A Distinct Saponin Profile Drives an Olfactory-Mediated Aggregation in the Aquacultivated Sea Cucumber Holothuria scabra
Abstract
:1. Introduction
2. Results
2.1. Spatial Distribution of Adults in Sea Pens
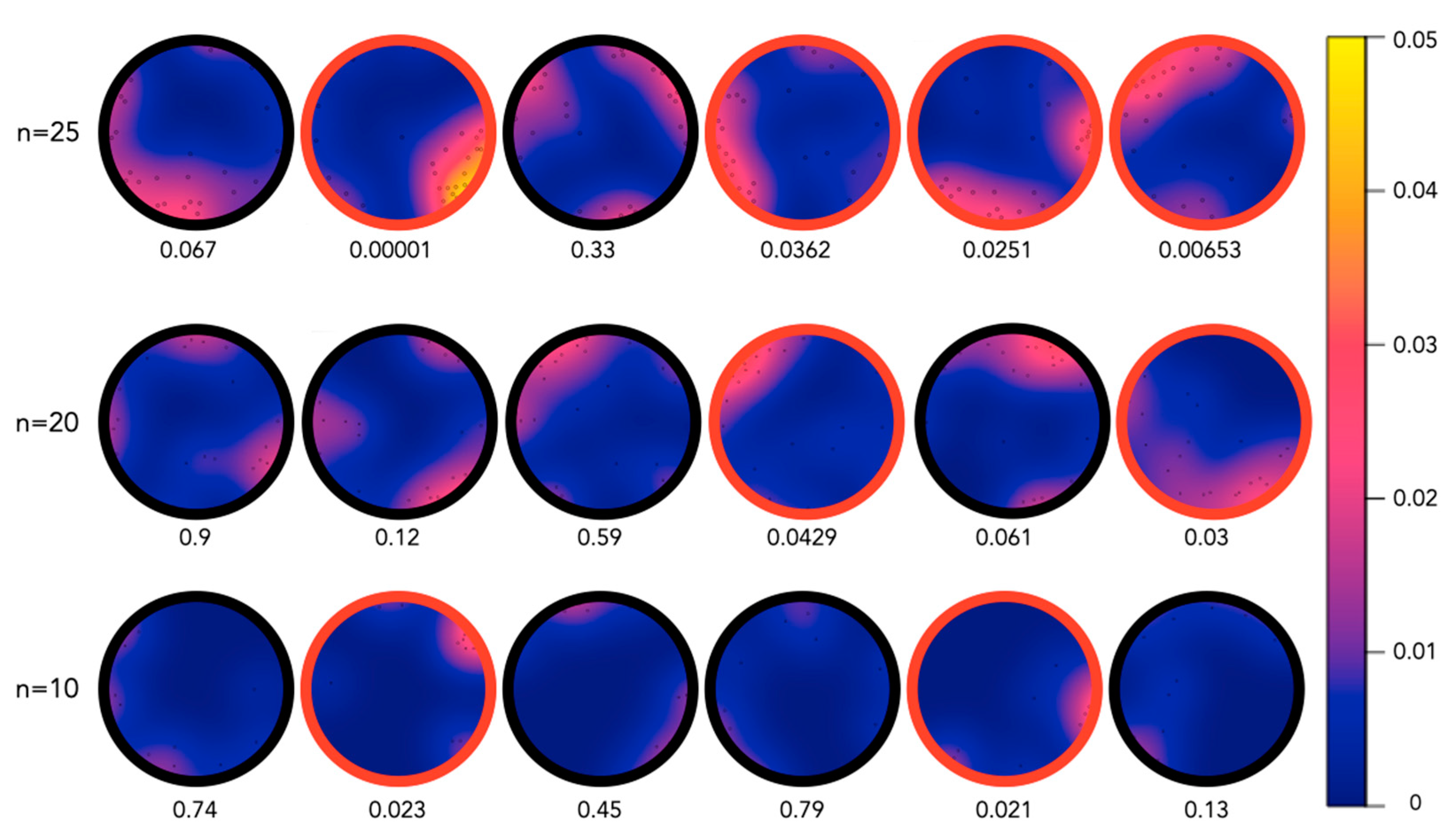
2.2. Aggregation of Juveniles in Circular Aquaria
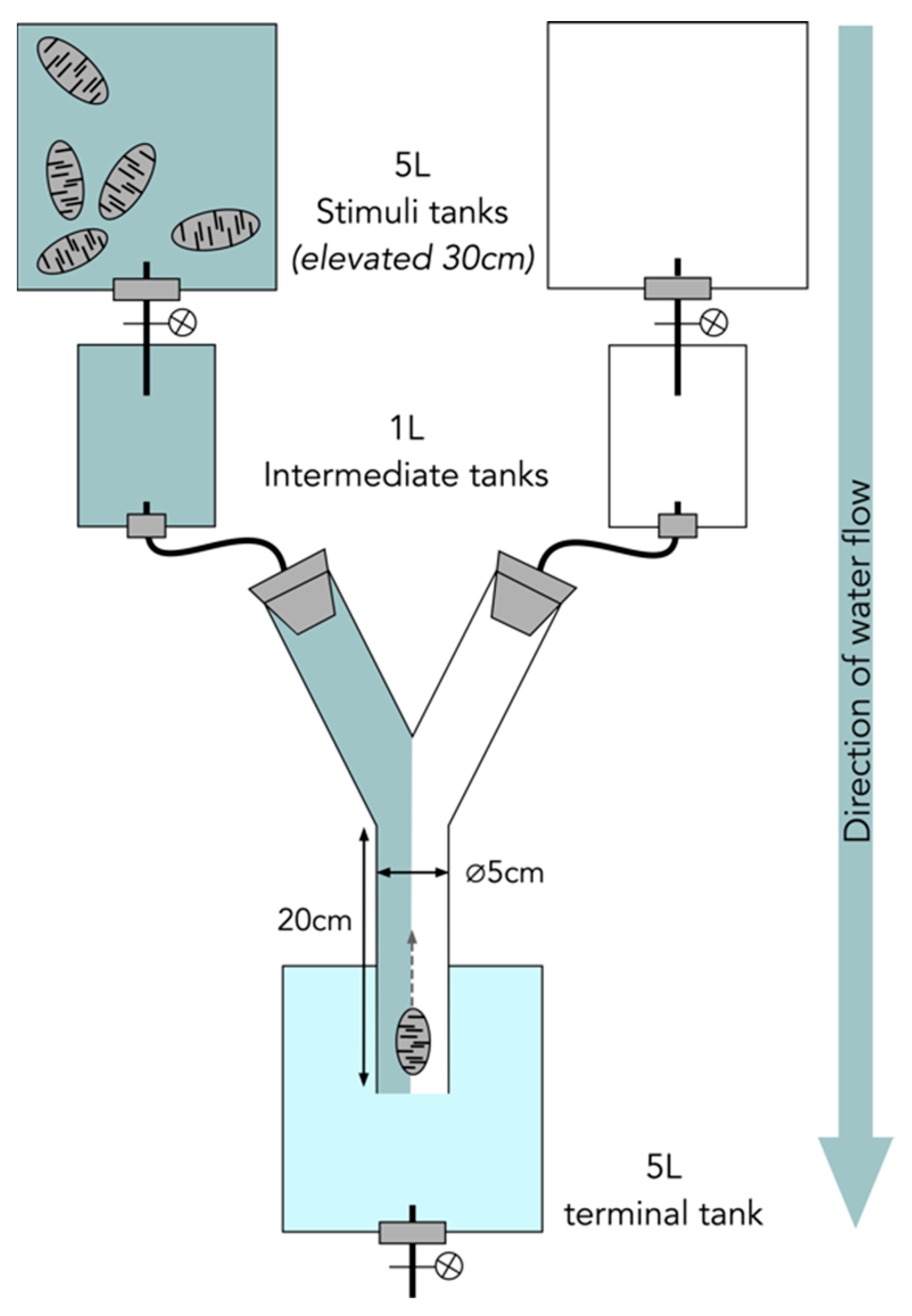
2.3. Olfactory Behavioral Assays
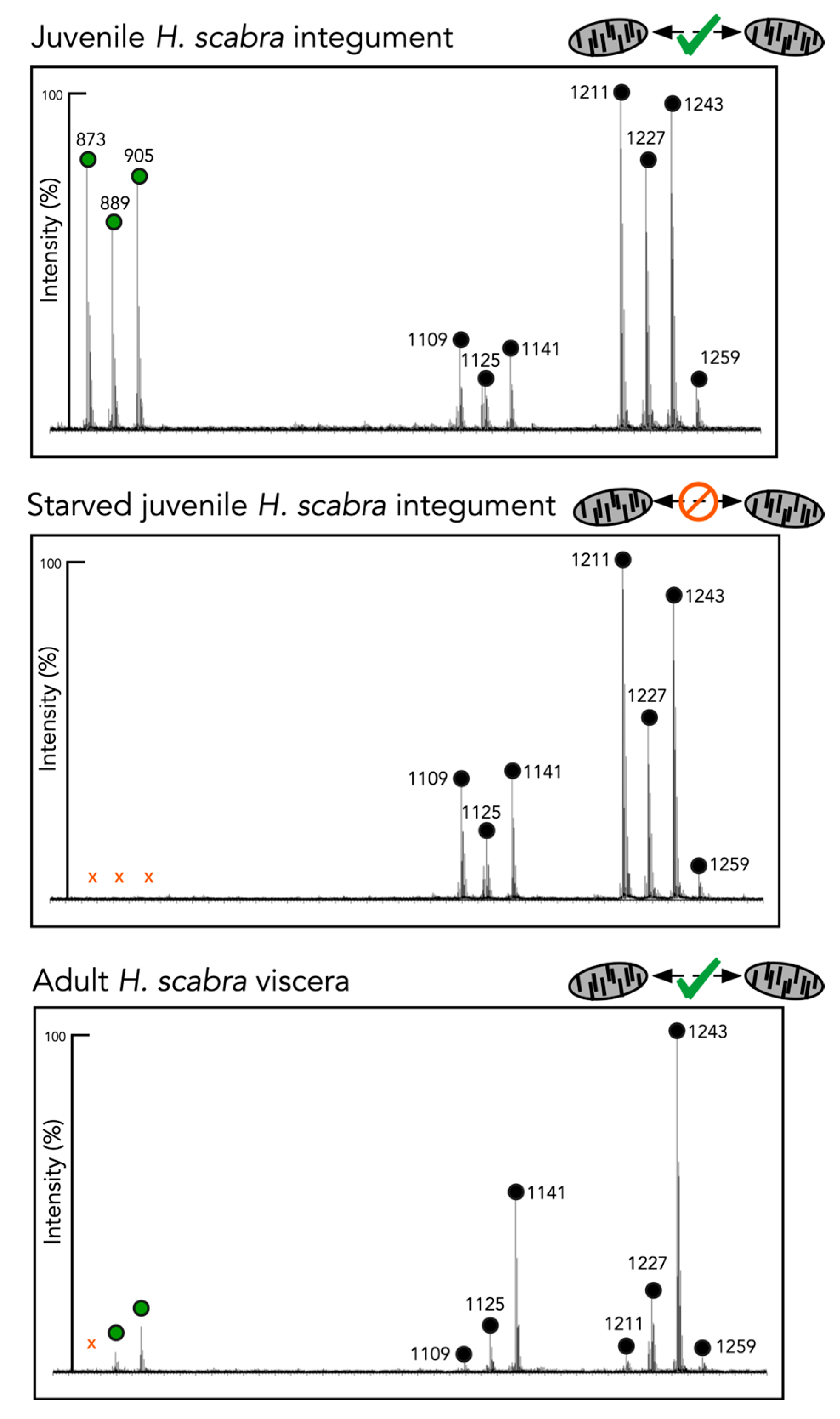
2.4. Chemical Analyses of Different Tissues and Extracts
3. Discussion
4. Materials and Methods
4.1. Organisms
4.2. Spatial Distribution of Individuals in Sea Pens
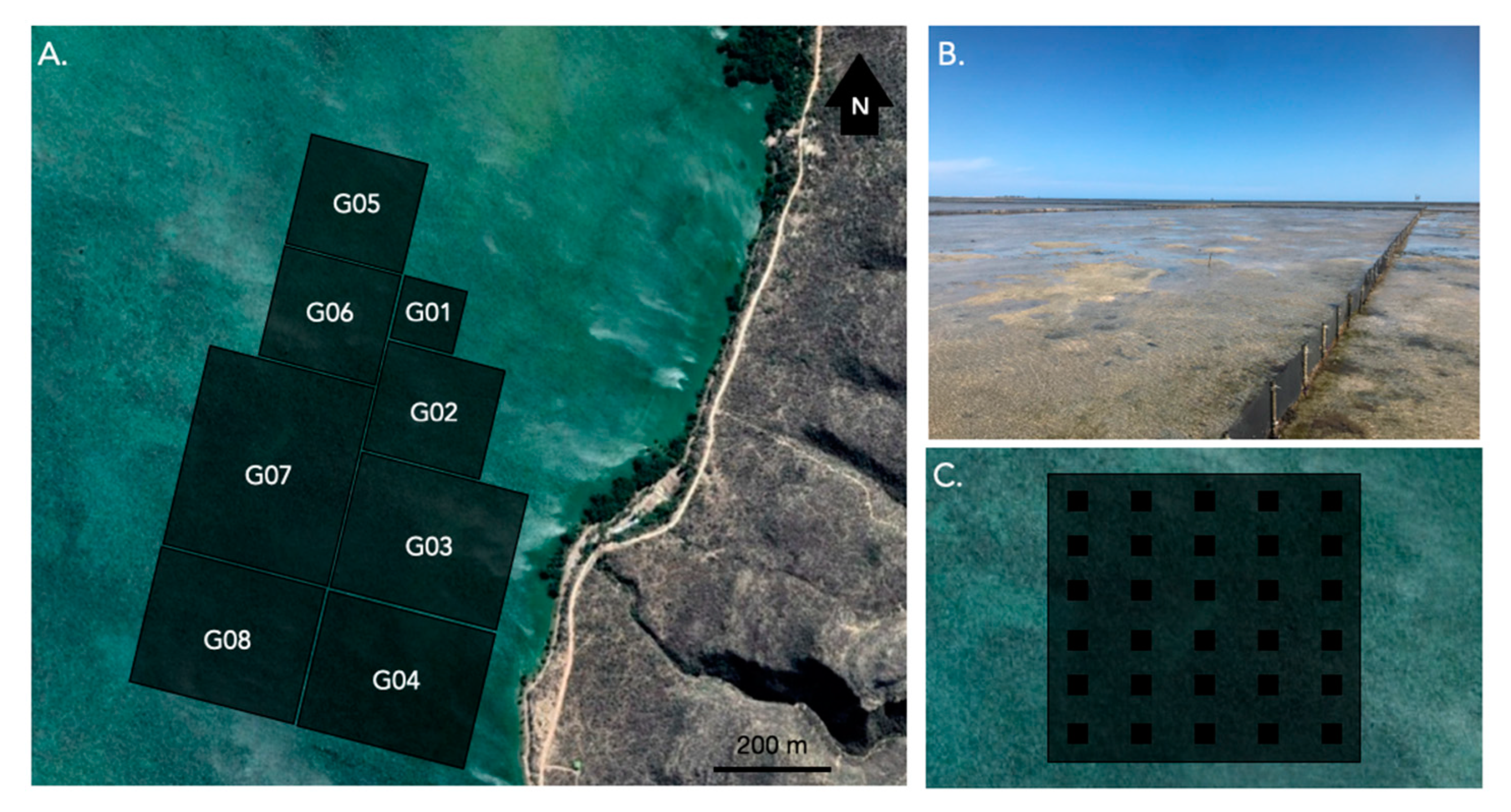
4.3. Aquaria Aggregation Experiments
4.4. Olfactory Behavioral Assay Experimental Setup
- H. scabra juvenile-conditioned water: a total of 5 juveniles of 15 g each were placed into 5 L of FFSW for 1 h.
- High-concentration H. scabra juvenile-conditioned water: a total of 15 juveniles were placed into 5 L of FFSW for 1 h.
- H. scabra adult-conditioned water: one adult of H. scabra (350 g) was placed into 5 L of FFSW for 1 h.
- Diluted H. scabra adult-conditioned water: One adult of H. scabra (350 g) was placed into 5 L of FFSW for 1 hour. 2.5 L of this conditioned water was then diluted twice.
- H. leucospilota adult-conditioned water: one adult of H. leucospilota (250 g) was placed into 5 L of FFSW for 1 h.
- Conditioned water of starved H. scabra juveniles: A total of 5 juveniles were placed into 5 L of FFSW without food for 1 week prior to the experiment, and oxygenation was maintained. Prior to experiments, 5 L of water was conditioned by the 5 starved individuals for 1 h.
- Denatured conditioned water of H. scabra juveniles: a total of 5 L of FFSW was conditioned for 1 hour with 5 juveniles, and the water was then boiled for 30 min before being used in experiments.
- Conditioned water of fecal matter of H. scabra juveniles: A total of 5 juveniles were placed into 5 L of FFSW overnight without food. The next day, the animals were removed, and the water changed whilst maintaining the fecal matter in the aquarium. The FFSW water was conditioned with the fecal matter for one hour before being used in experiments.
- Saponin extract of adult H. scabra viscera: Saponin extraction and purification were performed using the previously described method [60] on H. scabra viscera at UMons, Belgium. In brief, an overnight maceration of dry ground intestinal tissue in 70% methanol was conducted. The extract was then filtered and partitioned sequentially against hexane, dichloromethane, and chloroform. The methanol extract was then dried, resuspended in isobutanol, and partitioned against water to remove salts. The isobutanol fraction was then dried and resuspended in milli-Q water for further analyses or experiments. During the olfactory experiments, an approximate concentration of 200 mg L−1 was used, corresponding approximately to the quantity of saponin in sea-cucumber-conditioned water [61].
- Saponin extract of H. scabra juvenile-conditioned water: A total of 3 L of FFSW conditioned for 1 H by 5 juveniles was passed through a column of Amberlite XAD-4 (Sigma–Aldrich, St. Louis, MO, USA) as previously described [19,60,61]. Although this method is limited in its extraction ability, this is the selected method for in-field extractions, as solvents are limited. A concentration of 200 mg L−1 was used.
- Sediment-/food-conditioned water: A total of 50 g of the sediment used to feed the sea cucumbers in inland growing basins of IOT was placed into 5 L of FFSW. The water was conditioned with the sediment for 1 H before being used in olfactory experiments.
4.5. Mass Spectrometry Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Philippe, A.-S.; Jeanson, R.; Pasquaretta, C.; Rebaudo, F.; Sueur, C.; Mery, F. Genetic Variation in Aggregation Behaviour and Interacting Phenotypes in Drosophila. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2016, 283, 20152967–20152968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.J.; Campbell, A.C. An Investigation into the Behavioural and Ecological Bases for Periodic Infestations of Asterias rubens. In Echinodermata; Keegan, B.F., O’Connor, B.D.S., Eds.; CRC Press: Boca Raton, FL, USA, 1984; p. 596. [Google Scholar]
- Ormond, R.F.G.; Campbell, A.C.; Head, S.H.; Moore, R.J.; Rainbow, P.R.; Saunders, A.P. Formation and Breakdown of Aggregations of the Crown-of-Thorns Starfish Acanthaster Planci (L.). Nature 1973, 246, 167–169. [Google Scholar] [CrossRef]
- Broom, D.M. Aggregation Behaviour of the Brittle-Star Ophiothrix fragilis. J. Mar. Biol. Assoc. UK 1975, 55, 191–197. [Google Scholar] [CrossRef]
- Bernstein, B.B.; Schroeter, S.C.; Mann, K.H. Sea Urchin (Strongylocentrotus Droebachiensis) Aggregation Behavior Investigated by a Subtital Multifactorial Experiment. Can. J. Fish. Aquat. Sci. 1983, 40, 1975–1986. [Google Scholar] [CrossRef]
- Levitan, D.R.; Sewell, M.A.; Chia, F.-S. How Distribution and Abundance Influence Fertilization Success in the Sea Urchin Strongylocentrotus franciscanus. Ecology 1992, 73, 248–254. [Google Scholar] [CrossRef]
- Mercier, A.; Battaglene, S.C.; Hamel, J.-F. Periodic Movement, Recruitment and Size-Related Distribution of the Sea Cucumber Holothuria Scabra in Solomon Islands. Hydrobiologia 2000, 440, 81–100. [Google Scholar] [CrossRef]
- Frey, D.L.; Gagnon, P. Spatial Dynamics of the Green Sea Urchin Strongylocentrotus droebachiensis in Food-depleted Habitats. Mar. Ecol. Prog. Ser. 2016, 552, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Barkai, A. The Effect of Water Movement on the Distribution and Interaction of Three Holothurian Species on the South African West Coast. J. Exp. Mar. Biol. Ecol. 1991, 153, 241–254. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L.-A. Aquatic Chemical Ecology. In Chemical Ecology in Aquatic Systems; Oxford University Press: Oxford, UK, 2012; pp. 272–278. ISBN 9780199583096. [Google Scholar]
- Kost, C. Chemical Communication. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 557–575. ISBN 978-0-08-045405-4. [Google Scholar] [CrossRef]
- Mercier, A.; Hamel, J.-F. Perivisceral Coelomic Fluid as a Mediator of Spawning Induction in Tropical Holothurians. Invertebr. Reprod. Dev. 2002, 41, 223–234. [Google Scholar] [CrossRef]
- Soong, K.; Chang, D.; Chao, S.M. Presence of Spawn-Inducing Pheromones in Two Brittle Stars (Echinodermata: Ophiuroidea). Mar. Ecol. Prog. Ser. 2005, 292, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Zacarías-Soto, M.; Olvera-Novoa, M.A.; Pensamiento-Villarauz, S.; Sánchez-Tapia, I. Spawning and Larval Development of the Four-Sided Sea Cucumber, Isostichopus Badionotus (Selenka 1867), under Controlled Conditions. J. World Aquac. Soc. 2013, 44, 694–705. [Google Scholar] [CrossRef]
- Caballes, C.F.; Pratchett, M.S. Environmental and Biological Cues for Spawning in the Crown-of-Thorns Starfish. PLoS ONE 2017, 12, e0173964. [Google Scholar] [CrossRef] [PubMed]
- Marquet, N.; Hubbard, P.C.; da Silva, J.P.; Afonso, J.; Canário, A.V.M. Chemicals Released by Male Sea Cucumber Mediate Aggregation and Spawning Behaviours. Sci. Rep. 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.C.; Coppard, S.; D’Abreo, C.; Tudor-Thomas, R. Escape and Aggregation Responses of Three Echinoderms to Conspecific Stimuli. Biol. Bull. 2001, 201, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, L.; Caulier, G.; Lepoint, G.; Gerbaux, P.; Eeckhaut, I. Echinometra Mathaei and Its Ectocommensal Shrimps: The Role of Sea Urchin Spinochrome Pigments in the Symbiotic Association. Sci. Rep. 2018, 8, 17540. [Google Scholar] [CrossRef] [Green Version]
- Caulier, G.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. When a Repellent Becomes an Attractant: Harmful Saponins Are Kairomones Attracting the Symbiotic Harlequin Crab. Sci. Rep. 2013, 3, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, S.; Ichiba, T.; Reimer, J.D.; Tanaka, J. Chemoattraction of the Pearlfish Encheliophis vermicularis to the Sea Cucumber Holothuria leucospilota. Chemoecology 2014, 24, 121–126. [Google Scholar] [CrossRef]
- Claereboudt, E.J.S.; Caulier, G.; Decroo, C.; Colson, E.; Gerbaux, P.; Claereboudt, M.R.; Schaller, H.; Flammang, P.; Deleu, M.; Eeckhaut, I. Triterpenoids in Echinoderms: Fundamental Differences in Diversity and Biosynthetic Pathways. Mar. Drugs 2019, 17, 352. [Google Scholar] [CrossRef] [Green Version]
- Demeyer, M.; Wisztorski, M.; Decroo, C.; Winter, J.D.; Caulier, G.; Hennebert, E.; Eeckhaut, I.; Fournier, I.; Flammang, P.; Gerbaux, P. Inter- and Intra-Organ Spatial Distributions of Sea Star Saponins by MALDI Imaging. Anal. Bioanal. Chem. 2015, 407, 8813–8824. [Google Scholar] [CrossRef]
- Van Dyck, S.V.; Caulier, G.; Todesco, M.; Gerbaux, P.; Fournier, I.; Wisztorski, M.; Flammang, P. The Triterpene Glycosides of Holothuria forskali: Usefulness and Efficiency as a Chemical Defense Mechanism against Predatory Fish. J. Exp. Biol. 2011, 214, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, Y.; Franco, C. Acetylated Triterpene Glycosides and Their Biological Activity from Holothuroidea Reported in the Past Six Decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamyab, E.; Rohde, S.; Kellermann, M.Y.; Schupp, P.J. Chemical Defense Mechanisms and Ecological Implications of Indo-Pacific Holothurians. Molecules 2020, 25, 4808. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, I.; Caulier, G.; Brasseur, L.; Flammang, P.; Gerbaux, P.; Parmentier, E. Effects of Holothuroid Ichtyotoxic Saponins on the Gills of Free-Living Fishes and Symbiotic Pearlfishes. Biol. Bull. 2015, 228, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sroyraya, M.; Kaewphalug, W.; Anantachoke, N.; Poomtong, T.; Sobhon, P.; Srimongkol, A.; Suphamungmee, W. Saponins Enriched in the Epidermal Layer of Holothuria leucospilota body Wall. Microsc. Res. Tech. 2018, 81, 1182–1190. [Google Scholar] [CrossRef]
- Chantal, C. Les Holothuries Aspidochirotes Du Lagon de Nouvelle Calédonie: Biologie, Écologie et Exploitation; Institut Francais de Recherche Scientifique pour le Developpement en Cooperation: Marseille, France, 1989. [Google Scholar]
- James, D.B. Seed Production in Sea Cucumbers. Aqua Int. 1994, 1, 15–26. [Google Scholar]
- Lawrence, A.J.; Ahmed, M.; Hanafy, M.; Gabr, H.; Ibrahim, A.; Gab-Alla, A. Status of the Sea Cucumber Fishery in the Red Sea, the Egyptian Experience; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Al-Rashdi, K.M.; Claereboudt, M.R.; Al-Busaidi, S.S. Density and Size Distribution of the Sea Cucumber, Holothuria scabra (Jaeger, 1935), at Six Exploited Sites in Mahout Bay, Sultanate of Oman. J. Agric. Mar. Sci. JAMS 2017, 12, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lavitra, T.; Rasolofonirina, R.; Grosjean, P.; Jangoux, M.; Eeckhaut, I. The Effect of Food Quality and Rearing Density on the Growth and Survival of Epibenthic Juveniles of the Sea Cucumber Holothuria scabra. West. Indian Ocean. J. Mar. Sci. 2010, 8, 56678. [Google Scholar] [CrossRef]
- Eeckhaut, I.; Wayenberghe, K.V.; Nicolas, F.; Delroisse, J. Skin Ulcerations in Holothuria scabra Can Be Induced by Various Types of Food. SPC Beche-De-Mer Inf. Bull. 2019, 39, 31–35. [Google Scholar]
- Delroisse, J.; Wayneberghe, K.V.; Flammang, P.; Gillan, D.; Gerbaux, P.; Opina, N.; Todinanahary, G.G.B.; Eeckhaut, I. Epidemiology of a Skin Ulceration Disease (SKUD) in the Sea Cucumber Holothuria scabra with a Review on the SKUDs in Holothuroidea (Echinodermata). Sci. Rep. 2020, 10, 22150. [Google Scholar] [CrossRef]
- Bondoc, K.G.V.; Lee, H.; Cruz, L.J.; Lebrilla, C.B.; Juinio-Meñez, M.A. Chemical Fingerprinting and Phylogenetic Mapping of Saponin Congeners from Three Tropical Holothurian Sea Cucumbers. Comp. Biochem. Physiol. Part B 2013, 166, 182–193. [Google Scholar] [CrossRef]
- Caulier, G.; Flammang, P.; Rakotorioa, P.; Gerbaux, P.; Demeyer, M.; Eeckhaut, I. Preservation of the Bioactive Saponins of Holothuria scabra through the Processing of Trepang. Cah. Biol. Mar. 2013, 54, 685–690. [Google Scholar]
- Mercier, A.; Battaglene, S.C.; Hamel, J.-F. Daily Burrowing Cycle and Feeding Activity of Juvenile Sea Cucumbers. J. Exp. Mar. Biol. Ecol. 1999, 239, 125–156. [Google Scholar] [CrossRef]
- Plotieau, T.; Baele, J.-M.; Vaucher, R.; Hasler, C.-A.; Koudad, D.; Eeckhaut, I. Analysis of the Impact of Holothuria scabra Intensive Farming on Sediment. Cah. Biol. Mar. 2013, 54, 703–711. [Google Scholar]
- Williamson, J.E.; Duce, S.; Joyce, K.E.; Raoult, V. Putting Sea Cucumbers on the Map: Projected Holothurian Bioturbation Rates on a Coral Reef Scale. Coral Reefs 2021, 40, 559–569. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Animal Behavior, 2nd ed.; Cambridge University Press: Cambridge, UK, 2013; ISBN 9781139030748. [Google Scholar]
- Dal Bello, M.; Pérez-Escudero, A.; Schroeder, F.C.; Gore, J. Inversion of Pheromone Preference Optimizes Foraging in C. elegans. eLife 2021, 10, e58144. [Google Scholar] [CrossRef]
- Rono, E.; Njagi, P.G.N.; Bashir, M.O.; Hassanali, A. Concentration-Dependent Parsimonious Releaser Roles of Gregarious Male Pheromone of the Desert Locust, Schistocerca gregaria. J. Insect Physiol. 2008, 54, 162–168. [Google Scholar] [CrossRef]
- Hamel, J.-F.; Conand, C.; Pawson, D.L.; Mercier, A. The Sea Cucumber Holothuria scabra (Holothuroidea: Echinodermata): Its Biology and Exploitation as Beche-de-Mer. Adv. Mar. Biol. 2001, 41, 129–223. [Google Scholar] [CrossRef]
- Altamirano, J.P.; Recente, C.P.; Rodriguez, J.C., Jr. Substrate Preference for Burying and Feeding of Sandfish Holothuria scabra Juveniles. Fish. Res. 2016, 186, 514–523. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Schmid, B.; Tinevez, J.-Y.; White, D.J.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Rakotonjanahary, F.; Lavitra, T.; Fohy, N.; Eeckhaut, I. Essais d’optimisation de La Croissance Des Juvéniles d’Holothuria scabra Pendant La Phase de Pré-Grossissement. Bêche-De-Mer Bull. D’Inf. CPS 2016, 36, 76–81. [Google Scholar]
- Ahmed, Q.; Poot-Salazar, A.; Ali, Q.M.; Bat, L. Seasonal Variation in the Length-Weight Relationships and Condition Factor of Four Commercially Important Sea Cucumbers Species from Karachi Coast-Northern Arabian Sea. Nat. Eng. Sci. 2018, 3, 265–281. [Google Scholar] [CrossRef] [Green Version]
- Hamad, M.I.; Mwandya, A.W.; Munubi, R.S.; Chenyambuga, S. Comparative Growth and Survival Performance of Sea Cucumber (Holothuria scabra) in Co-Cultured Pen System with Commercial Macroalgae. Afr. J. Biol. Sci. 2019, 1, 32–41. [Google Scholar] [CrossRef]
- Juinio-Meñez, M.A.; Evangelio, J.C.; Miralao, S.J.A. Trial Grow-out Culture of Sea Cucumber Holothuria scabra in Sea Cages and Pens. Aquac. Res. 2012, 45, 1332–1340. [Google Scholar] [CrossRef]
- Juinio-Meñez, M.A.; Tech, E.D.; Ticao, I.P.; Gorospe, J.R.; Edullantes, C.M.A.; Rioja, R.A.V. Adaptive and Integrated Culture Production Systems for the Tropical Sea Cucumber Holothuria scabra. Fish. Res. 2016, 186, 502–513. [Google Scholar] [CrossRef]
- Baddeley, A.; Diggle, P.J.; Hardegen, A.; Lawrence, T.; Milne, R.K.; Nair, G. On Tests of Spatial Pattern Based on Simulation Envelopes. Ecol. Monogr. 2014, 84, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Landler, L.; Ruxton, G.D.; Malkemper, E.P. The Hermans–Rasson Test as a Powerful Alternative to the Rayleigh Test for Circular Statistics in Biology. BMC Ecol. 2019, 19, 30. [Google Scholar] [CrossRef] [Green Version]
- Fitak, R.R.; Johnsen, S. Bringing the Analysis of Animal Orientation Data Full Circle: Model-Based Approaches with Maximum Likelihood. J. Exp. Biol. 2017, 220, 3878–3882. [Google Scholar] [CrossRef] [Green Version]
- Baddeley, A.; Turner, R. Spatstat: An R Package for Analyzing Spatial Point Patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 February 2023).
- Van den Spiegel, D.; Eeckhaut, I.; Jangoux, M. Host Selection by Synalpheus stimpsoni (De Man), an Ectosymbiotic Shrimp of Comatulid Crinoids, Inferred by a Field Survey and Laboratory Experiments. J. Exp. Mar. Biol. Ecol. 1998, 225, 185–196. [Google Scholar] [CrossRef]
- Eeckhaut, I.; McHugh, D.; Mardulyn, P.; Tiedemann, R.; Monteyne, D.; Jangoux, M.; Milinkovitch, M.C. Myzostomida: A Link between Trochozoans and Flatworms? Proc. R. Soc. Lond. Ser. B. Biol. Sci. 2000, 267, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Vaïtilingon, D.; Eeckhaut, I.; Fourgon, D.; Jangoux, M. Population Dynamics, Infestation and Host Selection of Vexilla Vexillum, an Ectoparasitic Muricid of Echinoids, in Madagascar. Dis. Aquat. Org. 2004, 61, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Fourgon, D.; Jangoux, M.; Eeckhaut, I. Biology of a “Babysitting” Symbiosis in Brittle Stars: Analysis of the Interactions between Ophiomastix venosa and Ophiocoma scolopendrina. Invertebr. Biol. 2007, 126, 385–395. [Google Scholar] [CrossRef]
- Caulier, G.; Mezali, K.; Soualili, D.L.; Decroo, C.; Demeyer, M.; Eeckhaut, I.; Gerbaux, P.; Flammang, P. Chemical Characterization of Saponins Contained in the Body Wall and the Cuvierian Tubules of the Sea Cucumber Holothuria (platyperona) sanctori (Delle Chiaje, 1823). Biochem. Syst. Ecol. 2016, 68, 119–127. [Google Scholar] [CrossRef]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Elucidation of Molecular Diversity and Body Distribution of Saponins in the Sea Cucumber Holothuria forskali (Echinodermata) by Mass Spectrometry. Comp. Biochem. Physiol. Part B 2009, 152, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Decroo, C.; Colson, E.; Demeyer, M.; Lemaur, V.; Caulier, G.; Eeckhaut, I.; Cornil, J.; Flammang, P.; Gerbaux, P. Tackling Saponin Diversity in Marine Animals by Mass Spectrometry: Data Acquisition and Integration. Anal. Bioanal. Chem. 2017, 409, 3115–3126. [Google Scholar] [CrossRef] [PubMed]
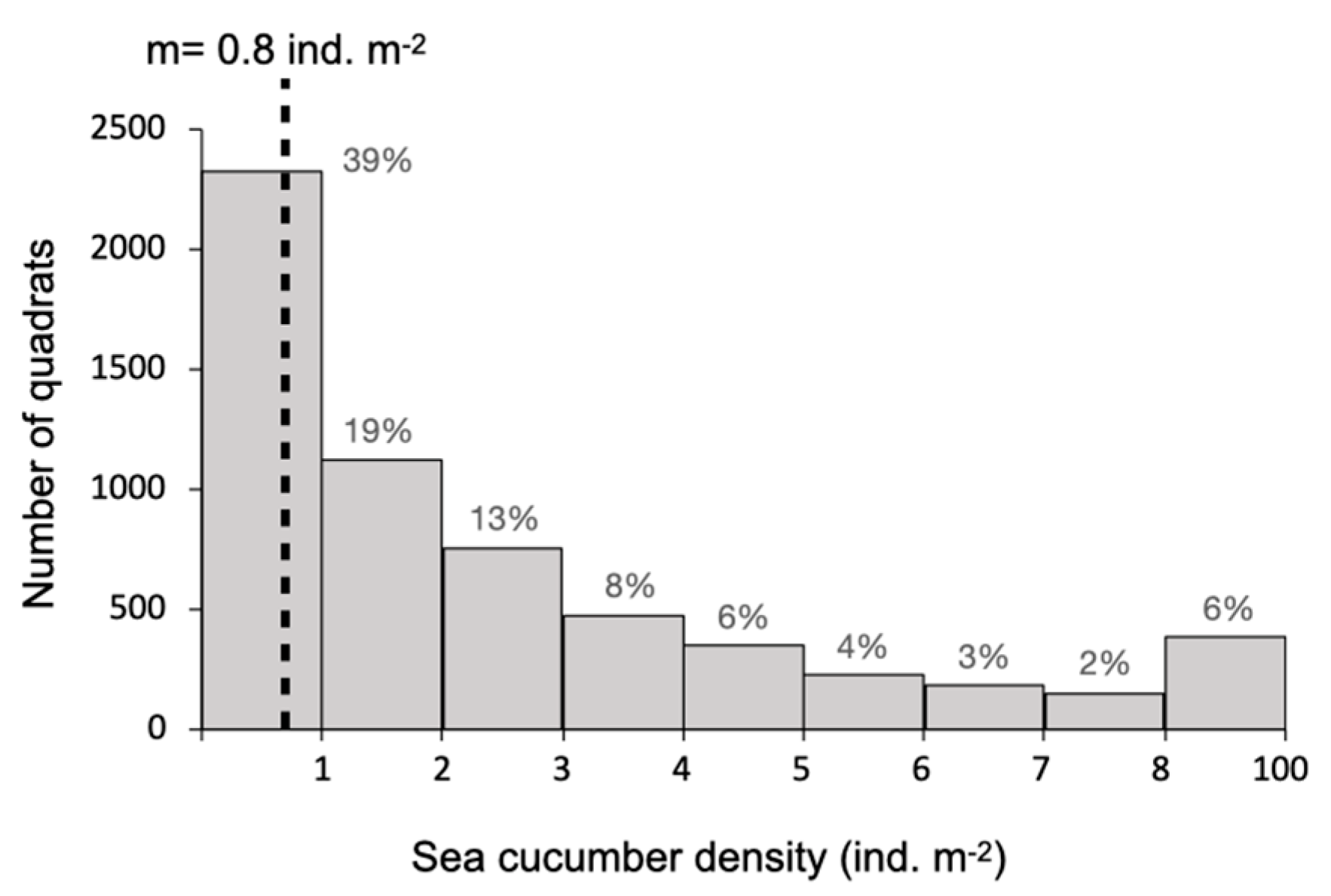



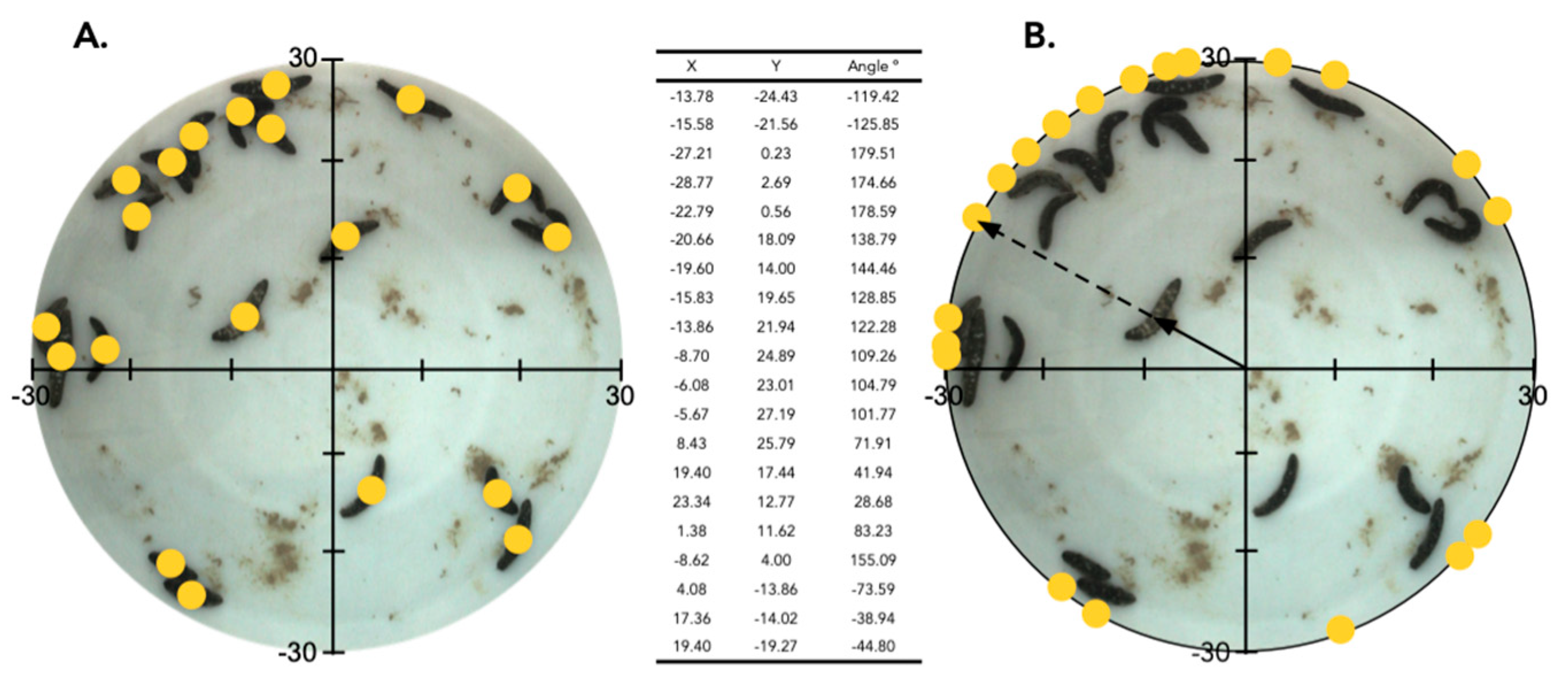
| Number of Significantly Aggregated Spatial Distributions among 70 Surveys | |
|---|---|
| Buried animals | 55 (78%) |
| Surface animals | 46 (66%) |
| Both buried and surface animals | 61 (87%) |
| [M + Na]+ m/z | No. of Sugar Residues | H. scabra Juvenile-Integument | H. scabra Juvenile Starved Integument | H. scabra Adult Viscera | H. scabra Adult Conditioned Water | H. leucospilota BW Mucus | |
|---|---|---|---|---|---|---|---|
| Holothurinoside N | 1317.1 | 5 | X | ||||
| Holothurinoside M | 1301.6 | 5 | X | ||||
| Girseaside A | 1289.6 | 5 | X | ||||
| Impatinside B | 1271.7 | 5 | X | ||||
| Holothurin A3 | 1259.3 | 4 | X | X | X | X | |
| Scabraside B Holothurin A 17- or 25- dehydroechinoside A | 1243.3 | 4 | X | X | X | X | X |
| Holothurin A2 | 1229.5 | 4 | X | ||||
| Scabraisde A Fuscocineroside B/C 24-dehydroechinoside A | 1227.5 | 4 | X | X | X | X | X |
| Unidentified | 1211 | 4 | X | ||||
| Desholothurin A Desholothurin A1 | 1141.5 | 4 | X | X | X | X | |
| Holothurinoside C | 1125.6 | 4 | X | X | X | ||
| Unidentified | 1109 | 4 | X | X | |||
| Leucospilotaside A | 921.4 | 2 | X | ||||
| Holothurin B4 Nobiliside B Holothurin B | 905.4 | 2 | X | X | X | ||
| Holothurin B3 | 889.4 | 2 | X | X | X | ||
| Unidentified | 873 | 2 | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claereboudt, E.J.S.; Claereboudt, M.R.; Savarino, P.; Caulier, G.; Gaumez, L.; Deleu, M.; Gerbaux, P.; Eeckhaut, I. A Distinct Saponin Profile Drives an Olfactory-Mediated Aggregation in the Aquacultivated Sea Cucumber Holothuria scabra. Mar. Drugs 2023, 21, 184. https://doi.org/10.3390/md21030184
Claereboudt EJS, Claereboudt MR, Savarino P, Caulier G, Gaumez L, Deleu M, Gerbaux P, Eeckhaut I. A Distinct Saponin Profile Drives an Olfactory-Mediated Aggregation in the Aquacultivated Sea Cucumber Holothuria scabra. Marine Drugs. 2023; 21(3):184. https://doi.org/10.3390/md21030184
Chicago/Turabian StyleClaereboudt, Emily J. S., Michel R. Claereboudt, Philippe Savarino, Guillaume Caulier, Loic Gaumez, Magali Deleu, Pascal Gerbaux, and Igor Eeckhaut. 2023. "A Distinct Saponin Profile Drives an Olfactory-Mediated Aggregation in the Aquacultivated Sea Cucumber Holothuria scabra" Marine Drugs 21, no. 3: 184. https://doi.org/10.3390/md21030184
APA StyleClaereboudt, E. J. S., Claereboudt, M. R., Savarino, P., Caulier, G., Gaumez, L., Deleu, M., Gerbaux, P., & Eeckhaut, I. (2023). A Distinct Saponin Profile Drives an Olfactory-Mediated Aggregation in the Aquacultivated Sea Cucumber Holothuria scabra. Marine Drugs, 21(3), 184. https://doi.org/10.3390/md21030184







