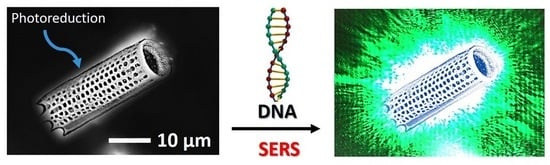
Photochemical Reduction of Silver Nanoparticles on Diatoms
Abstract
1. Introduction
2. Results
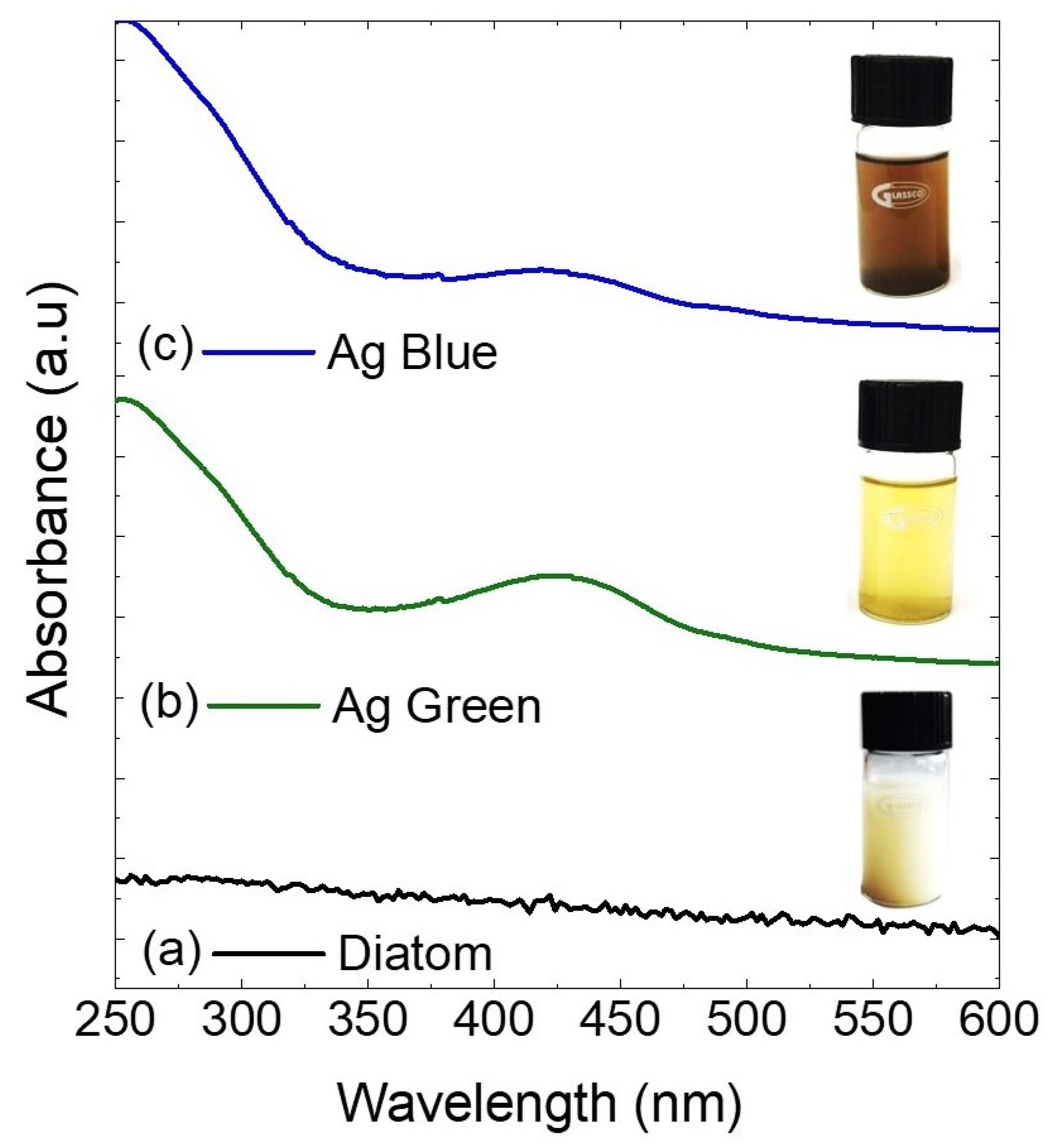
2.1. Ultraviolet-Visible Spectroscopy (UV-Vis)
2.2. X-ray Photoelectron Spectroscopy (XPS)
2.3. Scanning and Transmission Electron Microscopy (SEM and TEM)
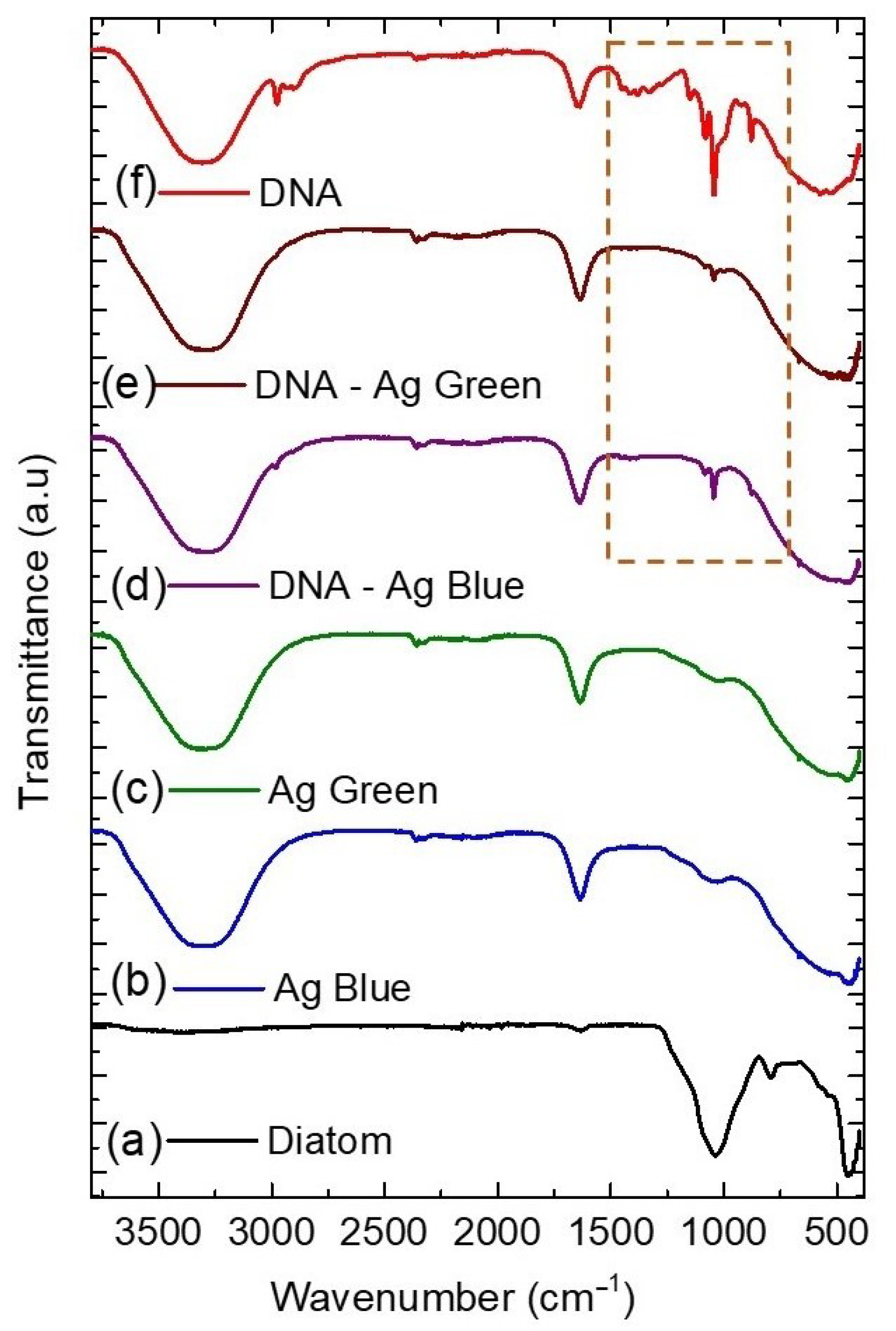
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
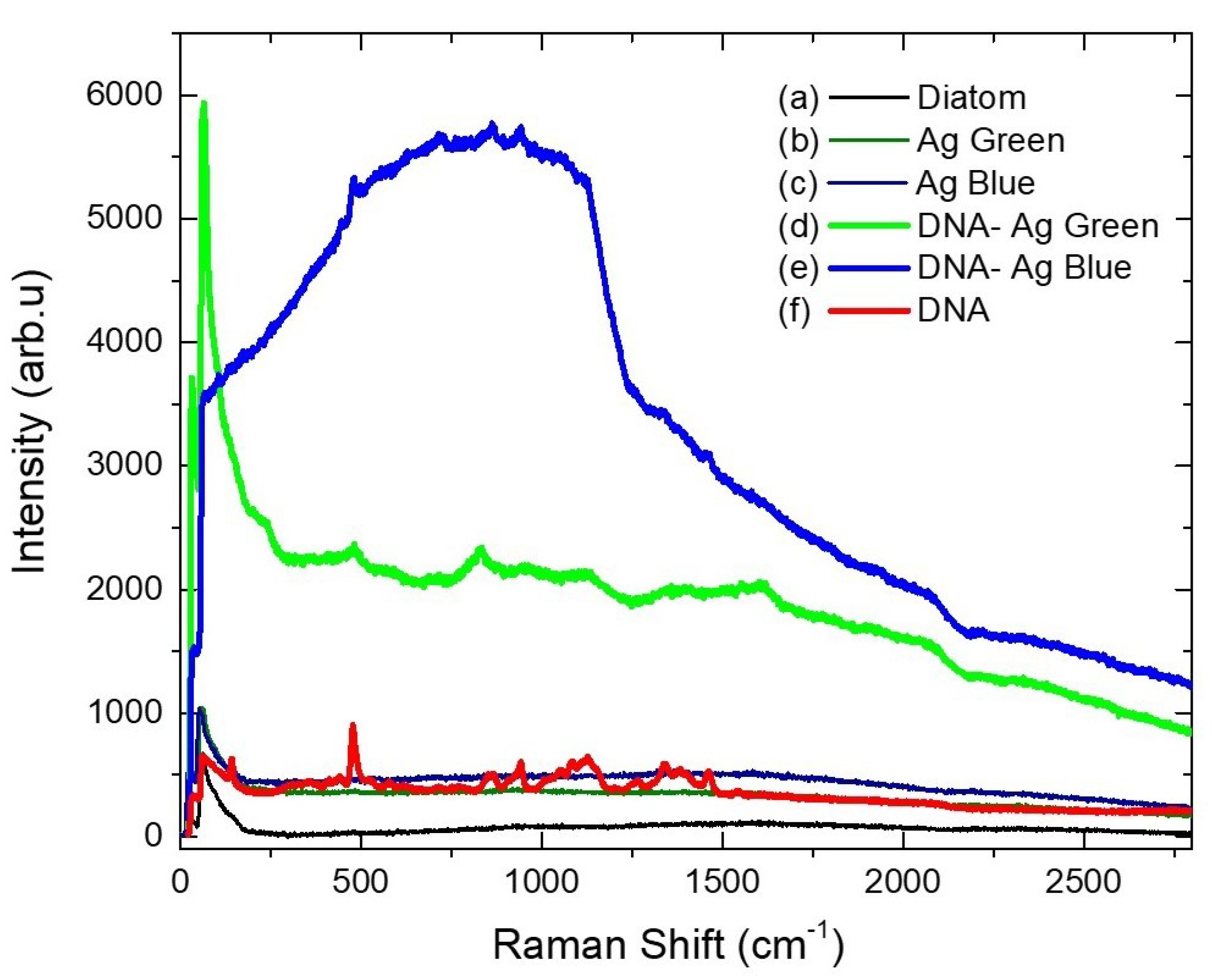
2.5. Raman Spectroscopy
2.6. Optical and Fluorescence Microscopy
3. Discussion
4. Materials and Methods
4.1. Chemicals
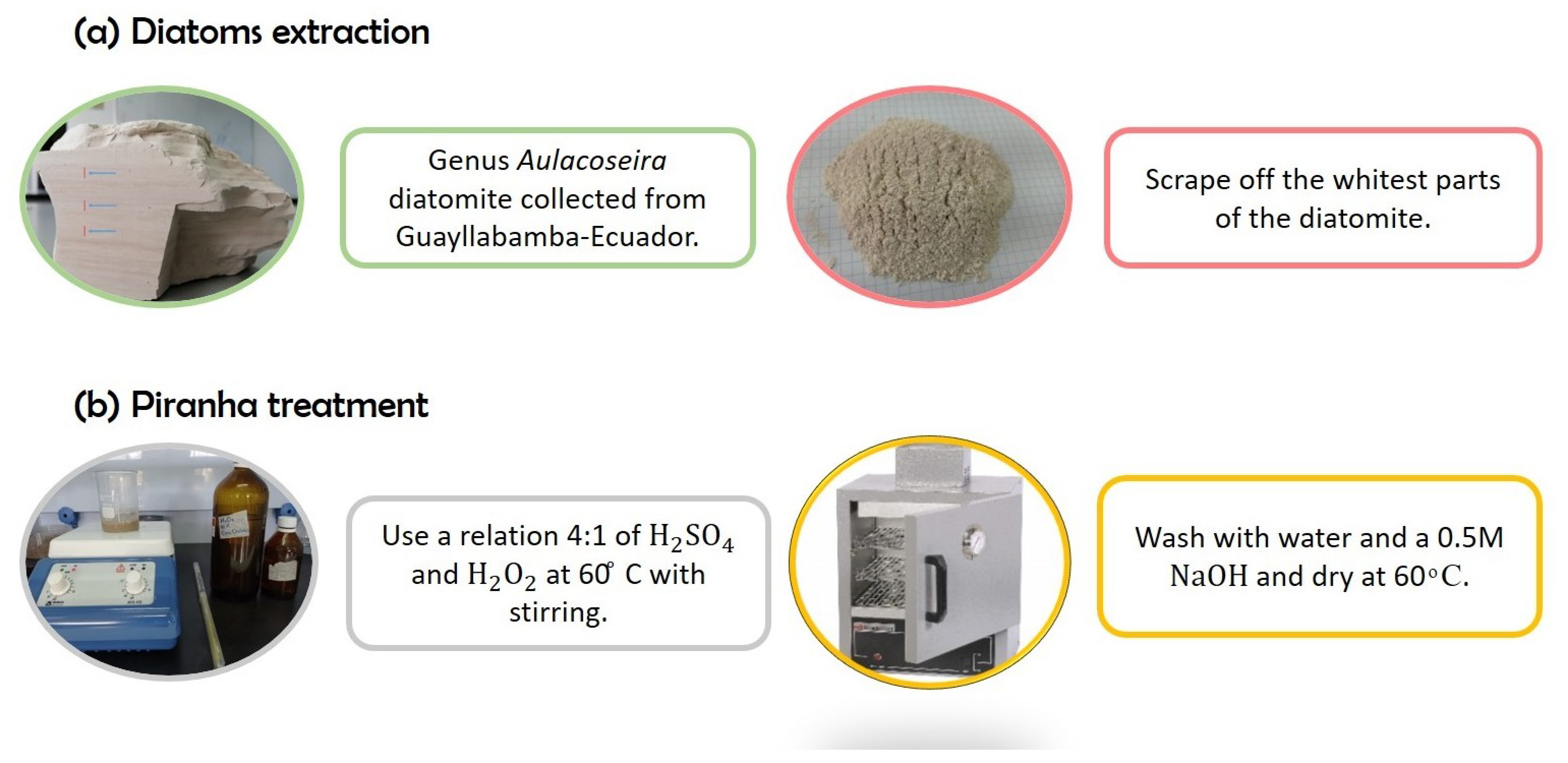
4.2. Diatom Extraction
4.3. Photoreduction of Silver Nanoparticles on Diatoms
4.4. DNA Purification and Functionalization
4.5. Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hussein, H.A.; Nazir, M.S.; Azra, N.; Qamar, Z.; Seeni, A.; Tengku Din, T.A.D.A.A.; Abdullah, M.A. Novel Drug and Gene Delivery System and Imaging Agent Based on Marine Diatom Biosilica Nanoparticles. Mar. Drugs 2022, 20, 480. [Google Scholar] [CrossRef]
- Rabiee, N.; Khatami, M.; Jamalipour Soufi, G.; Fatahi, Y.; Iravani, S.; Varma, R.S. Diatoms with Invaluable Applications in Nanotechnology, Biotechnology, and Biomedicine: Recent Advances. ACS Biomater. Sci. Eng. 2021, 7, 3053–3068. [Google Scholar] [CrossRef]
- Dao, T.N.T.; Seshadri Reddy, A.; Zhao, F.; Liu, H.; Koo, B.; Moniruzzaman, M.; Kim, J.; Shin, Y. Green Synthesis-Based Magnetic Diatoms for Biological Applications. ACS Sustain. Chem. Eng. 2021, 9, 3439–3451. [Google Scholar] [CrossRef]
- Briceño, S.; Chavez-Chico, E.A.; González, G. Diatoms decorated with gold nanoparticles by In-situ and Ex-situ methods for in vitro gentamicin release. Mater. Sci. Eng. C 2021, 123, 112018. [Google Scholar] [CrossRef]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors 2018, 18, 2357. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Green Synthesis of Silver Nanoparticles by the Cyanobacteria Synechocystis sp.: Characterization, Antimicrobial and Diabetic Wound-Healing Actions. Mar. Drugs 2022, 20, 56. [Google Scholar] [CrossRef]
- Bhaskar, S.; Srinivasan, V.; Ramamurthy, S.S. Nd2O3-Ag Nanostructures for Plasmonic Biosensing, Antimicrobial, and Anticancer Applications. ACS Appl. Nano Mater. 2023, 6, 1129–1145. [Google Scholar] [CrossRef]
- Qian, Z.S.; Chai, L.J.; Huang, Y.Y.; Tang, C.; Shen, J.J.; Chen, J.R.; Feng, H. A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens. Bioelectron. 2015, 68, 675–680. [Google Scholar] [CrossRef]
- Kong, X.; Squire, K.; Li, E.; LeDuff, P.; Rorrer, G.L.; Tang, S.; Chen, B.; McKay, C.P.; Navarro-Gonzalez, R.; Wang, A.X. Chemical and Biological Sensing Using Diatom Photonic Crystal Biosilica With In-Situ Growth Plasmonic Nanoparticles. IEEE Trans. Nanobiosci. 2016, 15, 828–834. [Google Scholar] [CrossRef]
- Kong, X.; Li, E.; Squire, K.; Liu, Y.; Wu, B.; Cheng, L.J.; Wang, A.X. Plasmonic nanoparticles-decorated diatomite biosilica: Extending the horizon of on-chip chromatography and label-free biosensing. J. Biophotonics 2017, 10, 1473–1484. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, R.; Yang, Y.; Huang, L.; Chen, N.; Xie, Q. Study of ion-imprinted adsorbent materials on diatom-based Cr(VI) Surfaces. Mater. Lett. 2022, 308, 131149. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Ren, F.; Campbell, J.; Wang, X.; Rorrer, G.L.; Wang, A.X. Enhancing surface plasmon resonances of metallic nanoparticles by diatom biosilica. Opt. Express 2013, 21, 15308. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; Stefano, L.D. Synthetic vs Natural: Diatoms Bioderived Porous Materials for the Next Generation of Healthcare Nanodevices. Adv. Healthc. Mater. 2016, 6, 1601125. [Google Scholar] [CrossRef]
- Ferrara, M.A.; Dardano, P.; Stefano, L.D.; Rea, I.; Coppola, G.; Rendina, I.; Congestri, R.; Antonucci, A.; Stefano, M.D.; Tommasi, E.D. Optical Properties of Diatom Nanostructured Biosilica in Arachnoidiscus sp: Micro-Optics from Mother Nature. PLoS ONE 2014, 9, e103750. [Google Scholar] [CrossRef]
- Zobi, F. Diatom Biosilica in Targeted Drug Delivery and Biosensing Applications: Recent Studies. Micro 2022, 2, 342–360. [Google Scholar] [CrossRef]
- Mishra, M.; Singh, S.K.; Bhardwaj, A.; Kumar, L.; Singh, M.K.; Sundaram, S. Development of a Diatom-Based Photoluminescent Immunosensor for the Early Detection of Karnal Bunt Disease of Wheat Crop. ACS Omega 2020, 5, 8251–8257. [Google Scholar] [CrossRef]
- Managò, S.; Tramontano, C.; Delle Cave, D.; Chianese, G.; Zito, G.; De Stefano, L.; Terracciano, M.; Lonardo, E.; De Luca, A.C.; Rea, I. SERS Quantification of Galunisertib Delivery in Colorectal Cancer Cells by Plasmonic-Assisted Diatomite Nanoparticles. Small 2021, 17, 2101711. [Google Scholar] [CrossRef]
- Mina-Villarreal, M.C.; Briceño, S.; Vizuete, K.; Alexis Debut, A.; Gonzalez, G. Growth of carbon nanotubes and carbon spheres on diatoms. J. Porous Mater. 2022, 68, 675–680. [Google Scholar] [CrossRef]
- Rea, I.; De Stefano, L. Recent Advances on Diatom-Based Biosensors. Sensors 2019, 19, 5208. [Google Scholar] [CrossRef]
- Chen, J.; Qin, G.; Chen, Q.; Yu, J.; Li, S.; Cao, F.; Yang, B.; Ren, Y. A synergistic combination of diatomaceous earth with Au nanoparticles as a periodically ordered, button-like substrate for SERS analysis of the chemical composition of eccrine sweat in latent fingerprints. J. Mater. Chem. C 2015, 3, 4933–4944. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Park, S.; Nichols, W.T. Self-assembled diatom substrates with plasmonic functionality. J. Korean Phys. Soc. 2014, 64, 1179–1184. [Google Scholar] [CrossRef]
- Tommasi, E.D.; Luca, A.C.D. Diatom biosilica in plasmonics: Applications in sensing, diagnostics and therapeutics. Biomed. Opt. Express 2022, 13, 3080–3101. [Google Scholar] [CrossRef]
- Chetia, L.; Kalita, D.; Ahmed, G.A. Synthesis of Ag nanoparticles using diatom cells for ammonia sensing. Sens. Bio-Sens. Res. 2017, 16, 55–61. [Google Scholar] [CrossRef]
- Alipour, L.; Hamamoto, M.; Nakashima, S.; Harui, R.; Furiki, M.; Oku, O. Infrared Microspectroscopy of Bionanomaterials (Diatoms) with Careful Evaluation of Void Effects. Appl. Spectrosc. 2016, 70, 427–442. [Google Scholar] [CrossRef]
- Taboury, J.A.; Liquier, J.; Taillandier, E. Characterization of DNA structures by infrared spectroscopy: Double helical forms of poly(dG-dC)•poly(dG-dC), poly(dD8G-dC)•poly(dD8G-dC), and poly(dG-dm5C)•poly(dG-dm5C). Can. J. Chem. 1985, 63, 1904–1909. [Google Scholar] [CrossRef]
- Ren, F.; Campbell, J.; Rorrer, G.L.; Wang, A.X. Surface-Enhanced Raman Spectroscopy Sensors From Nanobiosilica With Self-Assembled Plasmonic Nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 127–132. [Google Scholar] [CrossRef]
- Pannico, M.; Rea, I.; Chandrasekaran, S.; Musto, P.; Voelcker, N.H.; Stefano, L.D. Electroless Gold-Modified Diatoms as Surface-Enhanced Raman Scattering Supports. Nanoscale Res. Lett. 2016, 11, 315. [Google Scholar] [CrossRef]
- Grzelczak, M.; Liz-Marzán, L.M. The relevance of light in the formation of colloidal metal nanoparticles. Chem. Soc. Rev. 2014, 43, 2089–2097. [Google Scholar] [CrossRef]
- Onesto, V.; Villani, M.; Coluccio, M.L.; Majewska, R.; Alabastri, A.; Battista, E.; Schirato, A.; Calestani, D.; Coppedé, N.; Cesarelli, M.; et al. Silica diatom shells tailored with Aunnnanoparticles enable sensitive analysis of molecules for biological, safety and environment applications. Nanoscale Res. Lett. 2018, 13, 94. [Google Scholar] [CrossRef]
- Jaworska, A.; Pyrak, E.K.A. Comparison of the efficiency of generation of Raman radiation by various Raman reporters connected via DNA linkers to different plasmonic nanostructures. Vib. Spectrosc. 2019, 101, 34–39. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Langer, J.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Shiohara, A.; Wang, Y.; Liz-Marzán, L.M. Recent approaches toward creation of hot spots for SERS detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Saade, J.; de Araújo, C.B. Synthesis of silver nanoprisms: A photochemical approach using light emission diodes. Mater. Chem. Phys. 2014, 148, 1184–1193. [Google Scholar] [CrossRef]
- Benalcázar, J.; Lasso, E.D.; Ibarra-Barreno, C.M.; Arcos Pareja, J.A.; Vispo, N.S.; Chacón-Torres, J.C.; Briceño, S. Photochemical Optimization of a Silver Nanoprism/Graphene Oxide Nanocomposite’s Antibacterial Properties. ACS Omega 2022, 7, 46745–46755. [Google Scholar] [CrossRef]
- Arcos-Pareja, J.; Lasso, E.D.; Ibarra-Barreno, C.M.; Benalcázar, J.; Robalino, K.; Debut, A.; Briceño, S.; Chacón-Torres, J.C. Revealing the Synthesis of Triangular Silver Nanoplates: A Study of the Photochemical Growth Mechanism around the pH and Trisodium Citrate Variations. Phys. Status Solidi (b) 2021, 258, 2100189. [Google Scholar] [CrossRef]




| Element | Atomic Concentration (%) | Assignments | ||
|---|---|---|---|---|
| Diatom | Ag Green | Ag Blue | ||
| O1s | 69.7 | 68.7 | 63.7 | Si-O, Ag-O, C-O |
| Si2p | 19.1 | 19.0 | 18.9 | Si-O |
| C1s | 6.5 | 5.0 | 11.0 | C-O |
| Ag3d | 0 | 1.1 | 1.8 | Ag-O |
| Diatom | Ag Blue | Ag Green | DNA-Ag Blue | DNA-Ag Green | DNA | Assignment |
|---|---|---|---|---|---|---|
| - | 3291 | 3291 | 3291 | 3291 | 3291 | O–H stretching |
| - | - | - | 2974 | - | 2974 | N-H stretch |
| - | - | - | - | - | 2913 | C-H stretch |
| - | 1645 | 1645 | 1645 | 1645 | 1645 | O–H/C=O stretch |
| - | - | - | 1044 | 1044 | 1044 | C-O/C-N |
| 1034 | 1034 | 1034 | - | - | - | Si-O stretch |
| DNA | DNA-Ag Blue | DNA-Ag Green | Diatom | Assignment |
|---|---|---|---|---|
| 480 | 480 | 480 | - | N-H |
| 950 | 950 | 950 | - | C-C |
| 1126 | 1126 | 1126 | - | C-N |
| 1463 | 1463 | 1463 | - | N-H |
| 1463 | 1463 | 1463 | - | C-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Valencia, A.; Briceño, S.; Reinoso, C.; Vizuete, K.; Debut, A.; Caetano, M.; González, G. Photochemical Reduction of Silver Nanoparticles on Diatoms. Mar. Drugs 2023, 21, 185. https://doi.org/10.3390/md21030185
León-Valencia A, Briceño S, Reinoso C, Vizuete K, Debut A, Caetano M, González G. Photochemical Reduction of Silver Nanoparticles on Diatoms. Marine Drugs. 2023; 21(3):185. https://doi.org/10.3390/md21030185
Chicago/Turabian StyleLeón-Valencia, Adrián, Sarah Briceño, Carlos Reinoso, Karla Vizuete, Alexis Debut, Manuel Caetano, and Gema González. 2023. "Photochemical Reduction of Silver Nanoparticles on Diatoms" Marine Drugs 21, no. 3: 185. https://doi.org/10.3390/md21030185
APA StyleLeón-Valencia, A., Briceño, S., Reinoso, C., Vizuete, K., Debut, A., Caetano, M., & González, G. (2023). Photochemical Reduction of Silver Nanoparticles on Diatoms. Marine Drugs, 21(3), 185. https://doi.org/10.3390/md21030185