Alcalase-Assisted Mytilus edulis Hydrolysate: A Nutritional Approach for Recovery from Muscle Atrophy
Abstract
:1. Introduction
2. Results
2.1. Proximate Composition Analysis
2.2. Effect of EMHs on the Viability, Proliferation, and Dexamethasone-Induced Proliferation of C2C12
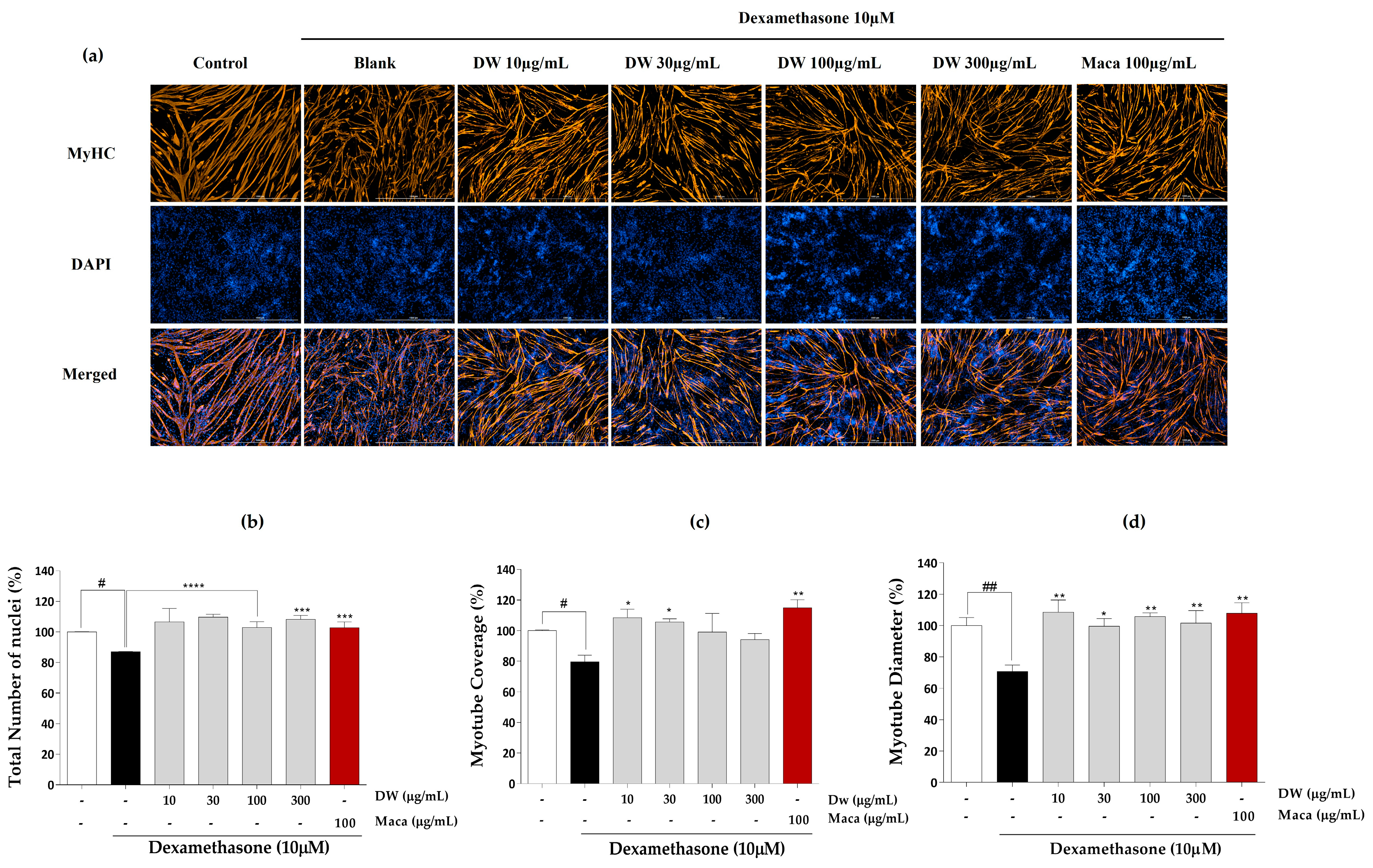
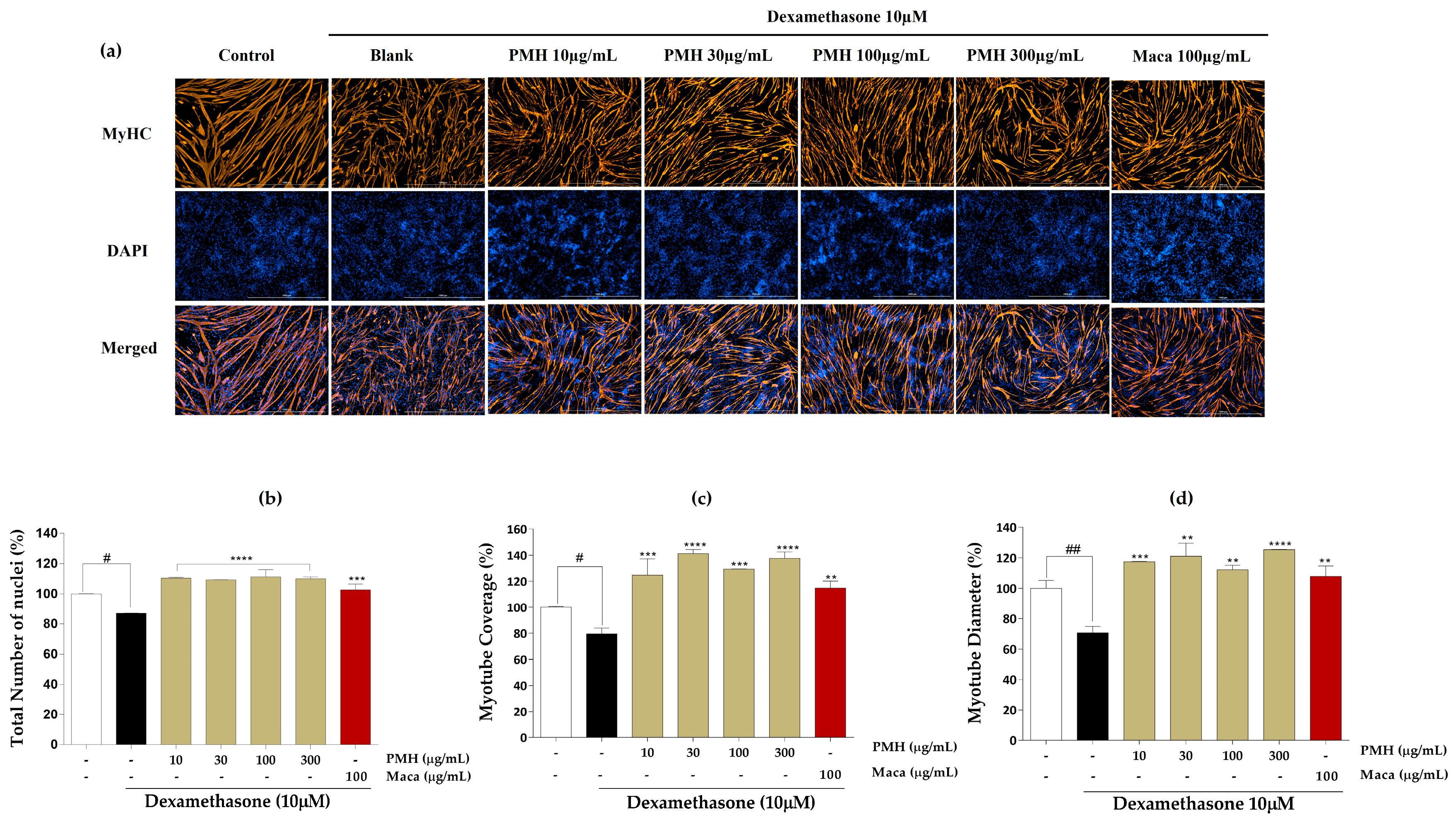
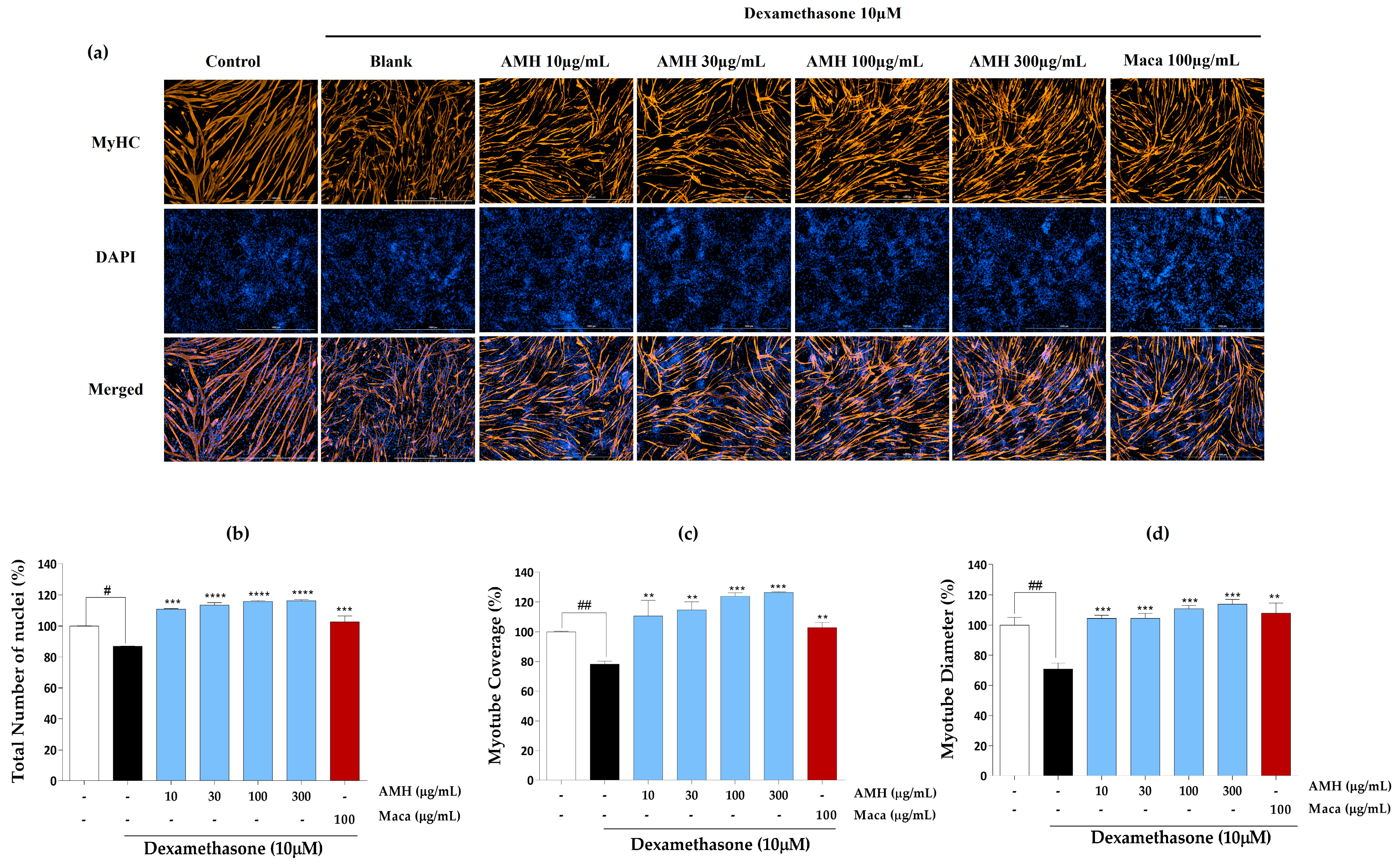
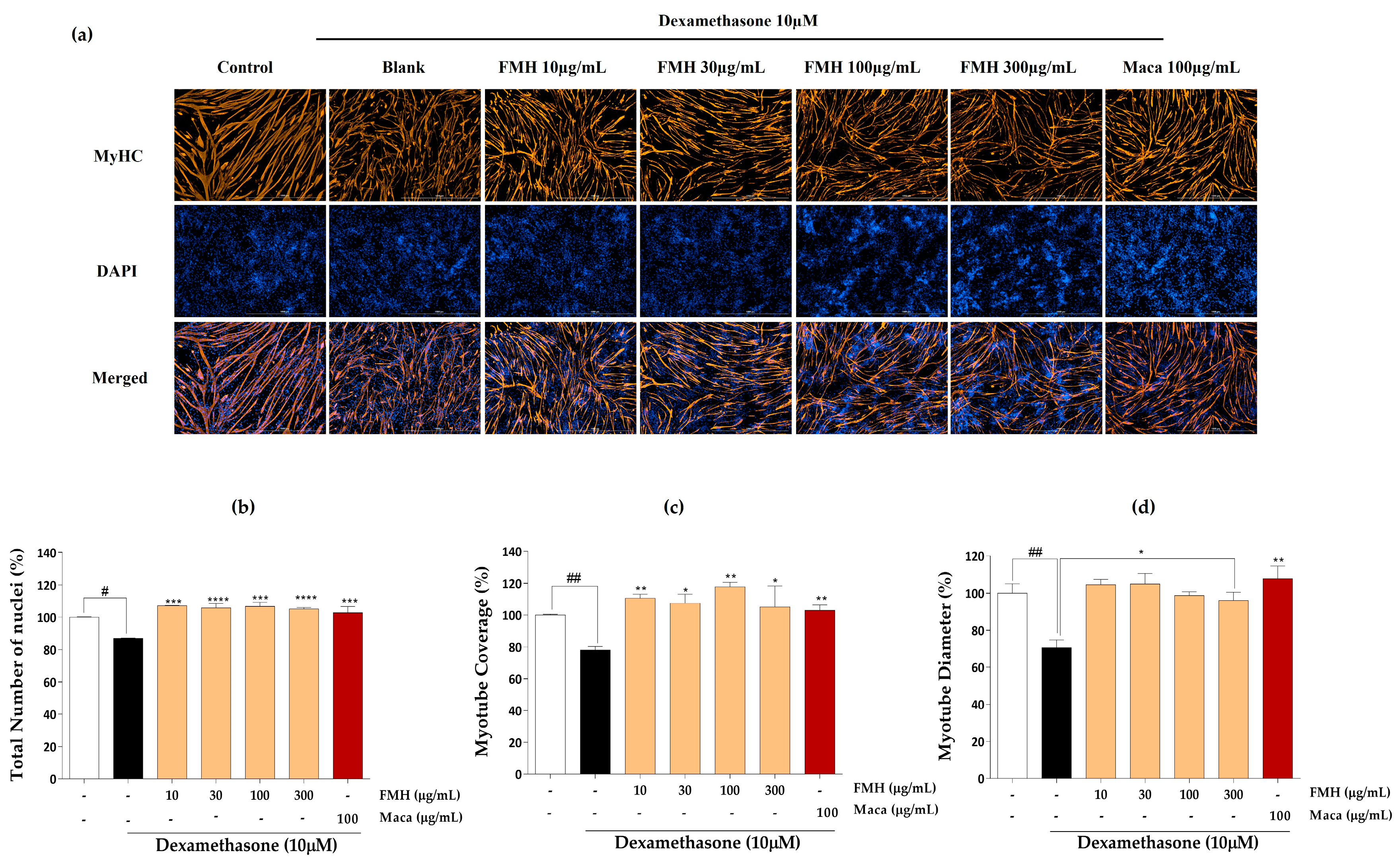
2.3. Effect of EMHs on Differentiation of C2C12 Myotubes
2.4. Effect of AMH on the Expression of Proteins Responsible for Muscle Hypertrophy and Atrophy
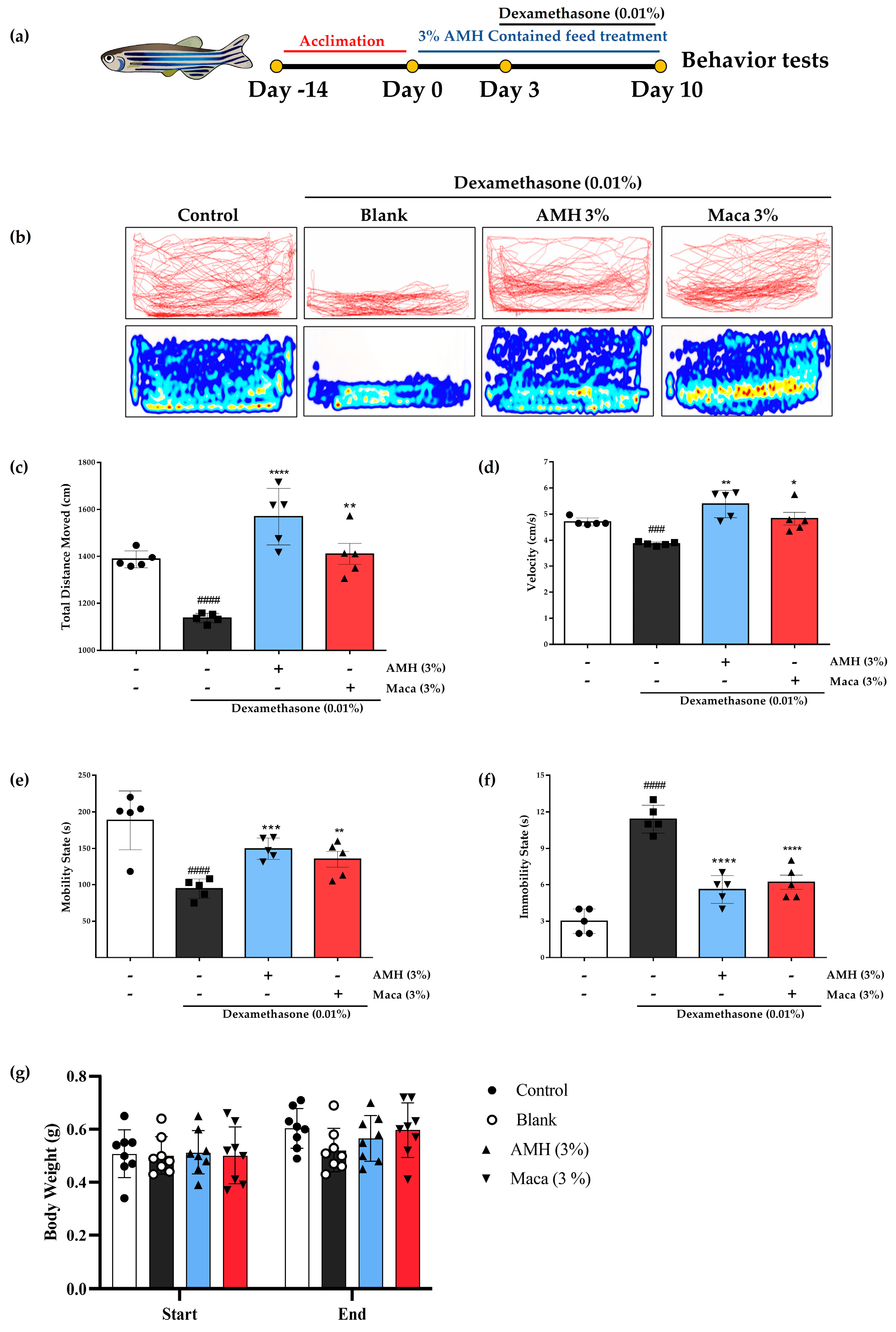
2.5. Recovery of Dex-Induced Muscle Atrophy in Zebrafish by AMH Administration
2.6. Amino Acid Composition of AMH36
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Cell Lines and Cell Culture
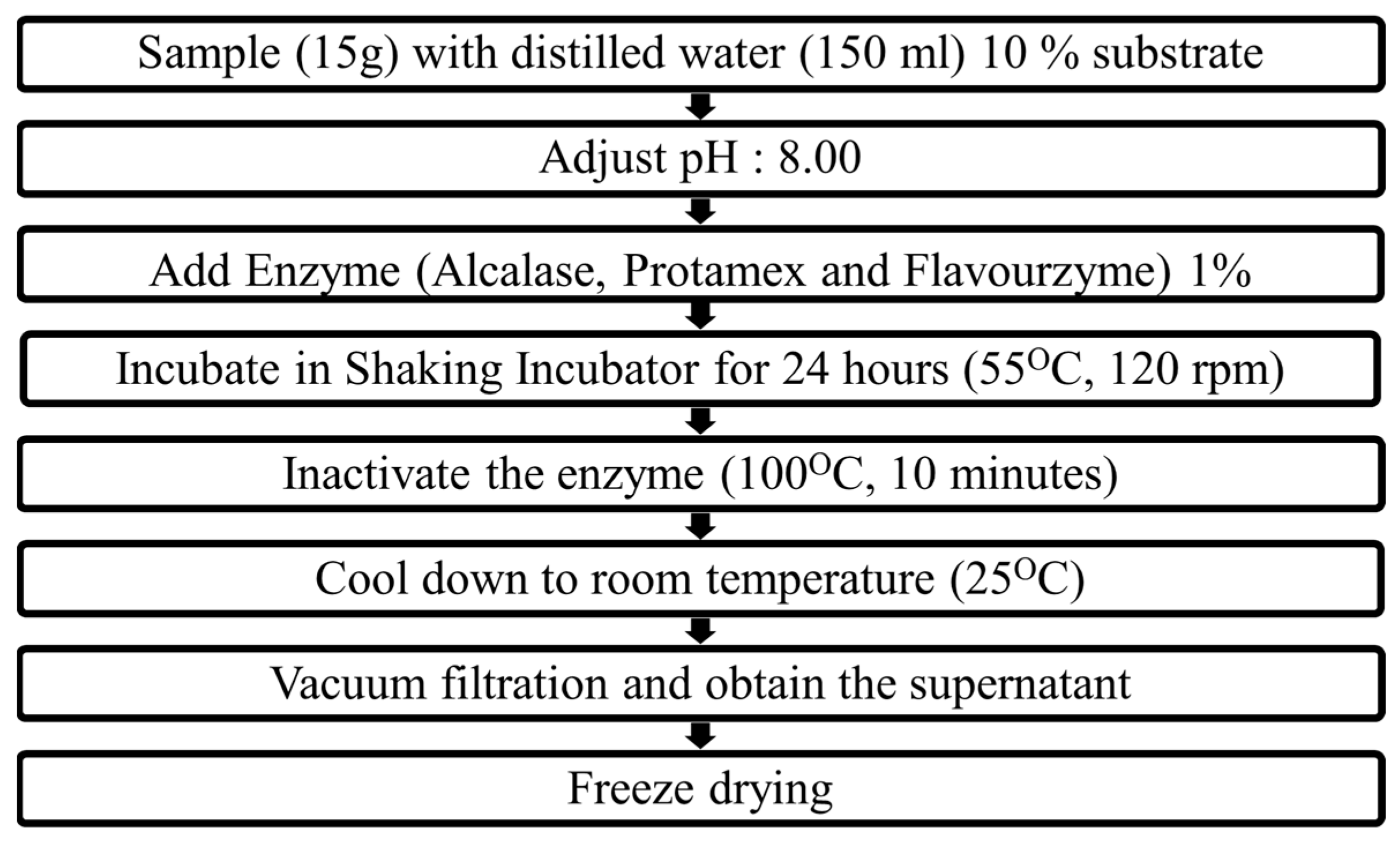
5.3. Preparation of Enzyme-Assisted Hydrolysates of M. edulis
5.4. Proximate Composition Analysis
5.4.1. Measurement of Hydrolysate Yield
5.4.2. Measurement of Protein Content
5.4.3. Measurement of Lipid Content
5.4.4. Measurement of the Ash Content
5.5. Evaluation of Cytotoxicity
5.6. Evaluation of Cell Proliferation
5.7. Immunofluorescence Staining
5.8. Western Blot Analysis
5.9. Zebrafish Experiments
5.10. Determination of Amino Acid Composition of AMH
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Felipe, A.; López-Soriano, F.J. Molecular mechanisms involved in muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Int. J. Biochem. Cell Biol. 2005, 37, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; von Haehling, S.; Kalantar-Zadeh, K.; Morley, J.E.; Anker, S.D.; Lainscak, M. Cachexia as a major public health problem: Frequent, costly, and deadly. J. Cachex-Sarcopenia Muscle 2013, 4, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.; Sun, P.Y.; Davies, K.J.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Glass, D.; Roubenoff, R. Recent advances in the biology and therapy of muscle wasting. Ann. N. Y. Acad. Sci. 2010, 1211, 25–36. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Gökoğlu, N. Molluscan Shellfish. In Shellfish Processing and Preservation; Gökoğlu, N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–250. [Google Scholar] [CrossRef]
- Monfort, M.-C. The European market for mussels. Globefish Res. Programme 2014, 115, 2014. [Google Scholar]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive value, health benefits, and consumer safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef]
- Ibañez, E.; Ibáñez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria, and Invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Hayes, M., Ed.; Springer: Boston, MA, USA, 2012; pp. 55–98. [Google Scholar]
- Park, S.Y.; Ahn, C.-B.; Je, J.-Y. Antioxidant and Anti-Inflammatory Activities of Protein Hydrolysates from Mytilus Edulis and Ultrafiltration Membrane Fractions. J. Food Biochem. 2014, 38, 460–468. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M.-E. Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products. Mar. Drugs 2013, 11, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W.; Park, J.Y.; Seo, J.-K.; Kim, D.-G. Purification and characterization of an antimicrobial peptide mytichitin-chitin binding domain from the hard-shelled mussel, Mytilus coruscus. Fish Shellfish. Immunol. 2018, 83, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Kim, S.-K. Isolation and characterisation of an anticoagulant oligopeptide from blue mussel, Mytilus edulis. Food Chem. 2009, 117, 687–692. [Google Scholar] [CrossRef]
- Gray, J.; Armstrong, G.; Farley, H. Opportunities and constraints in the functional food market. Nutr. Food Sci. 2003, 33, 213–218. [Google Scholar] [CrossRef]
- Cobb, C.S.; Ernst, E. Systematic review of a marine nutriceutical supplement in clinical trials for arthritis: The effectiveness of the New Zealand green-lipped mussel Perna canaliculus. Clin. Rheumatol. 2006, 25, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Yang, H.-W.; Je, J.-G.; Lee, H.-G.; Kim, G.H.; Jeon, Y.-J. The potent antioxidant effect of Neutrase-assisted hydrolysate from heat-resistant Pyropia yezoensis by molecular weight change. Algal Res. 2023, 69, 102894. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; McLellan, T.M.; Lieberman, H.R. The Effects of Protein Supplements on Muscle Mass, Strength, and Aerobic and Anaerobic Power in Healthy Adults: A Systematic Review. Sports Med. 2015, 45, 111–131. [Google Scholar] [CrossRef]
- Ryu, B.; Je, J.-G.; Jeon, Y.-J.; Yang, H.-W. Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells 2021, 10, 2879. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Yoshikawa, M.; Sugimoto, T.; Tomoo, K.; Okada, Y.; Hashimoto, T. Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. Int. J. Mol. Sci. 2022, 23, 6825. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, J.A.; Eric, C.R. Characterization of casein phosphopeptides prepared using alcalase: Determination of enzyme specificity. Enzym. Microb. Technol. 1996, 19, 202–207. [Google Scholar]
- Kun, W.; Susan, D.A. Modification of interactions between selected volatile flavour compounds and salt-extracted pea protein isolates using chemical and enzymatic approaches. Food Hydrocoll. 2016, 61, 567–577. [Google Scholar]
- Gbogouri, G.; Linder, M.; Fanni, J.; Parmentier, M. Influence of Hydrolysis Degree on the Functional Properties of Salmon Byproducts Hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Zheng, H.; Shen, X.; Bu, G.; Luo, Y. Effects of pH, temperature and enzyme-to-substrate ratio on the antigenicity of whey protein hydrolysates prepared by Alcalase. Int. Dairy J. 2008, 18, 1028–1033. [Google Scholar] [CrossRef]
- Zhang, Y.; Dutilleul, P.; Orsat, V.; Simpson, B.K. Alcalase assisted production of novel high alpha-chain gelatin and the functional stability of its hydrogel as influenced by thermal treatment. Int. J. Biol. Macromol. 2018, 118, 2278–2286. [Google Scholar] [CrossRef]
- Kimberle, P.d.S.; Carolina, M.-S.; Ana, I.S.B.; Luciana, R.B.G. Modifying alcalase activity and stability by immobilization onto chitosan aiming at the production of bioactive peptides by hydrolysis of tilapia skin gelatin. Process. Biochem. 2020, 97, 27–36. [Google Scholar] [CrossRef]
- Yang, A.; Long, C.; Xia, J.; Tong, P.; Cheng, Y.; Wang, Y.; Chen, H. Enzymatic characterisation of the immobilised Alcalase to hydrolyse egg white protein for potential allergenicity reduction. J. Sci. Food Agric. 2017, 97, 199–206. [Google Scholar] [CrossRef]
- Waglay, A.; Karboune, S. Enzymatic generation of peptides from potato proteins by selected proteases and characterization of their structural properties. Biotechnol. Prog. 2016, 32, 420–429. [Google Scholar] [CrossRef]
- Fernández, A.; Grienke, U.; Soler-Vila, A.; Guihéneuf, F.; Stengel, D.B.; Tasdemir, D. Seasonal and geographical variations in the biochemical composition of the blue mussel (Mytilus edulis L.) from Ireland. Food Chem. 2015, 177, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dare, P.; Edwards, D. Seasonal changes in flesh weight and biochemical composition of mussels (Mytilus edulis L.) in the Conwy Estuary, North Wales. J. Exp. Mar. Biol. Ecol. 1975, 18, 89–97. [Google Scholar] [CrossRef]
- Karayücel, S.; Kaya, Y.; Karayücel, İ. Effect of environmental factors on biochemical composition and condition index in the Medieterranean Mussel (Mytilus gallaprovincialis Lamarck, 1819) in the Sinop Region. Turk. J. Vet. Anim. Sci. 2003, 27, 1391–1396. [Google Scholar]
- Di Pasquale, M.G. Amino Acids and Proteins for the Athlete: The Anabolic Edge; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Matsui, T.; Soya, H. Brain Glycogen Decrease and Supercompensation with Prolonged Exhaustive Exercise. In Social Neuroscience and Public Health: Foundations for the Science of Chronic Disease Prevention; Hall, P.A., Ed.; Springer: New York, NY, USA, 2013; pp. 253–264. [Google Scholar]
- Millard-Stafford, M.; Childers, W.L.; Conger, S.A.; Kampfer, A.J.; Rahnert, J.A. Recovery Nutrition: Timing and Composition after Endurance Exercise. Curr. Sports Med. Rep. 2008, 7, 193–201. [Google Scholar] [CrossRef]
- Moore, D.R. Nutrition to Support Recovery from Endurance Exercise: Optimal Carbohydrate and Protein Replacement. Curr. Sports Med. Rep. 2015, 14, 294–300. [Google Scholar] [CrossRef]
- Phillips, S.M.; van Loon, L.J. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, Y.; Su, Y.; Chen, M. 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for skeletal muscle tissue engineering. Mater. Sci. Eng. C 2020, 116, 111070. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Soundharrajan, I.; Kim, D.H.; Hwang, I.; Choi, K.C. 4-hydroxy-3-methoxy cinnamic acid accelerate myoblasts differentiation on C2C12 mouse skeletal muscle cells via AKT and ERK 1/2 activation. Phytomedicine 2019, 60, 152873. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, H.-S.; Cho, M.; Jeon, Y.-J. Enzymatic Hydrolysates of Hippocampus abdominalis Regulates the Skeletal Muscle Growth in C2C12 Cells and Zebrafish Model. J. Aquat. Food Prod. Technol. 2019, 28, 264–274. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Jeon, Y.-J.; Kim, Y.-T.; Je, J.-Y. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process. Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Xu, Y.; Galanopoulos, M.; Sismour, E.; Ren, S.; Mersha, Z.; Lynch, P.; Almutaimi, A. Effect of enzymatic hydrolysis using endo- and exo-proteases on secondary structure, functional, and antioxidant properties of chickpea protein hydrolysates. J. Food Meas. Charact. 2020, 14, 343–352. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Reznick, A.Z. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free. Radic. Biol. Med. 2016, 98, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-K.; Lin, H.-Y.; Chen, C.-J.; Jhuo, C.-F.; Liao, K.-Y.; Chen, W.-Y.; Tzen, J.T. Promotion of myotube differentiation and attenuation of muscle atrophy in murine C2C12 myoblast cells treated with teaghrelin. Chem. Interactions 2020, 315, 108893. [Google Scholar] [CrossRef] [PubMed]
- Grand, T.I. Body weight: Its relation to tissue composition, segment distribution, and motor function. I. Interspecific comparisons. Am. J. Phys. Anthropol. 1977, 47, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Kang, S.-I.; Lee, S.-W.; Amarasiri, R.P.G.S.K.; Nagahawatta, D.P.; Roh, Y.; Wang, L.; Ryu, B.; Jeon, Y.-J. Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement. Antioxidants 2023, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Tatpati, L.L.; Irving, B.A.; Tom, A.; Bigelow, M.L.; Klaus, K.; Short, K.R.; Nair, K.S. The Effect of Branched Chain Amino Acids on Skeletal Muscle Mitochondrial Function in Young and Elderly Adults. J. Clin. Endocrinol. Metab. 2010, 95, 894–902. [Google Scholar] [CrossRef]
- Pasin, G.; Miller, S. US Whey products and sports nutrition. In Sports Nutrition; U.S. Dairy Export Counci: Arlington, VA, USA, 2000; pp. 1–6. [Google Scholar]
- Wang, X.; Proud, C.G. The mTOR Pathway in the Control of Protein Synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.M.; Kee, N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006, 1, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Noë, S.; Corvelyn, M.; Willems, S.; Costamagna, D.; Aerts, J.-M.; Van Campenhout, A.; Desloovere, K. The Myotube Analyzer: How to assess myogenic features in muscle stem cells. Skelet. Muscle 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Gilani, G.S. Amino acid analysis. Curr. Protoc. Protein Sci. 2009, 58, 11.9.1–11.9.37. [Google Scholar] [CrossRef]


| Sample | Hydrolysate Yield (%) | Proximate Composition (%) | |||
|---|---|---|---|---|---|
| Protein | Lipid | Polysaccharide | Ash | ||
| DW | 44.40 ± 1.60 | 43.61 ± 1.36 | 5.86 ± 0.56 | 30.20 ± 1.38 | 20.32 ± 0.15 |
| PMH | 72.90 ± 2.90 *** | 56.18 ± 0.77 ** | 6.48 ± 0.18 | 21.44 ± 1.27 ** | 15.90 ± 0.03 *** |
| AMH | 76.37 ± 0.77 *** | 57.33 ± 2.40 ** | 6.62 ± 0.03 | 21.61 ± 0.53 ** | 14.70 ± 0.28 *** |
| FMH | 63.22 ± 2.02 *** | 51.95 ± 0.75 * | 6.19 ± 0.17 | 23.51 ± 0.80 ** | 18.51 ± 0.09 *** |
| Amino Acid (AA) | mg/100 g |
|---|---|
| Arginine (ARG) | 3062 |
| Histidine (HIS) | 824 |
| Isoleucine (ISO) | 1977 |
| Leucine (LEU) | 3016 |
| Lysine (LYS) | 3718 |
| Methionine (MET) | 896 |
| Phenylalanine (PHE) | 1694 |
| Threonine (THR) | 231 |
| Tryptophan (TRY) | 394 |
| Valine (VAL) | 2276 |
| Alanine (ALA) | 2668 |
| Cystine (CYS) | 32 |
| Glutamic acid (GLA) | 5883 |
| Proline (PRO) | 1918 |
| Serine (SER) | 2482 |
| Tyrosine (TYR) | 1332 |
| Glycine (GLY) | 3732 |
| Aspartic acid (ASA) | 4656 |
| Total | 43,158 |
| EAA | 20,167 (46.73% of Total AAs) |
| Non-EAA | 22,991 (53.27% of Total AAs) |
| NCAA | 10,539 (24.42% of Total AAs) |
| AAA | 342 (7.92% of Total AAs) |
| BCAA | 7269 (16.84% of Total AAs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarasiri, R.P.G.S.K.; Hyun, J.; Lee, S.-W.; Kim, J.; Jeon, Y.-J.; Lee, J.-S. Alcalase-Assisted Mytilus edulis Hydrolysate: A Nutritional Approach for Recovery from Muscle Atrophy. Mar. Drugs 2023, 21, 623. https://doi.org/10.3390/md21120623
Amarasiri RPGSK, Hyun J, Lee S-W, Kim J, Jeon Y-J, Lee J-S. Alcalase-Assisted Mytilus edulis Hydrolysate: A Nutritional Approach for Recovery from Muscle Atrophy. Marine Drugs. 2023; 21(12):623. https://doi.org/10.3390/md21120623
Chicago/Turabian StyleAmarasiri, R. P. G. S. K., Jimin Hyun, Sang-Woon Lee, Jin Kim, You-Jin Jeon, and Jung-Suck Lee. 2023. "Alcalase-Assisted Mytilus edulis Hydrolysate: A Nutritional Approach for Recovery from Muscle Atrophy" Marine Drugs 21, no. 12: 623. https://doi.org/10.3390/md21120623
APA StyleAmarasiri, R. P. G. S. K., Hyun, J., Lee, S.-W., Kim, J., Jeon, Y.-J., & Lee, J.-S. (2023). Alcalase-Assisted Mytilus edulis Hydrolysate: A Nutritional Approach for Recovery from Muscle Atrophy. Marine Drugs, 21(12), 623. https://doi.org/10.3390/md21120623






