Emerging Technologies to Extract Fucoxanthin from Undaria pinnatifida: Microwave vs. Ultrasound Assisted Extractions
Abstract
1. Introduction
2. Results and Discussion
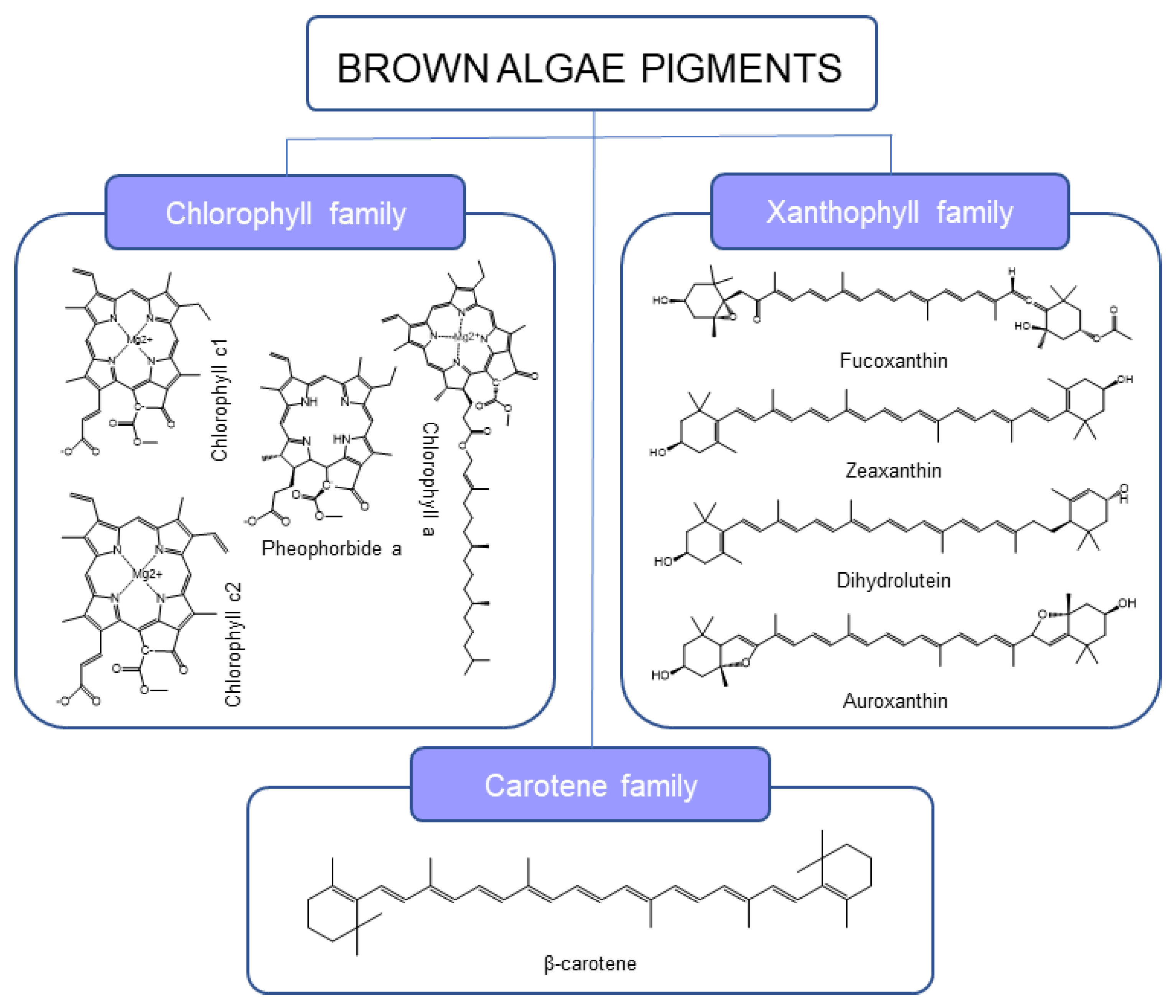
2.1. Pigment Identification in Moderate Conditions
2.2. SAE as Reference Method of Extraction
- Regarding the extraction of Fx, the content slightly differed between the two tested extractions. The 2 h extraction obtained 3.68 mg Fx/g A dw and 21.90 mg Fx/g E dw, whereas the 4 h extraction obtained higher values of 4.58 mg Fx/g A dw and 30.80 mg Fx/g E dw. These results are in agreement with previously published data regarding Fx thermal resistance [39]. Previous studies with SAE obtained much lower yields with 0.05 mg/g dw from U. pinnatifida using EtOH as a solvent for 12 h [18]. Other brown algae obtained better results with different solvents, as seen in Table 1 and Figure 2. When using ethyl acetate at 80 °C, Feldmannia mitchelliae obtained 5.50 mg/g dw in 16 h [19], and Sargassum swartzii C. Agardh obtained 0.17 mg/g dw in 6 h [34]. Using n-hexane, 0.45 mg/g dw of Fx was obtained from Saccharina japonica at 40 °C for 16 h [33].
- For chlorophyll a, the difference was even smaller, obtaining 1.00 mg Chl/g A dw and 45.60 mg chl/g E dw in 2 h and 1.26 mg Chl/g A dw and 45.89 mg Chl/g E dw in 4 h. The difference obtained is probably not relevant to justify doubling the time and the energy spent on the extraction, especially when scaling up this process.
- For β-carotene, the obtained results were 0.24 mg β-car/g A dw and 5.22 mg β-car/g E at the 2 h and 0.22 mg β-car/g A dw and 4.31 mg β-car/g E dw at 4 h extraction. These last results are the only ones where the 4-h extraction performed slightly worse, suggesting that β-carotene might be the least thermos-resistant pigment of the three in the study, which resulted in its degradation, leading to lower yields.
2.3. Optimization by MAE and UAE
2.3.1. Variable Selection for the Experimental Design
2.3.2. Experimental Data for All Response Criteria from CCCD and Theoretical RSM Analysis
2.3.3. Response Patterns and Optimal Conditions
- For the extraction yield ( ), the optimal variable conditions were 10.27 min, 12.00 bar and 20% of EtOH to obtain a response of 60.25%.
- For the Fx concentration ( ), the optimal variable conditions were 3.00 min, 2.00 bar and 100% of EtOH to obtain a response of 10.01 mg Fx/g A.
- For the Fx concentration ratio in the extract ( ), the optimal variable conditions were 3.00 min, 2.00 bar and 100% of EtOH to obtain a response of 58.83 mg Fx/g E.
- For the extraction yield ( ), the optimal variable conditions were 21.63 min, 388.68 W and 35% of EtOH to obtain a response of 54.13%.
- For the Fx concentration ( ), the optimal variable conditions were 30.46 min, 374.46 W and 76.55% of EtOH to obtain a response of 20.91 mg Fx/g A.
- For the Fx concentration ratio in the extract ( ), the optimal variable conditions were 35.16 min, 300.00 W and 100% of EtOH to obtain a response of 124.39 mg Fx/g E.
3. Materials and Methods
3.1. Samples Collection
3.2. Conventional Heat-Assisted Extraction for Pigment Identification
3.3. Soxhlet-Assisted Extraction as Reference Method
3.4. Chemical Analysis through HPLC-DAD
3.5. Extraction Yield
3.6. Optimization of the Extraction Process from Undaria Pinnatifida
3.6.1. Microwave-Assisted Extraction (MAE)
3.6.2. Ultrasound-Assisted Extraction (UAE)
3.6.3. Experimental Design, Analysis Model and Statistical Evaluation
Mathematical Model
Procedure for Optimization of Variables
Numerical Methods, Statistical Analysis, and Graphic Illustrations
- Determination of the coefficients: the parametric estimates were obtained by minimizing the sum of the quadratic differences between the obtained and predicted values, using the least squares method (quasi-Newton) by the “Solver” macro in Microsoft Excel, which allowed the rapid analysis of a hypothesis and its consequences.
- Significance of the coefficients: to obtain significance values, the confidence intervals of the parameters were calculated using “SolverAid”. The model was simplified by discarding the non-statistically significant terms for the p-value (p > 0.05).
- Consistency of the model: it was carried out through Fisher’s F test (α = 0.05) to determine if the constructed models were adequate to describe the data obtained.
- Other statistical evaluation criteria: to re-verify the uniformity of the model, the following criteria were applied: “SolverStat” to evaluate the prediction uncertainties of parameters and models, as well as the R² value, interpreted as the proportion of versatility of each dependent variable explained by the model.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedro, B.; Guedes, L.; André, R.; Gaspar, H.; Vaz, P.; Ascensão, L.; Melo, R.; Luísa Serralheiro, M. Undaria pinnatifida (U. pinnatifida) bioactivity: Antioxidant, gastro-intestinal motility, cholesterol biosynthesis and liver cell lines proteome. J. Funct. Foods 2021, 83, 104567. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.J.; Jeon, Y.J.; Ryu, B.M. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Gomez, L.; Tiwari, B.; Garcia-Vaquero, M. Emerging Extraction Techniques: Microwave-Assisted Extraction; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128179437. [Google Scholar]
- Queffelec, J.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Microwave hydrothermal processing of Undaria pinnatifida for bioactive peptides. Bioresour. Technol. 2021, 342, 125882. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.J.; Kim, Y.E.; Kim, J.E.; Park, J.; Kim, Y.H.; Song, K.M.; Lee, N.H. Production of Undaria pinnatifida sporophyll extract using pilot-scale ultrasound-assisted extraction: Extract characteristics and antioxidant and anti-inflammatory activities. Algal Res. 2020, 51, 102039. [Google Scholar] [CrossRef]
- Seaweed, C.E. Edible Seaweed and Microalgae—Regulatory Status in France and Europe—2019 Update; Centre d’Etude et de Valorisation des Algues: Saint Brieuc, France, 2020; pp. 1–15. [Google Scholar]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Eun, J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, A.S.; Sofian-Seng, N.S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Xu, D.; Chow, J.; Weber, C.C.; Packer, M.A.; Baroutian, S.; Shahbaz, K. Evaluation of deep eutectic solvents for the extraction of fucoxanthin from the alga Tisochrysis lutea—COSMO-RS screening and experimental validation. J. Environ. Chem. Eng. 2022, 10, 108370. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of natural deep eutectic solvents for extraction of hydrophilic and lipophilic compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N.P. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; Mackenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and Its Metabolites in Edible Brown Algae Cultivated in Deep Seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef]
- Chen, C.R.; Lin, D.M.; Chang, C.M.J.; Chou, H.N.; Wu, J.J. Supercritical carbon dioxide anti-solvent crystallization of fucoxanthin chromatographically purified from Hincksia mitchellae P.C. Silva. J. Supercrit. Fluids 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC—Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Extraction|Microwave-Assisted Extraction, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780081019832. [Google Scholar]
- Nuchdang, S.; Phruetthinan, N.; Paleeleam, P.; Domrongpokkaphan, V.; Chuetor, S.; Chirathivat, P.; Phalakornkule, C. Soxhlet, microwave-assisted, and room temperature liquid extraction of oil and bioactive compounds from palm kernel cake using isopropanol as solvent. Ind. Crops Prod. 2022, 176, 114379. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Ashokkumar, M. Sandra Kentish and Muthupandian Ashokkumar The Physical and Chemical Effects of Ultrasound. In Ultrasound Technologies for Food Bioprocessing; Feng, H., Barbosa-Cánovas, G., Weiss, J., Eds.; Springer Science & Business Media: New York, NY, USA, 2011; pp. 1–12. ISBN 9788578110796. [Google Scholar]
- Leong, T.; Ashokkumar, M.; Kentish, S. The Growth of Bubbles in an Acoustic Field by Rectified Diffusion. In Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016; ISBN 9789812872784. [Google Scholar]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC—Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Ran, X.G.; Wang, L.Y. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by-products. J. Sci. Food Agric. 2014, 94, 585–590. [Google Scholar] [CrossRef]
- Shi, Y.; Sheng, J.; Yang, F.; Hu, Q. Purification and identification of polysaccharide derived from Chlorella pyrenoidosa. Food Chem. 2007, 103, 101–105. [Google Scholar] [CrossRef]
- Mise, T.; Ueda, M.; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011, 3, 73–76. [Google Scholar]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Sadhasivam, G.; Kandasamy, S.; Poomani, K.; Thayumanavan, P. Purification of fucoxanthin from Sargassum wightii Greville and understanding the inhibition of angiotensin 1-converting enzyme: An in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 148, 696–703. [Google Scholar] [CrossRef]
- Raguraman, V.; MubarakAli, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: Purification, characterization and biomedical application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Lourenço-Lopes, C.; Silva, A.; Pereira, A.G.; Fraga-Corral, M.; Zhao, C.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Pigment Composition of Nine Brown Algae from the Iberian Northwestern Coastline: Influence of the Extraction Solvent. Mar. Drugs 2022, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Sonambekar, A.A.; Daksh, S.; Tomar, L. A rare presentation of methanol toxicity. Ann. Indian Acad. Neurol. 2013, 16, 249. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Soria-Lopez, A.; Nuñes-Estevez, B.; Barral-Martinez, M.; Silva, A.; Li, N.; Liu, C.; Simal-Gandara, J.; Prieto, M.A. Fucoxanthin’s Optimization from Undaria pinnatifida Using Conventional Heat Extraction, Bioactivity Assays and In Silico Studies. Antioxidants 2022, 11, 1296. [Google Scholar] [CrossRef]
- Irvani, N.; Hajiaghaee, R.; Zarekarizi, A.R. A review on biosynthesis, health benefits and extraction methods of fucoxanthin, particular marine carotenoids in algae. J. Med. Plants 2018, 17, 6–30. [Google Scholar]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.W.S.; Tan, K.M.; Chew, L.Y.; Kong, K.W.; Yan, S.W. Application of Two-Level Full Factorial Design for the Extraction of Fucoxanthin and Antioxidant Activities from Sargassum siliquosum and Sargassum polycystum. J. Aquat. Food Prod. Technol. 2018, 27, 446–463. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2017, 29, 1027–1036. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, E.A.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, Y.; Wang, Q.; Gao, X.; Gong, Q. Effects of temperature and salinity on the growth and biochemical composition of the brown alga Sargassum fusiforme (Fucales, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3061–3068. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale Preparation of Biofunctional Fucoxanthin from Waste Parts of Brown Sea Algae Laminaria japonica. Food Sci. Technol. Res. 2008, 14, 573–582. [Google Scholar] [CrossRef]
- Verma, P.; Kumar, M.; Mishra, G.; Sahoo, D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017, 226, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Shikov, A.N. Biochemical composition, antiradical potential and human health risk of the Arctic edible brown seaweed Fucus spiralis L. J. Appl. Phycol. 2023, 35, 365–380. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Garcia-Perez, P.; Carreira-Casais, A.; Silva, A.; Simal-Gandara, J.; Prieto, M.A. A HPLC-DAD method for identifying and estimating the content of fucoxanthin, β-carotene and chlorophyll a in brown algal extracts. Food Chem. Adv. 2022, 1, 100095. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. Fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef]
- Pereira, A.G.; Lourenç, C. Optimized ultrasound-assisted extraction of antioxidants from Himanthalia elongata by Response Surface Methodology. Chem. Proc. 2021, 43, 1–4. [Google Scholar]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of ergosterol from Agaricus bisporus L. by-products using response surface methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]



| Technique | Solvent | Conditions | Method | Fx | Ref. | |
|---|---|---|---|---|---|---|
| Undaria pinnatifida | ||||||
| HAE | EtOH | RT, 1 h | HPLC-DAD | 0.70 | [16] | |
| 0.07 * | ||||||
| MeOH | 2.08 | [31] | ||||
| 4.96 * | ||||||
| RT, 96 h | HPLC-DAD, 1H NMR and 13C NMR | 2.67 | [17] | |||
| SAE | EtOH | 78 °C, 12 h | HPLC-UV | 0.05 | [18] | |
| MAE | EtOH | 60 °C, 10 min | HPLC, 1H NMR and LC-MS | 0.73 | [23] | |
| Hp, AcO, W | 50 °C, 10 min | LC-ESI-MS, HPLC, 1H-NMR | 0.90 | |||
| UAE | MeOH | 25 °C, 3 h | HPLC | 0.03 | [6] | |
| Other Species | ||||||
| HAE | Cladosiphon okamuranus | MeOH | RT, 1 h | HPLC-DAD | 0.27 | [31] |
| Dictyota dicotoma | RT, 24 h | 6.42 | [14] | |||
| Fucus distichus | RT, 12 h | 0.90 | [32] | |||
| Saccharina japonica | RT, 15 min | 0.07 | [33] | |||
| Saccharina sculpera | RT, 12 h | 0.70 | [32] | |||
| Sargassum horneri | RT, 12 h | 3.70 | [32] | |||
| Sargassum thunbergii | RT, 12 h | 1.80 | [32] | |||
| SAE | Feldmannia mitchelliae | EA | 80 °C, 16 h | HPLC | 5.50 | [19] |
| Saccharina japonica | n-Hx | 40 °C, 16 h | HPLC-DAD | 0.45 | [33] | |
| Sargassum swartzii C. Agardh | EA | 80 °C, 6 h | FT-IR, 1H-NMR, 13C-NMR | 0.17 | [34] | |
| MAE | Laminaria japonica | Hp, AcO, W | 50 °C, 10 min | LC-ESI-MS, HPLC, 1H-NMR | 0.04 | [23] |
| Sargassum fusiforme | 0.02 | [23] | ||||
| UAE | Padina tetrastromatica | EtOH | 50 Hz, 30 min | HPLC-DAD | 0.75 | [35] |
| Coded Values | Natural Values | Experimental Responses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAE | UAE | MAE | UAE | ||||||||||||
| X1 | X2 | X3 | X1: t | X2: P | X3: S | X1: t | X2: Pw | X3: S | Y1 | Y2 | Y2/Y1 | Y1 | Y2 | Y2/Y1 | |
| min | Bar | % | min | W | % | % | (mg Fx/g A) | (mg Fx/g E) | % | (mg Fx/g A) | (mg Fx/g E) | ||||
| 1 | −1 | −1 | −1 | 7.1 | 4 | 36.2 | 15.1 | 181.1 | 48.2 | 37.17 | 0.05 | 0.13 | 9.55 | 0.80 | 8.40 |
| 2 | −1 | −1 | 1 | 7.1 | 4 | 83.8 | 15.1 | 181.1 | 86.8 | 35.77 | 8.83 | 24.70 | 8.93 | 3.64 | 40.73 |
| 3 | −1 | 1 | −1 | 7.1 | 10 | 36.2 | 15.1 | 418.9 | 48.2 | 50.63 | 0.01 | 0.02 | 45.79 | 7.75 | 16.93 |
| 4 | −1 | 1 | 1 | 7.1 | 10 | 83.8 | 15.1 | 418.9 | 86.8 | 43.68 | 2.18 | 5.00 | 32.88 | 15.66 | 47.61 |
| 5 | 1 | −1 | −1 | 18.9 | 4 | 36.2 | 44.9 | 181.1 | 48.2 | 50.42 | 0.03 | 0.05 | 9.14 | 0.91 | 9.94 |
| 6 | 1 | −1 | 1 | 18.9 | 4 | 83.8 | 44.9 | 181.1 | 86.8 | 39.48 | 6.74 | 17.08 | 13.29 | 5.39 | 40.54 |
| 7 | 1 | 1 | −1 | 18.9 | 10 | 36.2 | 44.9 | 418.9 | 48.2 | 51.22 | 0.00 | 0.00 | 30.24 | 4.16 | 13.76 |
| 8 | 1 | 1 | 1 | 18.9 | 10 | 83.8 | 44.9 | 418.9 | 86.8 | 41.62 | 0.70 | 1.68 | 23.05 | 12.53 | 54.36 |
| 9 | 1.68 | 0 | 0 | 23 | 7 | 60 | 5.0 | 300.0 | 67.5 | 49.07 | 0.89 | 1.82 | 20.05 | 7.02 | 35.03 |
| 10 | −1.68 | 0 | 0 | 3 | 7 | 60 | 55.0 | 300.0 | 67.5 | 24.75 | 5.40 | 21.81 | 34.05 | 15.94 | 46.80 |
| 11 | 0 | −1.68 | 0 | 13 | 2 | 60 | 30.0 | 100.0 | 67.5 | 43.53 | 5.79 | 13.31 | 9.80 | 3.70 | 37.77 |
| 12 | 0 | 1.68 | 0 | 13 | 12 | 60 | 30.0 | 500.0 | 67.5 | 52.55 | 0.18 | 0.34 | 24.38 | 16.15 | 66.26 |
| 13 | 0 | 0 | −1.68 | 13 | 7 | 20 | 30.0 | 300.0 | 35.0 | 55.29 | 0.00 | 0.00 | 52.05 | 0.09 | 0.17 |
| 14 | 0 | 0 | 1.68 | 13 | 7 | 100 | 30.0 | 300.0 | 100.0 | 16.24 | 1.62 | 10.00 | 11.14 | 13.68 | 122.85 |
| 15 | −1.68 | −1.68 | −1.68 | 3 | 2 | 20 | 5.0 | 100.0 | 35.0 | 45.43 | 0.58 | 1.27 | 13.94 | 0.08 | 0.57 |
| 16 | −1.68 | −1.68 | 1.68 | 3 | 2 | 100 | 5.0 | 100.0 | 100.0 | 13.61 | 9.53 | 70.04 | 5.47 | 6.79 | 124.07 |
| 17 | −1.68 | 1.68 | −1.68 | 3 | 12 | 20 | 5.0 | 500.0 | 35.0 | 55.91 | 0.00 | 0.00 | 47.46 | 0.55 | 1.16 |
| 18 | −1.68 | 1.68 | 1.68 | 3 | 12 | 100 | 5.0 | 500.0 | 100.0 | 14.90 | 2.29 | 15.34 | 8.86 | 10.03 | 113.11 |
| 19 | 1.68 | −1.68 | −1.68 | 23 | 2 | 20 | 55.0 | 100.0 | 35.0 | 52.08 | 0.41 | 0.79 | 33.52 | 0.08 | 0.24 |
| 20 | 1.68 | −1.68 | 1.68 | 23 | 2 | 100 | 55.0 | 100.0 | 100.0 | 15.14 | 10.18 | 67.27 | 5.70 | 9.20 | 161.48 |
| 21 | 1.68 | 1.68 | −1.68 | 23 | 12 | 20 | 55.0 | 500.0 | 35.0 | 58.43 | 0.00 | 0.00 | 45.33 | 0.48 | 1.07 |
| 22 | 1.68 | 1.68 | 1.68 | 23 | 12 | 100 | 55.0 | 500.0 | 100.0 | 29.82 | 0.00 | 0.00 | 15.09 | 20.89 | 138.49 |
| 23 | 0 | 0 | 0 | 13 | 7 | 60 | 30.0 | 300.0 | 67.5 | 47.37 | 4.88 | 10.30 | 43.65 | 20.36 | 46.65 |
| 24 | 0 | 0 | 0 | 13 | 7 | 60 | 30.0 | 300.0 | 67.5 | 48.03 | 5.04 | 10.49 | 45.40 | 15.64 | 34.46 |
| 25 | 0 | 0 | 0 | 13 | 7 | 60 | 30.0 | 300.0 | 67.5 | 50.80 | 5.14 | 10.12 | 51.55 | 18.46 | 35.81 |
| 26 | 0 | 0 | 0 | 13 | 7 | 60 | 30.0 | 300.0 | 67.5 | 51.58 | 5.13 | 9.94 | 39.50 | 18.46 | 46.73 |
| 27 | 0 | 0 | 0 | 13 | 7 | 60 | 30.0 | 300.0 | 67.5 | 50.64 | 5.00 | 9.87 | 46.69 | 20.52 | 43.95 |
| 28 | 0 | 0 | 0 | 13 | 7 | 60 | 30.0 | 300.0 | 67.5 | 50.04 | 5.13 | 10.26 | 45.66 | 17.48 | 38.29 |
| MAE | UAE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y2/Y1 | Y1 | Y2 | Y2/Y1 | ||||||||
| % | (mg Fx/g A) | (mg Fx/g E) | % | (mg Fx/g A) | (mg Fx/g E) | ||||||||
| A: PARAMETRIC AND STATISTICAL ANALYSIS | |||||||||||||
| Intercept | b0 | 49.87 | ±1.68 | 4.26 | ±0.12 | 11.00 | ±0.50 | 45.41 | ±2.41 | 18.49 | ±1.33 | 39.12 | ±5.83 |
| Linear Effect | b1 | -- | −0.31 | ±0.06 | −2.09 | ±0.35 | −4.47 | ±3.28 | -- | -- | -- | ||
| b2 | 2.61 | ±0.81 | −1.38 | ±0.06 | −6.54 | ±0.35 | 15.24 | ±3.28 | 4.76 | ±1.81 | -- | ||
| b3 | -- | 2.79 | ±0.20 | 6.54 | ±0.35 | -- | 3.40 | ±0.54 | -- | ||||
| Quadratic Effect | b11 | −3.87 | ±1.31 | -- | -- | −6.49 | ±1.70 | −2.48 | ±0.94 | −5.36 | ±4.54 | ||
| b22 | -- | −0.69 | ±0.10 | -- | −10.01 | ±1.70 | −3.03 | ±0.94 | -- | ||||
| b33 | −4.27 | ±1.31 | −0.79 | ±0.10 | -- | −4.88 | ±1.70 | −4.10 | ±0.94 | 8.05 | ±4.54 | ||
| Cubic Effect | b111 | 1.08 | ±0.32 | -- | -- | 1.79 | ±1.29 | -- | -- | ||||
| b222 | -- | -- | -- | −3.86 | ±1.29 | −1.09 | ±0.71 | -- | |||||
| b333 | −3.73 | ±0.32 | −0.56 | ±0.08 | -- | −3.04 | ±0.39 | -- | 13.01 | ±1.11 | |||
| Interactive Linear Effect | b12 | -- | -- | -- | -1.03 | ±0.70 | -- | -- | |||||
| b13 | -- | -- | -- | -- | 0.55 | ±0.38 | 2.21 | ±2.00 | |||||
| b23 | -- | −0.77 | ±0.04 | −5.20 | ±0.25 | −1.61 | ±0.70 | 0.68 | ±0.38 | -- | |||
| b123 | 0.48 | ±0.36 | -- | -- | 0.70 | ±0.43 | -- | -- | |||||
| Interactive Quadratic Effect | b1122 | -- | -- | -- | −6.26 | ±4.73 | −4.91 | ±2.61 | -- | ||||
| b1133 | -- | -- | -- | -- | -- | -- | |||||||
| b2233 | -- | 0.36 | ±0.05 | 0.95 | ±0.12 | -- | -- | -- | |||||
| b112233 | 0.39 | ±0.21 | -- | -- | 3.85 | ±1.48 | 2.39 | ±0.82 | 0.95 | ±0.74 | |||
| Statistics (R²) | 0.9426 | 0.8752 | 0.8695 | 0.9395 | 0.9034 | 0.9225 | |||||||
| B: OPTIMAL VARIABLE CONDITIONS | |||||||||||||
| X1: t (min) | 10.27 | ±1.60 | 3.00 | ±0.87 | 3.00 | ±0.87 | 21.63 | ±2.33 | 30.46 | ±2.76 | 35.16 | ±2.96 | |
| X2: P (bar) or Pw (W) | 12.00 | ±1.73 | 2.00 | ±0.71 | 2.00 | ±0.71 | 388.68 | ±9.86 | 374.46 | ±9.68 | 300.00 | ±8.66 | |
| X3: S (%) | 20.00 | ±2.24 | 100.00 | ±5.00 | 100.00 | ±5.00 | 35.00 | ±2.96 | 76.55 | ±4.37 | 100.00 | ±5.00 | |
| Optimal Response | 60.25 | ±4.99 | 10.01 | ±0.77 | 58.83 | ±3.86 | 54.13 | ±4.57 | 20.91 | ±1.66 | 124.39 | ±2.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço-Lopes, C.; Carreira-Casais, A.; Carperna, M.; Barral-Martinez, M.; Chamorro, F.; Jiménez-López, C.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Emerging Technologies to Extract Fucoxanthin from Undaria pinnatifida: Microwave vs. Ultrasound Assisted Extractions. Mar. Drugs 2023, 21, 282. https://doi.org/10.3390/md21050282
Lourenço-Lopes C, Carreira-Casais A, Carperna M, Barral-Martinez M, Chamorro F, Jiménez-López C, Cassani L, Simal-Gandara J, Prieto MA. Emerging Technologies to Extract Fucoxanthin from Undaria pinnatifida: Microwave vs. Ultrasound Assisted Extractions. Marine Drugs. 2023; 21(5):282. https://doi.org/10.3390/md21050282
Chicago/Turabian StyleLourenço-Lopes, Catarina, Anxo Carreira-Casais, Maria Carperna, Marta Barral-Martinez, Franklin Chamorro, Cecilia Jiménez-López, Lucia Cassani, Jesus Simal-Gandara, and Miguel A. Prieto. 2023. "Emerging Technologies to Extract Fucoxanthin from Undaria pinnatifida: Microwave vs. Ultrasound Assisted Extractions" Marine Drugs 21, no. 5: 282. https://doi.org/10.3390/md21050282
APA StyleLourenço-Lopes, C., Carreira-Casais, A., Carperna, M., Barral-Martinez, M., Chamorro, F., Jiménez-López, C., Cassani, L., Simal-Gandara, J., & Prieto, M. A. (2023). Emerging Technologies to Extract Fucoxanthin from Undaria pinnatifida: Microwave vs. Ultrasound Assisted Extractions. Marine Drugs, 21(5), 282. https://doi.org/10.3390/md21050282













