Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes
Abstract
1. Introduction
2. Natural PBPs
2.1. APC
2.2. PC
2.3. PE
2.4. Phycobiliprotein Conjugates
2.4.1. Crosslinkage of PBPs
2.4.2. Crosslinkage of PBPs and Dyes
2.5. Labeling of PBPs
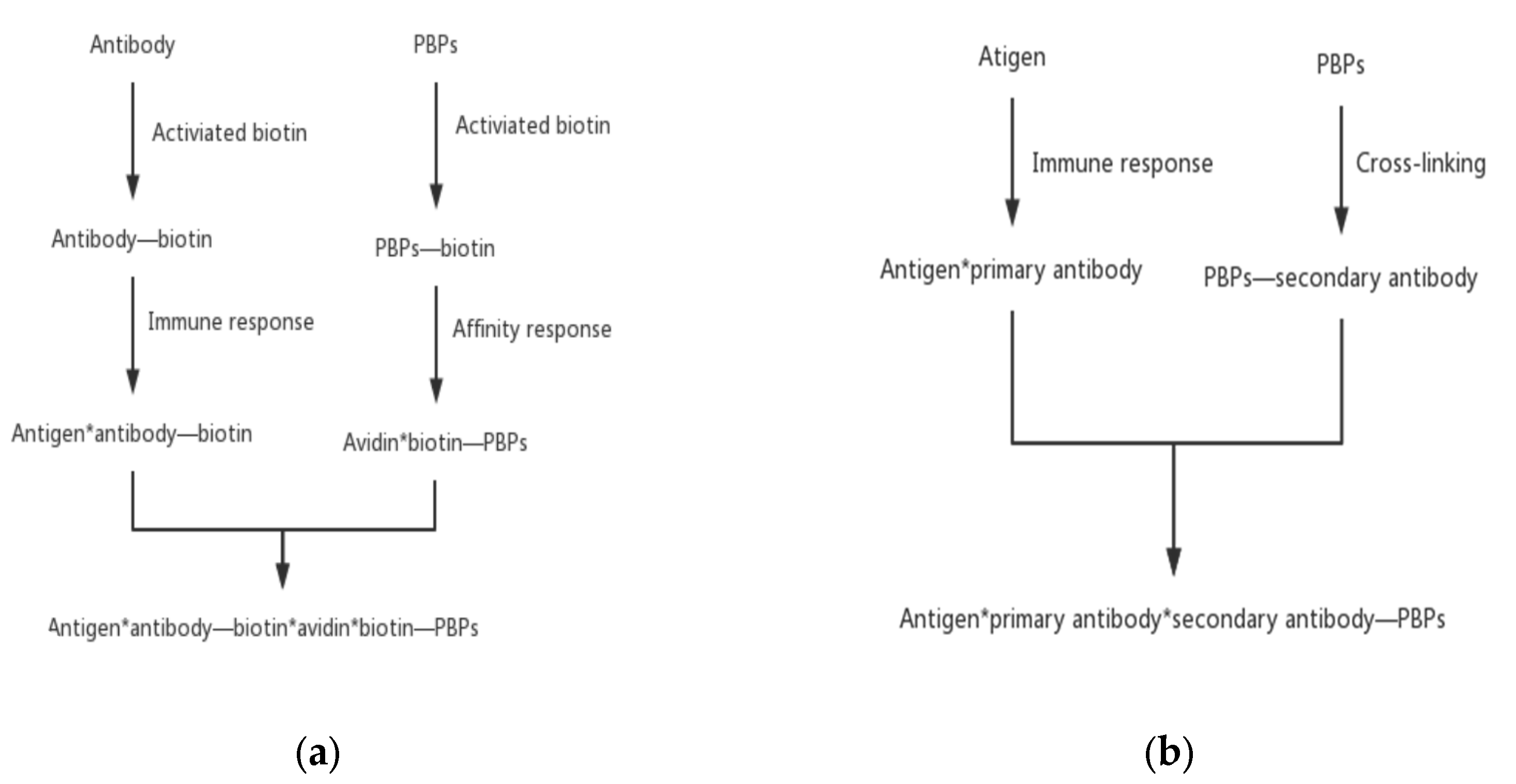
2.5.1. Direct Labeling
2.5.2. Indirect Labeling
3. Recombinant PBPs
3.1. Recombinant PBPs Equipped with a Strep2 Tag
3.2. Recombinant PBPs Fusion with Streptavidin
4. Far-Red Fluorescent Proteins Evolved from PBPs
5. Application of PBPs Fluorescent Probes
5.1. Biological Detections
5.1.1. Biomolecule Detection
5.1.2. Immunofluorescence Analysis
5.1.3. Detection in Other Fields
5.2. Detection of Ions
5.3. Fluorescent Image
| Types | Fields of Application | Specific Example | Reference |
|---|---|---|---|
| APC | Immunofluorescence analysis | Flow cytometric detected anti-neutrophil antibodies. | [79] |
| APC, together with PBXL-3 and conjugated avidin molecule accessed as a fluorochrome for FCM immunodetection of surface antigens on immune cells. | [80] | ||
| Recombinant PBP fluorescent probe (SLA) detected AFP. | [39] | ||
| Fluorescent image | BDFPs used as biomarkers in several mammalian cells in vivo. | [89] | |
| SmURFP and a BPH FP created a far-red and near-infrared fluorescent cell cycle indicator. | [64] | ||
| PC | Fluorescent image | Polypyrrole nanoparticles prepared from the albumin–phycocyanin complex killed MDA-MB-231 cells in a dual way under laser irradiation. | [10] |
| Biomolecule detection | PC and HAS complexes monitored drug transport processes. | [76] | |
| Immunofluorescence analysis | Two dual-functional SA–PBPs (SA–PCA–PCB and SA–PCA–PCB) detected AFP and CEA. | [48] | |
| Other | PC trimers applied to the LED-CCD fluorescent density strip qualitative detection system. | [9] | |
| PC-based fluorescence monitoring of cyanobacterial complexes online determined live cell concentrations | [83] | ||
| Detection of ions | CPC fluorescent probe detected Hg2+ ion in seafood. | [85] | |
| CPC biosensor detected Hg2+ in low concentrations. | [86] | ||
| PC-AgNPs detected Cu2+ ions in diverse water bodies. | [87] | ||
| PE | Biomolecule detection | Using liquid phase microarray detect vibrio combined with recombinant streptavidin-phycoerythrin specifically. | [47] |
| PE integrated with Cy5/Cy7 dye facilitated detection and visualization in molecular biology. | [72,73] | ||
| C-PE/graphene oxide composites detect dsDNA in nanomolar quantities. | [74] | ||
| Using the PDADMAC-mediated R-PE/BHQ2-ssDNA interaction, the fluorescent biosensor detected the target DNA. | [75] | ||
| R-PE probe cross-linked SPDP detected bursal disease virus. | [77] | ||
| Immunofluorescence analysis | Flow cytometric detected anti-neutrophil antibodies. | [79] | |
| R-PE labeled Mannheimia haemolytica can be monitored by observing fluorescence. | [81] | ||
| Detection of ions | R-PE-AgNPs traced Cu2+ ions in diverse aqueous media. | [88] |
6. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liang, X.; Wang, C.H.; Lu, X.; Pang, Y.Y.; Jiang, H.; Liu, X.L. Stability and Fluorescence Characteristics of C-phycocyanin in Spirulina platensis. Mod. Food Sci. Technol. 2020, 32, 89–96. [Google Scholar]
- Anwer, K.; Sonani, R.; Madamwar, D.; Singh, P.; Khan, F.; Bisetty, K.; Ahmad, F.; Hassan, M.I. Role of N-terminal residues on folding and stability of C-phycoerythrin: Simulation and urea-induced denaturation studies. J. Biomol. Struct. Dyn. 2015, 33, 121–133. [Google Scholar] [CrossRef]
- Kupka, M.; Scheer, H. Unfolding of C-phycocyanin followed by loss of non-covalent chromophore-protein interactions: 1. Equilibrium experiments. Bba-Bioenergetics 2008, 1777, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Wang, L.J.; Niu, J.F.; Cong, X.Z.; Wang, G.C. Research Progress of Phycobiliproteins from Seaweed. Sci. Technol. Food Ind. 2022, 43, 442–451. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, J.N.; Duan, K.H.; Tian, R.H. Purification of Spirulina phycocyanin by two-step salting-out. J. Inn. Mong. Agric. Univ. 2019, 40, 60–66. [Google Scholar]
- Wu, K.; Wang, J.Q.; Zhao, B.B.; Fang, Y.; Zhang, F.Y. Ultraviolet-visible spectral characteristics and mechanism analysis of purification of phycobiliprotein by column chromatography. Spectrosc. Spectr. Anal. 2020, 40, 117–122. [Google Scholar]
- Yang, L.H.; Ruan, X.; Qu, H.G.; Feng, P.Y.; Hu, X.M. The stability research on the anabaena phycocyanin as a coloring agent for food. Food Ferment. Ind. 2010, 36, 129–133. [Google Scholar]
- Wen, P.; Hu, T.G.; Wen, Y.; Linhardt, R.J.; Zong, M.H.; Zou, Y.X.; Wu, H. Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers. Food Funct. 2019, 10, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Mo, L.D.; Zhang, W.Y.; Duan, Y.J.; Huang, J.D.; Chen, C.Q.; Gao, Y.M.; Shi, X.A.; Li, F.; Yang, J.M.; et al. Phycocyanin fluorescent probe from Arthrospira platensis: Preparation and application in LED-CCD fluorescence density strip qualitative detection system. J. Appl. Phycol. 2019, 31, 1107–1115. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Bui, N.Q.; Jang, B.; Phan, T.T.V.; Jung, W.K.; Kim, Y.M.; Lee, K.D.; Oh, J. Photo-based PDT/PTT dual model killing and imaging of cancer cells using phycocyanin-polypyrrole nanoparticles. Eur. J. Pharm. Biopharm. 2018, 123, 20–30. [Google Scholar] [CrossRef]
- Sidler, W.A. Phycobilisome and Phycobiliprotein Structures. In The Molecular Biology of Cyanobacteria. Advances in Photosynthesis; Bryant, D.A., Ed.; Springer: Dordrecht, The Netherlands, 1994; Volume 1. [Google Scholar] [CrossRef]
- Peng, P.P.; Dong, L.L.; Sun, Y.F.; Zeng, X.L.; Ding, W.L.; Scheer, H.; Yang, X.; Zhao, K.H. The structure of allophycocyanin B from Synechocystis PCC 6803 reveals the structural basis for the extreme redshift of the terminal emitter in phycobilisomes. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 2558–2569. [Google Scholar] [CrossRef]
- Madhyastha, H.; Madhyastha, R.; Chakraborty, E.; Banerjee, K.; Shah, K.; Nakajima, Y.; Chauhan, N.S.; Sudhakaran, S.L.; Ohe, K.; Muthukaliannan, G.K.; et al. Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NF kappa B Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 3184. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.N.; Bryant, D.A. Allophycocyanin B (lambdamax 671, 618 nm): A new cyanobacterial phycobiliprotein. Arch. Microbiol. 1975, 104, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Wang, L.; Qu, Y.; Fu, X.; Zhao, M.; Wang, S.; Sun, L. Isolation, purification and properties of an R-phycocyanin from the phycobilisomes of a marine red macroalga Polysiphonia urceolata. PLoS ONE 2014, 9, e87833. [Google Scholar] [CrossRef]
- Liang, Y.; Hu, N.X.; Huang, X.L.; Tian, C.Y.; Yan, Y.Y.; Wang, S. Effects of Nitrogen Sources on Exopolysaccharide and Phycobiliprotein Contents of Porphyridium sp. and Chroomonas placoidea. Period. Ocean. Univ. China 2020, 50, 31–39. [Google Scholar]
- Kursar, T.A.; van der Meer, J.; Alberte, R.S. Light-Harvesting System of the Red Alga Gracilaria tikvahiae: II Phycobilisome Characteristics of Pigment Mutants. Plant Physiol. 1983, 73, 361–369. [Google Scholar] [CrossRef]
- de Marsac, N.T. Phycobiliproteins and phycobilisomes: The early observations. Photosynth. Res. 2003, 76, 193–205. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant potential of nature’s “something blue”: Something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends Food Sci. Tech. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. Int. J. Mol. Sci. 2018, 19, 220. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Lee, P.T.; Yeh, H.Y.; Lung, W.Q.C.; Huang, J.; Chen, Y.J.; Chen, B.; Nan, F.H.; Lee, M.C. R-Phycoerythrin from Colaconema formosanum (Rhodophyta), an Anti-Allergic and Collagen Promoting Material for Cosmeceuticals. Appl. Sci. 2021, 11, 9425. [Google Scholar] [CrossRef]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Seong, Y.; Nguyen, D.X.; Wu, Y.; Thakur, A.; Harding, F.; Nguyen, T.A. Novel PE and APC tandems: Additional near-infrared fluorochromes for use in spectral flow cytometry. Cytometry 2022, 101, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.N.; Stryer, L. Fluorescent tandem phycobiliprotein conjugates. Emission wavelength shifting by energy transfer. Biophys. J. 1983, 43, 383–386. [Google Scholar] [CrossRef]
- Mujumdar, R.B.; Ernst, L.A.; Mujumdar, S.R.; Lewis, C.J.; Waggoner, A.S. Cyanine dye labeling reagents: Sulfoindocyanine succinimidyl esters. Bioconjug. Chem. 1993, 4, 105–111. [Google Scholar] [CrossRef]
- Southwick, P.L.; Ernst, L.A.; Tauriello, E.W.; Parker, S.R.; Mujumdar, R.B.; Mujumdar, S.R.; Clever, H.A.; Waggoner, A.S. Cyanine dye labeling reagents—Carboxymethylindocyanine succinimidyl esters. Cytometry 1990, 11, 418–430. [Google Scholar] [CrossRef]
- Tian, Y.; Pappas, D. Energy transfer and light tolerance studies in a fluorescent tandem phycobiliprotein conjugate. Appl. Spectrosc. 2011, 65, 991–995. [Google Scholar] [CrossRef]
- Lansdorp, P.M.; Smith, C.; Safford, M.; Terstappen, L.W.; Thomas, T.E. Single laser three color immunofluorescence staining procedures based on energy transfer between phycoerythrin and cyanine 5. Cytometry 1991, 12, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Bolton, G.; Sehy, D.; Lay, G.; Campbell, D.; Huang, C.M. A novel dye that facilitates three-color analysis of PBMC by flow cytometry. Ann. N. Y. Acad. Sci. 1993, 677, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A.S.; Ernst, L.A.; Chen, C.H.; Rechtenwald, D.J. PE-CY5: A new fluorescent antibody label for three-color flow cytometry with a single laser. Ann. N. Y. Acad. Sci. 1993, 677, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Kantor, A.B.; Parks, D.R.; Herzenberg, L.A. Cy7PE and Cy7APC: Bright new probes for immunofluorescence. Cytometry 1996, 24, 191–197. [Google Scholar] [CrossRef]
- Wang, G.C.; Ma, S.Y.; Zeng, C.K. Comparative studies on the efficiency of energy transfer of two R-phycoerythrin-C-phycocyanin conjugates constructed from sodium periodate and glutaraldehyde respectively. Prog. Biochem. Biophys. 2004, 31, 273–277. [Google Scholar]
- Gambetta, G.A.; Lagarias, J.C. Genetic engineering of phytochrome biosynthesis in bacteria. Proc. Natl. Acad. Sci. USA 2001, 98, 10566–10571. [Google Scholar] [CrossRef] [PubMed]
- Kohchi, T.; Mukougawa, K.; Frankenberg, N.; Masuda, M.; Yokota, A.; Lagarias, J.C. The Arabidopsis HY2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. Plant Cell 2001, 13, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.H.; Su, P.; Böhm, S.; Song, B.; Zhou, M.; Bubenzer, C.; Scheer, H. Reconstitution of phycobilisome core-membrane linker, LCM, by autocatalytic chromophore binding to ApcE. Biochim. Biophys. Acta 2005, 1706, 81–87. [Google Scholar] [CrossRef]
- Li, W.J.; Pu, Y.; Gao, N.; Tang, Z.; Song, L.; Qin, S. Efficient purification protocol for bioengineering allophycocyanin trimer with N-terminus Histag. Saudi J. Biol. Sci. 2017, 24, 451–458. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.X.; Zhao, J.; Jiang, P. Fusion proteins of streptavidin and allophycocyanin alpha subunit for immunofluorescence assay. Biochem. Eng. J. 2017, 125, 97–103. [Google Scholar] [CrossRef]
- Biswas, A.; Vasquez, Y.M.; Dragomani, T.M.; Kronfel, M.; Williams, S.; Alvey, R.M.; Bryant, D.; Schluchter, W. Biosynthesis of cyanobacterial phycobiliproteins in Escherichia coli: Chromophorylation efficiency and specificity of all bilin lyases from Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 2010, 76, 2729–2739. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, P. Metabolic engineering of Escherichia coli for efficient biosynthesis of fluorescent phycobiliprotein. Microb. Cell Fact. 2019, 18, 58. [Google Scholar] [CrossRef]
- Moosmeier, M.A.; Bulkescher, J.; Reed, J.; Schnölzer, M.; Heid, H.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Transtactin: A universal transmembrane delivery system for Strep-tag II-fused cargos. J. Cell Mol. Med. 2010, 14, 1935–1945. [Google Scholar] [CrossRef]
- Busby, M.; Stadler, L.K.; Ko Ferrigno, P.; Davis, J.J. Optimisation of a multivalent Strep tag for protein detection. Biophys. Chem. 2010, 152, 170–177. [Google Scholar] [CrossRef][Green Version]
- Cai, Y.A.; Murphy, J.T.; Wedemayer, G.J.; Glazer, A.N. Recombinant phycobiliproteins-Recombinant C-phycocyanins equipped with affinity tags, oligomerization, and biospecific recognition domains. Anal. Biochem. 2001, 290, 186–204. [Google Scholar] [CrossRef]
- Romay, C.; González, R.; Ledón, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.A.; Lau, C.; Chen, G.; Lu, J. Hybridization-initiated exonuclease resistance strategy for simultaneous detection of multiple microRNAs. Talanta 2018, 190, 248–254. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, P.; Zhao, J. Expression of fusion protein of streptavidin and phycobiliprotein and its application in Luminex suspension chip. Biotechnology 2021, 31, 134–138. [Google Scholar]
- Ge, B.S.; Lin, X.J.; Chen, Y.; Wang, X.F.; Chen, H.X.; Jiang, P.; Huang, F. Combinational biosynthesis of dual-functional streptavidin-phycobiliproteins for high-throughput-compatible immunoassay. Process Biochem. 2017, 58, 306–312. [Google Scholar] [CrossRef]
- Glazer, A.N.; Stryer, L. Phycobiliprotein-avidin and phycobiliprotein-biotin conjugates. Methods Enzymol. 1990, 184, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Z.; McKeown, M.R.; Ng, H.L.; Aguilera, T.A.; Shaner, N.C.; Campbell, R.E.; Adams, S.R.; Gross, L.A.; Ma, W.; Alber, T.; et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 2009, 16, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Strack, R.L.; Hein, B.; Bhattacharyya, D.; Hell, S.W.; Keenan, R.J.; Glick, B.S. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry 2009, 48, 8279–8281. [Google Scholar] [CrossRef] [PubMed]
- Morozova, K.S.; Piatkevich, K.D.; Gould, T.J.; Zhang, J.; Bewersdorf, J.; Verkhusha, V.V. Far-Red Fluorescent Protein Excitable with Red Lasers for Flow Cytometry and Superresolution STED Nanoscopy. Biophys. J. 2010, 99, L13–L15. [Google Scholar] [CrossRef]
- Shcherbo, D.; Shemiakina, I.I.; Ryabova, A.V.; Luker, K.E.; Schmidt, B.T.; Souslova, E.A.; Gorodnicheva, T.V.; Strukova, L.; Shidlovskiy, K.M.; Britanova, O.V.; et al. Near-infrared fluorescent proteins. Nat. Methods 2010, 7, 827–829. [Google Scholar] [CrossRef]
- Konig, K. Multiphoton microscopy in life sciences. J. Microsc. 2000, 200, 83–104. [Google Scholar] [CrossRef]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909. [Google Scholar] [CrossRef]
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.T. The fluorescent toolbox for assessing protein location and function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. Constructing and exploiting the fluorescent protein paintbox (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2009, 48, 5612–5626. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Weitzman, S.A.; Gordon, L.I. Inflammation and cancer: Role of phagocyte-generated oxidants in carcinogenesis. Blood 1990, 76, 655–663. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.A.; Tran, G.N.; Gross, L.A.; Crisp, J.L.; Shu, X.; Lin, J.Y.; Tsien, R.Y. A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein. Nat. Methods 2016, 13, 763–769. [Google Scholar] [CrossRef]
- Wu, C. Structural Study of a Far-Red Fluorescent Protein smURFP; Tianjin University: Tianjin, China, 2018. [Google Scholar]
- Hou, Y.N.; Ding, W.L.; Jiang, S.P.; Miao, D.; Tan, Z.Z.; Hu, J.L.; Scheer, H.; Zhao, K.H. Bright near-infrared fluorescence bio-labeling with a biliprotein triad. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.N.; Ding, W.L.; Hu, J.L.; Jiang, X.X.; Tan, Z.Z.; Zhao, K.H. Very Bright Phycoerythrobilin Chromophore for Fluorescence Biolabeling. ChemBioChem 2019, 20, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Q.; Ding, W.L.; Tan, Z.Z.; Tang, Q.Y.; Zhao, K.H. A Large Stokes Shift Fluorescent Protein Constructed from the Fusion of Red Fluorescent mCherry and Far-Red Fluorescent BDFP1.6. ChemBioChem 2019, 20, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Kannaujiya, V.K.; Sundaram, S.; Sinha, R.P. Phycobiliproteins: Recent Developments and Future Applications; Springer: Singapore, 2017. [Google Scholar]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Leney, A.C.; Tschanz, A.; Heck, A.J.R. Connecting color with assembly in the fluorescent B-phycoerythrin protein complex. Febs. J. 2018, 285, 178–187. [Google Scholar] [CrossRef]
- De Rosa, S.C.; Brenchley, J.M.; Roederer, M. Beyond six colors: A new era in flow cytometry. Nat. Med. 2003, 9, 112–117. [Google Scholar] [CrossRef]
- Hulspas, R.; Dombkowski, D.; Preffer, F.; Douglas, D.; Kildew-Shah, B.; Gilbert, J. Flow Cytometry and the Stability of Phycoerythrin-Tandem Dye Conjugates. Cytom. Part A 2009, 75a, 966–972. [Google Scholar] [CrossRef]
- Ghosh, T.; Mondal, A.; Bharadwaj, S.V.V.; Mishra, S. A naturally fluorescent protein C-phycoerythrin and graphene oxide bio-composite as a selective fluorescence ‘turn off/on’ probe for DNA quantification and characterization. Int. J. Biol. Macromol. 2021, 185, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.K.; Ren, N.N.; Lu, Y.F.; Jia, M.; Wang, R.N.; Zhang, J.L. A poly (diallyldimethylammonium chloride)-mediated R-phycoerythrin/DNA hybrid system as a fluorescent biosensor for DNA detection. Microchem. J. 2020, 152, 104314. [Google Scholar] [CrossRef]
- Seyedi, S.; Parvin, P.; Jafargholi, A.; Jelvani, S.; Shahabi, M.; Shahbazi, M.; Mohammadimatin, P.; Moafi, A. Fluorescence properties of Phycocyanin and Phycocyanin-human serum albumin complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118468. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y. Application prospect of phycobiliprotein fluorescent probe in the detection of animal blight. Contemp. Anim. Husb. 2017, 35, 50–51. [Google Scholar]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed. Res. Int. 2013, 2013, 742859. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q. Establishment of the anti-neutrophil antibody detection by flow cytometry method and its primary clinical application. Shanghai J. Med. Lab. Sci. 2011, 26, 826–828. [Google Scholar]
- Telford, W.G.; Moss, M.W.; Morseman, J.P.; Allnutt, F.C. Cyanobacterial stabilized phycobilisomes as fluorochromes for extracellular antigen detection by flow cytometry. J. Immunol. Methods 2001, 254, 13–30. [Google Scholar] [CrossRef]
- Batista, C.F.; Souza, F.N.; Santos, K.R.; Ramos Sanchez, E.M.; Reis, L.C.; Bertagnon, H.G.; Blagitz, M.G.; Gomes, R.C.; Lage, A.P.; Heinemann, M.B.; et al. R-Phycoerythrin-labeled Mannheimia haemolytica for the simultaneous measurement of phagocytosis and intracellular reactive oxygen species production in bovine blood and bronchoalveolar lavage cells. Vet. Immunol. Immunop. 2018, 196, 53–59. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, J.; Frank, J.F. Role of Cellulose and Colanic Acid in Attachment of Shiga Toxin-Producing Escherichia coli to Lettuce and Spinach in Different Water Hardness Environments. J. Food Prot. 2015, 78, 1461–1466. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Sode, K.; Horikoshi, K.; Takeyama, H.; Nakamura, N.; Matsunaga, T. On-line monitoring of marine cyanobacterial cultivation based on phycocyanin fluorescence. J. Biotechnol. 1991, 21, 209–217. [Google Scholar] [CrossRef]
- Hou, Y.H.; Yan, M.H.; Wang, Q.F.; Wang, Y.F.; Xu, Y.F.; Wang, Y.T.; Li, H.Y.; Wang, H. C-phycocyanin from Spirulina maxima as a Green Fluorescent Probe for the Highly Selective Detection of Mercury(II) in Seafood. Food Anal. Method 2017, 10, 1931–1939. [Google Scholar] [CrossRef]
- Bhayani, K.; Mitra, M.; Ghosh, T.; Mishra, S. C-Phycocyanin as a potential biosensor for heavy metals like Hg2+ in aquatic systems. RSC Adv. 2016, 6, 111599–111605. [Google Scholar] [CrossRef]
- Wei, N.N.; Hou, Y.H.; Lu, Z.B.; Yu, H.T.; Wang, Q.F. Synthesis of silver nanoparticles stabilized with C-phycocyanin and for fluorimetric detection of copper ions. Iop. C Ser. Earth Env. 2018, 108, 022030. [Google Scholar] [CrossRef]
- Xu, Y.F.; Hou, Y.H.; Wang, Y.T.; Li, T.; Song, C.; Wei, N.; Wang, Q. Sensitive and selective detection of Cu2+ ions based on fluorescent Ag nanoparticles synthesized by R-phycoerythrin from marine algae Porphyra yezoensis. Ecotox. Environ. Safe 2019, 168, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.L.; Miao, D.; Hou, Y.N.; Jiang, S.P.; Zhao, B.Q.; Zhou, M.; Scheer, H.; Zhao, K.H. Small monomeric and highly stable near-infrared fluorescent markers derived from the thermophilic phycobiliprotein, ApcF2. BBA-Mol. Cell Res. 2017, 1864, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Haynes, R.D.; Corbel, S.Y.; Li, P.P.; González-González, E.; Burg, J.S.; Ataie, N.J.; Lam, A.J.; Cranfill, P.J.; Baird, M.A.; et al. Non-invasive intravital imaging of cellular differentiation with a bright red-excitable fluorescent protein. Nat. Methods 2014, 11, 572–578. [Google Scholar] [CrossRef]
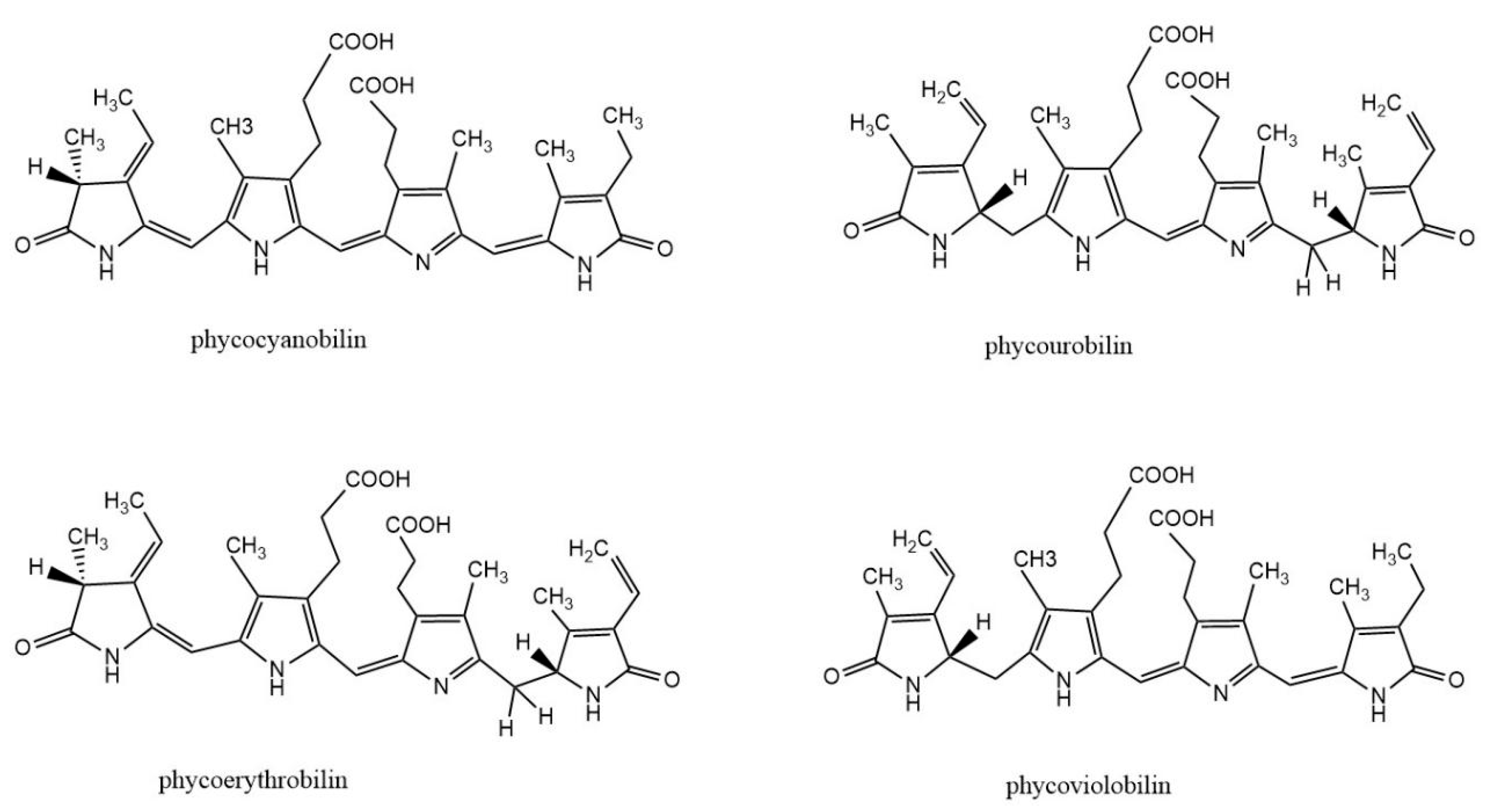



| PBPs | Sources for PBP Gene | Hosts | Phycobilins | Lyases |
|---|---|---|---|---|
| Apo-ApcA, Apo-ApcB | Synechococcos sp. PCC 7002 | E. coli | PCB | - |
| Apo-APC | Asterocapsa nidulans UTEX 625 | E. coli | PCB | - |
| Apo-CpcA | Asterocapsa nidulans R2 | E. coli | PCB | - |
| Holo-CpcA | Synechocystis sp. PCC 6803 | E. coli | PCB | CpcE/CpcF |
| Holo-PecA | Anabaena sp. PCC 7120 | E. coli | PVB | PecE/PecF |
| Holo-ApcAB | Synechococcus sp. PCC 7002 | E. coli | PCB | CpcU/CpcS |
| Holo-ApcAB | Synechocystis sp. PCC 6803 | E. coli | PCB | CpcU/CpcS |
| Holo-ApcAB | Gracilaria chilensis | E. coli | PCB | CpcU/CpcS |
| Holo-ApcA | Synechococcus elongatus BP-1 | E. coli | PCB | CpcS |
| Holo-ApcB | Spirulina sp. | E. coli | PCB | CpcS |
| Holo-ApcA | Synechococcus elongatus BP-1 | E. coli | PCB | CpcS |
| Streptavidin-Holo-ApcA | Synechococcus elongatus BP-1 | E. coli | PCB or PEB | CpcS |
| Streptavidin-Holo-ApcA | Synechococcus elongatus BP-1 | E. coli | PEB | CpcS |
| Holo-ApcB | Synechococcus elongatus BP-1 | E. coli | PCB | CpcS |
| Holo-ApcF | Synechococcus sp. PCC 7002 | E. coli | PCB | CpcU/CpcS |
| Holo-CpcA | Synechocystis sp. PCC 6803 | E. coli | PCB | CpcE/CpcF |
| Holo-CpcB | Synechocystis sp. PCC 6803 | E. coli | PCB | CpcU/CpcS |
| Holo-CpcB | Synechocystis sp. PCC 6803 | E. coli | PCB | CpcT |
| Holo-CpcB | Synechococcus elongatus BP-1 | E. coli | PEB, PUB | CpcU, CpcT |
| Holo-CpcA | Synechocystis sp. PCC 6803 Synechococcus sp. PCC 7002 | E. coli | PCB, PEB, PΦB, PUB, PVB, PtVB | CpcE/CpcF PecE/PecF |
| Holo-CpeA | Microchaete diplosiphon UTEX481 | E. coli | PEB | CpeY |
| Holo-CpeB | Synechococcus sp. RS9916 | E. coli | PUB | MpeV |
| Holo-CpeB | Microchaete diplosiphon | E. coli | PEB | CpeF |
| Holo-CpeB | Prochlorococcus marinus MED4 | E. coli | PEB | CpeS |
| PcA/PcB | Gracilariopsis lemaneiformis | E. coli | PCB | CpcU/CpcS, CpcE/CpcF, CpcT |
| Holo-MpeA | Synechococcus sp. RS9916 | E. coli | PUB | MpeZ |
| Holo-C-PC equipped with different tags | Anabaena sp. PCC7120 | Anabaena sp. PCC 7120 | PCB | - |
| Holo-APC | Cyanophora paradoxa (Glaucophyta) | Synechococcus sp. PCC 7002 | PCB | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Deng, J.; Li, L.; Liu, Z.; Sun, S.; Xiong, P. Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes. Mar. Drugs 2023, 21, 572. https://doi.org/10.3390/md21110572
Chen H, Deng J, Li L, Liu Z, Sun S, Xiong P. Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes. Marine Drugs. 2023; 21(11):572. https://doi.org/10.3390/md21110572
Chicago/Turabian StyleChen, Huaxin, Jinglong Deng, Longqi Li, Zhe Liu, Shengjie Sun, and Peng Xiong. 2023. "Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes" Marine Drugs 21, no. 11: 572. https://doi.org/10.3390/md21110572
APA StyleChen, H., Deng, J., Li, L., Liu, Z., Sun, S., & Xiong, P. (2023). Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes. Marine Drugs, 21(11), 572. https://doi.org/10.3390/md21110572






