Unveiling the Therapeutic Potential and Healthcare Applications of Marine Therapy: A Systematic Review with Meta-Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Literature Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Assessment of Potential Publication Bias
2.6. Quality Assessment
3. Results
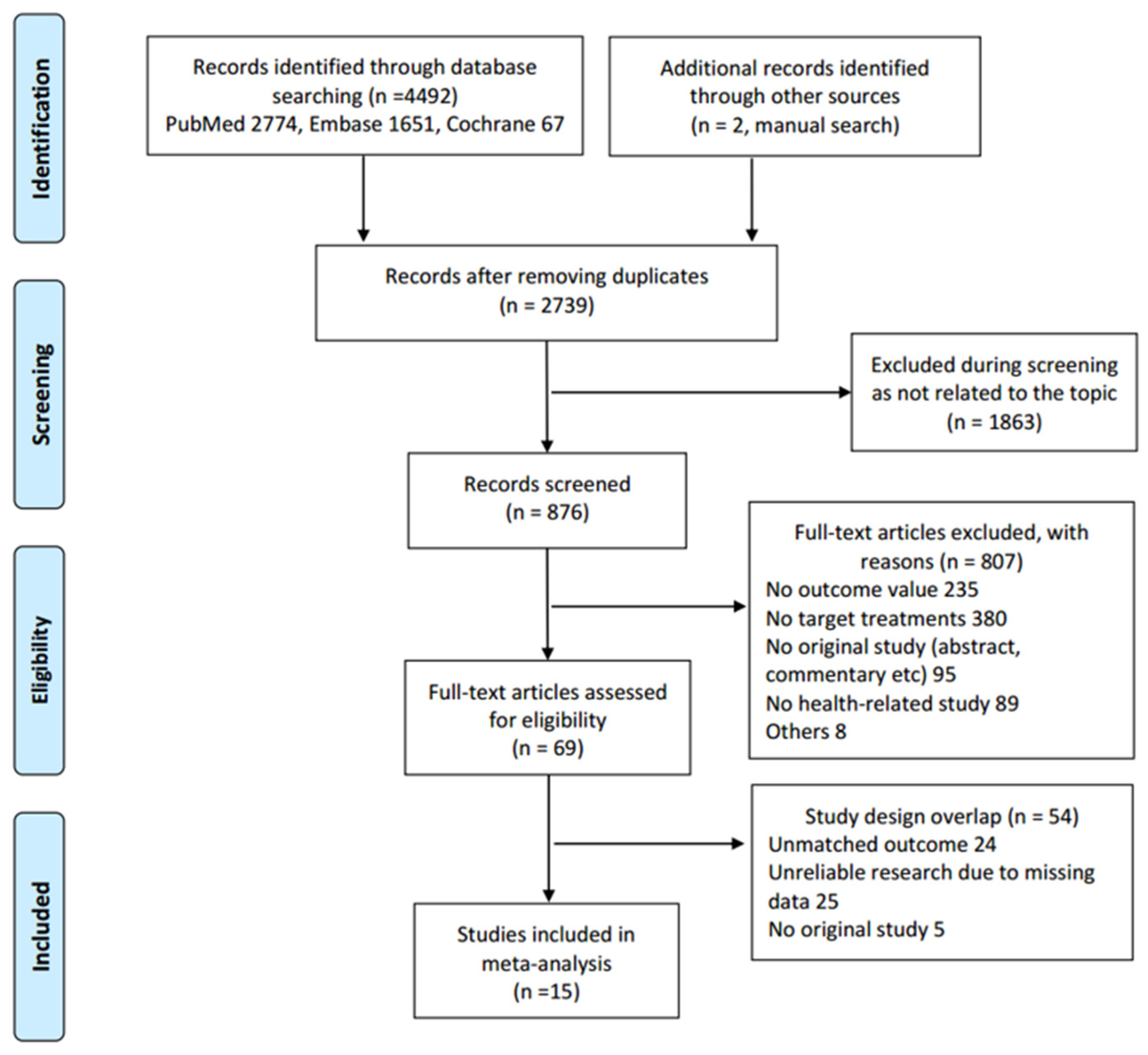
3.1. Study Selection
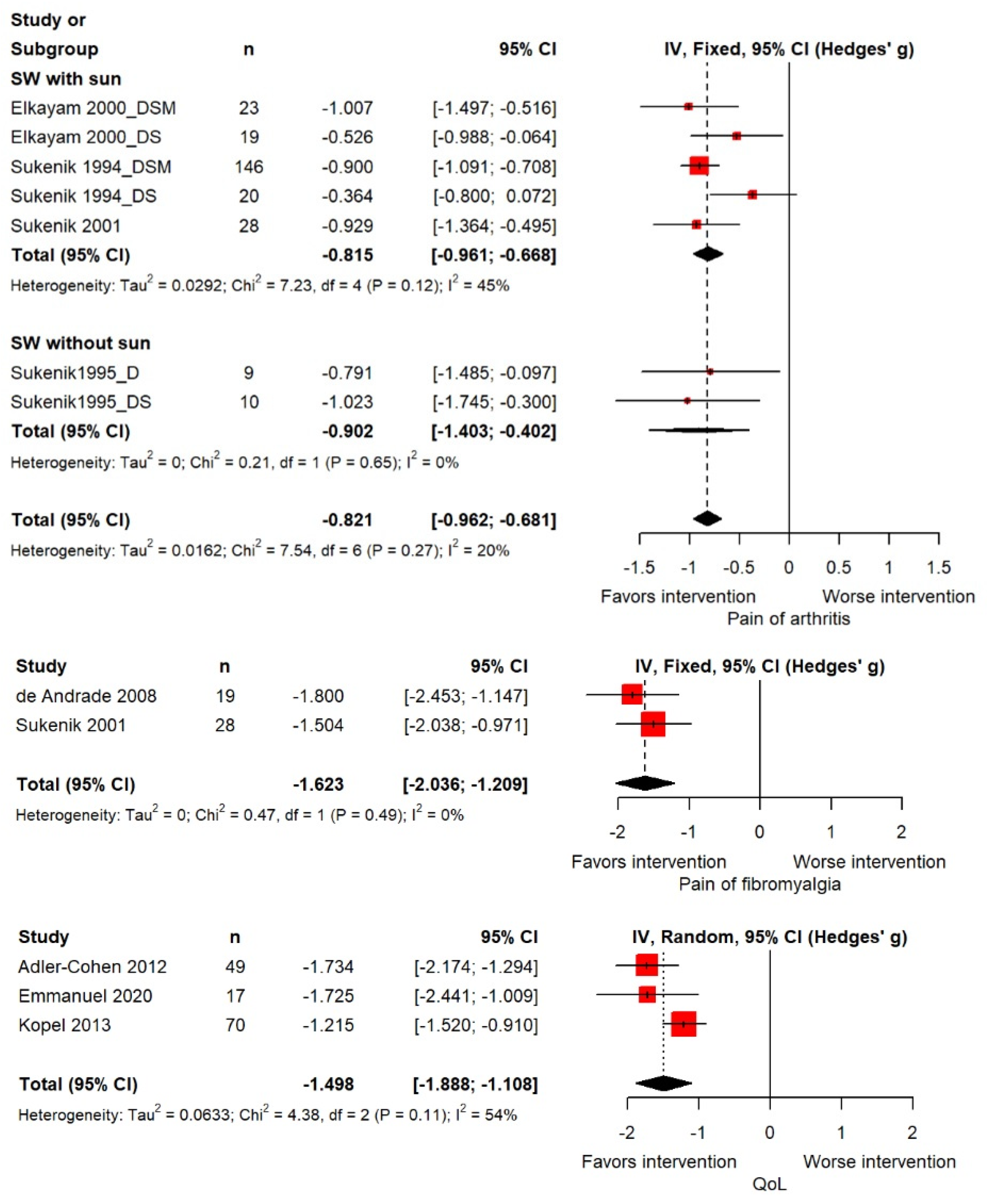
3.2. Outcome Findings
3.3. Moderator Analysis
3.4. Publication Bias
3.5. Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [CrossRef]
- Cleries, R.; Martínez, J.M.; Valls, J.; Pareja, L.; Esteban, L.; Gispert, R.; Moreno, V.; Ribes, J.; Borràs, J.M. Life expectancy and age-period-cohort effects: Analysis and projections of mortality in Spain between 1977 and 2016. Public Health 2009, 123, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 1 August 2023).
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; McDonough, N.; Austen, M.; Mee, L.; Moore, M.; Hess, P.; Depledge, M.H.; White, M.; Philippart, K.; Bradbrook, P.; et al. Oceans and Human Health: A rising tide of challenges and opportunities for Europe. Mar. Environ. Res. 2014, 99, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Short, R.E.; Cox, D.T.C.; Ling Tan, Y.; Bethel, A.; Eales, J.F.; Garside, R. Review of the evidence for oceans and human health relationships in Europe: A systematic map. Environ. Int. 2021, 146, 106275. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.L.; Johnson, S.T.; Boulé, N.G. Aquatic exercise for adults with type 2 diabetes: A meta-analysis. Acta Diabetol. 2017, 54, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Faíl, L.B.; Marinho, D.A.; Marques, E.A.; Costa, M.J.; Santos, C.C.; Marques, M.C.; Izquierdo, M.; Neiva, H.P. Benefits of aquatic exercise in adults with and without chronic disease-A systematic review with meta-analysis. Scand. J. Med. Sci. Sports 2022, 32, 465–486. [Google Scholar] [CrossRef]
- Costantino, M.; Marongiu, M.B.; Russomanno, G.; Conti, V.; Manzo, V.; Filippelli, A. Sulphureous mud-bath therapy and changes in blood pressure: Observational investigation. Clin. Ter. 2015, 166, 151–157. [Google Scholar] [CrossRef]
- Yang, B.; Qin, Q.Z.; Han, L.L.; Lin, J.; Chen, Y. Spa therapy (balneotherapy) relieves mental stress, sleep disorder, and general health problems in sub-healthy people. Int. J. Biometeorol. 2018, 62, 261–272. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Emmanuel, T.; Lybæk, D.; Johansen, C.; Iversen, L. Effect of Dead Sea Climatotherapy on Psoriasis; A Prospective Cohort Study. Front. Med. 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Kopel, E.; Levi, A.; Harari, M.; Ruzicka, T.; Ingber, A. Effect of the Dead Sea climatotherapy for psoriasis on quality of life. Isr. Med. Assoc. J. 2013, 15, 99–102. [Google Scholar] [PubMed]
- Finlay, A.Y.; Chernyshov, P.V.; Tomas Aragones, L.; Bewley, A.; Svensson, A.; Manolache, L.; Marron, S.; Suru, A.; Sampogna, F.; Salek, M.S.; et al. Methods to improve quality of life, beyond medicines. Position statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.J. Intervention meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019008. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Adler-Cohen, C.; Czarnowicki, T.; Dreiher, J.; Ruzicka, T.; Ingber, A.; Harari, M. Climatotherapy at the Dead Sea: An effective treatment modality for atopic dermatitis with significant positive impact on quality of life. Dermatitis 2012, 23, 75–80. [Google Scholar] [CrossRef]
- Cohen, A.D.; Shapiro, J.; Michael, D.; Hodak, E.; Van-Dijk, D.; Naggan, L.; Vardy, D.A. Outcome of “short-term” Dead Sea climatotherapy for psoriasis. Acta Derm.-Venereol. 2008, 88, 90–91. [Google Scholar] [CrossRef][Green Version]
- Cohen, A.D.; Van-Dijk, D.; Naggan, L.; Vardy, D.A. Effectiveness of climatotherapy at the Dead Sea for psoriasis vulgaris: A community-oriented study introducing the ‘Beer Sheva Psoriasis Severity Score’. J. Dermatol. Treat. 2005, 16, 308–313. [Google Scholar] [CrossRef]
- de Andrade, S.C.; de Carvalho, R.F.; Soares, A.S.; de Abreu Freitas, R.P.; de Medeiros Guerra, L.M.; Vilar, M.J. Thalassotherapy for fibromyalgia: A randomized controlled trial comparing aquatic exercises in sea water and water pool. Rheumatol. Int. 2008, 29, 147–152. [Google Scholar] [CrossRef]
- Elkayam, O.; Ophir, J.; Brener, S.; Paran, D.; Wigler, I.; Efron, D.; Even-Paz, Z.; Politi, Y.; Yaron, M. Immediate and delayed effects of treatment at the Dead Sea in patients with psoriatic arthritis. Rheumatol. Int. 2000, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Even-Paz, Z.; Gumon, R.; Kipnis, V.; Abels, D.; Efron, D. Dead Sea sun versus Dead Sea water in the treatment of psoriasis. J. Dermatol. Treat. 1996, 7, 83–86. [Google Scholar] [CrossRef]
- Harari, M.; Czarnowicki, T.; Fluss, R.; Ruzicka, T.; Ingber, A. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 554–559. [Google Scholar] [CrossRef]
- Harari, M.; Novack, L.; Barth, J.; David, M.; Friger, M.; Moses, S.W. The percentage of patients achieving PASI 75 after 1 month and remission time after climatotherapy at the Dead Sea. Int. J. Dermatol. 2007, 46, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Hodak, E.; Gottlieb, A.B.; Segal, T.; Politi, Y.; Maron, L.; Sulkes, J.; David, M. Climatotherapy at the Dead Sea is a remittive therapy for psoriasis: Combined effects on epidermal and immunologic activation. J. Am. Acad. Dermatol. 2003, 49, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, S.; Baradin, R.; Codish, S.; Neumann, L.; Flusser, D.; Abu-Shakra, M.; Buskila, D. Balneotherapy at the Dead Sea area for patients with psoriatic arthritis and concomitant fibromyalgia. Isr. Med. Assoc. J. 2001, 3, 147–150. [Google Scholar]
- Sukenik, S.; Flusser, D.; Codish, S.; Abu-Shakra, M. Balneotherapy at the Dead Sea area for knee osteoarthritis. Isr. Med. Assoc. J. 1999, 1, 83–85. [Google Scholar]
- Sukenik, S.; Giryes, H.; Halevy, S.; Neumann, L.; Flusser, D.; Buskila, D. Treatment of psoriatic arthritis at the Dead Sea. J. Rheumatol. 1994, 21, 1305–1309. [Google Scholar]
- Sukenik, S.; Neumann, L.; Flusser, D.; Kleiner-Baumgarten, A.; Buskila, D. Balneotherapy for rheumatoid arthritis at the Dead Sea. Isr. J. Med. Sci. 1995, 31, 210–214. [Google Scholar] [PubMed]
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Hosseinkhani, F.; Heinken, A.; Thiele, I.; Lindenburg, P.W.; Harms, A.C.; Hankemeier, T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 2021, 13, 1882927. [Google Scholar] [CrossRef]
- Rousset, L.; Halioua, B. Stress and psoriasis. Int. J. Dermatol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef]
- Coventry, P.A.; Brown, J.E.; Pervin, J.; Brabyn, S.; Pateman, R.; Breedvelt, J.; Gilbody, S.; Stancliffe, R.; McEachan, R.; White, P.L. Nature-based outdoor activities for mental and physical health: Systematic review and meta-analysis. SSM Popul. Health 2021, 16, 100934. [Google Scholar] [CrossRef]
- Tambyah, R.; Olcoń, K.; Allan, J.; Destry, P.; Astell-Burt, T. Mental health clinicians’ perceptions of nature-based interventions within community mental health services: Evidence from Australia. BMC Health Serv. Res. 2022, 22, 841. [Google Scholar] [CrossRef]
- Halverstam, C.P.; Lebwohl, M. Nonstandard and off-label therapies for psoriasis. Clin. Dermatol. 2008, 26, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Leibovici, V.; Sagi, E.; Siladji, S.; Greiter, J.C.; Greiter, F.; Holubar, K. Seasonal variation of UV radiation at the Dead Sea. Dermatologica 1987, 174, 290–292. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Design | Mean Age (Years) | Female Rate | No. of Participants | Treatments (n) | Disease | Outcome Measures | Treatment Duration (Weeks) |
|---|---|---|---|---|---|---|---|---|
| Emmanuel et al. [17] | Prospective cohort study | 52.2 | 35.3% | 17 | Seawater with sun (17) | Psoriasis | Severity (PASI), QoL (DLQI) | 4 |
| Kopel et al. [18] | Prospective study | 46.8 | 48.6% | 70 | Seawater with sun | Psoriasis and psoriatic arthritis | Severity (PASI), QoL (Skindex-29) | 4 |
| Adler-Cohen et al. [19] | Prospective study | 40.6 | 49.0% | 49 | Seawater with sun (49) | Atopic dermatitis | Severity (SCORAD), QoL (Skindex-29) | 4 |
| Harari et al. [20] | Retrospective study | 48.1 | 4.5% | 605 | Seawater with sun | Psoriasis | Severity (PASI) | 4 |
| Cohen et al. [21] | Prospective study | 52.5 | 44.7% | 85 | Seawater with sun | Psoriasis | Severity (PASI) | 2 |
| de Andrade et al. [22] | Prospective study | 48.5 | 50.0% | 19 | Aquatic exercise in the seawater | Fibromyalgia | Pain (no. of active joints) | 12 |
| Harari et al. [23] | Prospective study | 42 | 34.4% | 64 | Seawater with sun | Psoriasis | Severity (PASI) | 4 |
| Cohen et al. [24] | Prospective study | 48.5 | 42.9% | 70 | Seawater with sun | Psoriasis | Severity (PASI) | 2 |
| Hodak et al. [25] | Prospective study | 48.5 | 33.3% | 27 | Seawater with sun | Psoriasis | Severity (PASI) | 4 |
| Sukenik et al. [26] | Prospective study | 48.5 | 33.9% | 56 | Seawater with sun (28), seawater with sun, mud pack, and sulfur pool (28) | Psoriatic arthritis and fibromyalgia | Pain (no. of active joints) | 3 |
| Elkayam et al. [27] | Prospective study | 52 | 38.1% | 42 | Seawater with sun (19), seawater with sun, mud pack, and sulfur pool (23) | Psoriasis and psoriatic arthritis | Severity (PASI), pain (no. of active joints) | 4 |
| Sukenik et al. [28] | Prospective study | 63 | 88.9% | 17 | Seawater (10), seawater with sulfur pool (7) | Osteoarthritis_Kness | Severity (Lequesne index) | 2 |
| Even-Paz et al. [29] | Prospective study | 48 | 50.0% | 47 | Seawater (15), seawater with sun (32) | Psoriasis | Severity (PASI) | 4 |
| Sukenik et al. [30] | Prospective study | 60.1 | 86.1% | 9 | Seawater (9), seawater with sulfur pool (10) | Rheumatoid arthritis | Pain (no. of active joints) | 2 |
| Sukenik et al. [31] | Prospective study | 42.7 | 50.7% | 148 | Seawater with sun (18), seawater with sun, mud pack, and sulfur pool (130) | Psoriasis and psoriatic arthritis | Severity (PASI), pain (no. of active joints) | 3 |
| Severity | Pain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | k | Coef | SMD | 95% CI | p | k | Coef | SMD | 95% CI | p | ||
| No. of total participants | 14 | 0.000 | −0.002 | 0.002 | 0.979 | −0.001 | −0.005 | 0.002 | 0.451 | |||
| Treatment duration | 14 | −0.154 | −0.529 | 0.220 | 0.418 | 0.064 | −0.261 | 0.389 | 0.699 | |||
| Age | 14 | 0.041 | −0.028 | 0.109 | 0.249 | −0.009 | −0.044 | 0.026 | 0.610 | |||
| Female rate | 14 | 0.362 | −1.935 | 2.659 | 0.757 | −0.127 | −1.392 | 1.138 | 0.844 | |||
| Diseases | 0.056 | 0.815 | ||||||||||
| Psoriasis | 8 | −1.513 | −1.912 | −1.113 | ||||||||
| Atopic dermatitis | 1 | −2.015 | −2.500 | −1.530 | ||||||||
| Psoriasis and psoriatic arthritis | 5 | −1.252 | −1.647 | −0.856 | 4 | −0.8 | −0.955 | −0.644 | ||||
| Psoriatic arthritis and fibromyalgia | 1 | −0.929 | −1.364 | −0.495 | ||||||||
| Rheumatoid arthritis | 2 | −0.902 | −1.403 | −0.402 | ||||||||
| Treatments | 0.005 | 0.635 | ||||||||||
| Seawater | 1 | −0.604 | −1.129 | −0.079 | ||||||||
| Seawater with sun | 11 | −1.581 | −1.889 | −1.274 | 5 | −0.815 | −0.961 | −0.668 | ||||
| Seawater with sun and mud | 2 | −1.210 | −1.417 | −1.002 | 2 | −0.902 | −1.403 | −0.402 | ||||
| Quality assessment | 0.063 | 0.607 | ||||||||||
| Poor and fair | 9 | −1.649 | −2.050 | −1.248 | 1 | −0.929 | −1.364 | −0.495 | ||||
| Good | 5 | −1.218 | −1.430 | −1.005 | 6 | −0.809 | −0.957 | −0.66 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, S.R.; Shin, D.; Kim, S.-J.; Kim, Y.K.; Lee, K.J. Unveiling the Therapeutic Potential and Healthcare Applications of Marine Therapy: A Systematic Review with Meta-Analysis and Meta-Regression. Mar. Drugs 2023, 21, 604. https://doi.org/10.3390/md21120604
Shim SR, Shin D, Kim S-J, Kim YK, Lee KJ. Unveiling the Therapeutic Potential and Healthcare Applications of Marine Therapy: A Systematic Review with Meta-Analysis and Meta-Regression. Marine Drugs. 2023; 21(12):604. https://doi.org/10.3390/md21120604
Chicago/Turabian StyleShim, Sung Ryul, Dayeon Shin, Seong-Jang Kim, Young Kook Kim, and Kyung Ju Lee. 2023. "Unveiling the Therapeutic Potential and Healthcare Applications of Marine Therapy: A Systematic Review with Meta-Analysis and Meta-Regression" Marine Drugs 21, no. 12: 604. https://doi.org/10.3390/md21120604
APA StyleShim, S. R., Shin, D., Kim, S.-J., Kim, Y. K., & Lee, K. J. (2023). Unveiling the Therapeutic Potential and Healthcare Applications of Marine Therapy: A Systematic Review with Meta-Analysis and Meta-Regression. Marine Drugs, 21(12), 604. https://doi.org/10.3390/md21120604






