Penidihydrocitrinins A–C: New Polyketides from the Deep-Sea-Derived Penicillium citrinum W17 and Their Anti-Inflammatory and Anti-Osteoporotic Bioactivities
Abstract
:1. Introduction
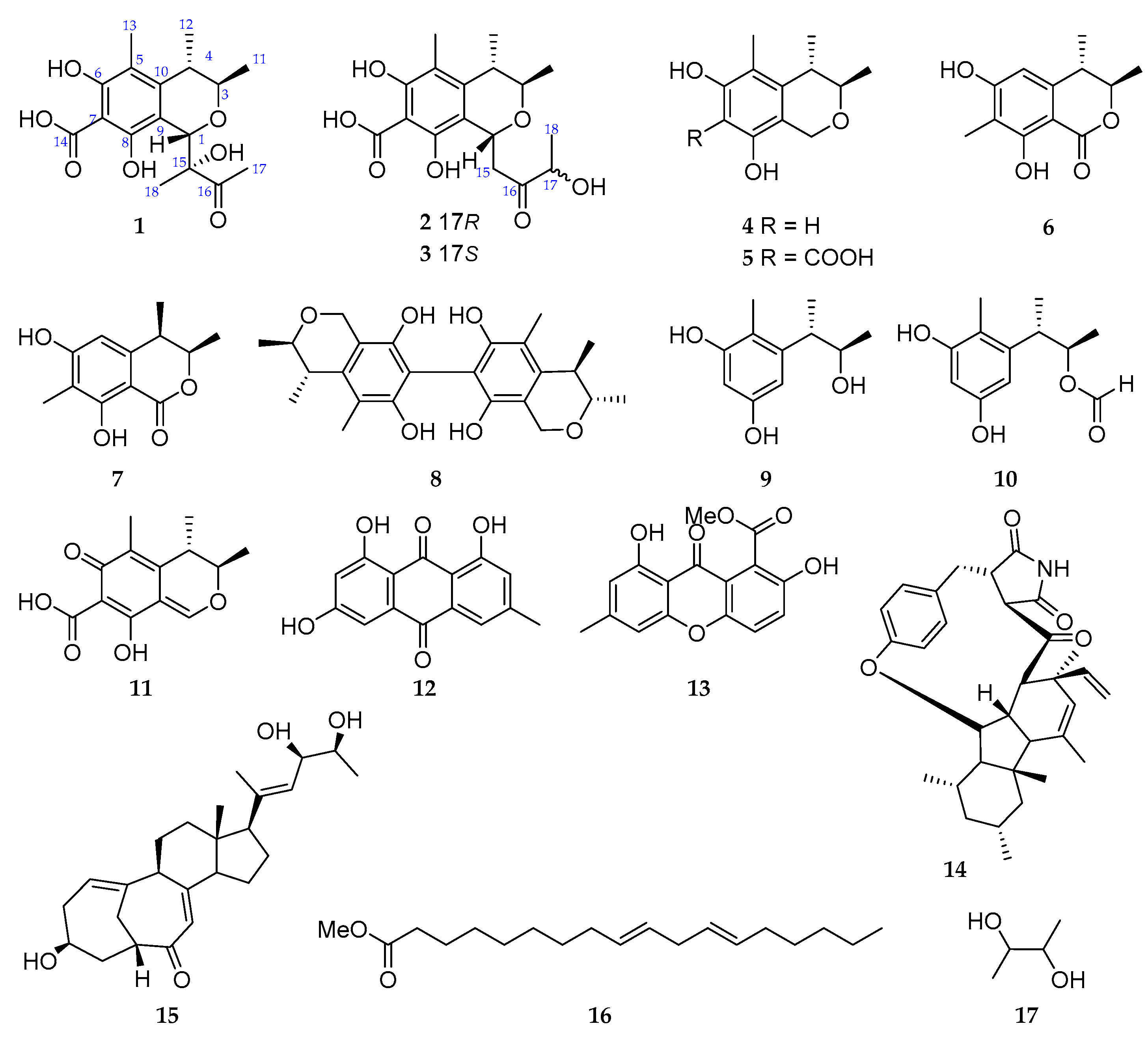
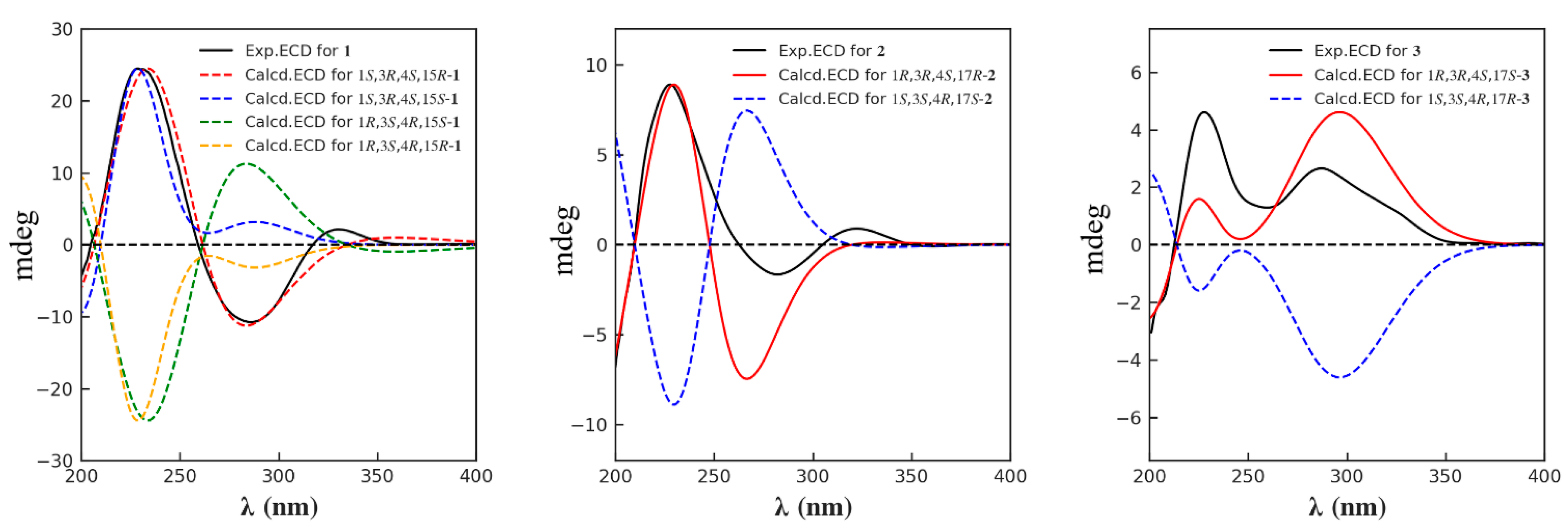
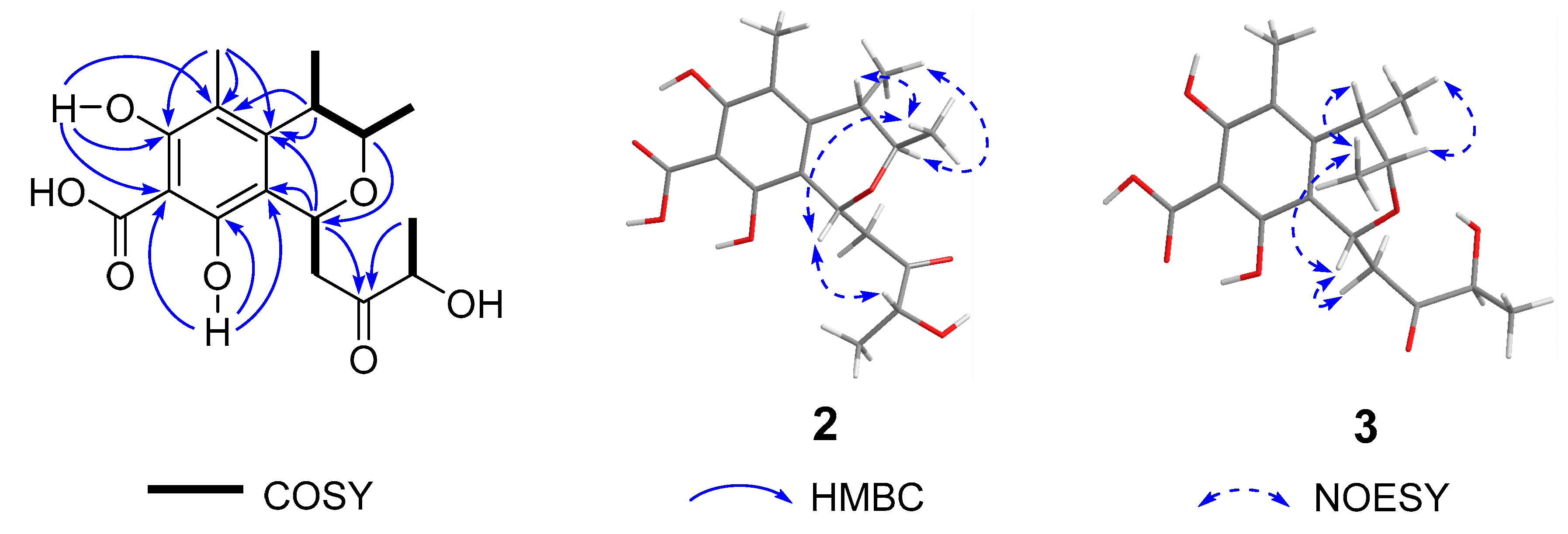
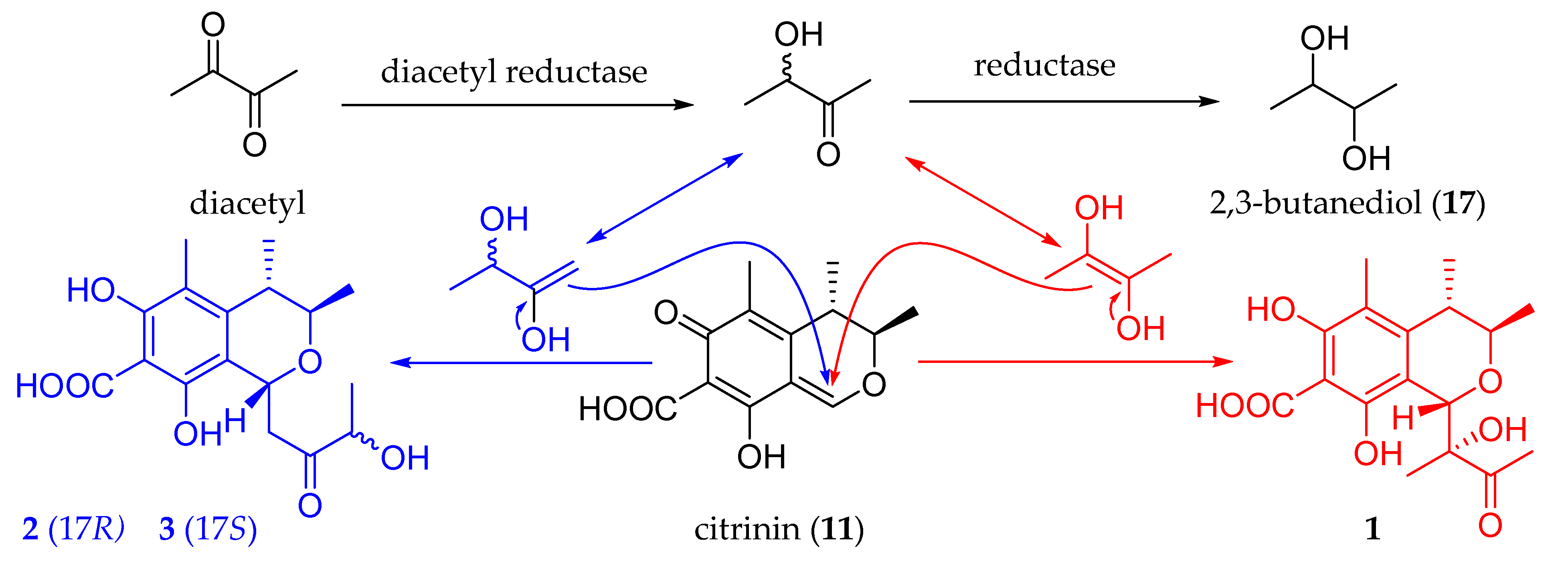
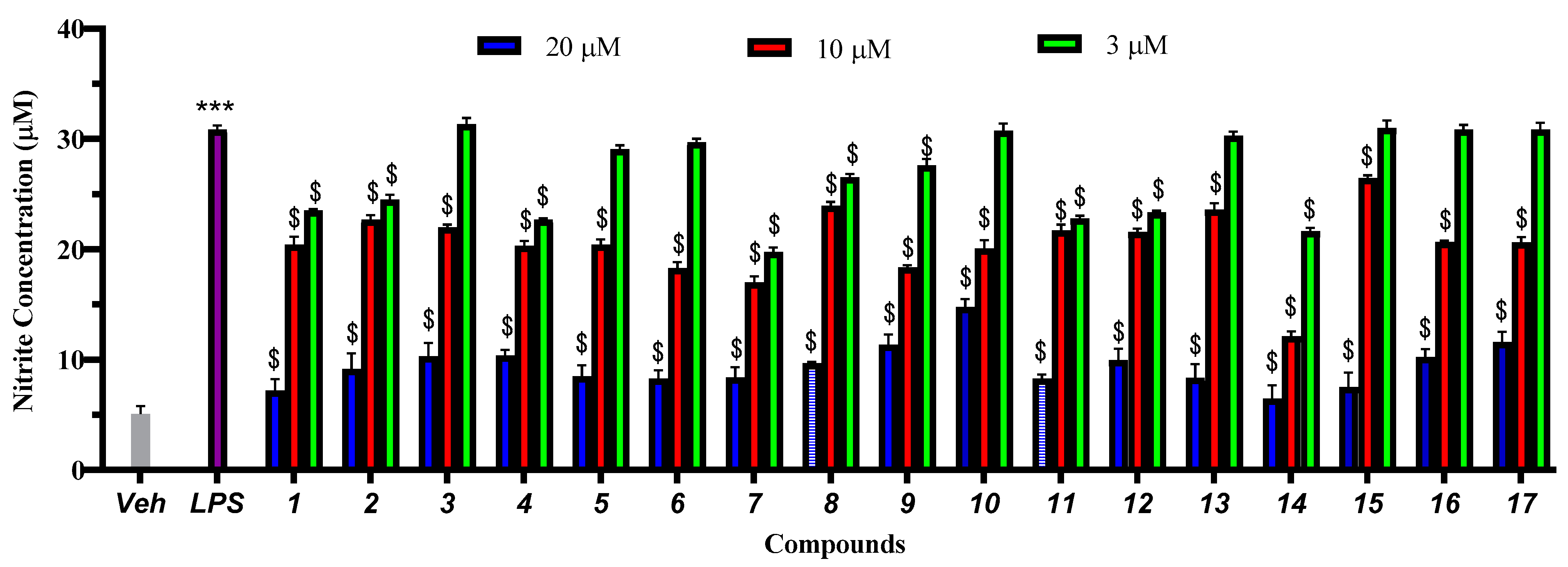
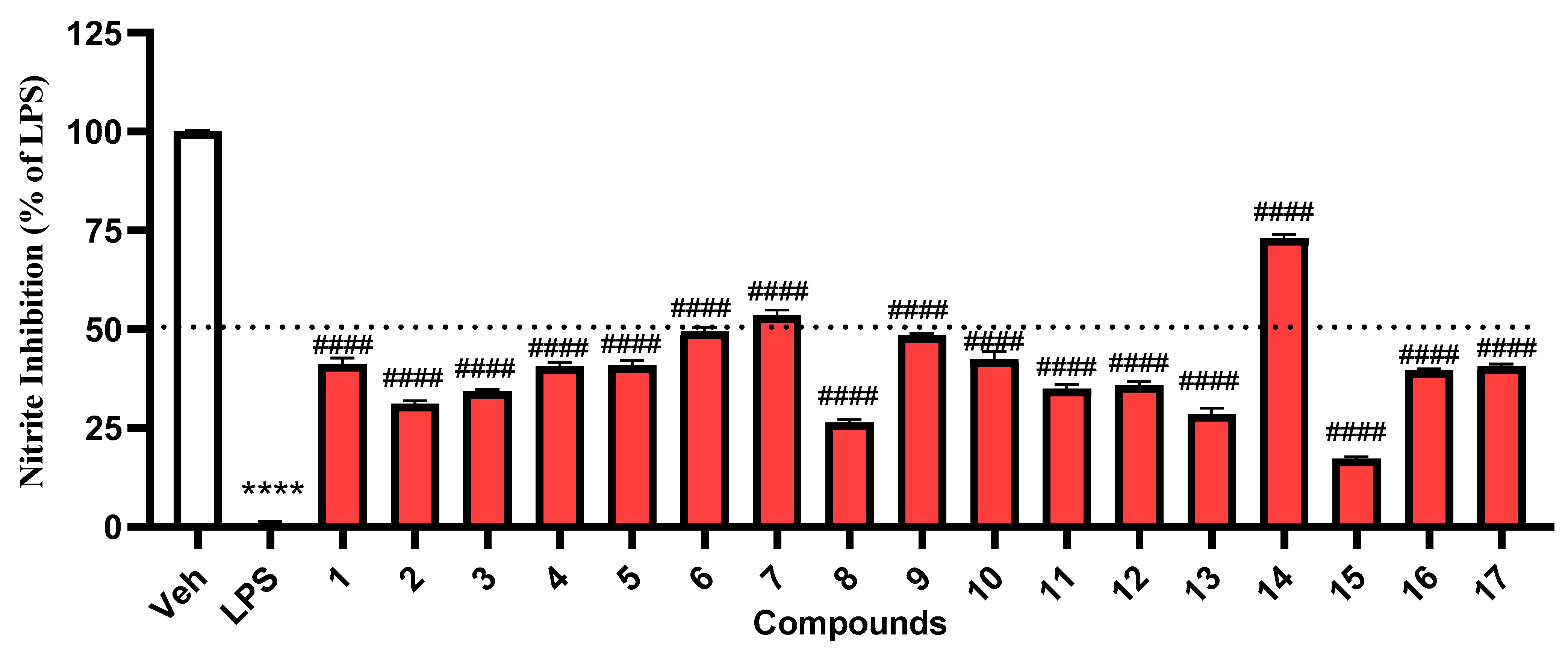
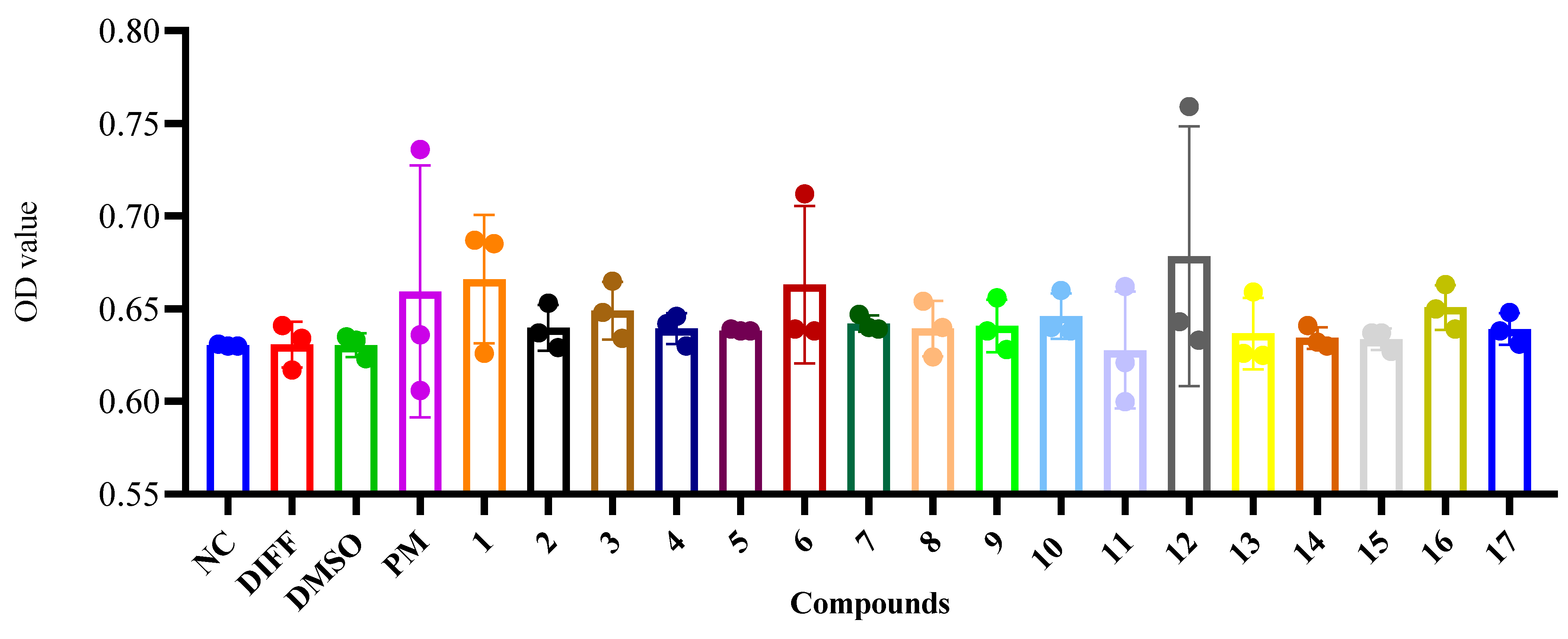
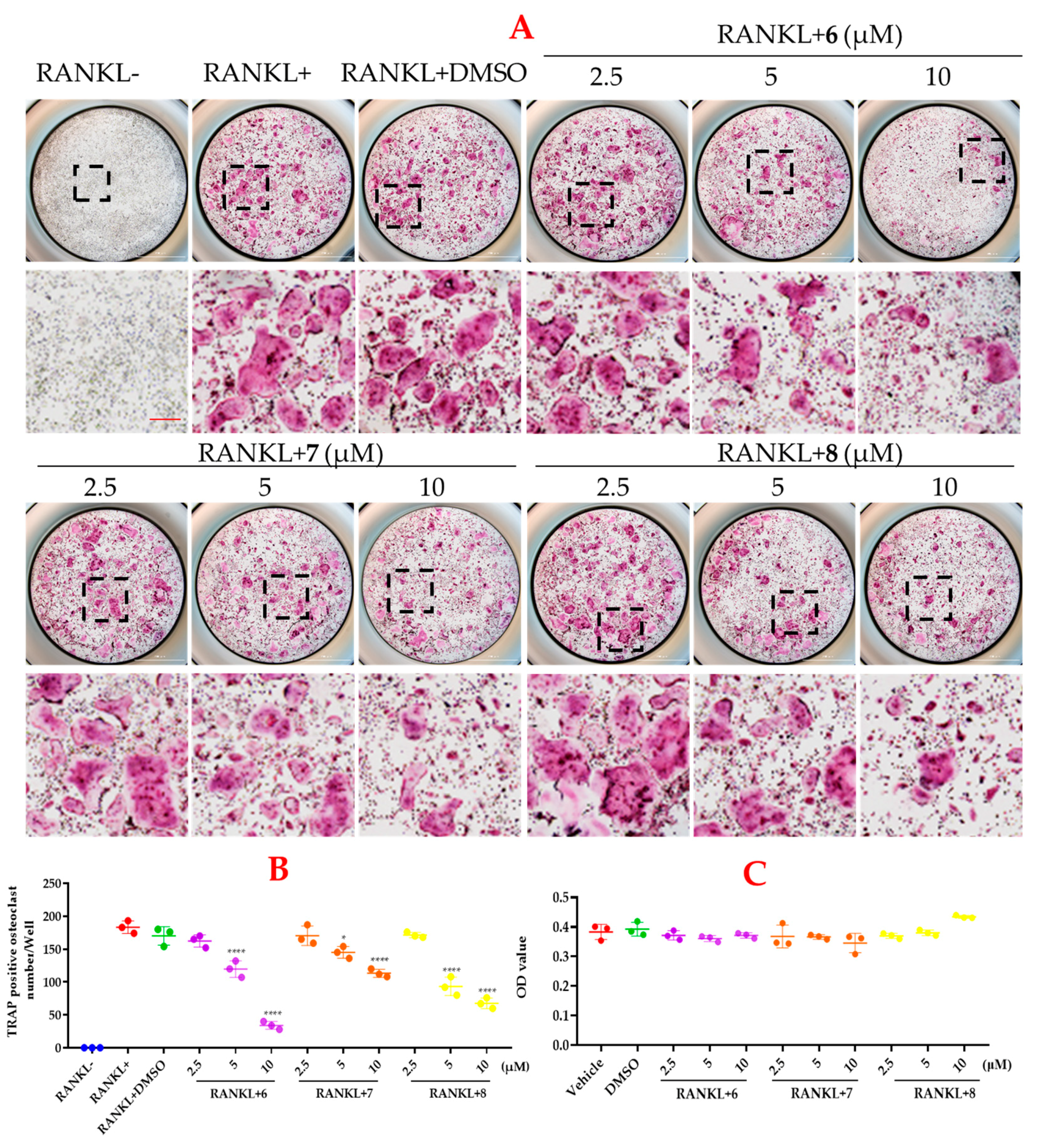
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Identification, Fermentation, and Extraction
3.3. Isolation and Purification
3.4. ECD Calculation
3.5. BV-2 Cell Culture and Compound Treatment
3.6. Quantification of Nitrite Levels
3.7. Cell Extraction and Culture
3.8. CCK-8 Assay
3.9. Osteoblast Differentiation and Mineralization
3.10. Osteoclast Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mudassir, S.; Zhang, Z.; Feng, Y.; Chang, Y.; Che, Q.; Gu, Q.; Zhu, T.; Zhang, G.; Li, D. Secondary metabolites from deep-sea derived microorganisms. Curr. Med. Chem. 2020, 27, 6244–6273. [Google Scholar] [CrossRef]
- Chooi, Y.H.; Tang, Y. Navigating the fungal polyketide chemical space: From genes to molecules. J. Org. Chem. 2012, 77, 9933–9953. [Google Scholar] [CrossRef]
- Niu, S.; Tang, X.X.; Fan, Z.; Xia, J.M.; Xie, C.L.; Yang, X.W. Fusarisolins A-E, polyketides from the marine-derived fungus Fusarium solani H918. Mar. Drugs 2019, 17, 125. [Google Scholar] [CrossRef]
- Pojer, F.; Ferrer, J.L.; Richard, S.B.; Nagegowda, D.A.; Chye, M.L.; Bach, T.J.; Noel, J.P. Structural basis for the design of potent and species-specific inhibitors of 3-hydroxy-3-methylglutaryl CoA synthases. Proc. Natl. Acad. Sci. USA 2006, 103, 11491–11496. [Google Scholar] [CrossRef]
- De Pascale, G.; Nazi, I.; Harrison, P.H.; Wright, G.D. β-Lactone natural products and derivatives inactivate homoserine transacetylase, a target for antimicrobial agents. J. Antibiot. 2011, 64, 483–487. [Google Scholar] [CrossRef]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, Y.H.; Shao, C.L.; Cao, F.; Wang, C.Y. Microketides A and B, polyketides from a gorgonian-derived Microsphaeropsis sp. fungus. J. Nat. Prod. 2020, 83, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Hsiao, G.; Chang, C.H.; Yang, Y.L.; Ju, Y.M.; Kuo, Y.H.; Lee, T.H. Polyketides with anti-neuroinflammatory activity from Theissenia cinerea. J. Nat. Prod. 2021, 84, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, Y.Y.; Chen, Z.Q.; Shen, N.X.; Shen, L.; Zhang, F.M.; Zhou, X.J.; Wang, C.Y. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2019, 82, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Xia, G.; Huang, H.; Li, H.; Ma, L.; Lu, Y.; He, L.; Xia, X.; She, Z. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 2015, 78, 1816–1822. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Zhu, T.; Kurtán, T.; Mándi, A.; Zhao, Z.; Li, J.; Gu, Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron 2011, 67, 7913–7918. [Google Scholar] [CrossRef]
- He, Z.H.; Xie, C.L.; Wu, T.; Yue, Y.T.; Wang, C.F.; Xu, L.; Xie, M.M.; Zhang, Y.; Hao, Y.J.; Xu, R.; et al. Tetracyclic steroids bearing a bicyclo[4.4.1] ring system as potent antiosteoporosis agents from the deep-sea-derived fungus Rhizopus sp. W23. J. Nat. Prod. 2023, 86, 157–165. [Google Scholar] [CrossRef]
- Hao, Y.J.; Zou, Z.B.; Xie, M.M.; Zhang, Y.; Xu, L.; Yu, H.Y.; Ma, H.B.; Yang, X.W. Ferroptosis inhibitory compounds from the deep-sea-derived fungus Penicillium sp. MCCC 3A00126. Mar. Drugs 2023, 21, 234. [Google Scholar] [CrossRef]
- He, Z.H.; Xie, C.L.; Wu, T.; Zhang, Y.; Zou, Z.B.; Xie, M.M.; Xu, L.; Capon, R.J.; Xu, R.; Yang, X.W. Neotricitrinols A-C, unprecedented citrinin trimers with anti-osteoporosis activity from the deep-sea-derived Penicillium citrinum W23. Bioorg. Chem. 2023, 139, 106756. [Google Scholar] [CrossRef]
- Xie, C.L.; Zhang, D.; Guo, K.Q.; Yan, Q.X.; Zou, Z.B.; He, Z.H.; Wu, Z.; Zhang, X.K.; Chen, H.F.; Yang, X.W. Meroterpenthiazole A, a unique meroterpenoid from the deep-sea-derived Penicillium allii-sativi, significantly inhibited retinoid X receptor (RXR)-α transcriptional effect. Chin. Chem. Lett. 2022, 33, 2057–2059. [Google Scholar] [CrossRef]
- Niu, S.; Xie, C.L.; Xia, J.M.; Liu, Q.M.; Peng, G.; Liu, G.M.; Yang, X.W. Botryotins A-H, tetracyclic diterpenoids representing three carbon skeletons from a deep-sea-derived Botryotinia fuckeliana. Org. Lett. 2020, 22, 580–583. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Zheng, S.; Zhang, Y.; Bao, Y. Genetic engineering of Bacillus sp. and fermentation process optimizing for diacetyl production. J. Biotechnol. 2019, 301, 2–10. [Google Scholar] [CrossRef]
- Wakana, D.; Hosoe, T.; Itabashi, T.; Okada, K.; de Campos Takaki, G.M.; Yaguchi, T.; Fukushima, K.; Kawai, K. New citrinin derivatives isolated from Penicillium citrinum. J. Nat. Med. 2006, 60, 279–284. [Google Scholar] [CrossRef]
- Deruiter, J.; Jacyno, J.M.; Davis, R.A.; Cutler, H.G. Studies on aldose reductase inhibitors from fungi. I. Citrinin and related benzopyran derivatives. J. Enzyme. Inhib. 1992, 6, 201–210. [Google Scholar] [CrossRef]
- Han, Z.; Mei, W.; Zhao, Y.; Deng, Y.; Dai, H. A new cytotoxic isocoumarin from endophytic fungus Penicillium SP. 091402 of the mangrove plant Bruguiera sexangula. Chem. Nat. Compd. 2010, 45, 805–807. [Google Scholar] [CrossRef]
- Kuramata, M.; Fujioka, S.; Shimada, A.; Kawano, T.; Kimura, Y. Citrinolactones A, B and C, and sclerotinin C, plant growth regulators from Penicillium citrinum. Biosci. Biotechnol. Biochem. 2007, 71, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Ouyang, M.A.; Tan, Q.W. New asperxanthone and asperbiphenyl from the marine fungus Aspergillus sp. Pest. Manag. Sci. 2009, 65, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Menta, A.B.; Yoneyama, K.; and Kitabatake, N. A major decomposition product, citrinin H2, from citrinin on heating with moisture. Biosci. Biotechnol. Biochem. 2002, 66, 206–210. [Google Scholar] [CrossRef]
- Chai, Y.J.; Cui, C.B.; Li, C.W.; Wu, C.J.; Tian, C.K.; Hua, W. Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 559–582. [Google Scholar] [CrossRef]
- Li, S.F.; Di, Y.T.; Wang, Y.H.; Tan, C.J.; Fang, X.; Zhang, Y.; Zheng, Y.T.; Li, L.; He, H.P.; Li, S.L.; et al. Anthraquinones and Lignans from Cassia occidentalis. Helv. Chim. Acta 2010, 93, 1795–1802. [Google Scholar] [CrossRef]
- Kim, S.; Le, T.C.; Han, S.A.; Hillman, P.F.; Hong, A.; Hwang, S.; Du, Y.E.; Kim, H.; Oh, D.C.; Cha, S.S.; et al. Saccharobisindole, neoasterric methyl ester, and 7-chloro-4(1H)-quinolone: Three new compounds isolated from the marine bacterium Saccharomonospora sp. Mar. Drugs 2021, 20, 35. [Google Scholar] [CrossRef]
- Oilawa, H. Biosynthesis of structurally unique fungal metabolite GKK1032A2: Indication of novel carbocyclic formation mechanism in polyketide biosynthesis. J. Org. Chem. 2003, 68, 3552–3557. [Google Scholar]
- Ren, J.M.; Yang, J.K.; Zhu, H.J.; Cao, F. Bioactive steroids from the marine-derived fungus Aspergillus flavus JK07-1. Chem. Nat. Compd. 2020, 56, 945–947. [Google Scholar] [CrossRef]
- Biswas, A.; Sharma, B.K.; Willett, J.L.; Erhan, S.Z.; Cheng, H.N. Room-temperature self-curing ene reactions involving soybean oil. Green Chem. 2008, 10, 290–295. [Google Scholar] [CrossRef]
- Uemura, Y.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Takeda, Y.; Kawahata, M.; Yamaguchi, K. Microtropins A–I: 6′-O-(2″S,3″R)-2″-ethyl-2″,3″-dihydroxybutyrates of aliphatic alcohol β-d-glucopyranosides from the branches of Microtropis japonica. Phytochemistry 2013, 87, 140–147. [Google Scholar] [CrossRef]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef]
- Rawji, K.S.; Mishra, M.K.; Michaels, N.J.; Rivest, S.; Stys, P.K.; Yong, V.W. Immunosenescence of microglia and macrophages: Impact on the ageing central nervous system. Brain 2016, 139, 653–661. [Google Scholar] [CrossRef]
- Costa, T.; Fernandez-Villalba, E.; Izura, V.; Lucas-Ochoa, A.M.; Menezes-Filho, N.J.; Santana, R.C.; de Oliveira, M.D.; Araujo, F.M.; Estrada, C.; Silva, V.; et al. Combined 1-deoxynojirimycin and ibuprofen treatment decreases microglial activation, phagocytosis and dopaminergic degeneration in MPTP-treated mice. J. Neuroimmune Pharmacol. 2021, 16, 390–402. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Ko, M.J.; Park, G.; Choi, Y.W. Anti-neuroinflammatory effect of emodin in LPS-stimulated microglia: Involvement of AMPK/Nrf2 activation. Neurochem. Res. 2016, 41, 2981–2992. [Google Scholar] [CrossRef]
- Song, T.; Chen, M.; Ge, Z.W.; Chai, W.; Li, X.C.; Zhang, Z.; Lian, X.Y. Bioactive penicipyrrodiether A, an adduct of GKK1032 analogue and phenol A derivative, from a marine-sourced fungus Penicillium sp. ZZ380. J. Org. Chem. 2018, 83, 13395–13401. [Google Scholar] [CrossRef]
- El-Desoky, A.H.H.; Tsukamoto, S. Marine natural products that inhibit osteoclastogenesis and promote osteoblast differentiation. J. Nat. Med. 2022, 76, 575–583. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q.F.; Yan, J.; Hu, R.; Jiang, H. Isoflurane preconditioning promotes the survival and migration of bone marrow stromal cells. Cell Physiol. Biochem. 2015, 36, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Yao, W.; Liu, R.; Lam, K.S.; Nolta, J.; Jia, J.; Panganiban, B.; Meng, L.; Zhou, P.; Shahnazari, M.; et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ou-Yang, H.; Yan, X.; Tang, B.W.; Fang, M.J.; Wu, Z.; Chen, J.W.; Qiu, Y.K. Open-ring butenolides from a marine-derived anti-neuroinflammatory fungus Aspergillus terreus Y10. Mar. Drugs 2018, 16, 428. [Google Scholar] [CrossRef]
- Niu, S.; Yang, L.; Zhang, G.; Chen, T.; Hong, B.; Pei, S.; Shao, Z. Phenolic bisabolane and cuparene sesquiterpenoids with anti-inflammatory activities from the deep-sea-derived Aspergillus sydowii MCCC 3A00324 fungus. Bioorg. Chem. 2020, 105, 104420. [Google Scholar] [CrossRef] [PubMed]


| No. | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 75.4 CH | 5.17 s | 64.8 CH | 5.08 (d, 9.7) | 64.8 CH | 5.08 (d, 9.2) |
| 3 | 74.0 CH | 3.98 (qd, 6.9, 1.8) | 71.7 CH | 3.82 (qd, 6.6, 1.5) | 71.7 CH | 3.82 (qd, 6.6, 1.5) |
| 4 | 36.9 CH | 2.62 (qd, 6.7, 1.8) | 35.1 CH | 2.52 m | 35.2 CH | 2.51 m |
| 5 | 114.0 C | 109.5 C | 109.6 C | |||
| 6 | 160.0 C | 158.5 C | 158.5 C | |||
| 7 | 102.2 C | 101.8 C | 101.8 C | |||
| 8 | 156.7 C | 155.8 C | 155.8 C | |||
| 9 | 110.3 C | 110.7 C | 110.9 C | |||
| 10 | 143.8 C | 139.7 C | 139.8 C | |||
| 11 | 18.3 CH3 | 1.11 (d, 6.4) | 18.2 CH3 | 1.01 (d, 6.5) | 18.3 CH3 | 0.99 (d, 6.5) |
| 12 | 20.1 CH3 | 1.28 (d, 6.9) | 20.1 CH3 | 1.17 (d, 6.8) | 20.2 CH3 | 1.17 (d, 7.2) |
| 13 | 10.2 CH3 | 2.04 s | 9.5 CH3 | 1.93 s | 9.6 CH3 | 1.92 s |
| 14 | 178.2 C | 175.6 C | 175.7 C | |||
| 15 | 83.2 C | 43.1 CH2 | 2.66 m 3.23 m | 43.0 CH2 | 2.72 m 3.16 m | |
| 16 | 211.3 C | 211.9 C | 212.1 C | |||
| 17 | 25.0 CH3 | 2.30 s | 72.2 CH | 4.11 m | 72.8 CH | 4.02 m |
| 18 | 20.7 CH3 | 1.10 s | 19.2 CH3 | 1.21 (d, 7.0) | 19.0 CH3 | 1.15 (d, 7.2) |
| 6-OH | 14.64 s | 14.62 s | ||||
| 8-OH | 15.16 s | 15.13 s | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xie, C.-L.; Wang, Y.; He, X.-W.; Xie, M.-M.; Li, Y.; Zhang, K.; Zou, Z.-B.; Yang, L.-H.; Xu, R.; et al. Penidihydrocitrinins A–C: New Polyketides from the Deep-Sea-Derived Penicillium citrinum W17 and Their Anti-Inflammatory and Anti-Osteoporotic Bioactivities. Mar. Drugs 2023, 21, 538. https://doi.org/10.3390/md21100538
Zhang Y, Xie C-L, Wang Y, He X-W, Xie M-M, Li Y, Zhang K, Zou Z-B, Yang L-H, Xu R, et al. Penidihydrocitrinins A–C: New Polyketides from the Deep-Sea-Derived Penicillium citrinum W17 and Their Anti-Inflammatory and Anti-Osteoporotic Bioactivities. Marine Drugs. 2023; 21(10):538. https://doi.org/10.3390/md21100538
Chicago/Turabian StyleZhang, Yong, Chun-Lan Xie, Yuan Wang, Xi-Wen He, Ming-Min Xie, You Li, Kai Zhang, Zheng-Biao Zou, Long-He Yang, Ren Xu, and et al. 2023. "Penidihydrocitrinins A–C: New Polyketides from the Deep-Sea-Derived Penicillium citrinum W17 and Their Anti-Inflammatory and Anti-Osteoporotic Bioactivities" Marine Drugs 21, no. 10: 538. https://doi.org/10.3390/md21100538
APA StyleZhang, Y., Xie, C.-L., Wang, Y., He, X.-W., Xie, M.-M., Li, Y., Zhang, K., Zou, Z.-B., Yang, L.-H., Xu, R., & Yang, X.-W. (2023). Penidihydrocitrinins A–C: New Polyketides from the Deep-Sea-Derived Penicillium citrinum W17 and Their Anti-Inflammatory and Anti-Osteoporotic Bioactivities. Marine Drugs, 21(10), 538. https://doi.org/10.3390/md21100538








