New Nostocyclophanes from Nostoc linckia
Abstract
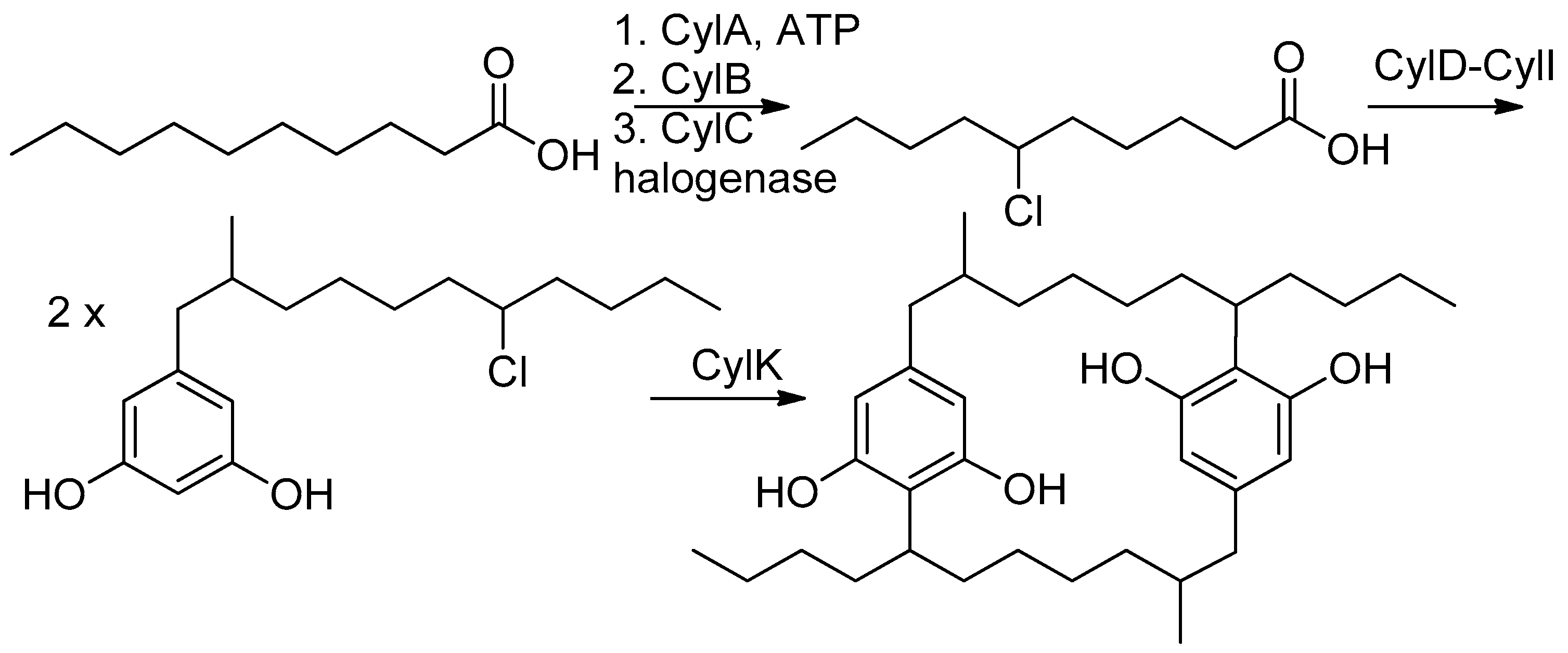
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemicals and Reagents
3.3. Biological Material
3.4. Extraction and Isolation of Metabolites
- Nostocyclophane E (1): amorphous white power; [α]D22 +5.7 (c 2.0, MeOH); UV (MeOH) λmax (log ε) 209 (2.5), 214 (2.5), 227 (1.3), 277 (0.2), 283 (0.2) nm; ECD (c 0.2 MeOH) λmax (Δ ε) 206 (0.80), 218 (−0.71), 240 (−0.20), 261 (−0.80), 284 (−0.090) nm; IR (film) νmax 3325, 2926, 2854, 1646, 1595, 1431, 1083, 1021 cm−1; see Table 1 and Table 2 for tabulated NMR spectroscopic data; HRESIMS m/z 617.3599 [M − H]− (calcd for C36H5435ClO6, 617.3609; Δ = −1.6 ppm).
- Nostocyclophane F (2): amorphous white power; [α]D22 −6.5 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 210 (2.5), 214 (2.5), 227 (1.4), 277 (0.3), 283 (0.2) nm; ECD (c 0.2 MeOH) λmax (Δ ε) 205 (1.10), 217 (−1.80), 235 (−0.67), 272 (−0.26), 280 (−0.25) nm; IR (film) νmax 3345, 2958, 2864, 1650, 1431, 1020 cm−1; see Table 1 and Table 2 for tabulated NMR spectroscopic data; HRESIMS m/z 603.3454 [M − H]− (calcd for C35H5235ClO6−, 603.3453; Δ = 0.3 ppm).
- Nostocyclophane G (3): amorphous white power; [α]D22 −25.6 (c 2.0, MeOH); UV (MeOH) λmax (log ε) 208 (0.9), 229 (0.3), 274 (0.1) nm; ECD (c 0.2 MeOH) λmax (Δ ε) 202 (0.9), 217 (−0.20), 237 (−0.89), 285 (−0.02) nm; IR (film) νmax 3390, 2932, 2859, 1588, 1429, 1085 cm−1; see Table 1 and Table 2 for tabulated NMR spectroscopic data; HRESIMS m/z 621.3102 [M − H]− (calcd for C35H5135Cl2O5−, 621.3114; Δ = −1.9 ppm).
- Nostocyclophane H (4): amorphous white power; [α]D22 −0.2 (c 2.0, MeOH); UV (MeOH) λmax (log ε) 209 (2.4), 227 (0.9), 274 (0.2), 283 (0.2) nm; ECD (c 0.2 MeOH) λmax (Δ ε) 207 (0.22), 218 (−0.25), 233 (−0.09), 273 (−0.35), 282 (−0.37) nm; IR (film) νmax 3399, 2932, 2873, 1589, 1428, 1089 cm−1; see Table 1 and Table 2 for tabulated NMR spectroscopic data; HRESIMS m/z 623.2901 [M − H]− (calcd for C34H4935Cl2O6−, 623.2906; Δ = −0.8 ppm).
- Nostocyclophane I (5): amorphous white power; [α]D22 +2.0 (c 1.0, MeOH); UV (MeOH) λmax (log ε) 209 (2.0), 227 (0.6), 275 (0.1), 283 (0.1) nm; ECD (c 0.2 MeOH) λmax (Δ ε) 201 (4.9), 215 (−5.0), 233 (−1.45), 271 (−0.35), 283 (−0.34) nm; IR (film) νmax 3341, 2927, 2863, 1614, 1595, 1429, 1093 cm−1; see Table 3 for tabulated NMR spectroscopic data; HRESIMS m/z 783.3642 [M − H]− (calcd for C41H6135Cl2O10−, 783.3642; Δ = −2.1 ppm).
- Nostocyclophane J (6): amorphous white power; [α]D22 +5.0 (c 1.0, MeOH); UV (MeOH) λmax (log ε) 214 (2.6), 228 (1.7), 278 (0.4), 282 (0.4) nm; ECD (c 0.2 MeOH) λmax (Δ ε) 207 (2.6), 217 (−2.2), 228 (−0.8), 273 (−0.44), 293 (−0.37) nm. IR (film) νmax 3374, 2950, 2864, 1604, 1081 cm−1; see Table 5 for tabulated NMR spectroscopic data; HRESIMS m/z 687.2973 [M − H]− (calcd for C36H5435Cl3O6−, 687.2986; Δ = −1.9 ppm).
3.5. Growth Inhibition Assays
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Prinsep, M.R.; Caplan, F.R.; Moore, R.E.; Patterson, G.M.L.; Smith, C.D. Tolyporphin, A Novel Multidrug Resistance Reversing Agent from the Blue-green Alga Tolypothrix nodosa. J. Am. Chem. Soc. 1992, 114, 385–387. [Google Scholar] [CrossRef]
- Moore, B.S.; Chen, J.L.; Patterson, G.M.L.; Moore, R.E.; Brinen, L.S.; Kato, Y.; Clardy, J. [7.7]Paracyclophanes from blue-green algae. J. Am. Chem. Soc. 1990, 112, 4061–4063. [Google Scholar] [CrossRef]
- Chen, J.L.; Moore, R.E.; Patterson, G.M.L. Structures of Nostocyclophanes A–D. J. Org. Chem. 1991, 56, 4360–4364. [Google Scholar] [CrossRef]
- May, D.S.; Chen, W.-L.; Lantvit, D.D.; Zhang, X.; Krunic, A.; Burdette, J.E.; Eustaquio, A.; Orjala, J. Merocyclophanes C and D from the Cultured Freshwater Cyanobacterium Nostoc sp. (UIC 10110). J. Nat. Prod. 2017, 80, 1073–1080. [Google Scholar] [CrossRef]
- Preisitsch, M.; Heiden, S.E.; Beerbaum, M.; Niedermeyer, T.H.J.; Schneefeld, M.; Herrmann, J.; Kumpfmüller, J.; Thürmer, A.; Neidhardt, I.; Wiesner, C.; et al. Effects of Halide Ions on the Carbamidocyclophane Biosynthesis in Nostoc sp. CAVN2. Mar. Drugs 2016, 14, 21. [Google Scholar] [CrossRef]
- Preisitsch, M.; Niedermeyer, T.H.J.; Heiden, S.E.; Neidhardt, I.; Kumpfmüller, J.; Wurster, M.; Harmrolfs, K.; Wiesner, C.; Enke, H.; Müller, R.; et al. Cylindrofridins A–C, Linear Cylindrocyclophane-Related Alkylresorcinols from the Cyanobacterium Cylindrospermum stagnale. J. Nat. Prod. 2016, 79, 106–115. [Google Scholar] [CrossRef]
- Bobzin, S.C.; Moore, R.E. Biosynthetic Origin of [7.7]paracyclophanes from Cyanobacteria. Tetrahedron 1993, 49, 7615–7626. [Google Scholar] [CrossRef]
- Nakamura, H.; Hamer, H.A.; Sirasani, G.; Balskus, E.P. Cylindrocyclophane Biosynthesis Involves Functionalization of an Unactivated Carbon Center. J. Am. Chem. Soc. 2012, 134, 18518–18521. [Google Scholar] [CrossRef]
- Moore, B.S.; Chen, J.L.; Patterson, G.M.L.; Moore, R.E. Structures of Cylindrocyclophanes A-F. Tetrahedron 1992, 48, 3001–3006. [Google Scholar] [CrossRef]
- Schultz, E.E.; Braffman, N.R.; Luescher, M.U.; Hager, H.H.; Balskus, E.P. Biocatalytic Friedel–Crafts Alkylation Using a Promiscuous Biosynthetic Enzyme. Angew. Chem. Int. Ed. 2019, 58, 3151–3155. [Google Scholar] [CrossRef] [PubMed]
- Braffman, N.R.; Ruskoski, T.B.; Davis, K.M.; Glasser, N.R.; Johnson, C.; Okafor, C.D.; Boal, A.K.; Balskus, E.P. Structural Basis for an Unprecedented Enzymatic Alkylation in Cylindrocyclophane Biosynthesis. eLife 2022, 11, e75761. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Mou, S.-B.; Xiao, W.; Zhou, H.; Hou, X.-D.; Wang, S.-J.; Wang, Q.; Gao, J.; Wei, Z.; Liu, L.; et al. Structural Basis for the Friedel–Crafts Alkylation in Cylindrocyclophane Biosynthesis. ACS Catal. 2022, 12, 2108–2117. [Google Scholar] [CrossRef]
- Thanh, N.V.; Thao, N.P.; Phong, N.V.; Cuong, N.X.; Nam, N.H.; Minh, C.V. A New [7.7]paracyclophane from Vietnamese Marine Snail Planaxis sulcatus (Born, 1780). Nat. Prod. Res. 2020, 34, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, J.A.; Poynton, E.F.; Iskakova, D.; McMann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; McCadden, C.A.; et al. The Natural Products Atlas 2.0: A Database of Microbially-derived Natural Products. Nucleic Acids Res. 2021, 50, D1317–D1323. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Schultz, E.E.; Balskus, E.P. A New Strategy for Aromatic Ring Alkylation in Cylindrocyclophane Biosynthesis. Nat. Chem. Biol. 2017, 13, 916–921. [Google Scholar] [CrossRef]
- Sekine, M.; Kimura, T.; Katayama, Y.; Takahashi, D.; Toshima, K. The direct and one-pot transformation of xylan into the biodegradable surfactants, alkyl xylosides, is aided by an ionic liquid. RSC Adv. 2013, 3, 19756–19759. [Google Scholar] [CrossRef]
- Lee, J.; Kobayashi, Y.; Tezuka, K.; Kishi, Y. Toward Creation of a Universal NMR Database for the Stereochemical Assignment of Acyclic Compounds: Proof of Concept. Org. Lett. 1999, 1, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of Dolastatin 10 From The Marine Cyanobacterium Symploca Species VP642 And Total Stereochemistry And Biological Evaluation Of Its Analogue Symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyj, L.; Tomer, K.B.; Bontems, R.J. The Isolation and Structure Of A Remarkable Marine Animal Antineoplastic Constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Moore, R.E.; Cheuk, C.; Yang, X.Q.G.; Patterson, G.M.L.; Bonjouklian, R.; Smitka, T.A.; Mynderse, J.S.; Foster, R.S.; Jones, N.D. Hapalindoles, Antibacterial and Antimycotic Alkaloids from the Cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 1036–1043. [Google Scholar] [CrossRef]

| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 1 | 4.04, dd (10.7, 3.9) | 4.03, dd (10.5, 3.9) | 4.04, dd (10.7, 4.1) | 4.03, dd (10.8, 3.9) |
| 2 | 2.07, ddd (11.0, 10.7, 3.9) | 2.07, ddd (11.1, 10.1, 3.8) | 2.08, ddd (11.2, 10.2, 3.3) | 2.07, ddd (13.2, 10.8, 3.9) |
| 1.82, m | 1.83, m | 1.82, m | 1.83, m | |
| 3 | 2.82, t (10.7) | 2.82, t (10.1) | 2.83, t (10.2) | 2.80, t (10.8) |
| 4 | 1.59, m | 1.59, m | 1.59, m | 1.61, m |
| 1.46, m | 1.45, m | 1.46, m | 1.46, m | |
| 5 | 1.36, m | 1.36, m | 1.36, m | 1.35, m |
| 0.52, m | 0.53, m | 0.58, m | 0.54, m | |
| 6 | 1.82, m | 1.82, m | 1.82, m | 1.83, m |
| 1.52, m | 1.50, m | 1.55, m | 1.52, m | |
| 7 | 3.07, m | 3.07, m | 3.09, m | 3.07, m |
| 10 | 6.09, s | 6.09, s | 6.06, s | 6.10, s |
| 12 | 6.13, s | 6.19, s | 6.15, s | 6.15, s |
| 14 | 3.69, dd (10.3, 4.4) | 4.05, dd (9.7, 4.0) | 2.40, m | 4.03, dd (10.8, 3.9) |
| 15 | 1.66, m | 1.60, m | 1.60, m | 2.07, ddd (13.2, 10.8, 3.9) |
| 1.30, m | 1.30, m | 1.83, m | ||
| 16 | 1.11, m | 1.11, m | 2.54, m | 2.80, t (10.8) |
| 1.00, m | 1.00, m | |||
| 17 | 0.98, m | 0.98, m | 1.59, m | 1.61, m |
| 0.51, m | 0.52, m | 1.46, m | 1.46, m | |
| 18 | 0.88, m | 0.89, m | 1.36, m | 1.35, m |
| 0.32, m | 0.32, m | 0.58, m | 0.54, m | |
| 19 | 1.82, m | 1.82, m | 1.82, m | 1.83, m |
| 1.52, m | 1.52, m | 1.55, m | 1.52, m | |
| 20 | 3.07, m | 3.07, m | 3.09, m | 3.07, m |
| 23 | 6.08, s | 6.13, s | 6.10, s | 6.10, s |
| 25 | 5.99, s | 5.95, s | 5.93, s | 6.15, s |
| 27 | 1.80, m | 1.80, m | 1.82, m | 1.80, m |
| 1.38, m | 1.30, m | 1.38, m | 1.35, m | |
| 28 | 1.18, m | 1.18, m | 1.18, m | 1.18, m |
| 29 | 1.25, m | 1.25, m | 1.25, m | 1.23, m |
| 30 | 0.77, t (7.2) | 0.76, t (7.2) | 0.77, t (7.2) | 0.76, t (7.2) |
| 31 | 1.80, m | 1.80, m | 1.82, m | 1.80, m |
| 1.38, m | 1.30, m | 1.38, m | 1.54, m | |
| 32 | 1.18, m | 1.18, m | 1.18, m | 0.69, t (7.4) |
| 33 | 1.25, m | 1.25, m | 1.25, m | |
| 34 | 0.77, t (7.2) | 0.76, t (7.2) | 0.77, t (7.2) | |
| 1-OMe | 3.03, s | 3.03, s | 3.03, s | 3.07, s |
| 14-OMe | 3.01, s | 3.07, s |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δC, Mult. | δC, Mult. | δC, Mult. | δC, Mult. | |
| 1 | 81.0, CH | 81.0, CH | 81.0, CH | 80.9, CH |
| 2 | 45.5, CH2 | 45.5, CH2 | 45.5, CH2 | 45.5, CH2 |
| 3 | 62.9, CH | 62.9, CH | 62.9, CH | 62.8, CH |
| 4 | 40.3, CH2 | 40.0, CH2 | 40.0, CH2 | 40.2, CH2 |
| 5 | 26.8, CH2 | 26.6, CH2 | 26.7, CH2 | 26.7, CH2 |
| 6 | 33.3, CH2 | 33.4, CH2 | 33.2, CH2 | 32.7, CH2 |
| 7 | 34.8, CH | 34.9, CH | 34.7, CH | 34.9, CH |
| 8 | 115.5, qC | 114.7, qC | 116.2, qC | 115.8, qC |
| 9 | 155.6, qC | 155.4, qC | 155.7, qC | 155.7, qC |
| 10 | 102.8, CH | 103.5, CH | 102.8, CH | 102.8, CH |
| 11 | 139.6, qC | 143.6, qC | 137.7, qC | 138.1, qC |
| 12 | 107.5, CH | 107.3, CH | 107.3, CH | 107.3, CH |
| 13 | 157.6, qC | 157.1, qC | 157.7, qC | 157.5, qC |
| 14 | 83.6, CH | 73.5, CH | 31.2, CH2 | 80.9, CH |
| 15 | 37.0, CH2 | 39.0, CH2 | 40.0, CH2 | 45.5, CH2 |
| 16 | 30.1, CH2 | 30.2, CH2 | 64.2, CH | 62.8, CH |
| 17 | 30.2, CH2 | 30.7, CH2 | 40.0, CH2 | 40.2, CH2 |
| 18 | 26.6, CH2 | 27.0, CH2 | 27.0, CH2 | 26.7, CH2 |
| 19 | 32.7, CH2 | 32.8, CH2 | 33.2, CH2 | 32.5, CH2 |
| 20 | 34.9, CH | 34.9, CH | 34.8, CH | 37.0, CH |
| 21 | 116.3, qC | 116.4, qC | 116.2, qC | 115.8, qC |
| 22 | 155.5, qC | 155.7, qC | 155.7, qC | 155.7, qC |
| 23 | 102.8, CH | 102.8, CH | 102.8, CH | 102.8, CH |
| 24 | 137.9, qC | 138.0, qC | 137.7, qC | 138.1, qC |
| 25 | 107.2, CH | 106.1, CH | 107.3, CH | 107.3, CH |
| 26 | 157.3, qC | 157.6, qC | 157.7, qC | 157.5, qC |
| 27 | 32.7, CH2 | 33.0, CH2 | 32.8, CH2 | 32.8, CH2 |
| 28 | 29.5, CH2 | 29.6, CH2 | 30.2, CH2 | 30.2, CH2 |
| 29 | 22.3, CH2 | 22.2, CH2 | 22.2, CH2 | 22.2, CH2 |
| 30 | 14.1, CH3 | 14.1, CH3 | 14.1, CH3 | 14.0, CH3 |
| 31 | 32.9, CH2 | 33.3, CH2 | 32.8, CH2 | 25.8, CH2 |
| 32 | 30.5, CH2 | 30.2, CH2 | 30.2, CH2 | 13.0, CH3 |
| 33 | 22.2, CH2 | 22.3, CH2 | 22.2, CH2 | |
| 34 | 14.1, CH3 | 14.1, CH3 | 14.1, CH3 | |
| 1-OMe | 55.7, CH3 | 55.7, CH3 | 55.8, CH3 | 55.7, CH3 |
| 14-OMe | 55.5, CH3 |
| Position | 5, Major | 5, Major | 5, Minor | 5, Minor |
|---|---|---|---|---|
| δH (J in Hz) | δc, Mult. | δH (J in Hz) | δc, Mult. | |
| 1 | 4.05, dd (10.7, 3.4) | 81.0, CH | 4.04, dd (10.7, 3.9) | 80.9, CH |
| 2 | 2.08, m | 45.6, CH2 | 2.06, m | 45.5, CH2 |
| 1.68, m | 1.80, m | |||
| 3 | 2.79, m | 62.7, CH | 2.82, m | 62.6, CH |
| 4 | 1.58, m | 39.6, CH2 | 1.47, m | 39.6, CH2 |
| 1.42, m | 1.36, m | |||
| 5 | 1.46, m | 26.7, CH2 | 1.34, m | 27.0, CH2 |
| 0.53, m | 0.45, m | |||
| 6 | 1.84, m | 32.4, CH2 | 2.12, m | 32.4, CH2 |
| 1.55, m | 1.58, m | |||
| 7 | 3.10, m | 35.1, CH | 3.08, m | 34.9, CH |
| 8 | 119.7, qC | 118.2, qC | ||
| 9 | 157.8, qC | 157.5, qC | ||
| 10 | 6.32, s | 100.8, CH | 6.42, s | 102.6, CH |
| 11 | 138.4, qC | 138.5, qC | ||
| 12 | 6.31, s | 107.4, CH | 6.30, s | 109.3, CH |
| 13 | 155.8, qC | 156.2, qC | ||
| 14 | 4.10, dd (10.7, 3.8) | 81.2, CH | 4.15, dd (10.7, 3.9) | 81.0, CH |
| 15 | 2.08, m | 45.9, CH2 | 2.08, m | 45.6, CH2 |
| 1.85, m | 1.80, m | |||
| 16 | 2.91, m | 63.1, CH | 2.79, m | 62.9, CH |
| 17 | 1.57, m | 40.2, CH2 | 1.47, m | 40.2, CH2 |
| 1.42, m | 1.36, m | |||
| 18 | 1.23, m | 26.2, CH2 | 1.27, m | 26.8, CH2 |
| 0.51, m | 0.54, m | |||
| 19 | 1.84, m | 33.1, CH2 | 1.85, m | 33.0, CH2 |
| 1.55, m | 1.50, m | |||
| 20 | 3.11, m | 35.0, CH | 3.11, m | 34.8, CH |
| 21 | 116.2, qC | 116.2, qC | ||
| 22 | 155.6, qC | 155.2, qC | ||
| 23 | 6.10, s | 102.7, CH | 6.09, s | 102.9, CH |
| 24 | 138.2, qC | 138.3, qC | ||
| 25 | 6.15, s | 107.0, CH | 6.14, s | 107.5, CH |
| 26 | 157.6, qC | 157.3, qC | ||
| 27 | 2.09, m | 32.7, CH2 | 1.72, m | 32.8, CH2 |
| 1.50, m | 1.35, m | |||
| 28 | 1.18, m | 30.2, CH2 | 1.18, m | 30.0, CH2 |
| 1.02, m | 1.02, m | |||
| 29 | 1.22, m | 22.3, CH2 | 1.22, m | 22.3, CH2 |
| 1.13, m | 1.13, m | |||
| 30 | 0.77, t (6.5) | 14.1, CH3 | 0.77, t (6.5) | 14.1, CH3 |
| 31 | 1.82, m | 32.5, CH2 | 1.72, m | 32.6, CH2 |
| 1.46, m | 1.35, m | |||
| 32 | 1.18, m | 30.2, CH2 | 1.18, m | 30.2, CH2 |
| 1.02, m | 1.02, m | |||
| 33 | 1.22, m | 22.3, CH2 | 1.22, m | 22.6, CH2 |
| 1.13, m | 1.12, m | |||
| 34 | 0.76, t (6.8) | 14.1, CH3 | 0.76, t (6.8) | 14.1, CH3 |
| 35 | 4.93, d (7.0) | 99.6, CH | 4.62, d (7.0) | 105.6, CH |
| 36 | 3.19, dd (8.8, 7.0) | 73.3, CH | 3.19, dd (8.8, 7.0) | 73.5, CH |
| 37 | 3.23, t (8.8) | 76.6, CH | 3.23, t (8.8) | 76.8, CH |
| 38 | 3.29, ddd (10.9, 8.8, 5.0) | 69.3, CH | 3.29, ddd (10.9, 8.8, 5.0) | 69.6, CH |
| 39 | 3.67, dd (10.9, 5.0) | 65.4, CH2 | 3.58, dd (10.9, 5.0) | 65.7, CH2 |
| 3.12, t (10.9) | 3.12, t (10.9) | |||
| 1-OMe | 3.04, s | 55.9, CH3 | 3.04, s | 55.9, CH3 |
| 14-OMe | 3.04, s | 55.8, CH3 | 3.04, s | 55.8, CH3 |
| Compound | GI50 (μM) |
|---|---|
| 1 | 0.72 |
| 2 | 0.94 |
| 3 | 5.1 |
| 4 | 1.2 |
| 5 | 1.7 |
| 6 | 8.2 |
| Nostocyclophane C | 0.95 |
| Position | 6 (DMSO-d6) | 6 (CDCl3) | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Mult. | δH (J in Hz) | δC, Mult. | |
| 1 | 4.03, m | 80.5, CH | 4.22, dd (8.7, 5.5) | 81.2, CH |
| 2 | 2.00, m | 45.6, CH2 | 2.14, ddd (15.3, 10.5, 5.3) | 45.3, CH2 |
| 1.83, m | 1.87, ddd (15.3, 8.7, 3.6) | |||
| 3 | 3.65, m | 61.0, CH | 3.52, m | 59.89, CH |
| 4 | 1.68, m | 37.5, CH2 | 1.74, m | 38.0, CH2 |
| 5 | 1.30, m | 24.2, CH2 | 1.30, m | 24.2, CH2 |
| 1.11, m | 1.20, m | |||
| 6 | 1.85, m | 32.7, CH2 | 1.84, m | 33.3, CH2 |
| 1.55, m | 1.62, m | |||
| 7 | 3.10, m | 33.9, CH | 3.09, m | 35.4, CH |
| 8 | 116.0, qC | 117.5, qC | ||
| 9 | 155.5, qC | 155.6, qC | ||
| 10 | 6.18, s | 104.5, CH | 6.43, s | 106.4, CH |
| 11 | 138.5, qC | 139.1, qC | ||
| 12 | 6.09, s | 104.5, CH | 6.43, s | 106.4, CH |
| 13 | 155.5, qC | 155.6, qC | ||
| 14 | 4.05, m | 80.5, CH | 4.27, dd (8.7, 5.3) | 81.3, CH |
| 15 | 2.14, m | 45.7, CH2 | 2.28, ddd (14.6, 9.7, 5.5) | 44.8, CH2 |
| 1.80, m | 1.96, ddd (14.6, 8.7, 3.5) | |||
| 16 | 3.35, m | 61.1, CH | 3.59, m | 59.88, CH |
| 17 | 1.68, m | 37.1, CH2 | 1.68, m | 37.8, CH2 |
| 18 | 1.64, m | 23.0, CH2 | 1.72, m | 23.2, CH2 |
| 1.33, m | 1.46, m | |||
| 19 | 1.64, m | 36.9, CH2 | 1.60, m | 37.9, CH2 |
| 20 | 3.97, m | 64.5, CH | 3.86, m | 64.0, CH |
| 21 | 6.09, s | 102.0, CH | 6.30, s | 102.6, CH |
| 22 | 158.5, qC | 157.0, qC | ||
| 23 | 6.11, s | 104.5, CH | 6.43, s | 106.4, CH |
| 24 | 142.9, qC | 143.5, qC | ||
| 25 | 6.18, s | 104.5, CH | 6.43, s | 106.4, CH |
| 26 | 158.5, qC | 157.0, qC | ||
| 27 | 1.74, m | 31.8, CH2 | 1.68, m | 38.2, CH2 |
| 1.48, m | 1.61, m | |||
| 28 | 1.00, m | 30.3, CH2 | 1.28, m | 30.6, CH2 |
| 1.11, m | 1.17, m | |||
| 29 | 1.24, m | 22.3, CH2 | 1.23, m | 22.0, CH2 |
| 1.17, m | ||||
| 30 | 0.77, t (7.1) | 14.1, CH3 | 0.83, t (7.1) | 14.1, CH3 |
| 31 | 1.64, m | 37.0, CH2 | 1.80, m 1.55, m | 32.3, CH2 |
| 32 | 1.40, m | 28.0, CH2 | 1.30, m | 28.7, CH2 |
| 33 | 1.25, m | 21.7, CH2 | 1.35, m 1.30, m | 22.2, CH2 |
| 34 | 0.85, t (7.2) | 13.9, CH3 | 0.90, t (7.2) | 13.9, CH3 |
| 1-OMe | 3.04, s | 55.8, CH3 | 3.20, s | 56.5, CH3 |
| 14-OMe | 3.05, s | 55.8, CH3 | 3.26, s | 56.6, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Philbin, C.S.; Wakano, C.; Yoshida, W.Y.; Williams, P.G. New Nostocyclophanes from Nostoc linckia. Mar. Drugs 2023, 21, 101. https://doi.org/10.3390/md21020101
Dai J, Philbin CS, Wakano C, Yoshida WY, Williams PG. New Nostocyclophanes from Nostoc linckia. Marine Drugs. 2023; 21(2):101. https://doi.org/10.3390/md21020101
Chicago/Turabian StyleDai, Jingqiu, Casey S. Philbin, Clay Wakano, Wesley Y. Yoshida, and Philip G. Williams. 2023. "New Nostocyclophanes from Nostoc linckia" Marine Drugs 21, no. 2: 101. https://doi.org/10.3390/md21020101
APA StyleDai, J., Philbin, C. S., Wakano, C., Yoshida, W. Y., & Williams, P. G. (2023). New Nostocyclophanes from Nostoc linckia. Marine Drugs, 21(2), 101. https://doi.org/10.3390/md21020101






