Abstract
A systematic chemical investigation of the deep-sea-derived fungus Aspergillus versicolor 170217 resulted in the isolation of six new (1–6) and 45 known (7–51) compounds. The structures of the new compounds were established on the basis of exhaustive analysis of their spectroscopic data and theoretical–statistical approaches including GIAO-NMR, TDDFT-ECD/ORD calculations, DP4+ probability analysis, and biogenetic consideration. Citriquinolinones A (1) and B (2) feature a unique isoquinolinone-embedded citrinin scaffold, representing the first exemplars of a citrinin–isoquinolinone hybrid. Dicitrinones K–L (3–4) are two new dimeric citrinin analogues with a rare CH-CH3 bridge. Biologically, frangula-emodin (32) and diorcinol (17) displayed remarkable anti-food allergic activity with IC50 values of 7.9 ± 3.0 μM and 13.4 ± 1.2 μM, respectively, while diorcinol (17) and penicitrinol A (20) exhibited weak inhibitory activity against Vibrio parahemolyticus, with MIC values ranging from 128 to 256 μM.
1. Introduction
Natural products play a dominant role in the discovery of drug leads for the treatment of human diseases [1,2]. Marine organisms living in harsh surroundings, such as high salt, high pressure, and hypoxic environments, are expected to produce unique secondary metabolites when compared with their terrestrial counterparts in terms of structural diversity and functional features, making them a promising storehouse of new bioactive entities for drug leads discovery [3,4]. Of all marine-derived fungi, the genus Aspergillus has been the most studied, as it is ubiquitous among almost all ecosystems and it is rich in bioactive secondary metabolites, with multifarious and intricate structures [5]. These include the citrinin family, a compound class that features a 6,8-dihydroxyl-3,4,5-trimethyl-chromene core and exhibits abundant structural diversity by decomposition, dimerization, and trimerization through various pathways [6,7]. Therefore, the biological behaviors of these compounds extend from anticancer to antimicrobial activities, influenza neuraminidase inhibitory and anti-osteoporosis effects. However, citrinin derivatives embedded with alkaloid moiety are rarely reported in the literature, with the exception of citrinidines A–E, whose structures involve a proline-derived unit [8].
As part of our ongoing investigation into the chemistry of deep-sea-derived fungi, we obtained an A. versicolor fungus from the intestinal contents of a whale Mesoplodon densirostris from the East China Sea which was stranded in Ningde, China. The primary chromatographic analysis of the extract from a PDA culture revealed the rich chemical diversity of the metabolites; therefore, we conducted an in-depth investigation of this strain to discover novel and active compounds. As a result, four new dimeric citrinin derivatives (1–4), one new isochromene derivative (5), and one new acetamide (6) (Figure 1), together with 45 known compounds, were obtained. The structures of the new compounds were established on the basis of exhaustive analysis of their spectroscopic data and theoretical–statistical approaches including GIAO-NMR, TDDFT-ECD/ORD calculations, DP4+ probability analysis, or biogenetic consideration. Intriguingly, citriquinolinones A–B (1–2) bear a citrinin scaffold embedded with an unusual isoquinolinone unit, which has not been previously reported in this compound class.
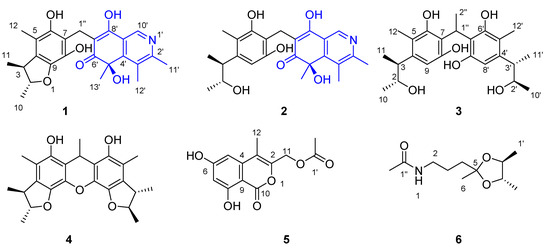
Figure 1.
The chemical structures of the new compounds 1–6 obtained from Aspergillus versicolor 170217.
By comparison of the NMR, MS, and optical rotation data with those published in the literature, the known compounds were determined to be viridicatol (7) [9], 4-(hydroxymethyl)benzoic acid (8) [10], p-hydroxybenzoic acid (9) [11], ferulic acid (10) [12], (2S,3S)-1-(4-hydroxyphenyl) butane-2,3-diol (11) [13], (2R,3S)-1-(4-hydroxyphenyl)butane-2,3-diol (12) [13], 4-((2S,3R)-3-hydroxybutan-2-yl)-3,6-dimethylbenzene-1,2-diol (13) [14,15], phenol A (14) [16], monodictyphenone (15) [17], dicitrinone F (16) [15], diorcinol (17) [18], verticilatin (18) [19], xerucitrinic acids A (19) [20], penicitrinol A (20) [21], penicitrinone B (21) [22], (+)-austrosene (22) [23], 3,6,8-trihydroxy-3,5,7-trimethylisochroman-1-one (23) [24], sescandelin (24) [25], sescandelin B (25) [25], dihydrocitrinone (26) [26,27], dihydroxy-3,4,7-trimethylisocoumarin (27) [28], lawsozaheer (28) [29], dyhydrocitrinin (29) [30], citrinin (30) [31], 5-methoxysterigmatocystin (31) [32], frangula-emodin (32) [33], citreorosein (33) [34], (E)-4-hydroxy-4-(3-hydroxybut-1-en-1-yl)-3,5,5-trimethylcyclohex-2-en-1-one (34) [35], citrinal A (35) [36], citrinal B (36) [36], macrolactin A (37) [37], quinolactacin A1 (38) [38], 7,8-epoxy-brevianamide Q (39) [39], sterigmatocystin (40) [40], (+)-brevianamide R (41) [39], (−)-brevianamide R (42) [39], brevianamide K (43) [41], epi-deoxybrevianamide E (44) [42], brevianamide W (45) [42], 2-(1,1-dimethyl-2-propen-1-yl)-1H-in-dole-3-carboxaldehyde (46) [43], 1H-indole-3-carbaldehyde (47) [44], (1H-indol-3-yl) oxoacetamide (48) [45], N-acetyramine (49) [46], cyclo-(Phe-Tyr) (50) [47], and glulisine A (51) [48]. Herein, we report the isolation, structures, and bioactivities of these isolates.
2. Results and Discussion
Compound 1 was isolated as a yellow powder. It was assigned a molecular formula C24H27NO6 due to its HRESIMS peak at m/z 424.1759 [M−H]−, requiring 12 degrees of unsaturation (DOU). The 1H and 13C NMR spectroscopic data (Figures S1 and S2, Table 1) revealed the presence of six methyls [δH 1.25 (3H, dd, J = 6.1 Hz, 1.7 Hz, 10-Me), 1.19 (3H, d, J = 6.8 Hz, 11-Me), 2.05 (3H, s, 12-Me), 2.67 (3H, s, 11′-Me), 2.70 (3H, s, 12′-Me), 1.60 (3H, s, 13′-Me); δC 21.2 (q, C-10), 19.8 (q, 11-Me), 12.3 (q, C-12), 20.0 (q, C-11′), 16.7 (q, C-12′), 29.3 (q, C-13′)]; one methylene [δH 3.70 (dd, J = 14.2 Hz, 2.4 Hz, H-1″), 3.60 (dd, J =14.2 Hz, 4.4 Hz, H2-1″); δC 20.2 (t, C-1″)]; three methines [δH 4.30 (dq, J = 6.1 Hz, 3.9 Hz, H-2); δH 2.90 (dq, J = 6.8 Hz, 3.9 Hz, H-3), 8.86 (s, H-10′); δC 87.6 (d, C-2), 45.3 (d, C-3), 137.6 (d, C-10′)]; fourteen non-hydrogenated carbons, including twelve olefinic [δC 130.4 (s, C-4), 114.0 (s, C-5), 148.0 (s, C-6), 117.6 (s, C-7), 138.2 (s, C-8), 140.9 (s, C-9), 155.8 (s, C-2′), 135.8 (s, C-3′), 159.0 (s, C-4′), 112.6 (s, C-7′), 175.3 (s, C-8′), 128.0 (s, C-9′)], one sp3 [δC 74.7 (s, C-5′)]; and one ketone at δC 196.2 (s, C-6′). Diagnostic HMBC correlations observed from H3-11 to C-2/3/4, H3-12 to C-4/5/6, H2-1″ to C-6/7/8, and from H-2/3 to C-9, together with the COSY cross-peaks H-2/H3-10/H-3/H3-11, supported a 2,3,5-trimethyl-6,8-dihydrobenzofuran fragment, A [49]. Another fragment, B, was recognized as an isoquinocitrinin owing to the HMBC correlations of H3-11′/C-2′/3′; H3-12′/C-3′/C-2′ and C-4′, H3-13′/C-5′/C-4′ and C-6′, H3-10′/C-2′ and C-4′/C-9′; as well as H-1″/C-6′/C-7′/C-8′ [50]. The HMBC cross-peaks to both fragments, starting from H2-1″, thus established the CH2 linkage between A and B (Figure 2).

Table 1.
1H (400 Hz) and 13C (100 Hz) NMR spectroscopic data of compounds 1 and 2 (δ in ppm, J in Hz within parentheses).
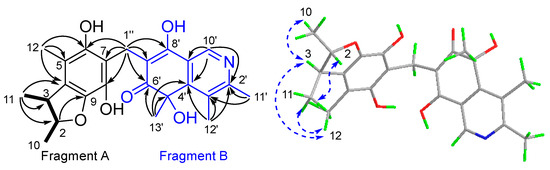
Figure 2.
The key 1H-1H COSY ( ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1.
) correlations of 1.
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1.
) correlations of 1.
The relative configuration of 1 was partially determined by NOESY experiments. The correlations observed from H-2 to H3-11 and H-3 to H3-10 indicated that 3-Me and 2-Me are trans-configured (Figure 2). To furtherly determine the relative configuration between C-5′ and C-2/3, we performed a quantum chemical calculation of NMR data for the two possible isomers of 1, 2R*,3S*,5′R* and 2R*,3S*,5′S*, along with a DP4+ probability analysis. As a result, the theoretical prediction of the chemical shift of the former showed a better correspondence with the experimental data, with an average probability of 83.04% (Figure 3). Finally, the absolute configuration of 1 was established by a TD-DFT based ECD calculation of the two enantiomers of 1 at the B3LYP/6-31+g (d, p) level, as well as comparison with the experimental data, which indicated the correct configuration as 2R,3S,5′R (Figure 4). Hence, the complete structure of 1 was assigned as shown and named citriquinolinone A. Of note, citriquinolinone A (1) represents the first exemplar of an isoquinolinone–citrinin hybrid.
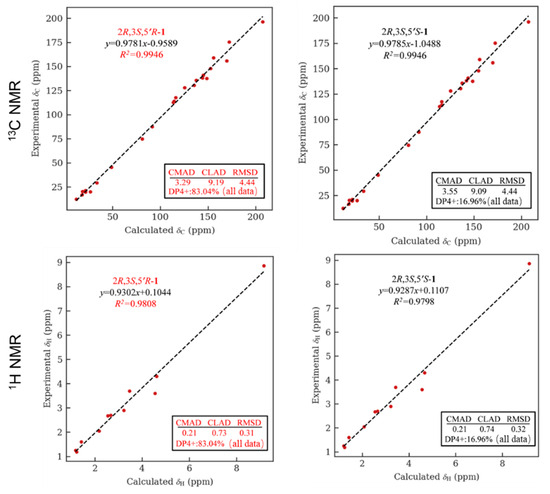
Figure 3.
NMR calculation for compound 1: regression analysis of experimental and calculated 13C and 1H NMR chemical shifts for 2R*,3S*,5′R*-1 and 2R*,3S*,5′S*-1, with the probability of each isomer analyzed by DP4+ analysis included.
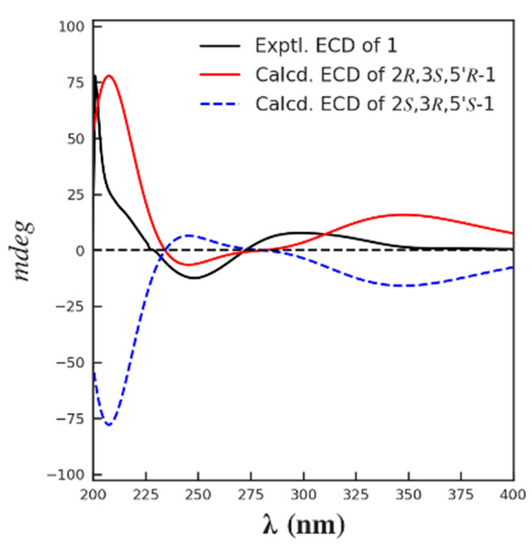
Figure 4.
The calculated and the experimental ECD spectra of compound 1.
Compound 2 was isolated as a yellow amorphous solid and assigned the molecular formula C24H29NO6 on the basis of the [M − H]− ionic peak at m/z 426.1996 in its negative HRESIMS spectrum, suggestive of a homologue of 1. Comparison of NMR data of 2 (Figures S8–S13, Table 1) and 1 revealed a high degree of similarity, including resonances accounting for the isoquinocitrinin substructure, namely fragment B of 1. The primary differences were attributed to the presence of an aromatic proton (δH 6.30, s; H-9), as well as the shielding of C-2 (ΔδC -5.5). HMBC correlations from H-1″ to C-6/7/8, H-9 to C-5/C-7, together with signals observed from H3-11 to C-4 and COSY correlations of H3-10/H-2/H-3/-H3-11, inferred the penta-substituted benzene fragment A. Considering the molecular formula, the planar structure of 2 was assigned as shown in Figure 5.

Figure 5.
The key 1H-1H COSY ( ) and HMBC (
) and HMBC ( ) correlations of compound 2.
) correlations of compound 2.
 ) and HMBC (
) and HMBC ( ) correlations of compound 2.
) correlations of compound 2.
As C-2/3 are not involved in a dihydrofuran ring, in this case, the NOE spectrum is useless for determining its relative configuration. However, compound 2 contains a citrinin moiety similar to that of 1, namely a phenol A unit; we therefore examined the biogenetic relationship between these two compounds. Capon et al. documented a plausible mechanism for the transformation from citrinin to 2,3,5-trimethyl-6,8-dihydrobenzofuran and phenol A, in which the stereo configurations of C-2/3 were retained in the whole process [27]. This, along with the co-occurrence of citrinin (30) and phenol A (14) in the same extract and a comparison of the chemical shift of fragment A of 2 with that of 14, permitted the appearance of relative/absolute configurations of C-2/3 in 2 same as those observed in 1, 14, and 30. To further assign the relative configuration between C-5′and C-2/3, the NMR data of 2 in MeOH was predicted using the GIAO based calculation, followed by DP4+ analysis. The comparison of the theoretical with the experimental data showed that the 2R*,3S*,5′R* isomer exhibited a 100% probability (Figure 6). Likewise, the absolute configuration of 2 was eventually established to be 2R,3S,5′R by comparing the calculated ECD spectrum with that of the experimental data (Figure S42). Therefore, the structure of 2 was assigned, and the compound was given the trivial name citriquinolinone B.

Figure 6.
NMR calculation for compound 2: regression analysis of experimental and calculated 13C NMR chemical shifts for 2R*,3S*,5′R*-2 and 2R*,3S*,5′S*-2, with the probability of each isomer analyzed by DP4+ analysis included.
Compound 3 was isolated as a yellow oil and assigned the molecular formula C24H34O6, based on the HRESI(-)MS peak at m/z 441.2250 [M+Na]+, requiring eight DOU. The 1H, 13C NMR, and DEPT spectroscopic data (Figures S15–S20 and Table 2) revealed the presence of 24 carbons, including seven methyls, seven methines, and 10 quaternary carbons. In the 13C NMR spectrum, all the carbon signals, except the C-1″ (δC 27.7, CH) and C-2″ (δC 17.8, CH3) examples, existed in pairs, suggesting that 3 was likely a dimeric compound. The comparison of the 1D and 2D NMR data of 3 with those of 16 showed that they closely resembled each other, with the primary difference being the replacement of a methylene in 2 with a methine and methyl in 3. Further confirmation was obtained using the COSY correlations of H3-10 (δH 1.10, d, J = 6.3 Hz)/H-2 (δH 3.80, dq, J = 6.5, 6.3 Hz)/H-3 (δH 3.02, dq, J = 6.8, 6.5 Hz)/H3-11 (δH 1.12, d, J = 6.8 Hz), and HMBC cross-peaks from H3-12 (δH 2.10, s) to C-4 (δC 143.2 s) C-5 (δC 117.6 s) and C-6 (δC 154.6 s), H3-11 to C-4 (δC 143.2 s), H-9 (δH 6.37, s) to C-3 (δC 42.9 s), C-5, and C-7 (δC 116.7 s). The COSY correlations of H-1″ (δH 4.9, m)/H3-2″ (δH 1.80, d, J = 7.5 Hz), along with the diagnostic HMBC correlations from H-2″ to C-7, H-1″ to C-6, C-7 and C-8 (δC 152.9 s), proved that the linkage between the two monomers were C-1″-2″, thereby assigning the planar structure of 3, as shown in Figure 7.

Table 2.
1H (400 Hz) and 13C (100 Hz) NMR data for compounds 3 and 4 (δ in ppm, J in Hz within parentheses).
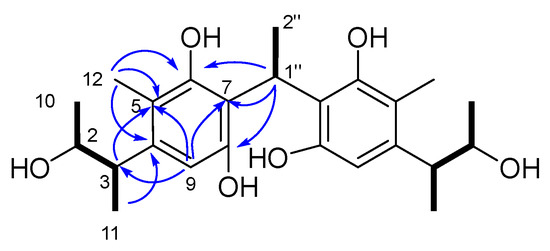
Figure 7.
The key 1H-1H COSY ( ) and HMBC (
) and HMBC ( ) correlations of 3.
) correlations of 3.
 ) and HMBC (
) and HMBC ( ) correlations of 3.
) correlations of 3.
The relative and absolute configuration of C-2/2′ and C-3/3′ were deduced in the same manner as that used for 1–2, based on a biogenetic consideration with citrinin (30) and phenol A (14). Finally, by comparison of its calculated and experimental ECD spectra (Figure 8), the absolute configuration of 3 was thus established as 2R,3S,2′R,3′S. Herein, 3 was given the trivial name dicitrinone K.
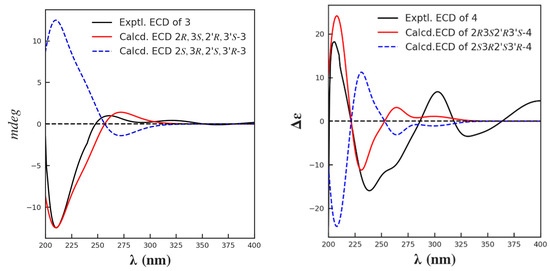
Figure 8.
The calculated and the experimental ECD spectra of compounds 3 and 4.
Compound 4 was isolated as a yellow amorphous solid. The molecular formula of 4 was deduced as C24H28O5 from the HRESI(−)MS signal m/z 395.1841 [M−H]−. The 1H, 13C NMR and DEPT spectroscopic data revealed 24 carbons, including seven methyl singlets, five methines, and 12 protonated carbons. Similar to those of 3, the carbon signals of 4 also existed in pairs, with the exception of C-1″ (δC 25.6 d) and C-2″ (δC 23.0 q). A detailed comparison of the 1D and 2D NMR data for 4 and 3 revealed that they were closely related, with the major difference being the replacement of an aromatic methine in 3 with a non-hydrogenated carbon (δC 141.9 s; C-9) in 4. The diagnostic HMBC correlations from H-2 (δH 4.40, m) to C-9 revealed the presence of a dihydrofuran ring. Further connections were confirmed by the COSY correlations of H3-10 (δH 1.32, m)/H-2 (δH 4.40, m)/H-3 (δH 3.05, m)/H3-11(δH 1.26, m), and H-2″ (δH 1.24, m)/H-1″ (δH 4.48, dd, J = 6.7, 13.4 Hz), as well as the HMBC correlations observed from H3-12 (δH 2.22, s) to C-4 (δC 130.2 s), C-5 (δC 113.0 s) and C-6 (δC 146.3 s), from H3-11 to C-4, from H-3 (δH 3.05, m) to C-4, C-9 (δC 141.9 s), C-5, and from H-1″ to C-7 (δC 116.4 s), C-8 (δC 136.5 s), C-6 (δC 146.3 s) (Figure 9). The relative configuration was determined based on the NOESY correlations of H-2/H3-11, H-3/H3-10. By comparison of its calculated and experimental ECD spectra, the absolute configuration was established as 2/2′R,3/3′S (Figure 8), and was given the trivial name dicitrinone L.

Figure 9.
The key 1H-1H COSY ( ), HMBC (
), HMBC ( ) and NOESY (
) and NOESY ( ) correlations of compound 4.
) correlations of compound 4.
 ), HMBC (
), HMBC ( ) and NOESY (
) and NOESY ( ) correlations of compound 4.
) correlations of compound 4.
Compound 5 was isolated as a yellow amorphous solid. Its molecular formula was established as C13H12O6, according to the pseudo molecular ion at m/z 263.0547 [M−H]− in its HRESI(-)MS spectrum, requiring eight DOU. The 1H-NMR and 13C NMRDEPT spectroscopic data exhibited 13 carbons (Table 3), including two methyls, one methene, two methines, and eight non-hydrogenated carbons (including one carbonyl and one ketone). According to the key HMBC correlations from H3-12 (δH 2.39, m) to C-3 (δC 110.5 s), C-2 (δC 156.4 s) and C-4 (δC 140.7 s), H3-2′ (δH 2.04, s) to C-1′ (δC 172.5 s), H2-11 (δH 5.13, s) to C-2, C-3 and C-1′, H-7 (δH 6.34, d, J = 1.6 Hz) to C-6 (δC 167.5 s), C-8 (δC 165.3 s), C-5 (δC 102.6 s) and C-9 (δC 99.7 s), H-5 (δH 6.49, d, J = 1.6 Hz) to C-6, C-3, C-7 (δC 101.8 d) and C-9, along with the consideration of the rest of the DOU, the structure of 5 was assigned, as shown in Figure 10. Therefore, 5 was identified as (6,8-dihydroxy-4-methyl-1-oxo-1H-isochromen-3-yl) methyl acetate.

Table 3.
1H (400 Hz) and 13C (100 Hz) NMR data for compounds 5 and 6 (δ in ppm, J in Hz within parentheses).
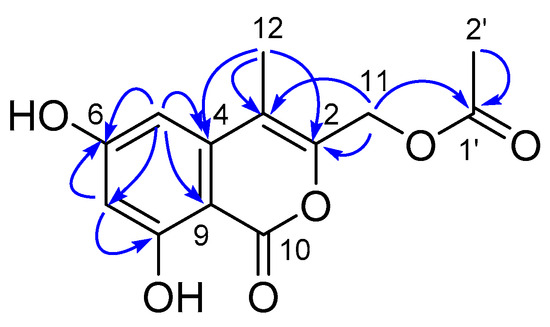
Figure 10.
The Key HMBC correlations of compound 5.
Compound 6 was obtained as a yellow oil. Its molecular formula was established as C11H21NO3 on the basis of the pseudo molecular ion at m/z 238.1435 [M + Na]+ in its HRESI(+)MS spectrum, requiring two degrees of unsaturation. The 13C NMR spectrum, in association with the DEPT spectrum, indicated 11 carbon signals ascribed to four methyls (δC 23.3 q, C-2″; 17.2 q, C-1′; 16.4 q, C-4′; and 25.8 q, C-6), two sp3 methylenes (δC 37.6 t, C-4; 23.7 t, C-3; and 39.6 t, C-2), two sp3 methines (δC 78.0 d, C-2′ and δC 79.0 d, C-3′), one sp3 quaternary carbon (δC 108.8 s, C-5), and one carbonyl (δC 170.0 s, C-1″). The 2D NMR spectra revealed COSY correlations of H2-2 (δH 3.25, dd, J = 12.7, 6.4 Hz)/H2-3 (1.67, m; 1.62, m)/H2-4 (1.66, m), H3-1′ (δH 1.23, d, J = 5.8 Hz)/H-2′ (δH 3.68, m)/H-3′ (δH 3.56, m)/H-4′ (δH 1.24, d, J = 5.7 Hz). These, along with the HMBC correlation from H2-3 and H3-6 (δH 1.30, s) to C-5, allowed for the assignment of the planar structure, as shown in Figure 11. For the stereochemistry, the key NOESY correlations of H3-1′/H-3′ and H3-4′/H-2′ established a trans-relationship between the two methyls. Additionally, a DFT calculation of the OR value of the two possible enantiomers was performed, in which the predicted OR of the 2’S3’S isomer showed the same sign as the experimental result (Table 4). Therefore, 6 was determined as N-(3-((2′S,3′S)-2′,3′,5′-trimethyl-1,3-dioxolan-2-yl)propyl)acetamide.
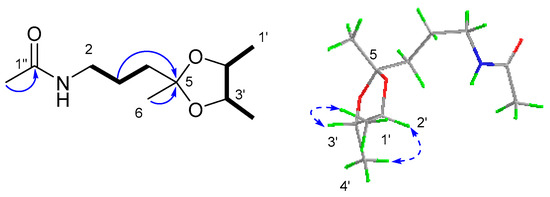
Figure 11.
The key 1H-1H COSY ( ), HMBC (
), HMBC ( ) and NOESY (
) and NOESY ( ) correlations of compound 6.
) correlations of compound 6.
 ), HMBC (
), HMBC ( ) and NOESY (
) and NOESY ( ) correlations of compound 6.
) correlations of compound 6.

Table 4.
The calculated and experimental specific rotation values of compound 6.
All isolates were tested for anti-food allergic activity under the concentration of 50 μM and for antibacterial activity up to 256 µM. As a result, diorcinol (17) and frangula-emodin (32) showed potent degranulation-inhibitory activity, with IC50 values of 13.4 and 7.9 μM, respectively, while diorcinol (17) and penicitrinol A (20) exhibited mild inhibition against Vibrio parahemolyticus, with MIC values of 128 μM and 256 μM, respectively (Table 5). No compounds were active against MRSA (methicillin-resistant Staphylococcus aureus).

Table 5.
Bioactivities of compounds from Aspergillus versicolor 170217.
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra were recorded on a Bruker 400 MHz spectrometer. The HRESIMS spectra were recorded on a Waters Xevo G2 Q-TOF mass spectrometer (Waters Corporation, Milford, MA, USA). Optical rotations were obtained with an Anton Par polarimeter (MCP100). ECD spectra were measured on a Chirascan spectrometer. The semi-preparative HPLC was conducted on an Agilent instrument (1260) equipped with a 1260 Diode Array Detector (DAD) and column COSMOSIL 5 C18-MS-II 10 ID × 250 mm (Nacalai Tesque, Japan). Column chromatography was performed on silica gel, Sephadex LH-20, and ODS. Analytical-grade solvents, purchased from Sinopharm Chemical Reagent Co., Ltd. or Xilong Scientific Co., Ltd., were used for solvent extractions Chromatography solvents were HPLC grade, supplied by Xilong Scientific Co., Ltd. or Sigma-Aldrich. Deuterated solvents were purchased from Cambridge Isotopes.
3.2. Fungal Identification, Fermentation, and Extraction
The fungus strain 170217 was isolated from the intestinal contents of a whale Mesoplodon densirostris stranded in Ningde of the East China Sea. It was identified to be an Aspergillus versicolor (GenBank accession number SUB13826338), as the 18S rRNA gene sequence alignment demonstrated that it was 100% identical to the Aspergillus versicolor TF34 (GenBank accession number MN515366.1). For scale-up fermentation, the A. versicolor 170217 was grown under static conditions at 25 °C in 85 × 1 L Erlenmeyer flasks, each containing 200 g oatmeal agar, including 100 g of oatmeal, 15% sea salt, and 120 mL of distilled H2O. After 30 days, the fermentation product was extracted, in triplicate, using 95% ethanol. Then, the organic solvent was combined and concentrated to a small volume. The latter was then extracted, in triplicate, using ethyl acetate. Finally, the solvent was removed under vacuum to provide the crude extract (153 g).
3.3. Isolation and Purification
The crude extract was separated into four fractions (Fr.1−Fr.4) via medium pressure liquid chromatography (MPLC, 680 mm × 58 mm) on silica gel, with a gradient of CH2Cl2-MeOH (100%→85%). Fraction Fr.2 (17 g) was separated into eight subfractions (Fr.2-1–Fr.2-8) by column chromatography (CC) over ODS (460 mm × 26 mm; MeOH-H2O, 30%→100%). Subfraction Fr.2-1 was purified by CC over Sephadex LH-20 (MeOH) and HPLC (MeOH-H2O, 45%→100%) to yield 5 (1.5 mg, 0.001%), 36 (19 mg, 0.012%), and 43 (2 mg, 0.001%). Subfraction Fr.2-2 was separated by CC over Sephadex LH-20 (MeOH) and CC on silica gel, followed by HPLC (MeOH-H2O, 55%→100%) to afford 27 (2.4 mg, 0.002%). Compound 35 (12 mg, 0.008%) was isolated from Fr.2-3 by CC over Sephadex LH-20 (MeOH) and HPLC (MeOH-H2O, 55%→100%). Subfraction Fr.2-4 was separated by CC over Sephadex LH-20 (MeOH) to obtain 30 (90 mg, 0.059%) and 20 (1 mg, 0.0006%). Subfraction Fr.2-5 was separated by CC over Sephadex LH-20 (MeOH) to yield 31 (6 mg, 0.0039%), and 32 (3mg, 0.002%) was isolated from Fr.2-6 by CC over Sephadex LH-20 (MeOH). Compound 4 (3 mg, 0.002%) was isolated from Fr.2-7 by CC over Sephadex LH-20 (MeOH) and HPLC (MeOH-H2O, 60%→100%). Subfraction Fr.2-8 was separated by CC over Sephadex LH-20 (MeOH) and HPLC (MeOH-H2O, 40%→100%) to obtain 17 (3 mg, 0.002%), 44 (15 mg, 0.01%), 45 (9 mg, 0.006%), and 46 (2.3 mg, 0.002%).
Fraction Fr.3 (42 g) was separated into 21 subfractions by CC over ODS (460 mm × 26 mm; MeOH-H2O, 10%→100%). Subfraction Fr.3-3 was purified by CC over Sephadex LH-20 (MeOH) and silica gel to yield 6 (5.8 mg, 0.045%), 13 (11 mg, 0.007%), 26 (22 mg, 0.014%), 10 (4 mg, 0.003%), and 48 (10 mg, 0.006%). Subfraction Fr.3-7 was purified by CC over Sephadex LH-20 (MeOH) and HPLC (MeOH-H2O, 60%→100%) to yield compound 1 (3 mg, 0.002%). Subfraction Fr.3-5 was purified by CC over Sephadex LH-20 (MeOH) and silica gel to yield 23 (4 mg, 0.003%) and 39 (1.5 mg, 0.001%). Compound 21 (4 mg, 0.003%) was obtained from Fr.3-6 by CC over Sephadex LH-20 (MeOH), 18 (2 mg, 0.001%) and 19 (2 mg, 0.001%) were obtained from Fr.3-11 by CC over Sephadex LH-20 (MeOH), and HPLC. Subfraction Fr.3-9 was purified by CC over Sephadex LH-20 (MeOH) and silica gel to yield 33 (4 mg, 0.003%), 37 (36 mg, 0.023%), 40 (12 mg, 0.008%), 41 (32 mg, 0.021%), and 42 (4 mg, 0.003%). Subfraction Fr.3-4 was purified by CC over Sephadex LH-20 (MeOH), silica gel, and HPLC to yield 24 (1.5 mg, 0.001%), 29 (76 mg, 0.05%), and 47 (4 mg, 0.003%).
Fraction Fr.4 (11.8 g) was separated into seven subfractions (Fr.4-1-Fr.4-7) by CC over ODS (460 mm × 26 mm; MeOH-H2O, 10%→100%). Subfraction Fr.4-1 was purified by CC over Sephadex LH-20 (MeOH) and silica gel to yield 8 (2.3 mg, 0.002%), 9 (18 mg, 0.012%), 11 (8 mg, 0.005%), 12 (2 mg, 0.001%), and 49 (3.5 mg, 0.002%). Subfraction Fr.4-2 was purified by CC over Sephadex LH-20 (MeOH) and HPLC to yield 28 (3.3 mg, 0.002%) and 7 (2 mg, 0.001%). Compound 15 (30 mg, 0.02%) was isolated from Fr.4-3 by CC over Sephadex LH-20 (MeOH). Compounds 14 (5 mg, 0.003%), 50 (2 mg, 0.001%), and 51 (26 mg, 0.017%) were isolated from Fr.4-4 by CC over Sephadex LH-20 (MeOH) and silica gel. Compound 25 (2.7 mg, 0.002%) was isolated from Fr.4-5 by CC over Sephadex LH-20 (MeOH). Subfraction Fr.4-6 was repeatedly separated by CC over Sephadex LH-20 (MeOH), silica gel to provide 2 (5 mg, 0.003%). Subfraction Fr.4-7 was subjected toCC over Sephadex LH-20 (MeOH). Final purification by HPLC afforded 16 (52.8 mg, 0.038%) and 3 (10 mg, 0.006%).
Citriquinolione A (1): Yellow powder; [α +44 (c 0.1, MeOH); UV (MeOH) λmax (logε) 271 (2.86) nm, 202 (3.43) nm; ECD (MeOH) (Δε) 252 (−3.18), 297 (+1.92) nm; 1H and 13C NMR data; see Table 1; HRESIMS m/z 424.1759 [M−H]− (calcd for C24H26NO6, 424.1760, ΔmDa −0.1).
Citriquinolione B (2): Yellow amorphous solid; [α −18 (c 0.15, MeOH); UV (MeOH) λmax (logε) 310 (2.10) nm, 351 (2.40) nm; ECD (MeOH) (Δε) 212 (−5.51), 285 (+ 0.40) nm; 1H and 13C NMR data; see Table 1; HRESIMS m/z 426.1996 [M−H]− (calcd for C24H28NO6, 426.1917, ΔmDa 7.9).
Dicitrinone K (3): Yellow oil; [α −26 (c 0.1, MeOH); UV (MeOH) λmax (logε) 278 (2.05) nm; ECD (MeOH) (Δε) 210 (−3.17), 261 (+ 0.25) nm; 1H and 13C NMR data; see Table 2; HRESIMS m/z 441.2250 [M+Na]+ (calcd for C24H33O6, 441.2253, ΔmDa −0.3).
Dicitrinone L (4): Yellow amorphous solid; [α +42 (c 0.1, MeOH); UV(MeOH) λmax (logε) 302 (2.56) nm; ECD (MeOH) (Δε) 239 (−3.82), 303 (+ 1.62) nm; 1H and 13C NMR data; see Table 2; HRESIMS m/z 395.1841 [M−H]− (calcd for C24H28O5, 395.1858, ΔmDa −1.7).
(6,8-Dihydroxy-4-methyl-1-oxo-1H-isochromen-3-yl) methyl acetate (5): Yellow amorphous solid; 1H and 13C NMR data, see Table 3; HRESIMS m/z 263.0547 [M−H]− (calcd for C13H11O6, 263.0556, ΔmDa −0.9).
N-(3-((2′S,3′S)-2′,3′,5′-Trimethyl-1,3-dioxolan-2-yl)propyl)acetamide (6): yellow oil; [α +18 (c 0.1, MeOH); 1H and 13C NMR data; see Table 4; HRESIMS m/z 238.1435 [M+Na]+ (calcd for C11H21NO3, 238.1419, ΔmDa 1.6).
3.4. Theoretical Calculations
Conformational analysis was first performed via random searching in the stochastic algorithm using the MMFF94 force field, with an energy cutoff of 7.0 kcal/mol and an RMSD threshold of 0.5 Å. The predominant conformers were relocated and confirmed at the B3LYP/6-31G(d) level. The theoretical ECD spectra were calculated using the time-dependent density functional theory (TD-DFT) in methanol. The ECD spectrum was obtained by averaging each conformer using the Boltzmann distribution theory. NMR calculation and conformational optimization were performed under the HF/6-31G(d) level; then NMR was calculated at the mPW1PW91/6-311G (2d, p) level. For ORD calculation, the conformations of the compounds were optimized at the B3LYP/6-31G(d) level to obtain the energy-minimized conformers. Then, the optimized conformers were subjected to the calculations of specific rotation value using the B3LYPspAug-cc-pVDZ level (λ = 589.3 nm). The calculated specific rotations were later obtained according to the Boltzmann weighting of each conformer.
3.5. Anti-Food Allergic Bioassay
The in vitro anti-food allergic assay was conducted following our previous protocol [51,52]. Briefly, the rat basophilic leukemia 2H3 (RBL-2H3) cells were incubated with dinitrophenyl (DNP)-immunoglobulin E (IgE) overnight. Then, the IgE-sensitized RBL-2H3 cells were pretreated with the tested compounds and stimulated with DNP-bovine serum albumin (BSA). The bioactivity was quantified by measuring the fluorescence intensity of the hydrolyzed substrate in a fluorometer. Loratadine, a commercially available anti-allergy medicine, was used as a positive control.
3.6. Antibacterial Bioassay
The antibacterial assay (MICs) was evaluated by a broth microdilution in 96-well plates, and two bacterial strains, MRSA and Vibrio parahemolyticus, were used as the test targets, following the methods included in the literature [53]. The tested compounds were prepared in 20% DMSO to obtain the mother solution with the initial concentration of 20 mM, and then were diluted 40-fold with PBS. The compound solution was subsequently diluted using the 2× dilution method in series to reach eight concentrations from 512 µM to 4 µM. The bacteria are diluted into 5 × 105 CFU/mL and added into the 96-well plates, and equal volumes of the compound solutions were added into each well. After culturing for 24 h at 37 °C, the plates were observed by the naked eye. Each experiment was repeated three times. An equal amount of DMSO was used as a negative control.
4. Conclusions
In the present study, the chemical investigation of the deep-sea-derived fungus A. versicolor 170217 led to the isolation of six new (1–6) and 45 known (7–51) compounds, enriching the diversity of secondary metabolites from the deep-sea-derived Aspergillus. The structures, including absolute configurations of new compounds, were elucidated by the analysis of comprehensive spectroscopic data, quantum chemical calculations, and biogenetic considerations. Biologically, compounds 32 and 17 showed remarkable anti-food allergic activity, with IC50 values of 7.9 ± 3.0 μM and 13.4 ± 1.2 μM, respectively, while displaying no cytotoxicity. Moreover, diorcinol (17) and penicitrinol A (20) exhibited mild inhibition against Vibrio parahemolyticus, with MIC values of 128 μM and 256 μM, respectively.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md21100504/s1, One-dimensional and two-dimensional NMR spectra of all new compounds, as well as the experimental and calculated ECD spectra of compound 2.
Author Contributions
X.-W.Y. designed the project; S.-H.L., Q.-X.Y., Z.-B.Z., Y.-J.H. and M.-M.X. isolated and purified all compounds; L.X. performed the fermentation; Y.Z. conducted the ECD calculations; Q.-M.L. and G.-M.L. conducted the anti-food allergic experiment; J.-Y.J. and Z.L. performed the antibacterial tests; S.-H.L., T.-Z.W. and X.-W.Y. analyzed the data and wrote the paper, while critical revision of the publication was performed by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC2804104), the Xiamen Southern Oceanographic Center (22GYY007HJ07), and the Key Laboratory of Marine Genetic Resources, Third Institute of Oceanography, Ministry of Natural Resources (QYZ-KYZX-22029).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank Xiao-Yong Zhang of South China Agricultural University for the support with fungal isolation and Kai Zhang of the Third Institute of Oceanography, Ministry of Natural Resources, for the assistance with taxonomy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luo, D.M.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Li, H.J.; Li, Q.Y.; Wu, Y.C. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Orfali, R.; Aboseada, M.A.; Abdel-Wahab, N.M.; Hassan, H.M.; Perveen, S.; Ameen, F.; Alturki, E.; Abdelmohsen, U.R. Recent updates on the bioactive compounds of the marine-derived genus Aspergillus. RSC Adv. 2021, 11, 17116–17150. [Google Scholar] [CrossRef]
- Yang, S.Q.; Li, X.M.; Li, H.L.; Meng, L.H.; Wang, B.G. New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- He, Z.H.; Xie, C.L.; Wu, T.; Zhang, Y.; Zou, Z.B.; Xie, M.M.; Xu, L.; Capon, R.J.; Xu, R.; Yang, X.W.; et al. unprecedented citrinin trimers with anti-osteoporosis activity from the deep-sea-derived Penicillium citrinum W23. Bioorg. Chem. 2023, 139, 106756. [Google Scholar] [CrossRef]
- Wei, J.; Chen, X.; Ge, Y.; Yin, Q.; Wu, X.; Tang, J.; Zhang, Z.; Wu, B. Citrinin monomer, trimer, and tetracyclic alkaloid derivatives from the hydrothermal vent-associated fungus Penicillium citrinum TW132-59. J. Org. Chem. 2022, 87, 13270–13279. [Google Scholar] [CrossRef]
- Liu, S.Z.; He, F.M.; Bin, Y.L.; Li, C.F.; Xie, B.Y.; Tang, X.X.; Qiu, Y.K. Bioactive compounds derived from the marine-derived fungus MCCC3A00951 and their influenza neuraminidase inhibition activity in vitro and in silico. Nat. Prod. Res. 2021, 35, 5621–5628. [Google Scholar] [CrossRef]
- Bala, G.; Dhananjaya, N. Chemical investigation of Mycale mytilorum and a study on toxicity and antidiabetic activity of 5-Octadecylpyrrole-2-carboxaldehyde. Bioorg. Med. Chem. 2000, 8, 27–36. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.R. Phenolic constituents from the fruits of Amomum tsao-ko (Zingiberaceae). Yunnan Zhiwu Yanjiu 2009, 31, 284–288. [Google Scholar] [CrossRef]
- Jin, X.; Shi, S.M.; Zhang, D.F.; Zhu, Z. Chemical constituents of Andrographis paniculata (II). Zhong Cao Yao 2014, 45, 164–169. [Google Scholar]
- Peng, X.P.; Wang, Y.; Liu, P.P.; Hong, K.; Chen, H.; Yin, X.; Zhu, W.M. Aromatic compounds from the halotolerant fungal strain of Wallemia sebi PXP-89 in a hypersaline medium. Arch. Pharm. Res. 2011, 34, 907–912. [Google Scholar] [CrossRef]
- Han, Z.; Mei, W.L.; Cui, H.B.; Zeng, Y.B.; Lin, H.P.; Hong, K.; Dai, H.F. Antibacterial constituents from the endophytic fungus Penicillium sp. of mangrove plant Cerbera manghas. Gaodeng Xuexiao Huaxue Xuebao 2008, 29, 749–752. [Google Scholar]
- Wang, L.; Li, C.; Yu, G.; Sun, Z.; Zhang, G.; Gu, Q.; Zhu, T.; Che, Q.; Guan, H.; Li, D. Dicitrinones E and F, citrinin dimers from the marine derived fungus Penicillium citrinum HDN-152-088. Tetrahedron Lett. 2019, 60, 151182–151187. [Google Scholar] [CrossRef]
- Song, T.; Chen, M.; Ge, Z.W.; Chai, W.; Li, X.C.; Zhang, Z.; Lian, X.Y. Bioactive penicipyrrodiether A, an adduct of GKK1032 analogue and phenol A derivative, from a marine-sourced fungus Penicillium sp. ZZ380. J. Org. Chem. 2018, 83, 13395–13401. [Google Scholar] [CrossRef]
- Krick, A.; Kehraus, S.; Gerhauser, C.; Klimo, K.; Nieger, M.; Maier, A.; Fiebig, H.H.; Atodiresei, I.; Raabe, G.; Fleischhauer, J.; et al. Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2007, 70, 353–360. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Kalinovsky, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. A new meroterpenoid from the marine fungus Aspergillus versicolor (Vuill.) Tirab. Russ. Chem. Bull. 2010, 59, 852–856. [Google Scholar] [CrossRef]
- Wei, P.Y.; Li, L.; Yang, C.G.; Luo, D.Q.; Zheng, Z.H.; Lu, X.H.; Shi, B.Z. A novel oxybis cresol verticilatin with highly varying degrees of biological activities from the insect pathogenic fungus Paecilomyces verticillatus. J. Asian Nat. Prod. Res. 2014, 16, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Laksanacharoen, P.; Rachtawee, P.; Pittayakhajonwut, P. Antimicrobial activity and cytotoxicity of polyketides isolated from the mushroom Xerula sp. BCC56836. RSC Adv. 2016, 6, 94510–94523. [Google Scholar] [CrossRef]
- Wakana, D.; Hosoe, T.; Itabashi, T.; Okada, K.; de Campos Takaki, G.M.; Yaguchi, T.; Fukushima, K.; Kawai, K. New citrinin derivatives isolated from Penicillium citrinum. J. Nat. Med. 2006, 60, 279–284. [Google Scholar] [CrossRef]
- Xu, L.L.; Cao, F.; Tian, S.S.; Zhu, H.J. Alkaloids and polyketides from the soil fungus Aspergillus terreus and their antibacterial activities. Chem. Nat. Compd. 2017, 53, 1212–1215. [Google Scholar] [CrossRef]
- Ebrahim, W.; El-Neketi, M.; Lewald, L.I.; Orfali, R.S.; Lin, W.; Rehberg, N.; Kalscheuer, R.; Daletos, G.; Proksch, P. Metabolites from the fungal endophyte Aspergillus austroafricanus in axenic culture and in fungal–bacterial mixed cultures. J. Nat. Prod. 2016, 79, 914–922. [Google Scholar] [CrossRef]
- Sassa, T.; Aoki, H.; Namiki, M.; Munakata, K. Plant growth promoting metabolites of Sclerotinia sclerotioum. Agric. Biol. Chem. 1968, 32, 1432–1439. [Google Scholar]
- Kimura, Y.; Nakadoi, M.; Shimada, A.; Nakajima, H.; Hamasaki, T. Biosyntheses of sescandelin and sescandelin B: New isocoumarin compounds produced by the fungus, Sesquicilium candelabrum. Biosci. Biotech. Biochem. 1994, 58, 1525–1526. [Google Scholar] [CrossRef]
- Xia, N.N.; Gao, J.P.; Cai, X.L.; She, Z.G. Secondary metabolites of mangrove endophytic fungus SK5 in the South China Sea. Zhong Yao Cai 2009, 32, 1843–1845. [Google Scholar]
- Clark, B.R.; Capon, R.J.; Lacey, E.; Tennant, S.; Gill, J.H. Citrinin revisited: From monomers to dimers and beyond. Org. Biomol. Chem. 2006, 4, 1520–1528. [Google Scholar] [CrossRef]
- Han, Z.; Mei, W.; Zhao, Y.; Deng, Y.; Dai, H. A new cytotoxic isocoumarin from endophytic fungus Penicillium sp. 091402 of the mangrove plant Bruguiera sexangula. Chem. Nat. Compd. 2009, 45, 805–807. [Google Scholar] [CrossRef]
- Abbas, Z.; Siddiqui, B.S.; Shahzad, S.; Sattar, S.; Begum, S.; Batool, A.; Choudhary, M.I. Lawsozaheer, a new chromone produced by an endophytic fungus Paecilomyces variotii isolated from Lawsonia Alba Lam. inhibits the growth of Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 4448–4453. [Google Scholar] [CrossRef]
- Zhou, Y.; Debbab, A.; Mándi, A.; Wray, V.; Schulz, B.; Müller, W.E.G.; Kassack, M.; Lin, W.; Kurtán, T.; Proksch, P.; et al. Alkaloids from the sponge-associated fungus Aspergillus sp. Eur. J. Org. Chem. 2013, 2013, 894–906. [Google Scholar] [CrossRef]
- Chai, Y.J.; Cui, C.B.; Li, C.W.; Wu, C.J.; Tian, C.K.; Hua, W. Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 559–582. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhu, T.; Du, L.; Zhao, B.; Li, D.; Gu, Q. Sterigmatocystins from the deep-sea-derived fungus Aspergillus versicolor. J. Antibiot. 2011, 64, 193–196. [Google Scholar] [CrossRef]
- Kharlamova, T.V. Reaction of frangula-emodin with α-bromoalkylmethketones. Chem. Nat. Compd. 2007, 43, 391–394. [Google Scholar] [CrossRef]
- Fredimoses, M.; Zhou, X.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Liu, J.; Liu, Y. Emerixanthone E, a new xanthone derivative from deep sea fungus Emericella sp SCSIO 05240. Nat. Prod. Res. 2019, 33, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Zheng, J.; Yu, Y.; Zhang, N.; Liu, H.W.; Sun, Y.P.; Liu, J.S. Chemical constituents of ethyl acetate fraction from Dioscorea bulbifera. Zhongguo Yaoxue Zazhi 2018, 53, 1815–1820. [Google Scholar]
- Wang, F.; Zhu, H.; Ma, H.; Jiang, J.; Sun, W.; Cheng, L.; Zhang, G.; Zhang, Y. Citrinal B, a new secondary metabolite from endophytic fungus Colletotrichum capsici and structure revision of citrinal A. Tetrahedron Lett. 2016, 57, 4250–4253. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Yan, X.; Zhu, P.; Chen, J.; Yang, R. Preparative isolation and purification of macrolactin antibiotics from marine bacterium Bacillus amyloliquefaciens using high-speed counter-current chromatography in stepwise elution mode. J. Chromatogr. A 2013, 1272, 15–19. [Google Scholar] [CrossRef]
- Kim, W.G.; Song, N.K.; Yoo, I.D. Quinolactacins Al and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. J. Antibiot. 2001, 54, 831–835. [Google Scholar] [CrossRef]
- Hu, J.S.; Li, Z.; Gao, J.Y.; He, H.T.; Dai, H.Q.; Xia, X.K.; Liu, C.H.; Zhang, L.X.; Song, F.H. New diketopiperazines from a marine-derived fungus strain Aspergillus versicolor MF180151. Mar. Drugs 2019, 17, 262. [Google Scholar] [CrossRef]
- Li, G.Y.; Li, L.M.; Yang, T.; Chen, X.Z.; Fang, D.M.; Zhan, G.L. Four new alkaloids, Brevianamides O–R, from the fungus Aspergillus versicolor. Helv. Chim. Acta 2010, 93, 2075–2080. [Google Scholar] [CrossRef]
- Li, G.Y.; Yang, T.; Luo, Y.G.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org. Lett. 2009, 11, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.T.; Huang, C.Y.; Li, J.L.; Chen, T.; Tang, J.; Liu, W.B.; Long, Y.H. Isolation, structural characterization and antidiabetic activity of new diketopiperazine alkaloids from mangrove endophytic fungus Aspergillus sp. 16-5c. Mar. Drugs 2021, 19, 402. [Google Scholar] [CrossRef]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Tian, X.P.; Li, J.; Zhang, W.M.; Zhang, S. Analysis of secondary metabolites produced by Eurotium sp. SCSIO F452 isolated from the South China Sea sediment. Chin. J. Mar. Drugs 2013, 32, 7–12. [Google Scholar]
- Wang, R.P.; Lin, H.W.; Li, L.Z.; Gao, P.Y.; Xu, Y.; Song, S.J. Monoindole alkaloids from a marine sponge Mycale fibrexilis. Biochem. Syst. Ecol. 2012, 43, 210–213. [Google Scholar] [CrossRef]
- Bao, B.; Zhang, P.; Lee, Y.; Hong, J.; Lee, C.O.; Jung, J.H. Monoindole alkaloids from a marine sponge Spongosorites sp. Mar. Drugs 2007, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Yu, M.M.; Luo, J.Y.; Huang, S.X.; Su, C.; Xue, Q.H.; Sun, Y.; Ma, Y.T. Isolation, identification and antimicrobial activity of secondary metabolites from a soil-derived Streptomyces from arid habitats of Qinghai. Tianran Chanwu Yanjiu Yu Kaifa 2015, 27, 1900–1904. [Google Scholar]
- Huang, Z.; Yang, R.; Guo, Z.; She, Z.; Lin, Y. A new xanthone derivative from mangrove endophytic fungus No. ZSU-H16. Chem. Nat. Compd. 2010, 46, 348–351. [Google Scholar] [CrossRef]
- Yang, S.Q.; Li, X.M.; Xu, G.M.; Li, X.; An, C.Y.; Wang, B.G. Antibacterial anthraquinone derivatives isolated from a mangrove-derived endophytic fungus Aspergillus nidulans by ethanol stress strategy. J. Antibiot. 2018, 71, 778–784. [Google Scholar] [CrossRef]
- Chen, C.H.; Shaw, C.Y.; Chen, C.C.; Tsai, Y.C. 2,3,4-Trimethyl-5,7-dihydroxy-2,3-dihydrobenzofuran, a novel antioxidant, from Penicillium citrinum F5. J. Nat. Prod. 2002, 65, 740–741. [Google Scholar] [CrossRef]
- Yao, G.; Sebisubi, F.M.; Voo, L.Y.C.; Ho, C.C.; Tan, G.T.; Chang, L.C. Citrinin derivatives from the soil filamentous fungus Penicillium sp. H9318. J. Braz. Chem. Soc. 2011, 22, 1125–1129. [Google Scholar] [CrossRef]
- Xing, C.P.; Chen, D.; Xie, C.L.; Liu, Q.; Zhong, T.H.; Shao, Z.; Liu, G.; Luo, L.Z.; Yang, X.W. Anti-food allergic compounds from penicillium griseofulvum MCCC 3A00225, a deep-sea-derived fungus. Mar. Drugs 2021, 19, 224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.M.; Yang, Y.; Maleki, S.J.; Alcocer, M.; Xu, S.S.; Shi, C.L.; Cao, M.J.; Liu, G.M. Anti-food allergic activity of sulfated polysaccharide from Gracilaria lemaneiformis is dependent on immunosuppression and inhibition of p38 MAPK. J. Agric. Food. Chem. 2016, 64, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hou, E.; Wen, T.; Yan, X.; Han, M.; Bai, L.-P.; Fu, X.; Liu, J.; Qin, S. Development of membrane-active honokiol/magnolol amphiphiles as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).