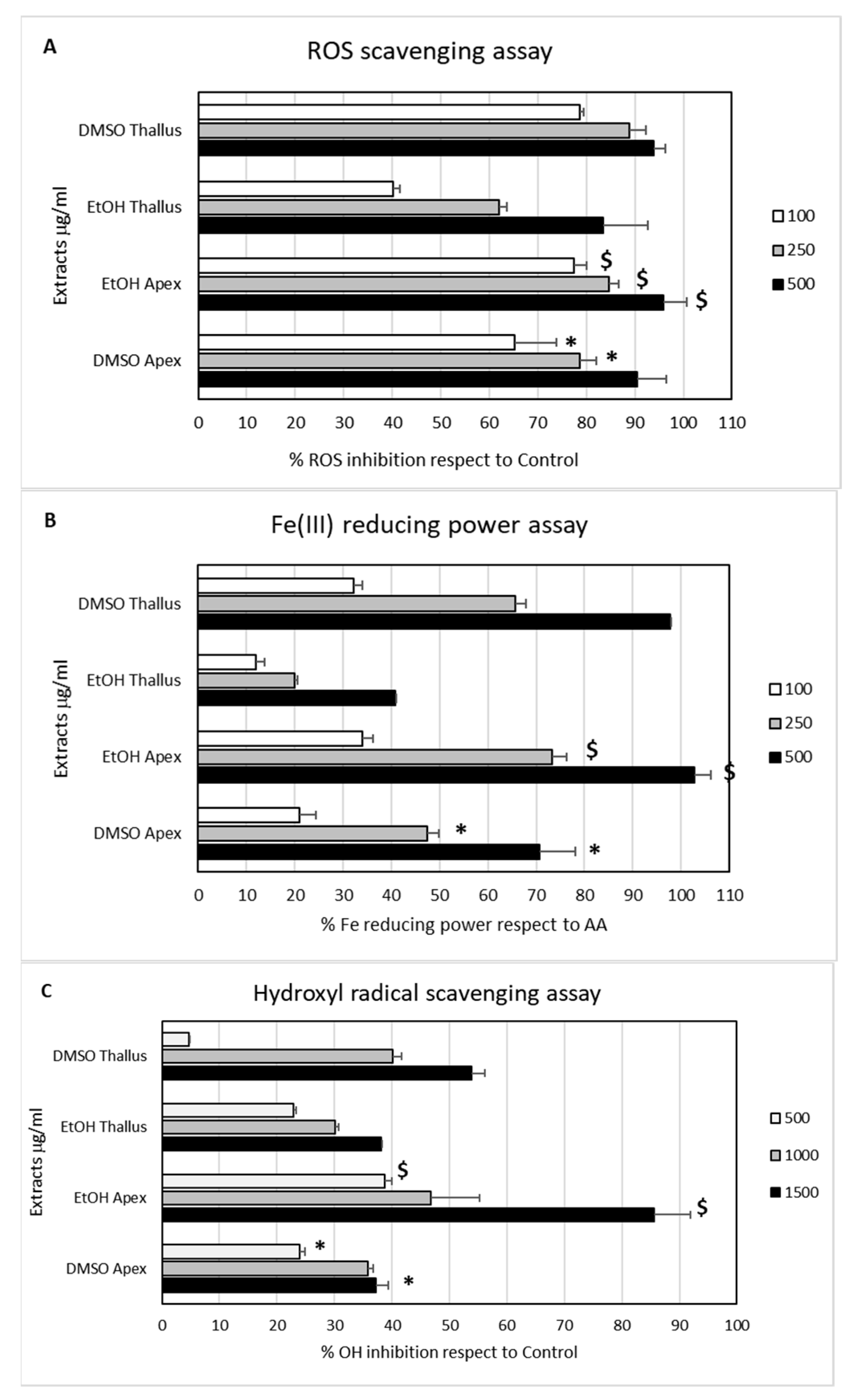
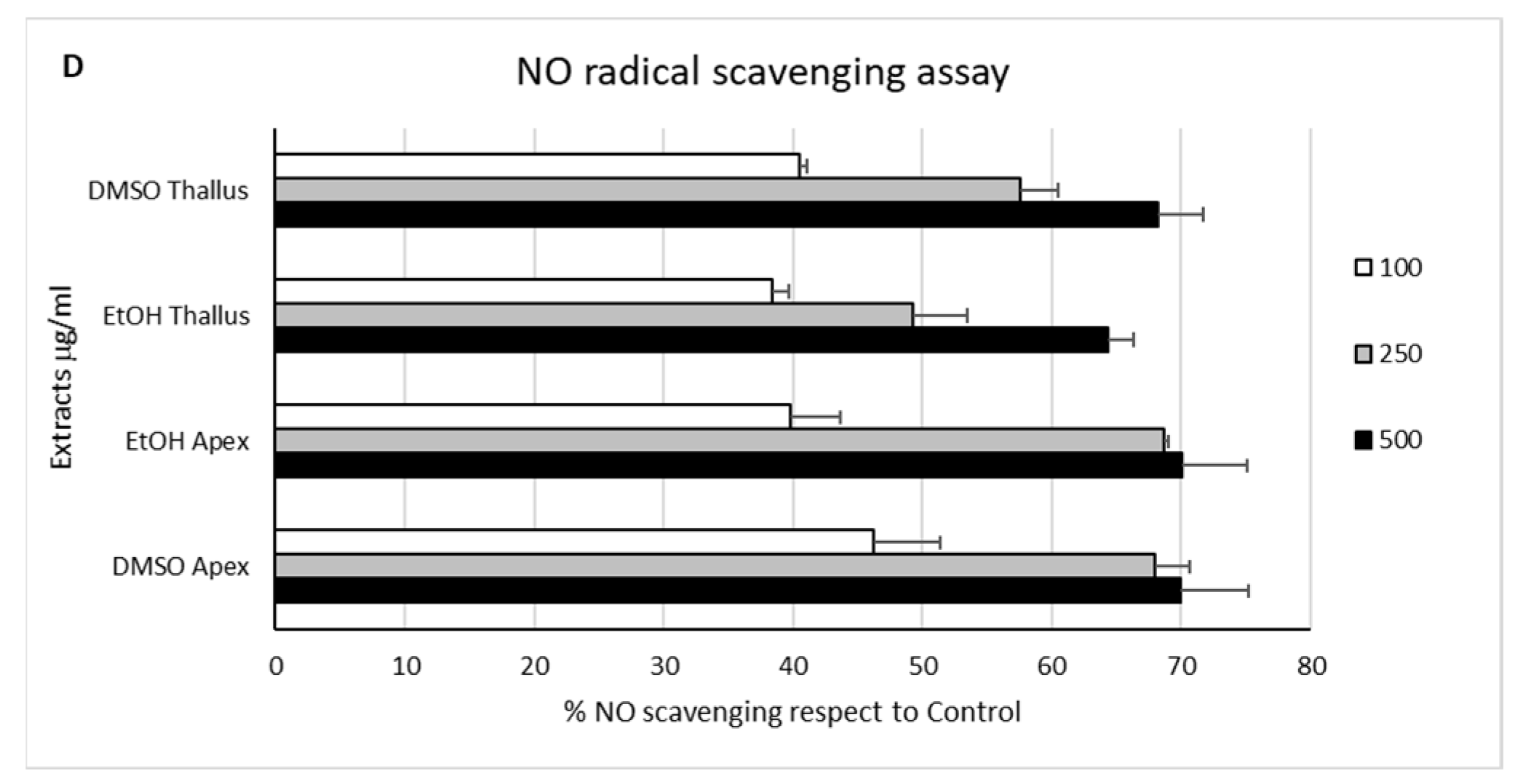
2.2. In Chemico Antioxidant Activity
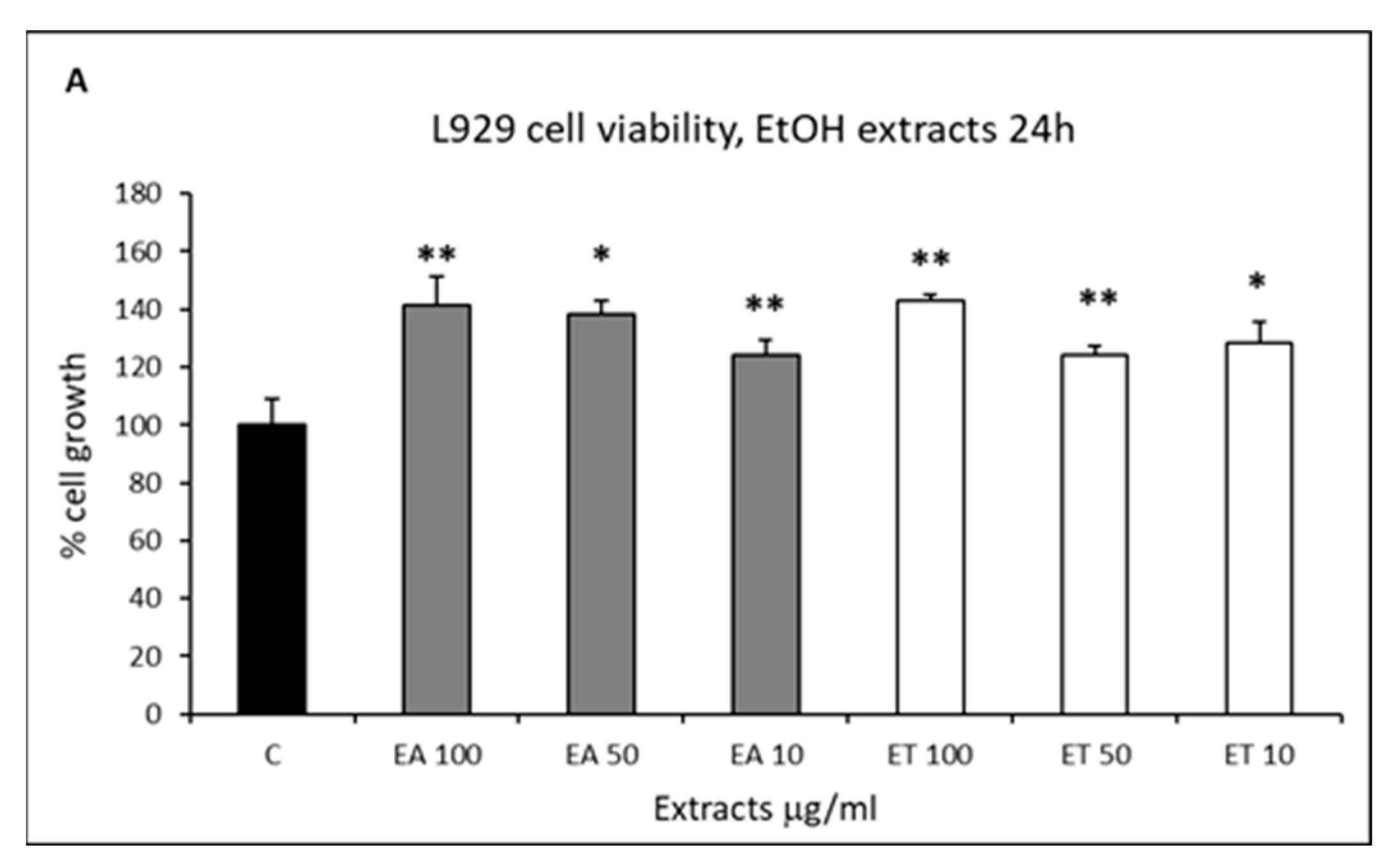
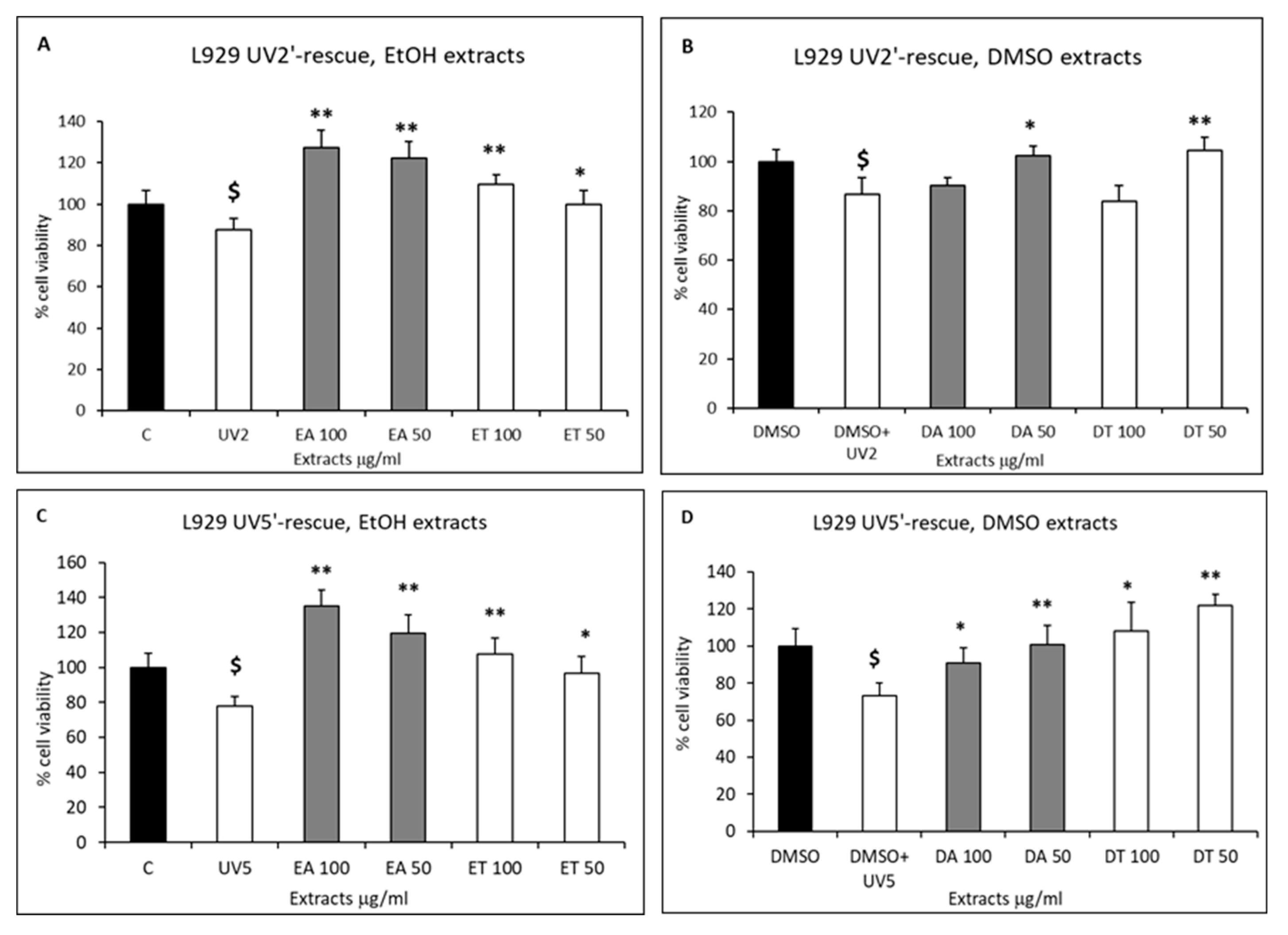
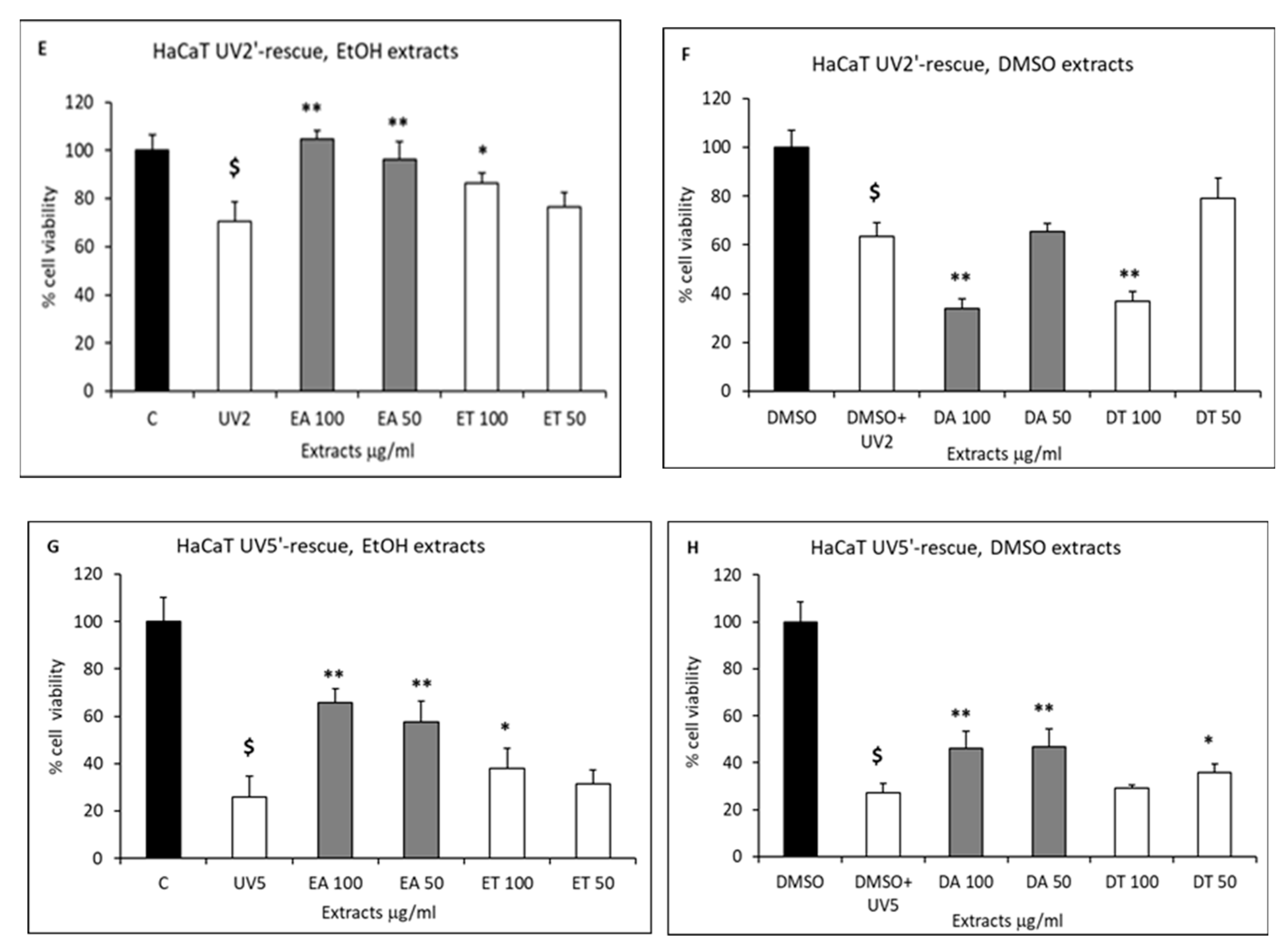
2.3. Cell Proliferation and Rescue from Stressful Stimuli
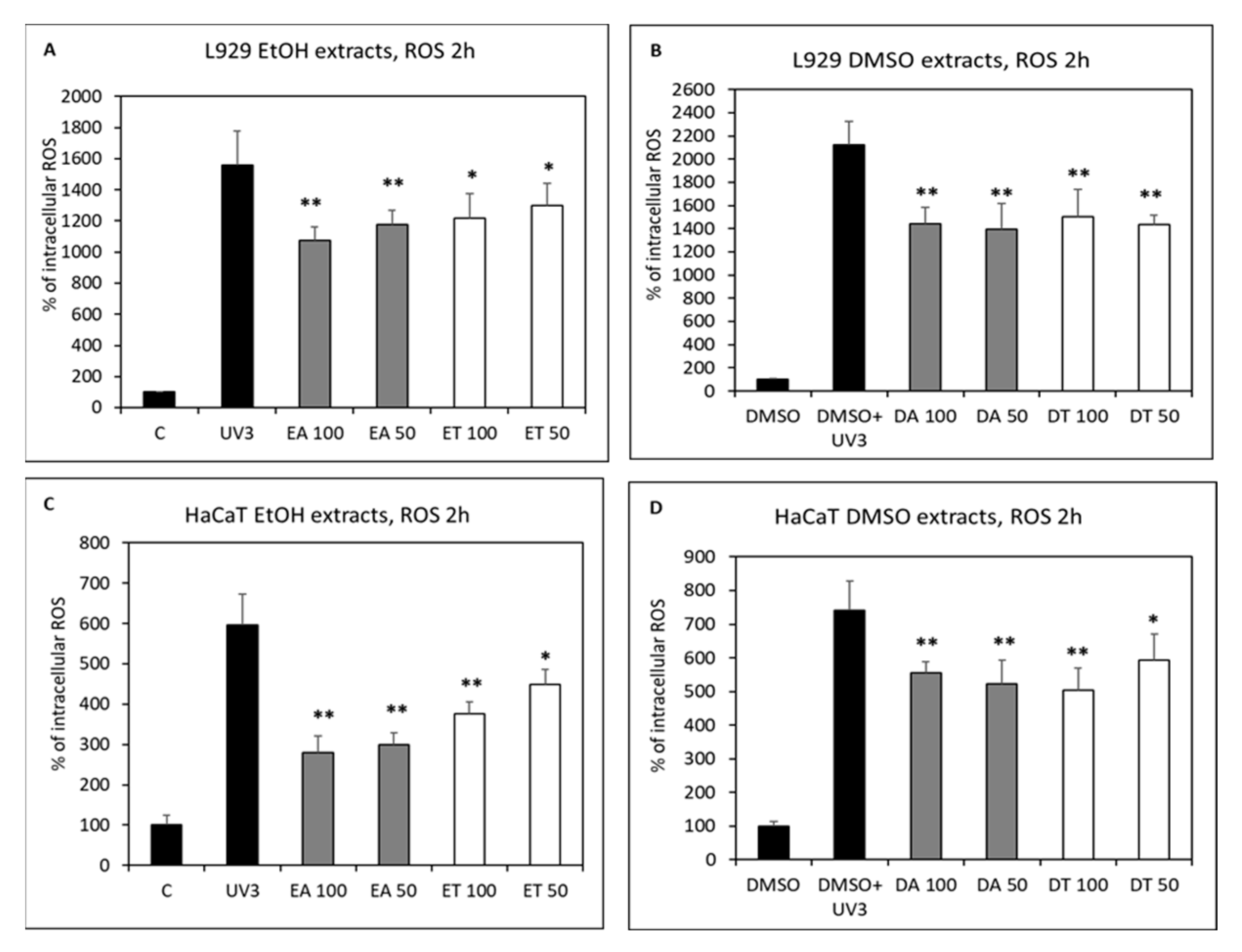
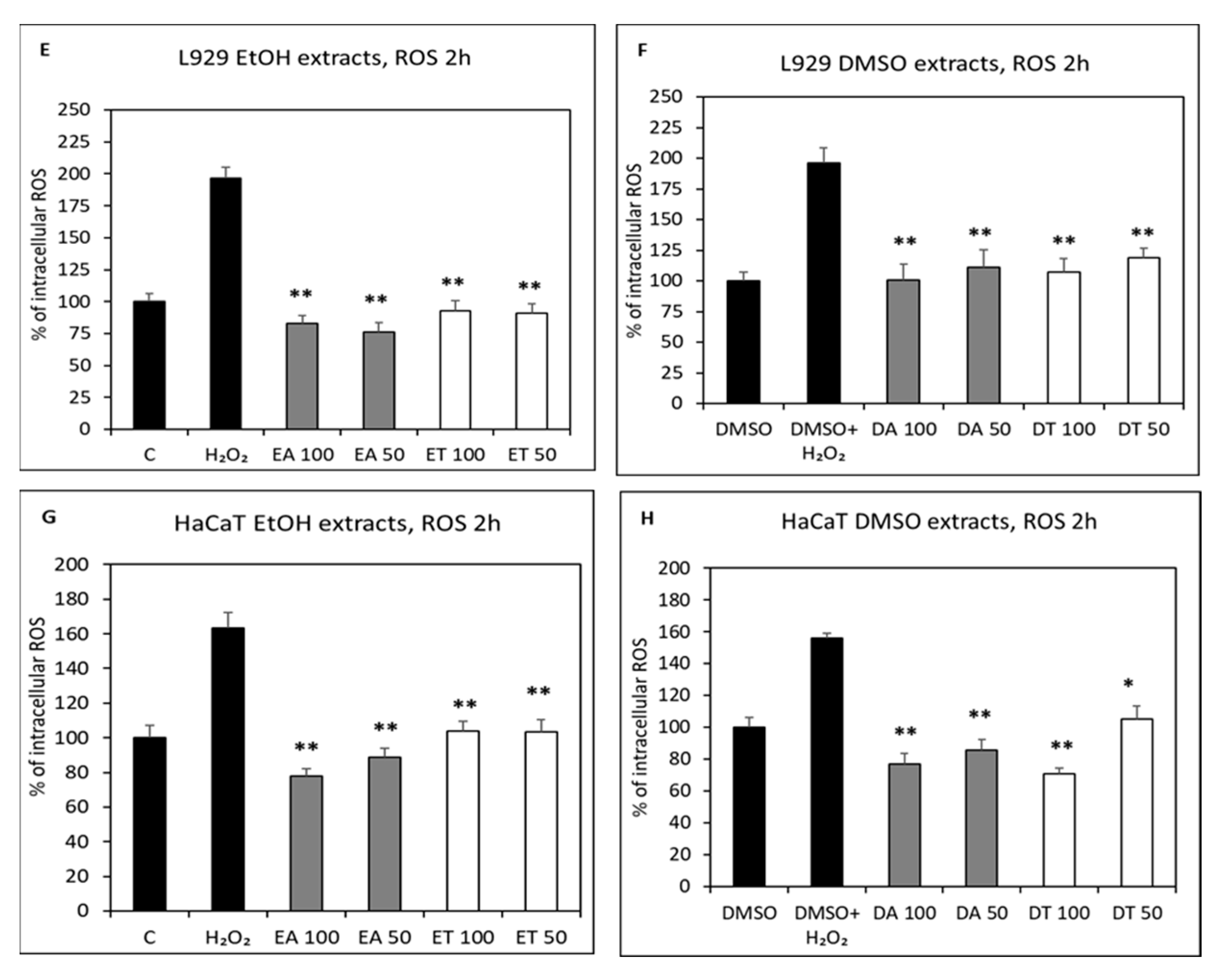
2.4. Intracellular Antioxidant Activity
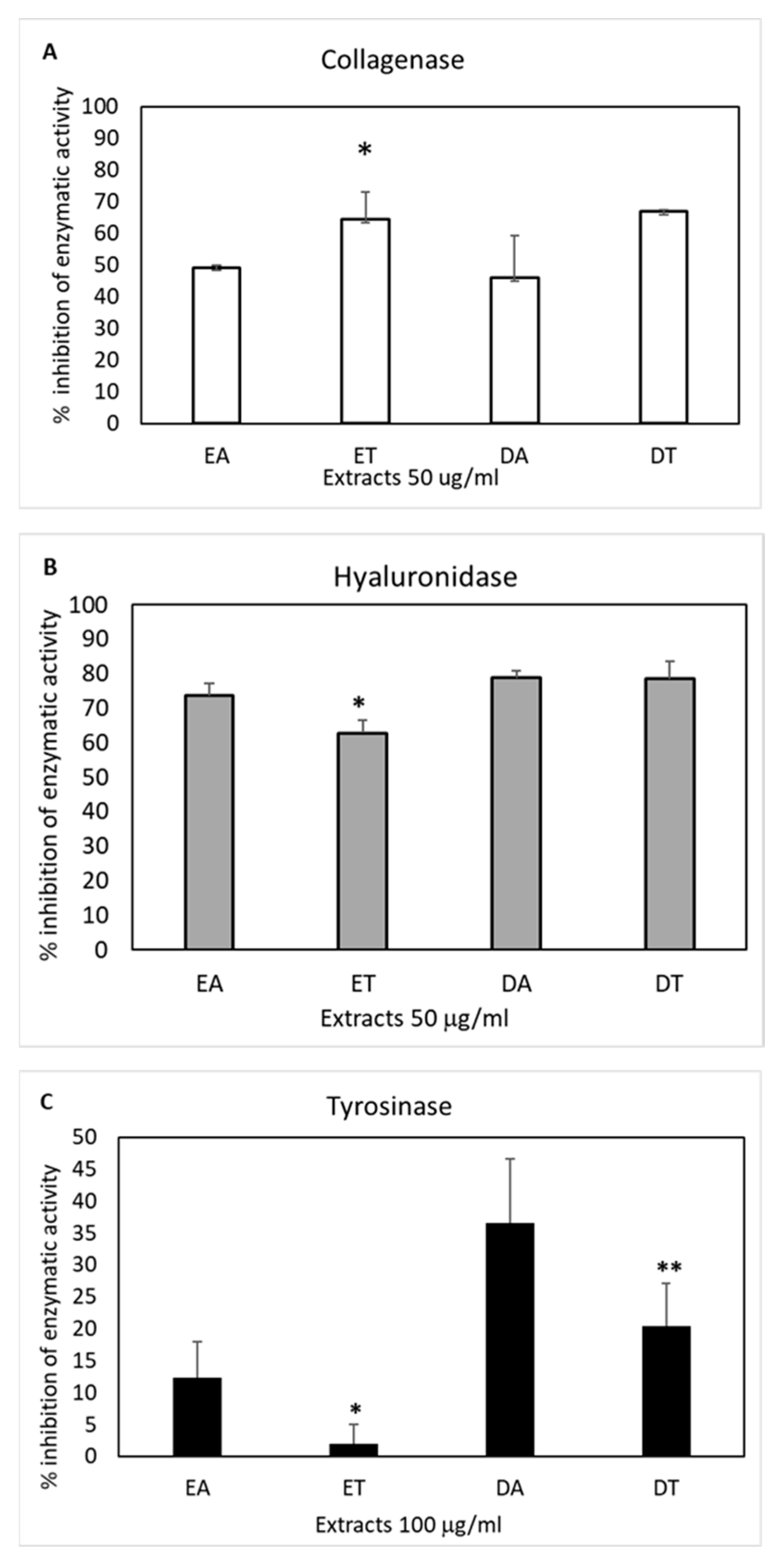
2.5. Skin Hydrolytic Enzyme Inhibition
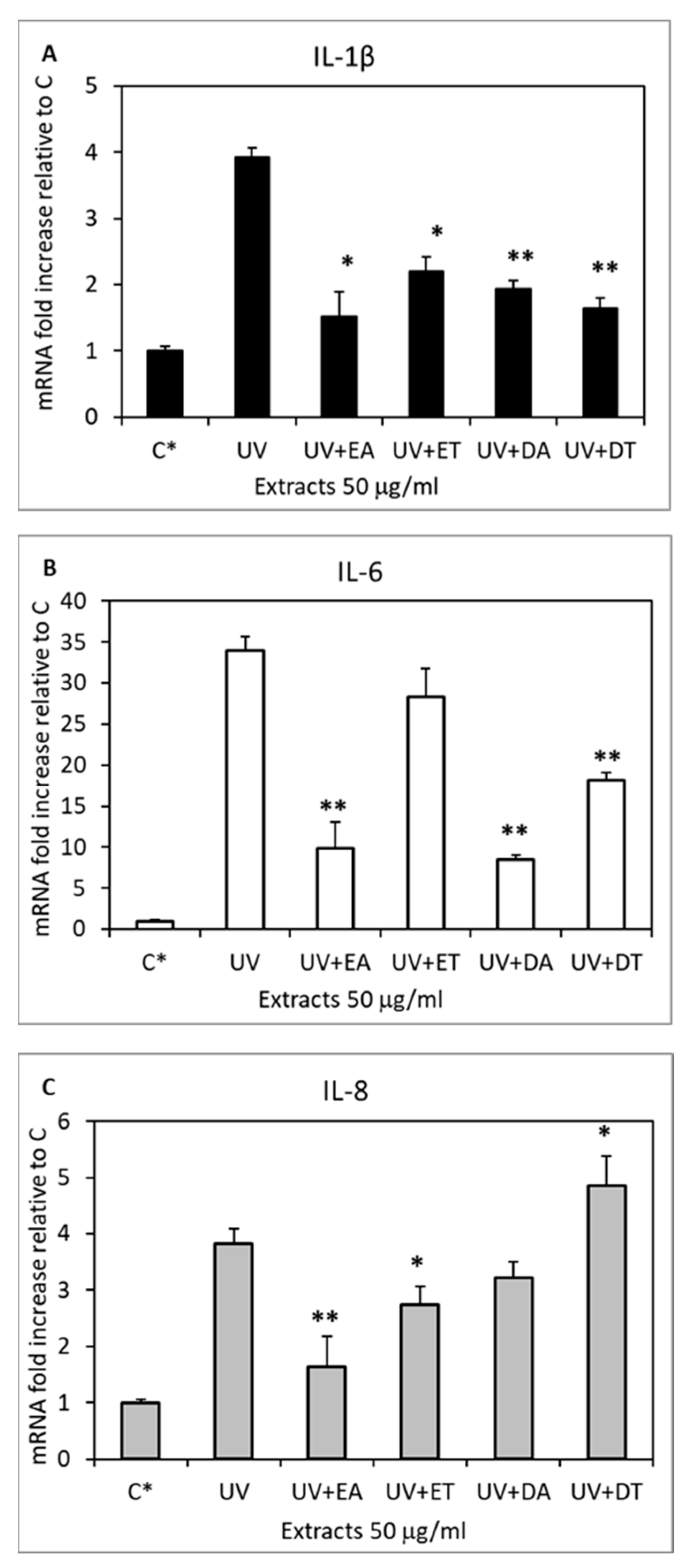
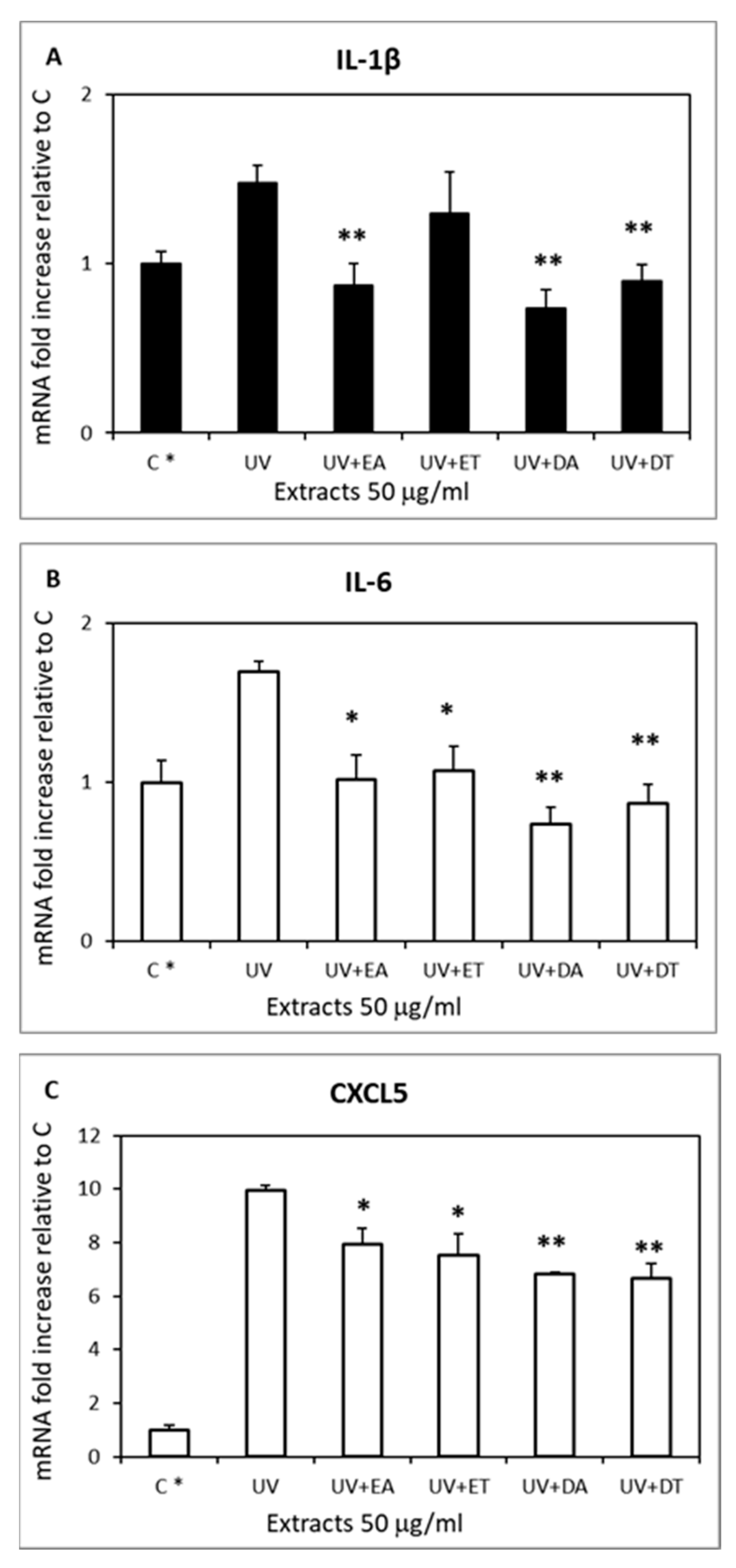
2.6. Skin Anti-Inflammatory Effect
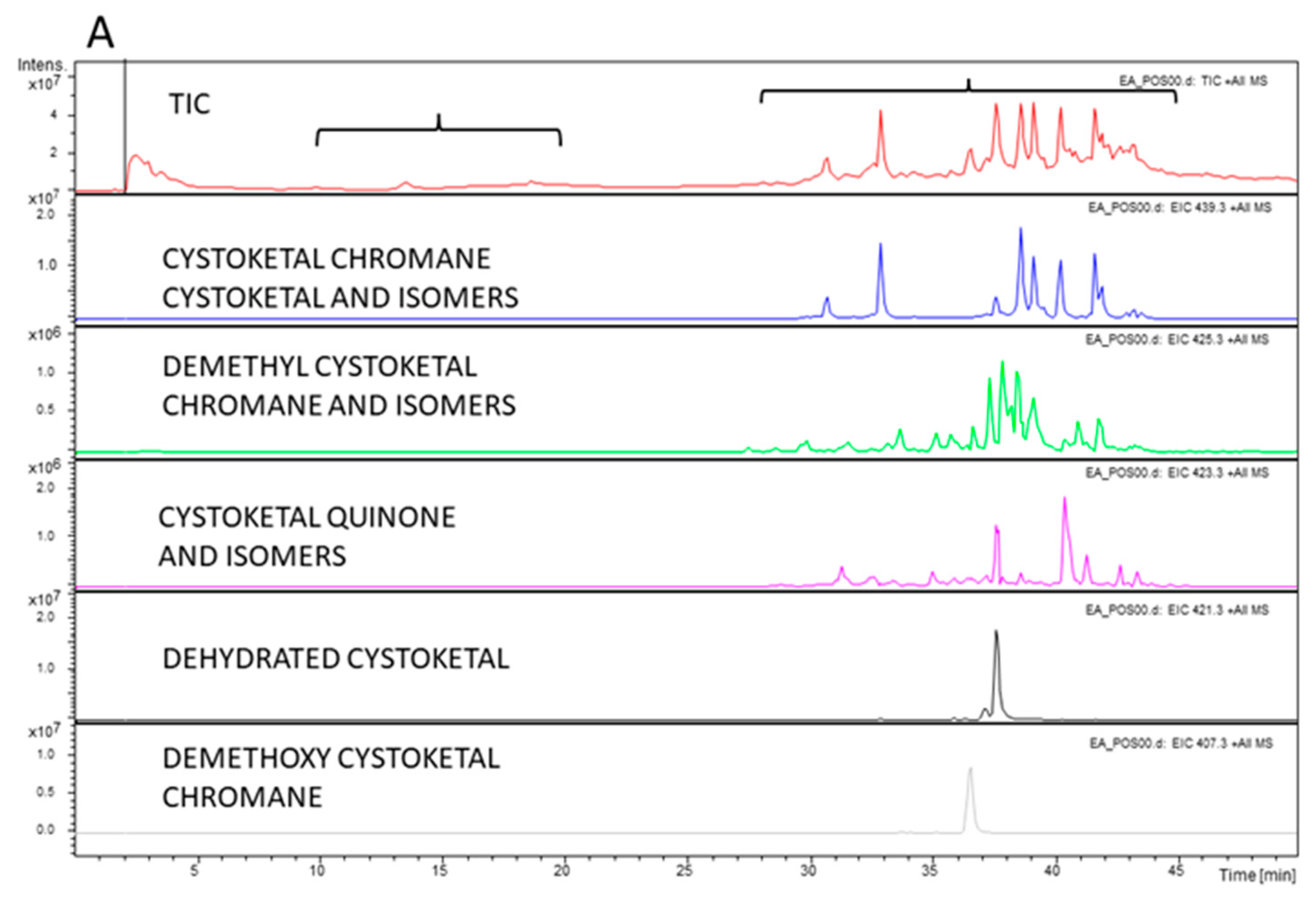
2.7. Chemical Characterization of E. amentacea Apices and Thalli Extracts
3. Materials and Methods
3.1. Chemicals
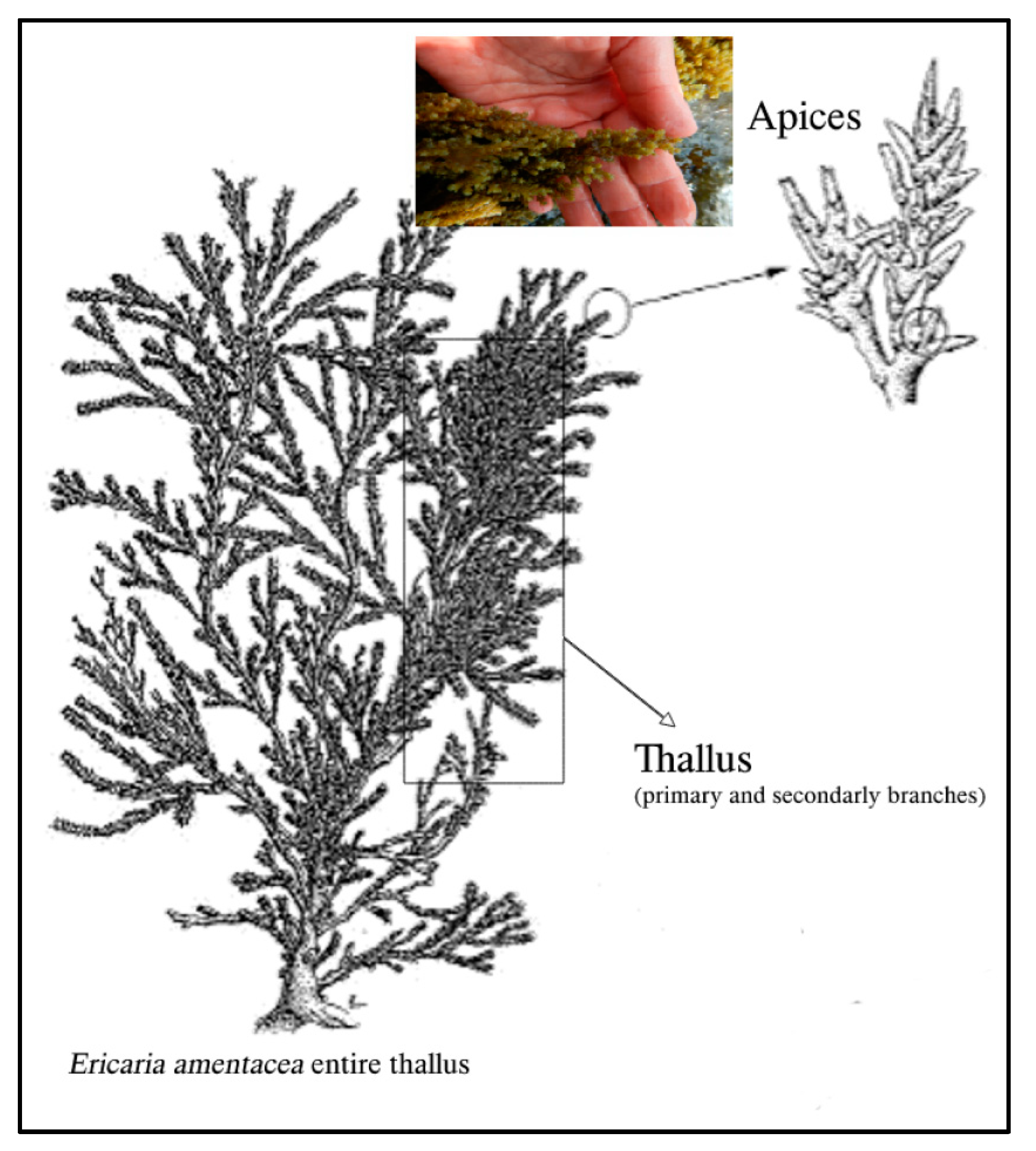
3.2. Algae Collection
3.3. Production of Extracts from E. amentacea
3.4. Total Phenolic and Flavonoid Contents
3.5. In Chemico Antioxidant Activity
3.5.1. DPPH Assay
3.5.2. Reducing Fe (III) Power Assay, Hydroxyl Radical and NO Scavenging Activities
3.6. Tyrosinase, Collagenase and Hyaluronidase Inhibition
3.7. Cell Cultures
3.8. Cell Toxicity and Rescue from UV and H2O2 Treatment
3.9. Inhibition of Intracellular ROS Production
3.10. Gene Expression Analyses
3.11. HPLC/MS Analyses
3.12. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guiry, M.D. How Many Species of Algae Are There? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- de Sousa, C.B.; Gangadhar, K.N.; Macridachis, J.; Pavão, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron Asymmetry 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Gaysinski, M.; Ortalo-Magné, A.; Thomas, O.P.; Culioli, G. Extraction, purification, and NMR analysis of terpenes from brown algae. In Methods in Molecular Biology Book Series; Humana Press: Clifton, NJ, USA, 2015; Volume 1308, pp. 207–223. [Google Scholar]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.-K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.-C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Kang, K.; Hye, J.H.; Dong, H.H.; Park, Y.; Seong, H.K.; Bong, H.L. Antioxidant and antiinflammatory activities of ventol, a phlorotannin-rich natural agent derived from Ecklonia cava, and its effect on proteoglycan degradation in cartilage ex-plant culture. Res. Commun. Mol. Pathol. Pharmacol. 2004, 115–116, 77–95. [Google Scholar]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-Tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.L.; Kelly, K. Prevention and Treatment of Skin Aging. Ann. N. Y. Acad. Sci. 2006, 1067, 323–331. [Google Scholar] [CrossRef]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative activities in some common seaweeds. Plant Foods Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef]
- Hirose, Y.; Yoshimi, N.; Suzui, M.; Kawabata, K.; Tanaka, T.; Mori, H. Expression of bcl-2, bax, and bcl-XL proteins in azoxymethane-induced rat colonic adenocarcinomas. Mol. Carcinog. 1997, 19, 25–30. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; De Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae——A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Stanoikovic, T.P.; Konic-Ristic, A.; Kljajic, Z.; Grozdanic-Stanisavljevic, N.; Srdic-Rajic, T.; Zdunic, G.; Savikin, K. Antioxidant, antiplatelet and cytotoxic activity of extract of Cystoseira amentacea from the coast of Montenegro (South-east Adriatic Sea). Digest J. Nanomat. Biostruct. 2014, 9, 869–880. [Google Scholar]
- Ruberto, G.; Baratta, M.T.; Biondi, D.M.; Amico, V. Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J. Appl. Phycol. 2001, 13, 403–407. [Google Scholar] [CrossRef]
- De La Fuente, G.; Pinteus, S.; Silva, J.; Alves, C.; Pedrosa, R. Antioxidant and antimicrobial potential of six Fucoids from the Mediterranean Sea and the Atlantic Ocean. J. Sci. Food Agric. 2022, 102, 5568–5575. [Google Scholar] [CrossRef]
- Mannino, A.M.; Vaglica, V.; Oddo, E. Seasonal variation in total phenolic content of Dyctiopteris polypodioides (Dictyotaceae) and Cystoseira amentacea (Sargassacee) from the Sicilian coast. Flora Mediterr. 2014, 24, 39–50. [Google Scholar] [CrossRef]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfì, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira amentacea var. stricta. Mar. Drugs 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Goutzourelas, N.; Kevrekidis, D.P.; Barda, S.; Malea, P.; Trachana, V.; Savvidi, S.; Kevrekidou, A.; Assimopoulou, A.N.; Goutas, A.; Liu, M.; et al. Antioxidant Activity and Inhibition of Liver Cancer Cells’ Growth of Extracts from 14 Marine Macroalgae Species of the Mediterranean Sea. Foods 2023, 12, 1310. [Google Scholar] [CrossRef]
- Falace, A.; Kaleb, S.; De La Fuente, G.; Asnaghi, V.; Chiantore, M. Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS ONE 2018, 13, e0193011. [Google Scholar] [CrossRef]
- De La Fuente, G.; Chiantore, M.; Asnaghi, V.; Kaleb, S.; Falace, A. First ex situ outplanting of the habitat-forming seaweed Cystoseira amentacea var. stricta from a restoration perspective. PeerJ 2019, 7, e7290. [Google Scholar] [CrossRef] [PubMed]
- Clausing, R.J.; De La Fuente, G.; Falace, A.; Chiantore, M. Accounting for environmental stress in restoration of intertidal foundation species. J. Appl. Ecol. 2023, 60, 305–318. [Google Scholar] [CrossRef]
- Celis-Plã¡, P.S.M.; Bouzon, Z.L.; Hall-Spencer, J.M.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Van Alstyne, K.; McCarthy, J.J.; Hustead, C.L.; Kearns, L.J. Phlorotannin allocation among tissues of northeastern pacific kelps and rockweeds. J. Phycol. 1999, 35, 483–492. [Google Scholar] [CrossRef]
- Rosa, G.P.; Peixoto, A.F.; Barreto, M.C.; Seca, A.M.L.; Pinto, D.C.G.A. Bio-Guided Optimization of Cystoseira abiesmarina Cosmeceuticals Extraction by Advanced Technologies. Mar. Drugs 2023, 21, 35. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Mekinić, I.G.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Kootstra, A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol. Biol. 1994, 26, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Carotenoids and Flavonoids Contribute to Nutritional Protection against Skin Damage from Sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Vizetto-Duarte, C.; Custódio, L.; Acosta, G.; Lago, J.H.G.; Morais, T.R.; Bruno de Sousa, C.; Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Lima, R.T.; et al. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamaricifolia as a source for antioxidant and anti-hepatocarcinoma compounds. PeerJ 2016, 4, e1704. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Vasincu, A.; Luca, S.V.; Neophytou, C.; Wolfram, E.; Opitz, S.E.; Sava, D.; Bucur, L.; Cioroiu, B.I.; Miron, A.; et al. Unravelling the potential of seaweeds from the Black Sea coast of Romania as bioactive compounds sources. Part I: Cystoseira barbata (Stackhouse) C. Agardh. Food Chem. Toxicol. 2019, 134, 110820. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxid. Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Junior, J.E.G.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, 15903. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.R.; Grether-Beck, S.; Krutmann, J.; Correia, P.; Gonçalves, J.E.; Sant’anna, B.; Kerob, D. Efficacy of a topical serum containing L-ascorbic acid, neohesperidin, pycnogenol, tocopherol, and hyaluronic acid in relation to skin aging signs. J. Cosmet. Dermatol. 2022, 21, 4462–4469. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The Inhibitory Effects of Eckol and Dieckol from Ecklonia stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown algaEcklonia stolonifera. Arch. Pharmacal. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef]
- Zhao, H.-C.; Xiao, T.; Chen, Y.-J. Ultraviolet-Induced Skin Inflammation. Int. J. Dermatol. Venereol. 2021, 4, 229–235. [Google Scholar] [CrossRef]
- Hol, J.; Wilhelmsen, L.; Haraldsen, G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J. Leukoc. Biol. 2010, 87, 501–508. [Google Scholar] [CrossRef] [PubMed]
- de los Reyes, C.; Ortega, M.J.; Zbakh, H.; Motilva, V.; Zubía, E. Cystoseira usneoides: A Brown Alga Rich in Antioxidant and Anti-inflammatory Meroditerpenoids. J. Nat. Prod. 2016, 79, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.; Mesguiche, V.; Piovetti, L.; Prost, M.; Peiffer, G. Meroditerpenes from the brown alga Cystoseira amentacea var. stricta collected off the French mediterranean coast. Phytochemistry 1996, 41, 1367–1371. [Google Scholar] [CrossRef]
- Mesguiche, V.; Valls, R.; Piovetti, L.; Banaigs, B. Meroditerpenes from Cystoseira amentacea var. stricta collected off the French Mediterranean coasts. Phytochemistry 1997, 45, 1489–1494. [Google Scholar] [CrossRef]
- Rahman, M.; Biswas, S.; Islam, K.J.; Paul, A.S.; Mahato, S.K.; Ali, A.; Halim, M.A. Antiviral phytochemicals as potent inhibitors against NS3 protease of dengue virus. Comput. Biol. Med. 2021, 134, 104492. [Google Scholar] [CrossRef]
- Laird, D.W.; van Altena, I.A. Tetraprenyltoluquinols from the brown alga Cystophora fibrosa. Phytochemistry 2006, 67, 944–955. [Google Scholar] [CrossRef]
- Ainane, T.A.; Fatouma, A.A.; Ayoub, A.; Ayoub, R.; Ahmed, B.; Abdelmjid, A. Methoxycystoketal Quinone: Natural Compound from Bioactive Diethyl Ether Extract of Brown Seaweed Cystoseira tamariscifolia. Pharmacol. Online Arch. 2021, 2, 583–589. Available online: https://pharmacologyonline.silae.it/files/archives/2021/vol2/PhOL_2021_2_A066_AINANE.pdf (accessed on 9 May 2023).
- Biju, J.; Sulaiman, C.T.; Sateesh, G.; Reddy, V.R.K. Total phenolics and flavonoids in selected medicinal plants in Kerala. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–408. [Google Scholar]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Steinbrink, D. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981, 113, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Bralley, E.; Greenspan, P.; Hargrove, J.L.; Hartle, D.K. Inhibition of Hyaluronidase Activity by Vitis rotundifolia. (Muscadine) Berry Seeds and Skins. Pharm. Biol. 2007, 45, 667–673. [Google Scholar] [CrossRef]
- Cannella, V.; Altomare, R.; Leonardi, V.; Russotto, L.; Di Bella, S.; Mira, F.; Guercio, A. In Vitro Biocompatibility Evaluation of Nine Dermal Fillers on L929 Cell Line. BioMed Res. Int. 2020, 2020, 8676343. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, T.O.; Popov, A.L.; Kolesnik, I.V.; Kolmanovich, D.D.; Baranchikov, A.E.; Shcherbakov, A.B.; Ivanov, V.K. Amorphous and crystalline cerium(iv) phosphates: Biocompatible ROS-scavenging sunscreens. J. Mater. Chem. B 2022, 10, 1775–1785. [Google Scholar] [CrossRef]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef]
- Pavelkova, R.; Matouskova, P.; Hoova, J.; Porizka, J.; Marova, I. Preparation and characterisation of organic UV filters based on combined PHB/liposomes with natural phenolic compounds. J. Biotechnol. 2020, 324, 100021. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Mirata, S.; Sionkowska, A.; Vicini, S.; Alloisio, M.; Castellano, M. Effect of Crosslinking Type on the Physical-Chemical Properties and Biocompatibility of Chitosan-Based Electrospun Membranes. Polymers 2021, 13, 831. [Google Scholar] [CrossRef]
- Dodero, A.; Donati, I.; Scarfì, S.; Mirata, S.; Alberti, S.; Lova, P.; Comoretto, D.; Alloisio, M.; Vicini, S.; Castellano, M. Effect of sodium alginate molecular structure on electrospun membrane cell adhesion. Mater. Sci. Eng. C 2021, 124, 112067. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth factor-β/Smad signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 162, 633–640. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z.; et al. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Vergani, L.; Ragazzoni, M.; Delpiano, L.; Grasselli, E.; Voci, A.; Giovine, M.; Scarfì, S. Different reactivity of primary fibroblasts and endothelial cells towards crystalline silica: A surface radical matter. Toxicology 2016, 361–362, 12–23. [Google Scholar] [CrossRef] [PubMed]


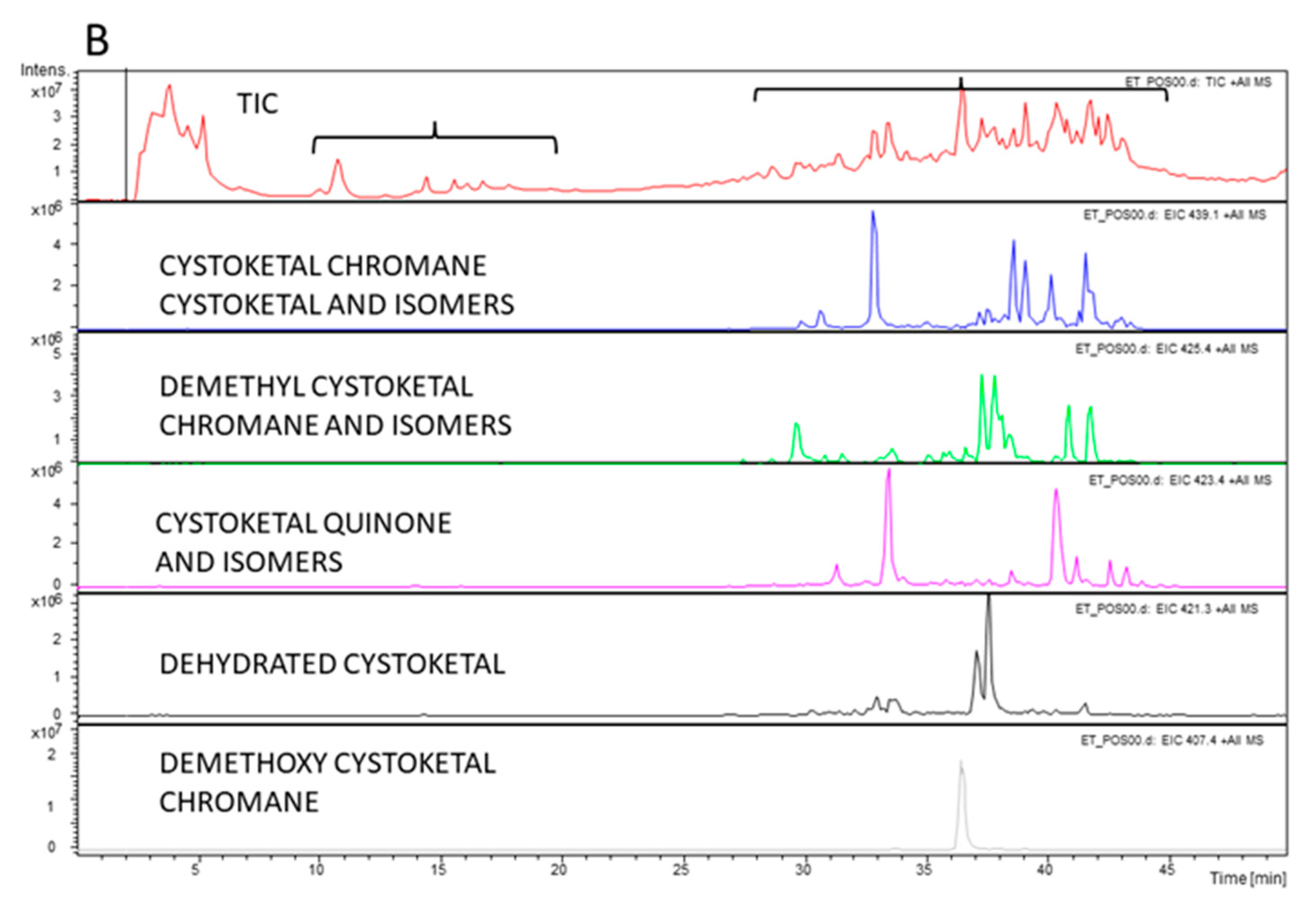

| Extract | TPC (μg/mg extr) | TFC (μg/mg extr) |
|---|---|---|
| EtOH Apex | 48.32 ± 0.54 | 5.60 ± 0.66 |
| EtOH Thallus | 18.47 ± 3.49 | 2.28 ± 0.27 |
| DMSO Apex | 39.40 ± 1.94 | 2.433 ± 0.35 |
| DMSO Thallus | 55.52 ± 6.08 | 4.45 ± 0.19 |
| Molecular Structure | Name | References |
|---|---|---|
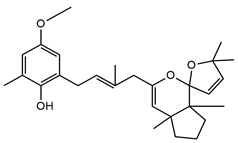 | Cystoketal | [56,57] |
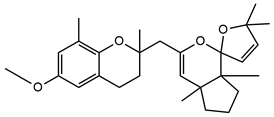 | Cystoketal Chromane | |
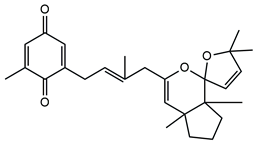 | Cystoketal quinone | [58] |
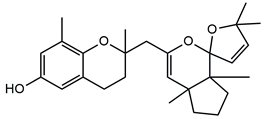 | Demethyl cystoketal chromane | |
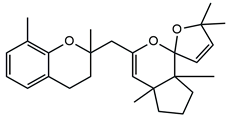 | Demethoxy cystoketal chromane | [4,36] |
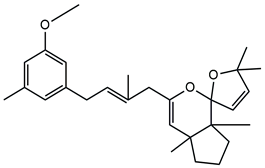 | Dehydrated cystoketal |
| Identified Compounds | Abundance in Apices | Abundance in Thalli |
|---|---|---|
| Cystoketal, cystoketal chromane and isomers | ++++ | + |
| Demethyl cystoketal chromane, and isomers | + | +++ |
| Cystoketal quinone, and isomers | + | +++ |
| Dehydrated cystoketal | ++++ | + |
| Demethoxy cystoketal chromane | + | ++ |
| GENE | GenBank (a.n.) | Forward | Reverse | Size (bp) |
|---|---|---|---|---|
| Human: | ||||
| IL-1β | NM_008361.4 | gCAgCACATCAACAAgAg | CAgCAggTTATCATCATCAT C | 184 |
| IL-6 | NM_031168.2 | ACCTgTCTATACCACTTC | gCATCATCgTTgTTCATA | 117 |
| IL-8 | NM_000584.4 | AATTCATTCTCTgTggTATC | CCAGGAATCTTgTATTgC | 127 |
| HPRT-1 | NM_000194.3 | ggTCAggCAgTATAATCCAAAg | TTCATTATAgTCAAgggCATATCC | 144 |
| Murine: | ||||
| CXCL5 | NM_009141.3 | TgCTTAACCgTAACTCCAA | ATCCAgACAgACCTCCTT | 129 |
| IL-1b | NM_008361.4 | gCAgCACATCAACAAgAg | CAgCAggTTATCATCATCATC | 184 |
| IL-6 | NM_031168.2 | ACCTgTCTATACCACTTC | gCATCATCgTTgTTCATA | 117 |
| GAPDH | NM_001289726.1 | TCTCCCTCACAATTTCCATCCCAg | gggTgCAGCgAACTT TATTgATgg | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirata, S.; Asnaghi, V.; Chiantore, M.; Salis, A.; Benvenuti, M.; Damonte, G.; Scarfì, S. Photoprotective and Anti-Aging Properties of the Apical Frond Extracts from the Mediterranean Seaweed Ericaria amentacea. Mar. Drugs 2023, 21, 306. https://doi.org/10.3390/md21050306
Mirata S, Asnaghi V, Chiantore M, Salis A, Benvenuti M, Damonte G, Scarfì S. Photoprotective and Anti-Aging Properties of the Apical Frond Extracts from the Mediterranean Seaweed Ericaria amentacea. Marine Drugs. 2023; 21(5):306. https://doi.org/10.3390/md21050306
Chicago/Turabian StyleMirata, Serena, Valentina Asnaghi, Mariachiara Chiantore, Annalisa Salis, Mirko Benvenuti, Gianluca Damonte, and Sonia Scarfì. 2023. "Photoprotective and Anti-Aging Properties of the Apical Frond Extracts from the Mediterranean Seaweed Ericaria amentacea" Marine Drugs 21, no. 5: 306. https://doi.org/10.3390/md21050306
APA StyleMirata, S., Asnaghi, V., Chiantore, M., Salis, A., Benvenuti, M., Damonte, G., & Scarfì, S. (2023). Photoprotective and Anti-Aging Properties of the Apical Frond Extracts from the Mediterranean Seaweed Ericaria amentacea. Marine Drugs, 21(5), 306. https://doi.org/10.3390/md21050306







