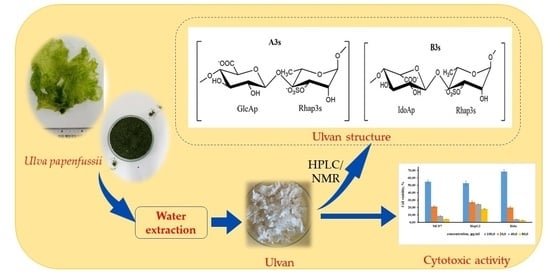
Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva papenfussii
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the Extracted Ulvan
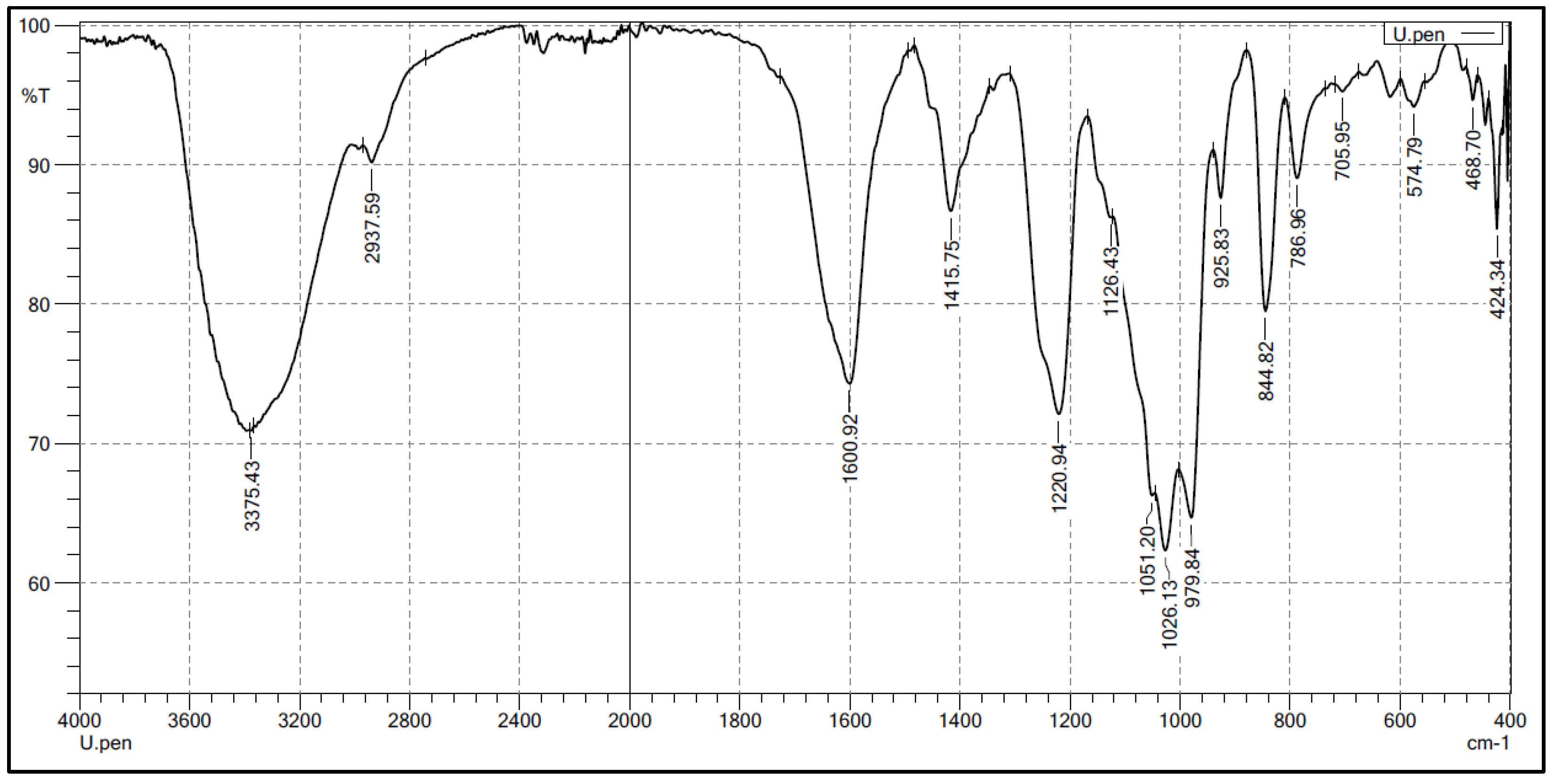
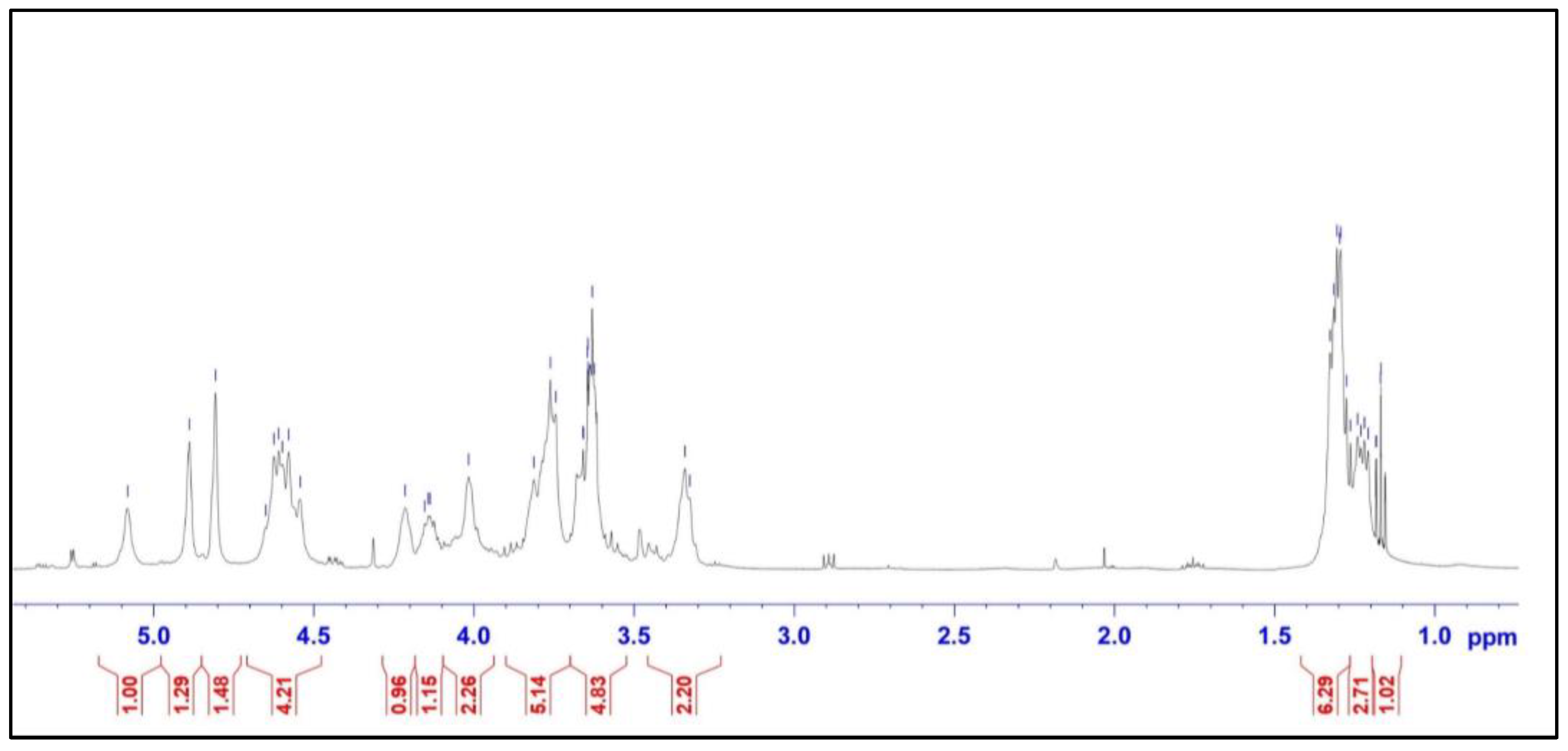
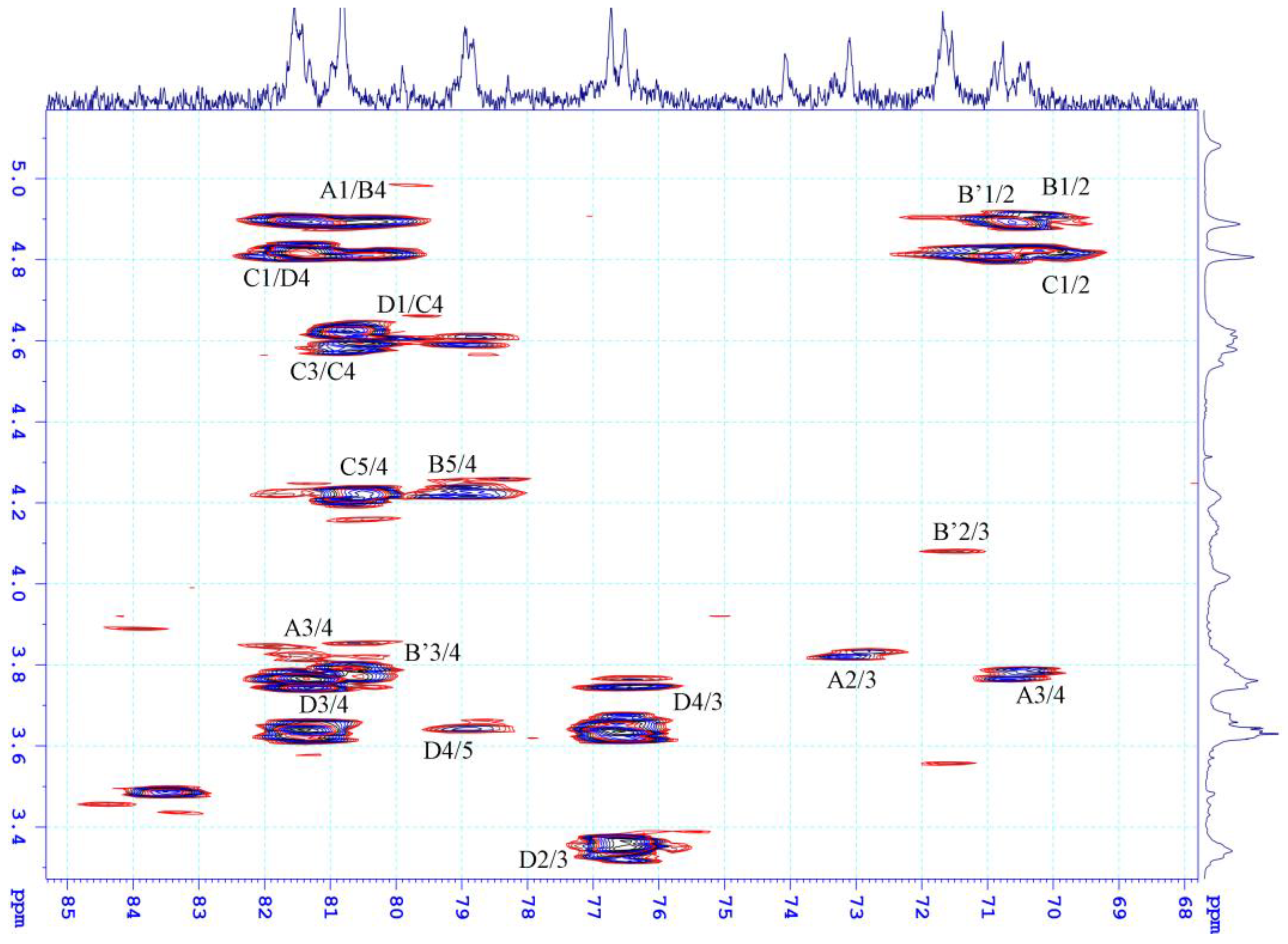
2.2. Structural Characteristics of the Extracted Ulvan from U. papenfussii
2.3. Anticancer Activities
2.4. Toxicity Estimation Based on QSAR
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Purification of Ulvan
4.3. Chemical Analysis of Ulvan
4.4. Structural Characterization of Ulvan
4.5. Cytotoxic Assays
4.6. Toxicity Prediction using QSAR Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Carnachan, S.M.; Magnusson, M.; Lawton, R.J.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Glasson, C.R.K. Are All Ulvans Equal? A Comparative Assessment of the Chemical and Gelling Properties of Ulvan from Blade and Filamentous Ulva. Carbohydr. Polym. 2021, 264, 118010. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Gaillard, C.; Sassi, J.F.; Leral, Y.; Lahaye, M. Ultrastructure of Ulvan: A Polysaccharide from Green Seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E. A Conformational Study on the Algal Polysaccharide Ulvan. Macromolecules 2002, 35, 6404–6411. [Google Scholar] [CrossRef]
- Lahaye, M.; Brunel, M.; Bonnin, E. Fine Chemical Structure Analysis of Oligosaccharides Produced by an Ulvan-Lyase Degradation of the Water-Soluble Cell-Wall Polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr. Res. 1997, 304, 325–333. [Google Scholar] [CrossRef]
- Lahaye, M.; Ray, B. Cell-Wall Polysaccharides from the Marine Green Alga Ulva “Rigida” (Ulvales, Chlorophyta)—NMR Analysis of Ulvan Oligosaccharides. Carbohydr. Res. 1996, 283, 161–173. [Google Scholar] [CrossRef]
- Ray, B.; Lahaye, M. Cell-Wall Polysaccharides from the Marine Green Alga Ulva “Rigida” (Ulvales, Chlorophyta). Extraction and Chemical Composition. Carbohydr. Res. 1995, 274, 251–261. [Google Scholar] [CrossRef]
- Ray, B.; Lahaye, M. Cell-Wall Polysaccharides from the Marine Green Alga Ulva “Rigida” (Ulvales, Chlorophyta). Chemical Structure of Ulvan. Carbohydr. Res. 1995, 274, 313–318. [Google Scholar] [CrossRef]
- Brading, J.W.E.; Georg-Plant, M.M.T.; Hardy, D.M. The Polysaccharide from the Alga Ulva lactuca. Purification, Hydrolysis, and Methylation of the Polysaccharide. J. Chem. Soc. 1954, 319–324. [Google Scholar] [CrossRef]
- Lahaye, M.; Axelos, M.A.V. Gelling Properties of Water-Soluble Polysaccharides from Proliferating Marine Green Seaweeds (Ulva Spp.). Carbohydr. Polym. 1993, 22, 261–265. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Function Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Quemener, B.; Lahaye, M.; Bobin-Dubigeon, C. Sugar Determination in Ulvans by a Chemical-Enzymatic Method Coupled to High Performance Anion Exchange Chromatography. J. Appl. Phycol. 1997, 9, 179–188. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, Conformation in Aqueous Solution and Antimicrobial Activity of Ulvan Extracted from Green Seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattová, E.; Taubner, T.; Choi, J.W.; Lee, C.W.; Park, J.K.; et al. Structural Features and Anti-Coagulant Activity of the Sulphated Polysaccharide SPS-CF from a Green Alga Capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef]
- Reis, S.E.; Andrade, R.G.C.; Accardo, C.M.; Maia, L.F.; Oliveira, L.F.C.; Nader, H.B.; Aguiar, J.A.K.; Medeiros, V.P. Influence of Sulfated Polysaccharides from Ulva lactuca L. upon Xa and IIa Coagulation Factors and on Venous Blood Clot Formation. Algal Res. 2020, 45, 101750. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef]
- Trentin, R.; Custódio, L.; Rodrigues, M.J.; Moschin, E.; Sciuto, K.; da Silva, J.P.; Moro, I. Exploring Ulva australis Areschoug for Possible Biotechnological Applications: In Vitro Antioxidant and Enzymatic Inhibitory Properties, and Fatty Acids Contents. Algal Res. 2020, 50, 101980. [Google Scholar] [CrossRef]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic Activity of High Sulfate Content Derivative of Polysaccharide Extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Qi, H.; Sheng, J. The Antihyperlipidemic Mechanism of High Sulfate Content Ulvan in Rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar] [CrossRef]
- Berri, M.; Slugocki, C.; Olivier, M.; Helloin, E.; Jacques, I.; Salmon, H.; Demais, H.; Le Goff, M.; Collen, P.N. Marine-Sulfated Polysaccharides Extract of Ulva Armoricana Green Algae Exhibits an Antimicrobial Activity and Stimulates Cytokine Expression by Intestinal Epithelial Cells. J. Appl. Phycol. 2016, 28, 2999–3008. [Google Scholar] [CrossRef]
- Deveau, A.M.; Miller-Hope, Z.; Lloyd, E.; Williams, B.S.; Bolduc, C.; Meader, J.M.; Weiss, F.; Burkholder, K.M. Antimicrobial Activity of Extracts from Macroalgae Ulva Lactuca against Clinically Important Staphylococci Is Impacted by Lunar Phase of Macroalgae Harvest. Lett. Appl. Microbiol. 2016, 62, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva Clathrata and Cladosiphon Okamuranus Seaweeds Both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chan, Y.L.; Li, T.L.; Wu, C.J. Inhibition of Japanese Encephalitis Virus Infection by the Sulfated Polysaccharide Extracts from Ulva Lactuca. Mar. Biotechnol. 2012, 14, 468–478. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and Cytotoxic Activity of Ulvan Extracted from Green Seaweed Ulva Lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Wang, J.; Liu, Z.; Zhao, S. Antitumor Activity of a Sulfated Polysaccharide from Enteromorpha intestinalis Targeted against Hepatoma through Mitochondrial Pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hong, P.; Cheng, Y.; Liao, M.; Li, S. Polysaccharides from Enteromorpha tubulosa: Optimization of Extraction and Cytotoxicity. J. Food Process. Preserv. 2018, 42, e13373. [Google Scholar] [CrossRef]
- Bussy, F.; Matthieu, L.G.; Salmon, H.; Delaval, J.; Berri, M.; Pi, N.C. Immunomodulating Effect of a Seaweed Extract from Ulva armoricana in Pig: Specific IgG and Total IgA in Colostrum, Milk, and Blood. Vet. Anim. Sci. 2019, 7, 100051. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of Biochemical and Immunomodulatory Activity of Sulphated Polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef]
- Costa, C.; Alves, A.; Pinto, P.R.; Sousa, R.A.; Borges Da Silva, E.A.; Reis, R.L.; Rodrigues, A.E. Characterization of Ulvan Extracts to Assess the Effect of Different Steps in the Extraction Procedure. Carbohydr. Polym. 2012, 88, 537–546. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.H.; Kim, S.K. Seaweed Polysaccharides and Their Potential Biomedical Applications. Starch-Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Arsianti, A.A.; Fadilah, F.; Fatmawaty, Y.; Wibisono, L.K.; Kusmardi, S.; Azizah, N.N.; Putrianingsih, R.; Murniasih, T.; Rasyid, A.; Pangestuti, R. Phytochemical Composition and Anticancer Activity of Seaweeds Ulva lactuca and Eucheuma cottonii against Breast MCF-7 and Colon HCT-116 Cells. Asian J. Pharm. Clin. Res. 2016, 9, 115–119. [Google Scholar] [CrossRef]
- Cho, M.L.; Yang, C.; Kim, S.M.; You, S.G. Molecular Characterization and Biological Activities of Watersoluble Sulfated Polysaccharides from Enteromorpha prolifera. Food Sci. Biotechnol. 2010, 19, 525–533. [Google Scholar] [CrossRef]
- Shao, P.; Pei, Y.; Fang, Z.; Sun, P. Effects of Partial Desulfation on Antioxidant and Inhibition of DLD Cancer Cell of Ulva fasciata Polysaccharide. Int. J. Biol. Macromol. 2014, 65, 307–313. [Google Scholar] [CrossRef]
- Thu, Q.T.M.; Bang, T.H.; Nu, N.T.; Luong, D.V.; Ly, B.M.; Van, T.T.T.; Thuy, T.T.T. Structural Determination of Ulvan from Green Seaweed Ulva reticulata Collected at Central Coast of Vietnam. Chem. Lett. 2015, 44, 788–790. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Huy, B.T.; Truong, H.B.; Bui, M.L.; Thanh, T.T.T.; Dao, D.Q. Structure Analysis of Sulfated Polysaccharides Extracted from Green Seaweed Ulva lactuca: Experimental and Density Functional Theory Studies. Monatshefte Chem. 2018, 149, 197–205. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In Vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phytother. Res. PTR 2013, 27, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.B.; Huy, B.T.; Ray, S.K.; Gyawali, G.; Lee, Y.I.; Cho, J.; Hur, J. Magnetic Visible-Light Activated Photocatalyst ZnFe2O4/BiVO4/g-C3N4 for Decomposition of Antibiotic Lomefloxacin: Photocatalytic Mechanism, Degradation Pathway, and Toxicity Assessment. Chemosphere 2022, 299, 134320. [Google Scholar] [CrossRef]
- WoRMS-World Register of Marine Species—Hydropuntia Eucheumatoides (Harvey) Gurgel & Fredericq. 2004. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=376385 (accessed on 29 August 2023).
- Robic, A.; Bertrand, D.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Determination of the Chemical Composition of Ulvan, a Cell Wall Polysaccharide from Ulva Spp. (Ulvales, Chlorophyta) by FT-IR and Chemometrics. J. Appl. Phycol. 2009, 21, 451–456. [Google Scholar] [CrossRef]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Structure and Interactions of Ulvan in the Cell Wall of the Marine Green Algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Lahaye, M.; Inizan, F.; Vigouroux, J. NMR Analysis of the Chemical Structure of Ulvan and of Ulvan-Boron Complex Formation. Carbohydr. Polym. 1998, 36, 239–249. [Google Scholar] [CrossRef]
- Lahaye, M. NMR Spectroscopic Characterisation of Oligosaccharides from Two Ulva Rigida Ulvan Samples (Ulvales, Chlorophyta) Degraded by a Lyase. Carbohydr. Res. 1998, 314, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Cimadevilla, E.A.C.; Kuhlenkamp, R.; Quemener, B.; Lognoné, V.; Dion, P. Chemical Composition and 13C NMR Spectroscopic Characterisation of Ulvans from Ulva (Ulvales, Chlorophyta). J. Appl. Phycol. 1999, 11, 1–7. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A Practical Perspective on Ulvan Extracted from Green Algae. J. Appl. Phycol. 2012, 25, 407–424. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Jin, W.; Zhang, H.; Zhang, Q. Structure-Activity Relationship of Sulfated Hetero/Galactofucan Polysaccharides on Dopaminergic Neuron. Int. J. Biol. Macromol. 2016, 82, 878–883. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Sims, I.M.; Carnachan, S.M.; de Nys, R.; Magnusson, M. A Cascading Biorefinery Process Targeting Sulfated Polysaccharides (Ulvan) from Ulva Ohnoi. Algal Res. 2017, 27, 383–391. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Bergé, J.P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-Assisted Extraction (EAE) for the Production of Antiviral and Antioxidant Extracts from the Green Seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.G.; Dabaghian, E.H.; Surayot, U. Water-Soluble Polysaccharides from Ulva intestinalis: Molecular Properties, Structural Elucidation and Immunomodulatory Activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Barthélemy, J.P.; Paquot, M.; Blecker, C.; Attia, H. Impact of Extraction Procedures on the Chemical, Rheological and Textural Properties of Ulvan from Ulva lactuca of Tunisia Coast. Food Hydrocoll. 2014, 40, 53–63. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Luiten, C.A.; Carnachan, S.M.; Daines, A.M.; Kidgell, J.T.; Hinkley, S.F.R.; Praeger, C.; Andrade Martinez, M.; Sargison, L.; Magnusson, M.; et al. Structural Characterization of Ulvans Extracted from Blade (Ulva ohnoi) and Filamentous (Ulva tepida and Ulva prolifera) Species of Cultivated Ulva. Int. J. Biol. Macromol. 2022, 194, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, H.; Wang, P.; Du, C.; Ye, H.; Zuo, S.; Guan, H.; Wang, P. Structural Characterization of Ulvan Extracted from Ulva Clathrata Assisted by an Ulvan Lyase. Carbohydr. Polym. 2020, 229, 115497. [Google Scholar] [CrossRef]
- Tabarsa, M.; Lee, S.J.; You, S. Structural Analysis of Immunostimulating Sulfated Polysaccharides from Ulva pertusa. Carbohydr. Res. 2012, 361, 141–147. [Google Scholar] [CrossRef]
- Tako, M.; Tamanaha, M.; Tamashiro, Y.; Uechi, S. Structure of Ulvan Isolated from the Edible Green Seaweed, Ulva pertusa. Adv. Biosci. Biotechnol. 2015, 06, 645–655. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a Fucoidan from the Brown Seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Bakunina, I.Y.; Nedashkovskaya, O.I.; Gorshkova, N.M.; Alexeeva, Y.V.; Zelepuga, E.A.; Zvaygintseva, T.N.; Nicolau, D.V.; Mikhailov, V.V. Ecophysiological Variabilities in Ectohydrolytic Enzyme Activities of Some Pseudoalteromonas Species, P. citrea, P. issachenkonii, and P. nigrifaciens. Curr. Microbiol. 2003, 46, 6–10. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Nguyen Tran, V.H.; Dieu Trang, V.T.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Thuy Cao, H.T.; Thanh Van, T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus Distichus subsp. Evanescens and Saccharina Latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Zeuner, B.; Muschiol, J.; Holck, J.; Lezyk, M.; Gedde, M.R.; Jers, C.; Mikkelsen, J.D.; Meyer, A.S. Substrate Specificity and Transfucosylation Activity of GH29 α-l-Fucosidases for Enzymatic Production of Human Milk Oligosaccharides. New Biotechnol. 2018, 41, 34–45. [Google Scholar] [CrossRef]
- Orf, G.M.; Fritz, J.S. Preparation and Chromatographic Applications of an Amide Resin. Anal. Chem. 1978, 50, 1328–1330. [Google Scholar] [CrossRef]
- Biel-Nielsen, T.L.; Li, K.; Sørensen, S.O.; Sejberg, J.J.P.; Meyer, A.S.; Holck, J. Utilization of Industrial Citrus Pectin Side Streams for Enzymatic Production of Human Milk Oligosaccharides. Carbohydr. Res. 2022, 519, 108627. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, C.; Grisoni, F.; Consonni, V.; Ballabio, D. Consensus versus IndivIdoApl QSARs in Classification: Comparison on a Large-Scale Case Study. J. Chem. Inf. Model. 2020, 60, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Gramatica, P. Principles of QSAR Models Validation: Internal and External. QSAR Comb. Sci. 2007, 26, 694–701. [Google Scholar] [CrossRef]



| Monomers | Composition (mol% of the Total Carbohydrates) | |
|---|---|---|
| Neutral monosaccharides | Rhamnose | 44.9 ± 1.5 1 |
| Galactose | 2.2 ± 0.2 1 | |
| Glucose | 5.3 ± 0.4 1 | |
| Xylose | 8.5 ± 0.4 1 | |
| Uronic acids | Glucuronic acid | 15.7 ± 1.0 1 |
| Iduronic acid | 23.4 ± 0.8 1 | |
| Composition | ||
| Sulfate (%) | 13.4 ± 0.6 2 | |
| Residue\Atom | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| A | →4)-β-D-IdoAp-(1- | 5.08 | 3.64 | 3.81 | 4.01 | 4.54 | |
| 105.46 | 73.1 | 74.08 | 81.55 | 73.1 | 177.54 | ||
| B | →4)-α-L-Rhap3S-(1- | 4.88 | 4.15 | 4.57 | 3.74 | 4.21 | 1.3 |
| 103.53 | 70.76 | 80.81 | 78.95 | 71.54 | 19.58 | ||
| B’ | →4)-α-L-Rhap-(1- | 4.88 | 4.1 | 4.01 | 3.76 | 4.01 | 1.30 |
| 100.00 | 71.54 | 70.76 | 80.81 | 70.76 | 19.5 | ||
| C | →4)-α-L-Rhap-3S-(1- | 4.80 | 4.21 | 4.59 | 3.76 | 4.21 | |
| 102.39 | 71.68 | 79.9 | 80.8 | 70.37 | 19.63 | ||
| D | →4)-β-D-GlcAp-(1- | 4.62 | 3.34 | 3.64 | 3.65 | 3.74 | |
| 105.81 | 76.33 | 76.51 | 81.55 | 78.95 | 177.54 | ||
| % Cell Inhibition | |||||||
|---|---|---|---|---|---|---|---|
| Ulvan | Ellipticine | ||||||
| Conc. (µg/mL) | MCF7 | HepG2 | Hela | Conc. (µg/mL) | MCF7 | HepG2 | Hela |
| 100 | 54.96 ± 2.30 | 52.95 ± 2.85 | 68.57 ± 2.43 | 10 | 107.02 ± 4.30 | 99.72 ± 4.70 | 94.18 ± 4.32 |
| 20 | 21.25 ± 1.32 | 27.21 ± 1.60 | 19.96 ± 1.35 | 2 | 76.76 ± 3.35 | 80.04 ± 3.24 | 88.03 ± 3.67 |
| 4 | 8.61 ± 0.53 | 24.45 ± 0.40 | 4.11 ± 0.30 | 0.4 | 50.24 ± 2.56 | 51.38 ± 2.31 | 47.89 ± 2.33 |
| 0.8 | 4.30 ± 0.03 | 18.11 ± 0.21 | 2.76 ± 0.23 | 0.08 | 23.32 ± 1.21 | 24.31 ± 1.33 | 25.67 ± 1.24 |
| IC50 | 85.48 ± 5.75 | 89.78 ± 6.55 | 66.95 ± 2.45 | IC50 | 0.41 ± 0.03 | 0.38 ± 0.02 | 0.36 ± 0.05 |
| Toxicity Endpoints | Unit | Structural Form | |
|---|---|---|---|
| A3s | B3s | ||
| 96 h P. promelas LC50 | mg/L | 3755.9 | N/A |
| 48 h D. magna LC50 | mg/L | 5661 | 421 |
| 48 h T. pyriformis IGC50 | mg/L | N/A | N/A |
| Oral rat LD50 | mg/kg | 2512.8 | N/A |
| Bioconcentration factor | 3.8 | 2.44 | |
| Developmental Toxicity | 0.5 (non-toxicant) | N/A | |
| Mutagenicity | N/A | 0.32 (negative) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.H.N.; Mikkelsen, M.D.; Truong, H.B.; Vo, H.N.M.; Pham, T.D.; Cao, H.T.T.; Nguyen, T.T.; Meyer, A.S.; Thanh, T.T.T.; Van, T.T.T. Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva papenfussii. Mar. Drugs 2023, 21, 556. https://doi.org/10.3390/md21110556
Tran VHN, Mikkelsen MD, Truong HB, Vo HNM, Pham TD, Cao HTT, Nguyen TT, Meyer AS, Thanh TTT, Van TTT. Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva papenfussii. Marine Drugs. 2023; 21(11):556. https://doi.org/10.3390/md21110556
Chicago/Turabian StyleTran, Vy Ha Nguyen, Maria Dalgaard Mikkelsen, Hai Bang Truong, Hieu Nhu Mai Vo, Thinh Duc Pham, Hang Thi Thuy Cao, Thuan Thi Nguyen, Anne S. Meyer, Thuy Thu Thi Thanh, and Tran Thi Thanh Van. 2023. "Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva papenfussii" Marine Drugs 21, no. 11: 556. https://doi.org/10.3390/md21110556
APA StyleTran, V. H. N., Mikkelsen, M. D., Truong, H. B., Vo, H. N. M., Pham, T. D., Cao, H. T. T., Nguyen, T. T., Meyer, A. S., Thanh, T. T. T., & Van, T. T. T. (2023). Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva papenfussii. Marine Drugs, 21(11), 556. https://doi.org/10.3390/md21110556