Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation
Abstract
1. Introduction
2. Coumarin Derivatives from Marine World: Synthesis and Activities
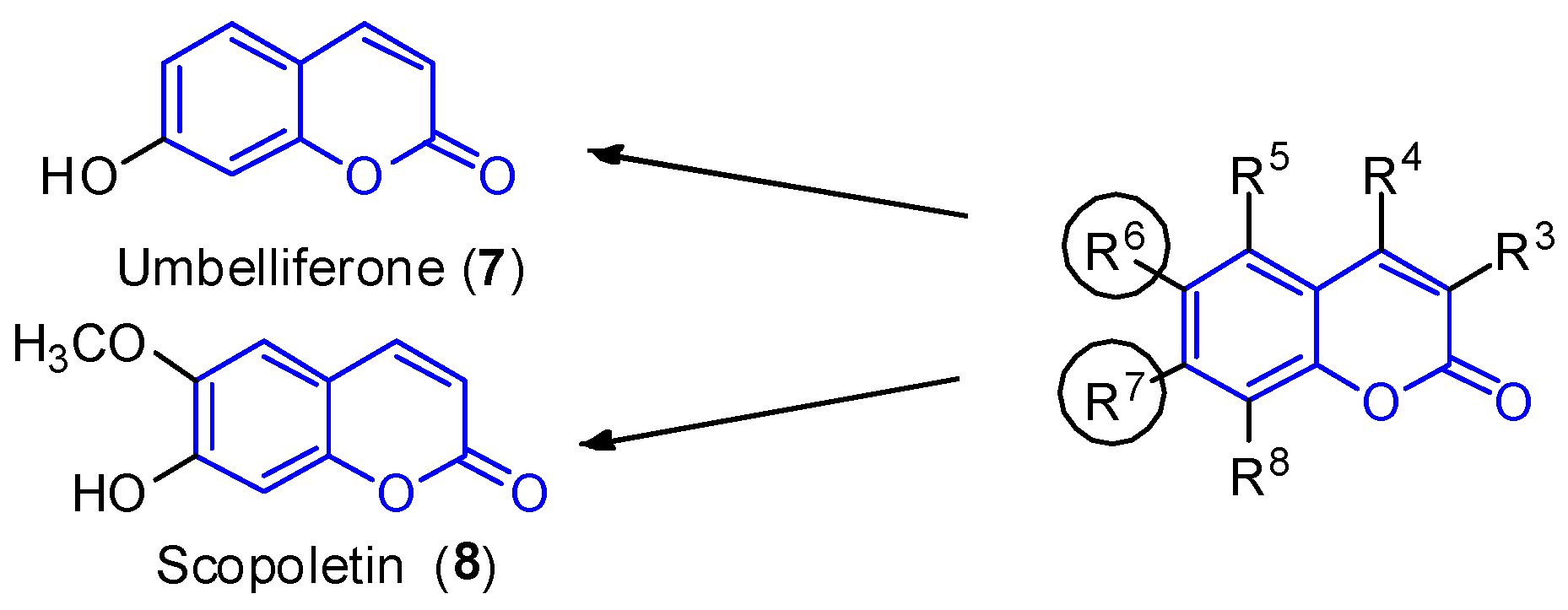
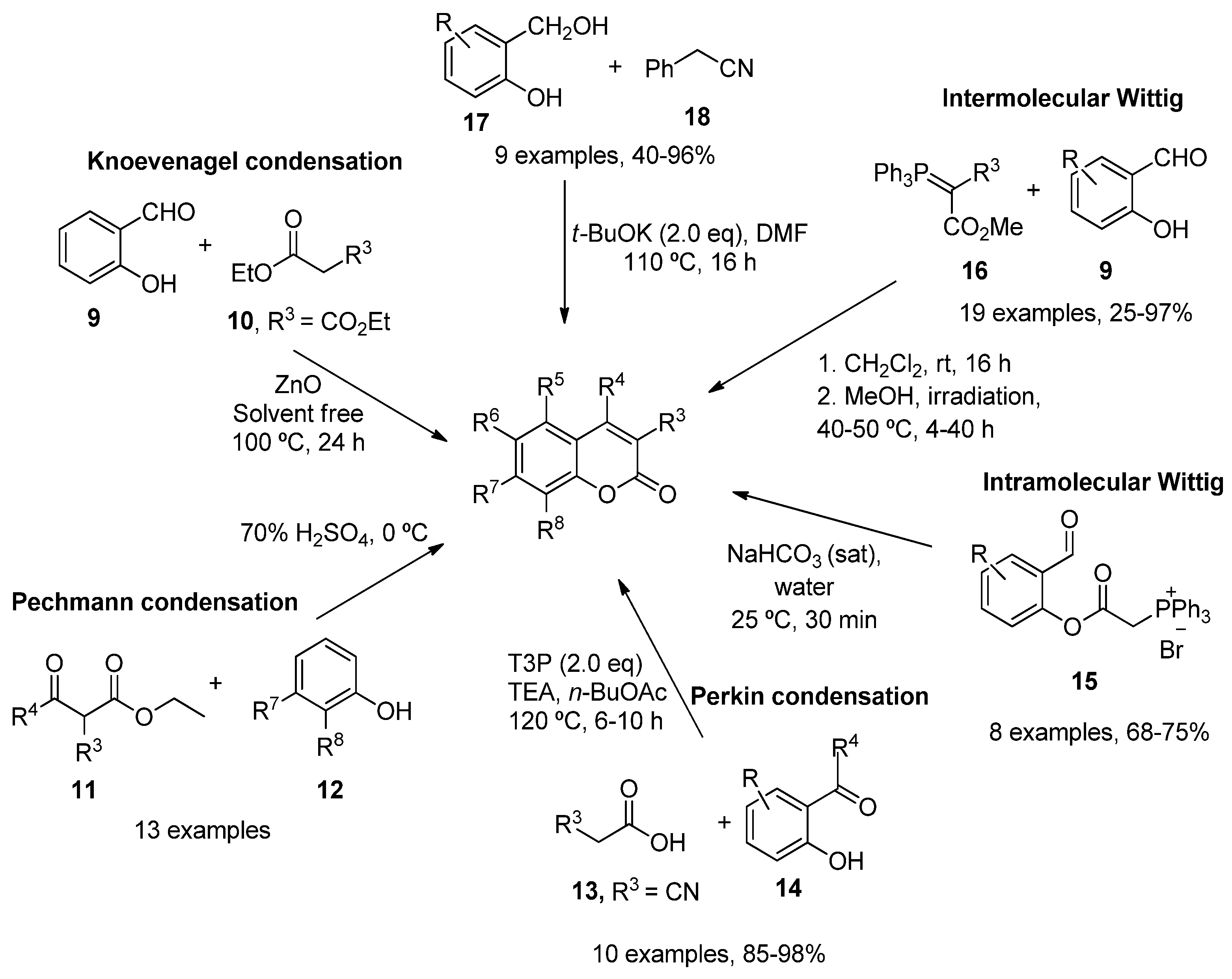
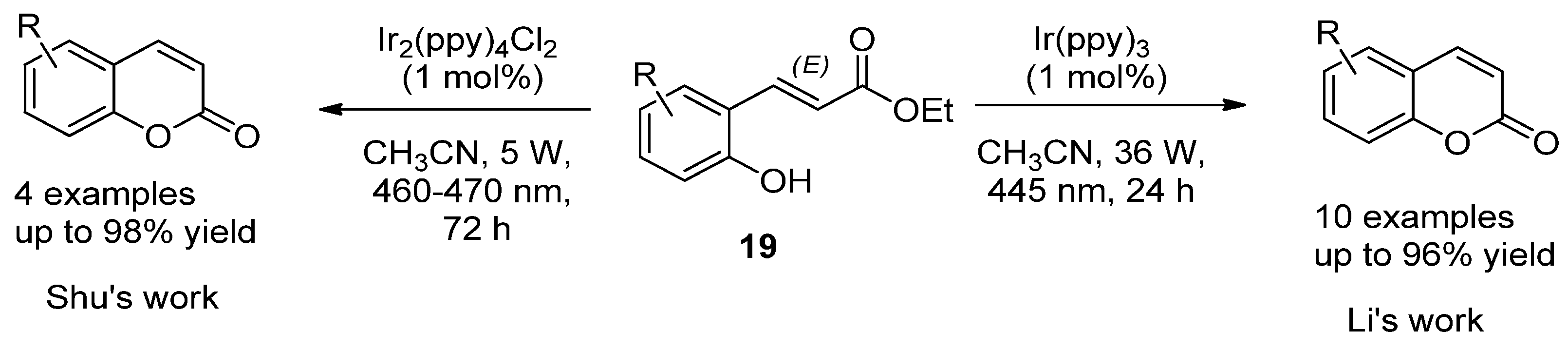
2.1. Simple Coumarins
2.2. 3-Substituted (Imino- and Amido-) Coumarins
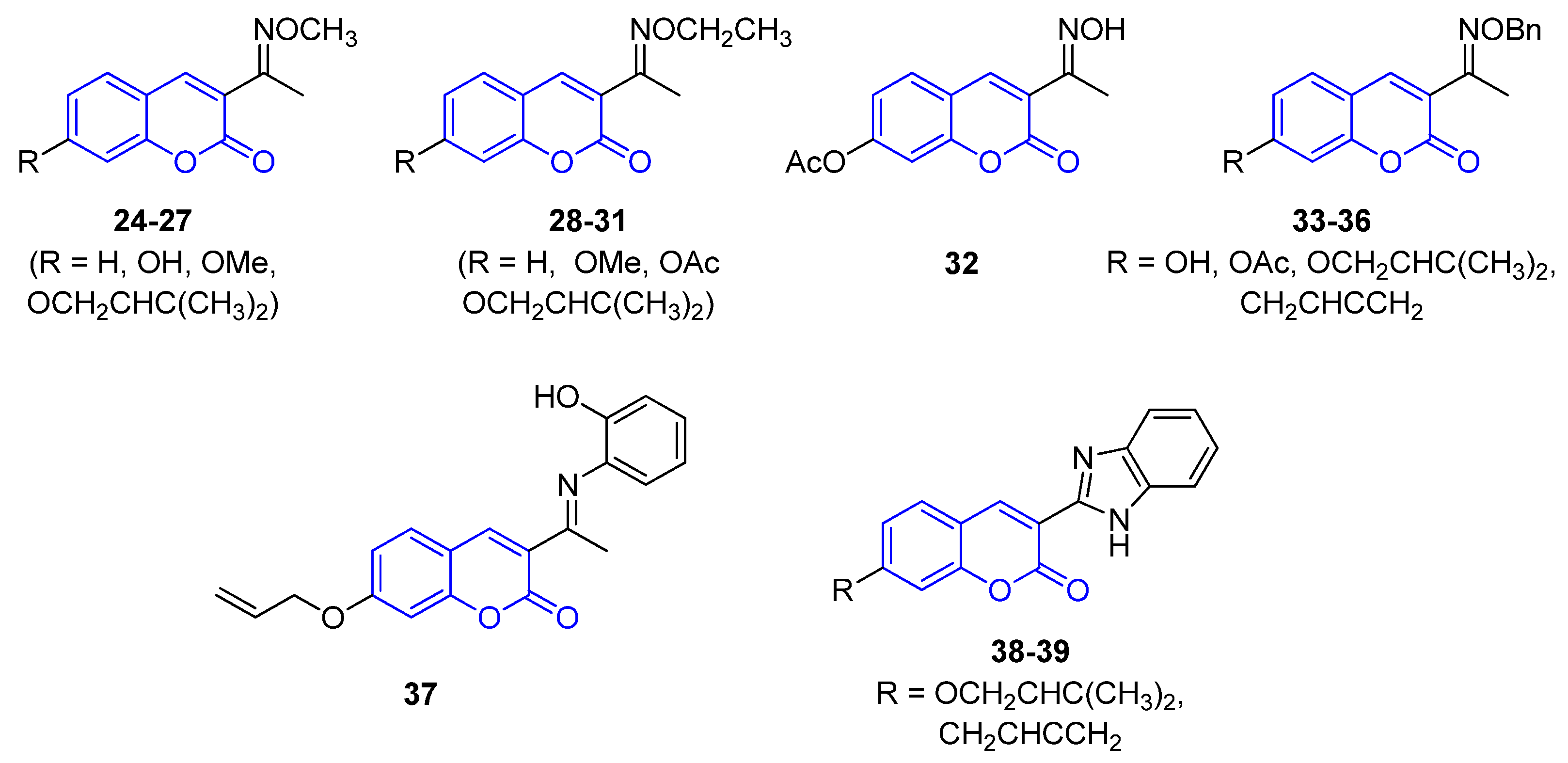
2.2.1. 3-Iminocoumarins
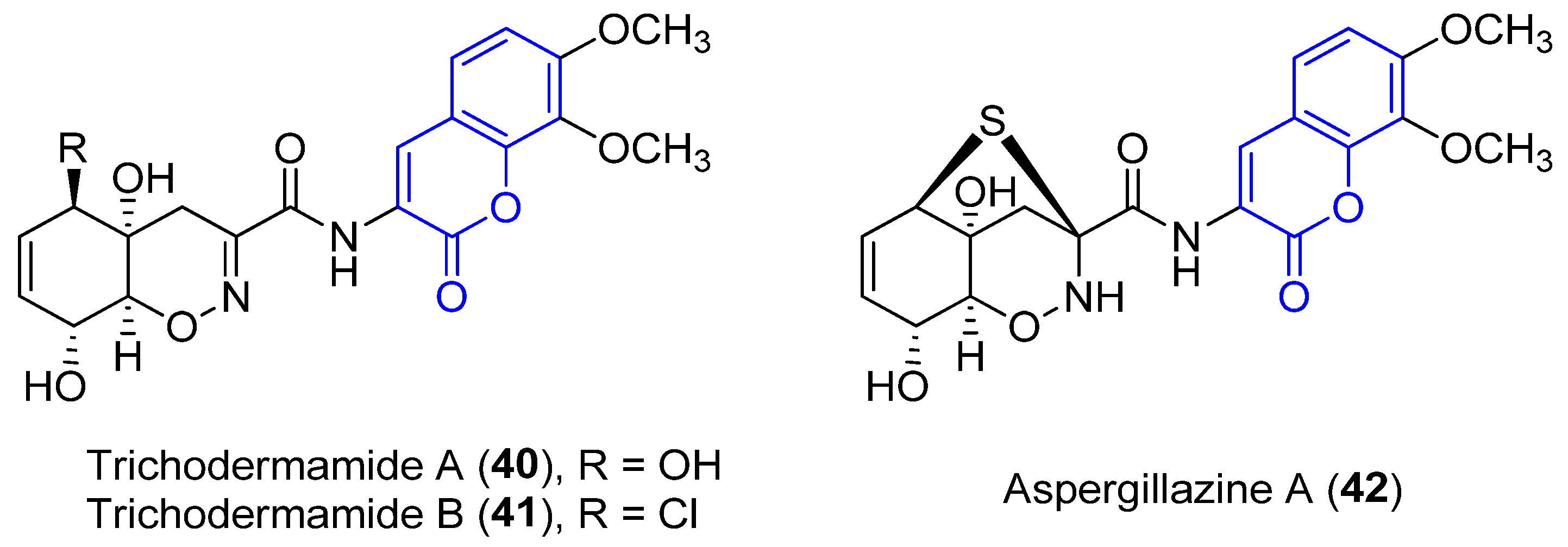
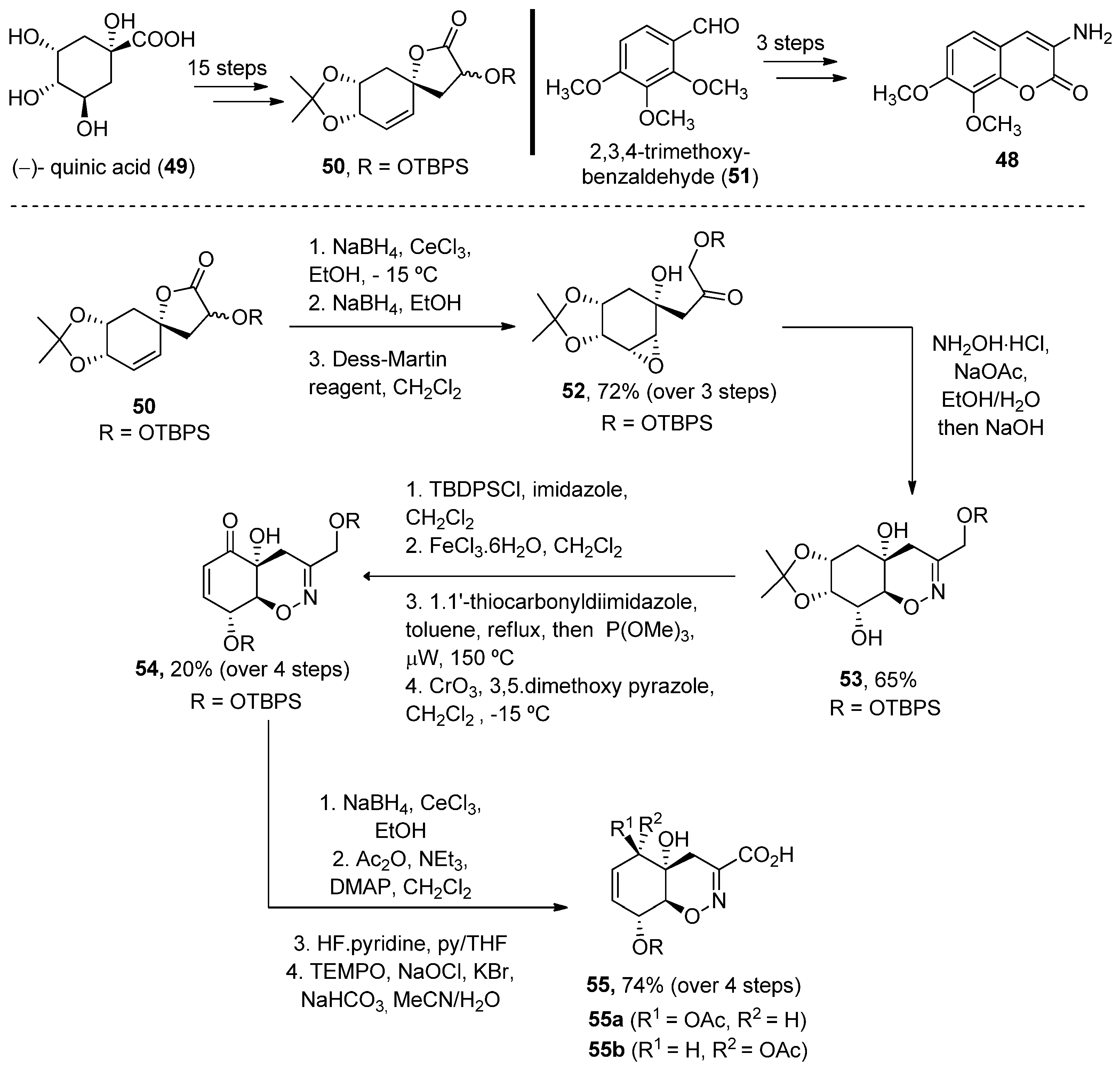
2.2.2. 3-Amidocoumarins
2.3. Tricyclic Coumarins
2.3.1. Furocoumarins
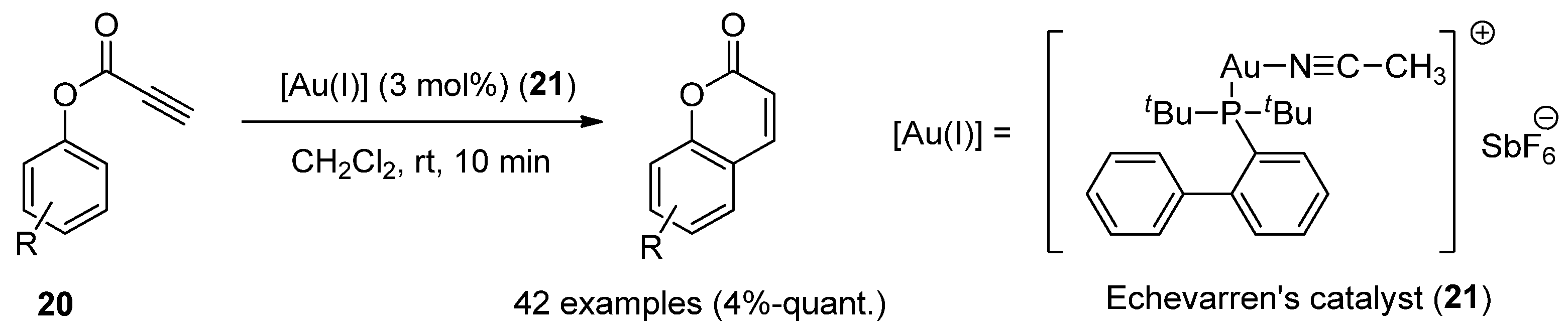
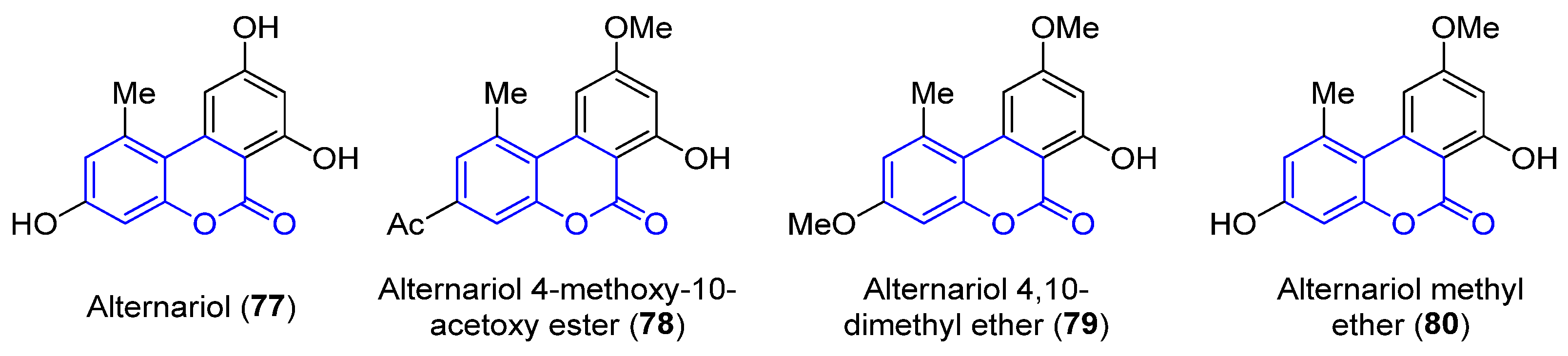
2.3.2. Benzo[c]coumarins
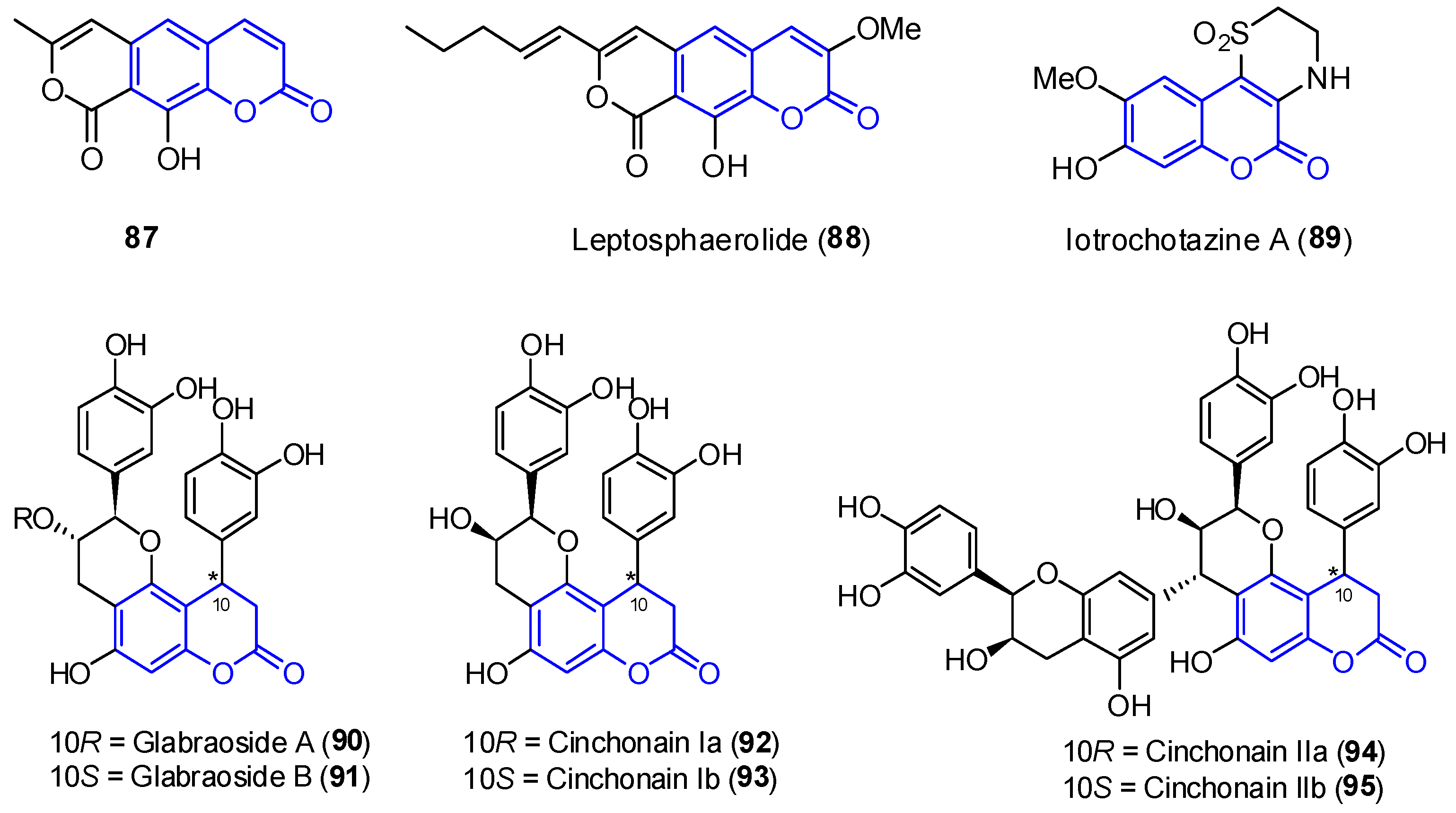
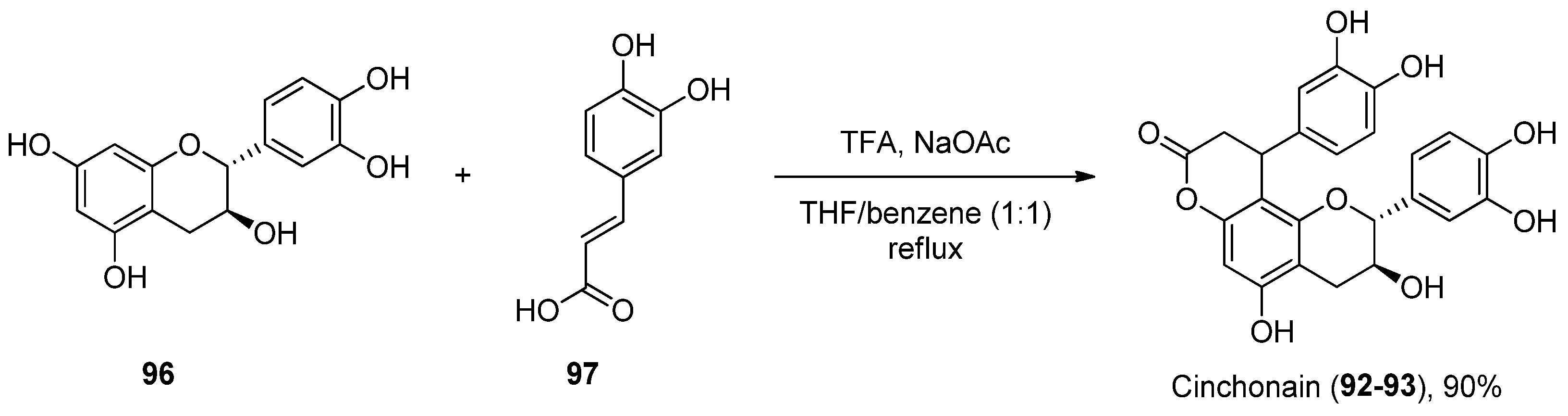
2.3.3. Other Tricyclic Coumarins
2.4. Pentacyclic Coumarins
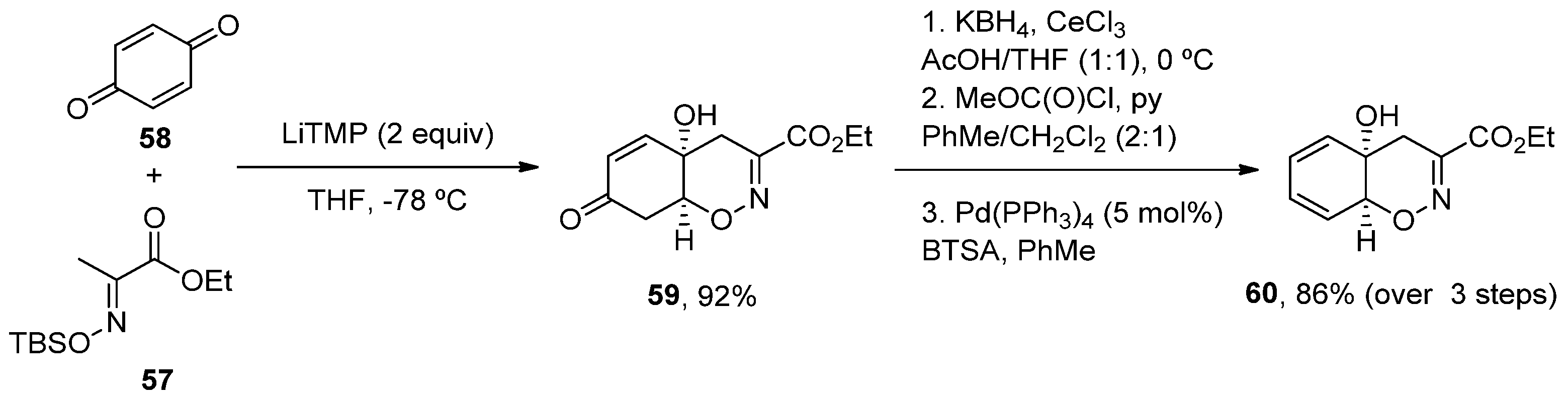
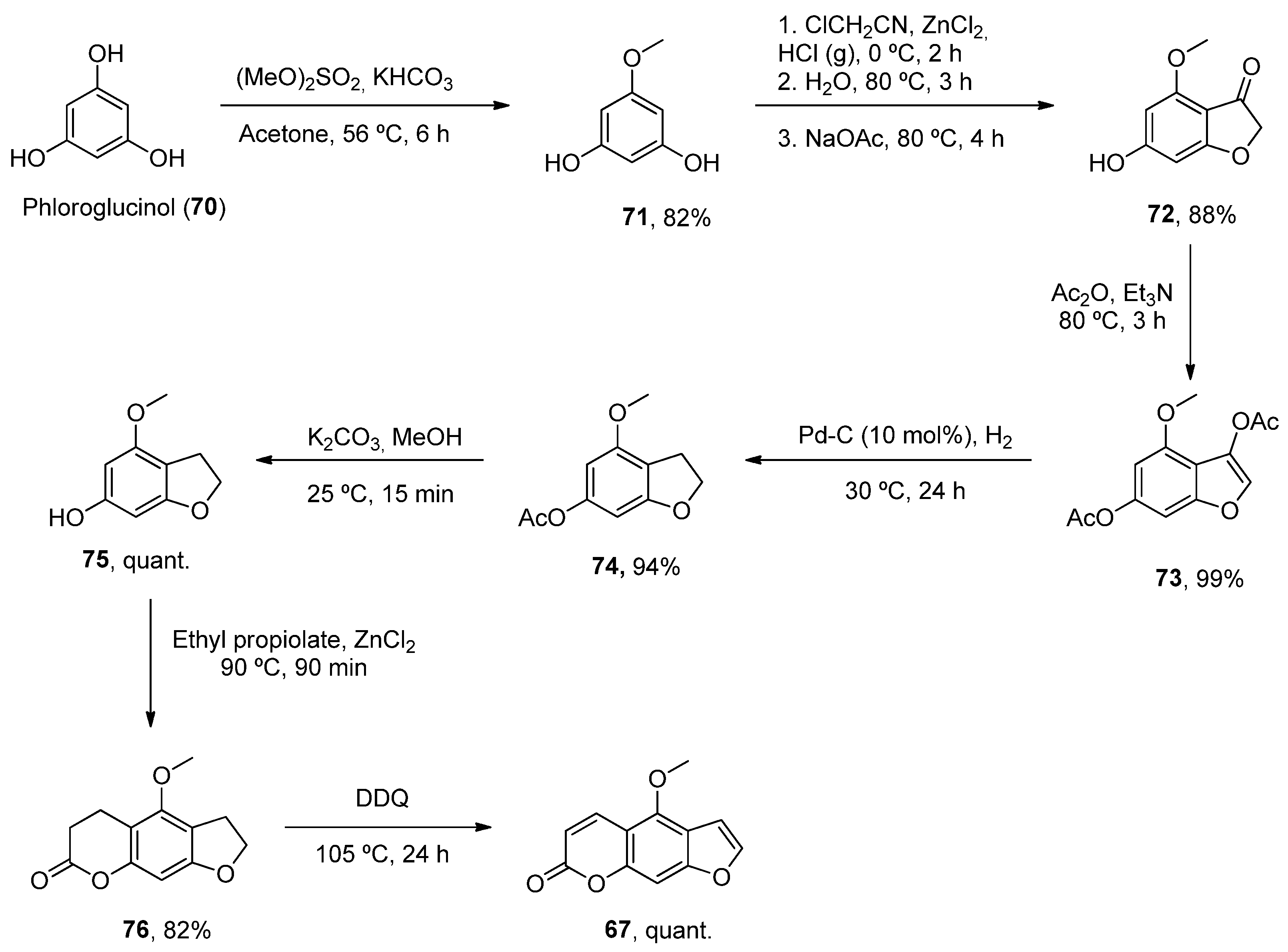
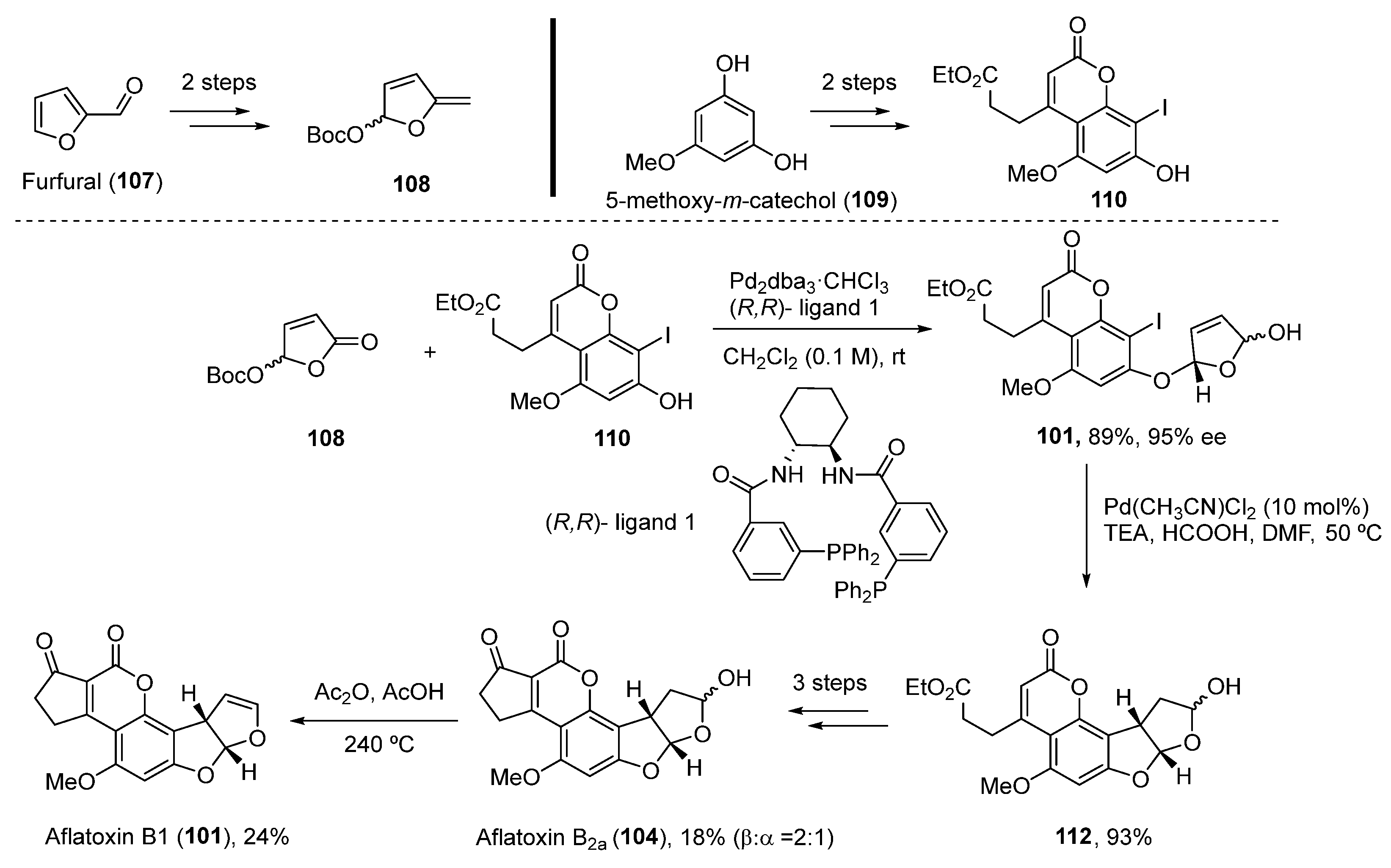
2.4.1. Aflatoxins
2.4.2. Lamellarins
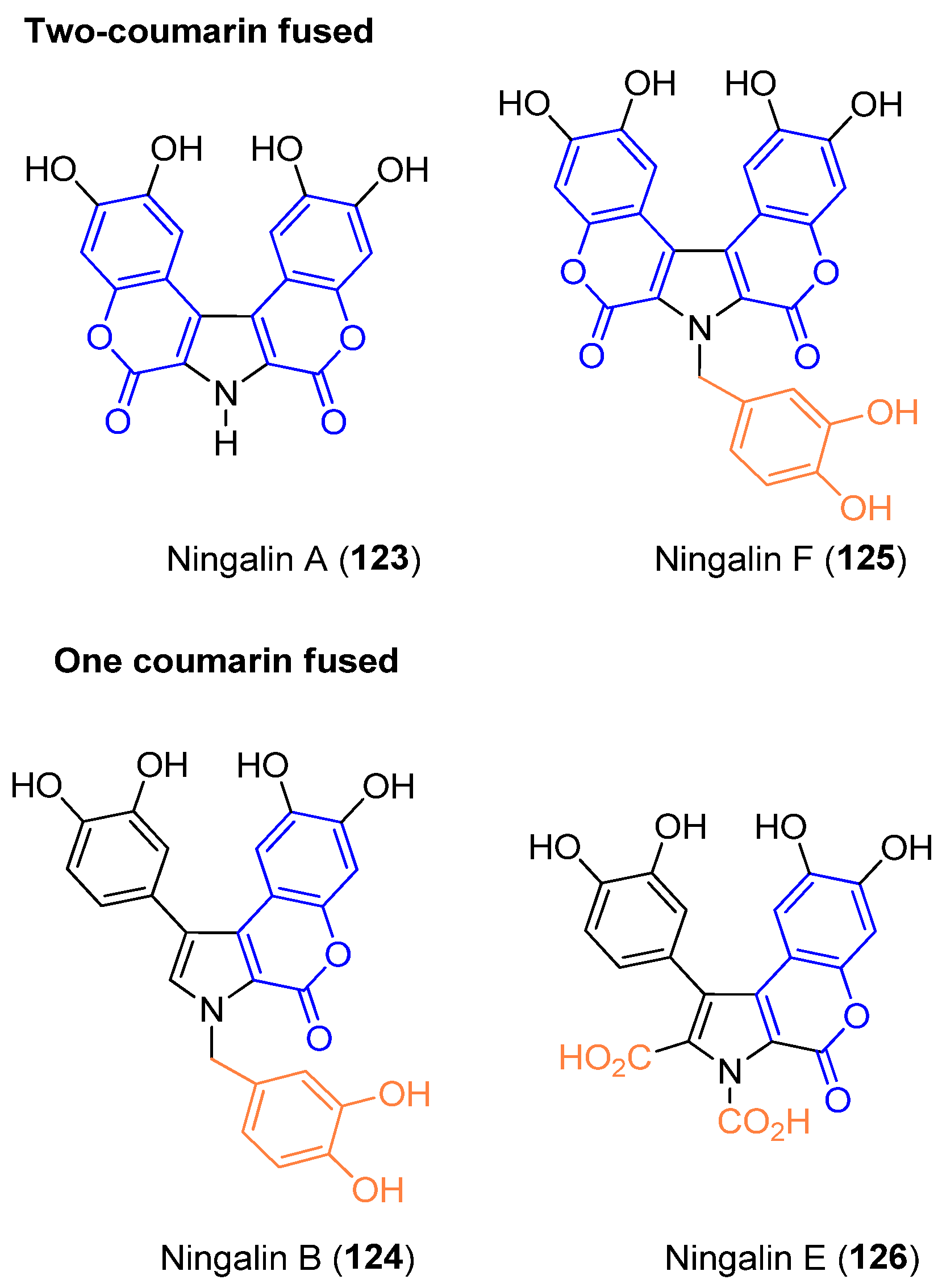
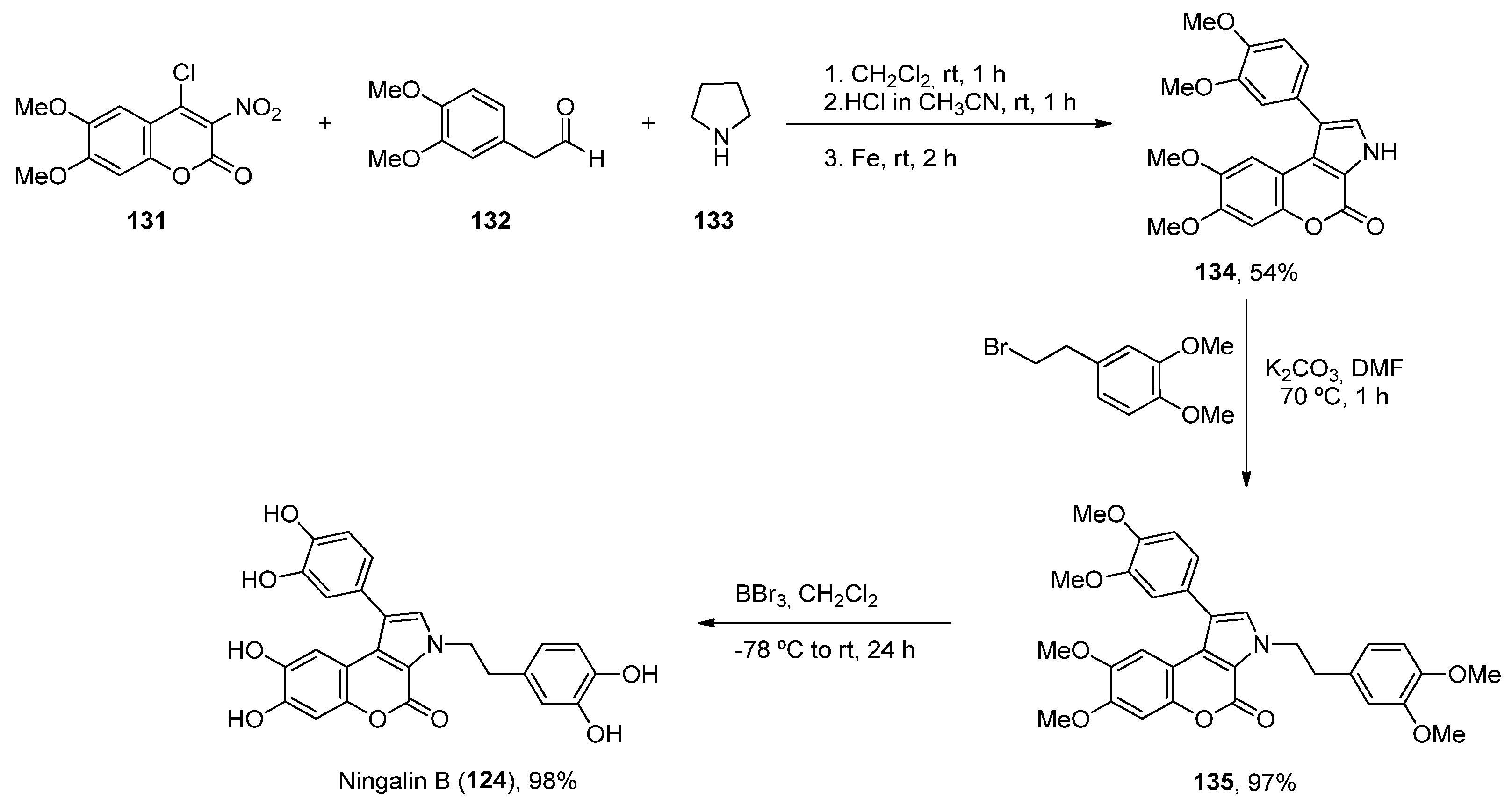
2.5. Ningalins
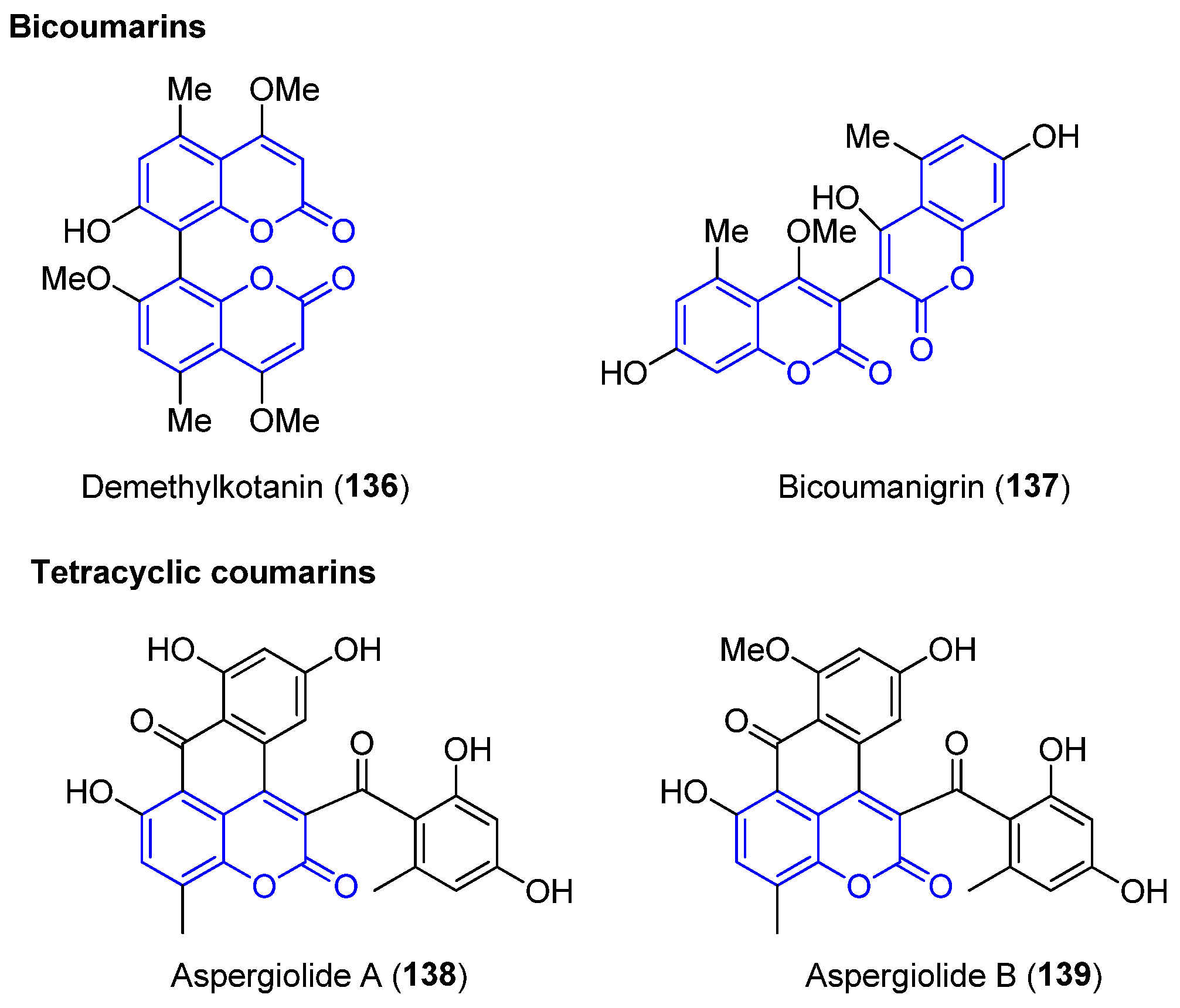
2.6. Other Coumarin Derivatives: Bicoumarins and Tetracyclics
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vazquez-Rodriguez, S.; Matos, M.J.; Borges, F.; Uriarte, E.; Santana, L. Bioactive coumarins from marine sources: Origin, structural features and pharmacological properties. Curr. Top. Med. Chem. 2015, 15, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Díez-Poza, C.; González-Andrés, P.; Barbero, A. The Tetrahydrofuran Motif in Polyketide Marine Drugs. Mar. Drugs 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef]
- Murray, R.D. The naturally occurring coumarins. Prog. Chem. Org. Nat. Prod. 2002, 1–619. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Matos, M.J. Coumarin and Its Derivatives—Editorial. Molecules 2021, 26, 6320. [Google Scholar] [CrossRef] [PubMed]
- Vekariya, R.H.; Patel, H.D. Recent advances in the synthesis of coumarin derivatives via Knoevenagel condensation: A review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Fotopoulos, I.; Hadjipavlou-Litina, D. Hybrids of coumarin derivatives as potent and multifunctional bioactive agents: A review. Med. Chem. 2020, 16, 272–306. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, 1900380. [Google Scholar] [CrossRef] [PubMed]
- Song, X.F.; Fan, J.; Liu, L.; Liu, X.F.; Gao, F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020, 353, 2000025. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Goud, N.S.; Kumar, P.; Bharath, R.D. Recent developments of target based coumarin derivatives as potential anticancer agents. Mini Rev. Med. Chem. 2020, 20, 1754–1766. [Google Scholar] [CrossRef]
- Menichelli, D.; Poli, D.; Antonucci, E.; Cammisotto, V.; Testa, S.; Pignatelli, P.; Palareti, G.; Pastori, D.; Clinics, I.F.o.A. Comparison of anticoagulation quality between acenocoumarol and warfarin in patients with mechanical prosthetic heart valves: Insights from the nationwide PLECTRUM study. Molecules 2021, 26, 1425. [Google Scholar] [CrossRef]
- Kang, J.K.; Hyun, C.-G. 4-Hydroxy-7-methoxycoumarin inhibits inflammation in LPS-activated RAW264. 7 macrophages by suppressing NF-κB and MAPK activation. Molecules 2020, 25, 4424. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, L.C. Coumarin derivatives in inflammatory bowel disease. Molecules 2021, 26, 422. [Google Scholar] [CrossRef] [PubMed]
- Moya-Alvarado, G.; Yañez, O.; Morales, N.; González-González, A.; Areche, C.; Núñez, M.T.; Fierro, A.; García-Beltrán, O. Coumarin-chalcone hybrids as inhibitors of MAO-B: Biological activity and in silico studies. Molecules 2021, 26, 2430. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, S.; Vilar, S.; Kachler, S.; Klotz, K.-N.; Uriarte, E.; Borges, F.; Matos, M.J. Adenosine Receptor Ligands: Coumarin–Chalcone Hybrids as Modulating Agents on the Activity of h ARs. Molecules 2020, 25, 4306. [Google Scholar] [CrossRef] [PubMed]
- Phutdhawong, W.; Chuenchid, A.; Taechowisan, T.; Sirirak, J.; Phutdhawong, W.S. Synthesis and biological activity evaluation of coumarin-3-carboxamide derivatives. Molecules 2021, 26, 1653. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Rzeski, W.; Jakubowicz-Gil, J. Antiglioma potential of coumarins combined with Sorafenib. Molecules 2020, 25, 5192. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics 2022, 11, 1156. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; Cai, X.; She, Z.; Lin, Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp. (ZH16). Nat. Prod. Res. 2012, 26, 1291–1295. [Google Scholar] [CrossRef]
- Lino, C.; Taveira, M.; Viana, G.; Matos, F. Analgesic and antiinflammatory activities of Justicia pectoralis Jacq and its main constituents: Coumarin and umbelliferone. Phytother. Res. 1997, 11, 211–215. [Google Scholar] [CrossRef]
- Stefanova, T.H.; Nikolova, N.J.; Toshkova, R.A.; Neychev, H.O. Antitumor and immunomodulatory effect of coumarin and 7-hydroxycoumarin against Sarcoma 180 in mice. J. Exp. Ther. Oncol. 2007, 6, 107–115. [Google Scholar] [PubMed]
- Yu, S.M.; Hu, D.H.; Zhang, J.J. Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 3869–3873. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhang, L.; Fu, X.L.; Chen, K.; Qian, B.C. Effect of scopoletin on PC3 cell proliferation and apoptosis. Acta Pharmacol. Sin. 2001, 22, 929–933. [Google Scholar] [PubMed]
- Hornick, A.; Lieb, A.; Vo, N.P.; Rollinger, J.M.; Stuppner, H.; Prast, H. The coumarin scopoletin potentiates acetylcholine release from synaptosomes, amplifies hippocampal long-term potentiation and ameliorates anticholinergic- and age-impaired memory. Neuroscience 2011, 197, 280–292. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Biorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A.; Abbas, S.Y. An overview on synthetic strategies to coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Shakil, M.R.; Meguerdichian, A.G.; Tasnim, H.; Shirazi-Amin, A.; Seraji, M.S.; Suib, S.L. Syntheses of ZnO with Different Morphologies: Catalytic Activity toward Coumarin Synthesis via the Knoevenagel Condensation Reaction. Inorg. Chem. 2019, 58, 5703–5714. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, X.; Liang, C.; Zhang, W. Design, synthesis, and antifungal evaluation of novel coumarin-pyrrole hybrids. J. Heterocycl. Chem. 2021, 58, 450–458. [Google Scholar] [CrossRef]
- Augustine, J.K.; Bombrun, A.; Ramappa, B.; Boodappa, C. An efficient one-pot synthesis of coumarins mediated by propylphosphonic anhydride (T3P) via the Perkin condensation. Tetrahedron Lett. 2012, 53, 4422–4425. [Google Scholar] [CrossRef]
- Belavagi, N.S.; Deshapande, N.; Sunagar, M.G.; Khazi, I.A.M. A practical one-pot synthesis of coumarins in aqueous sodium bicarbonate via intramolecular Wittig reaction at room temperature. RSC Adv. 2014, 4, 39667–39671. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Kumar, P. A novel synthesis of coumarins employing triphenyl(α-carboxymethylene)phosphorane imidazolide as a C-2 synthon. Tetrahedron Lett. 2009, 50, 236–238. [Google Scholar] [CrossRef]
- Pünkösti, Z.; Kele, P.; Herner, A. Synthesis of 7-Azido-3-Formylcoumarin—A Key Precursor in Bioorthogonally Applicable Fluorogenic Dye Synthesis. J. Heterocycl. Chem. 2018, 55, 1183–1188. [Google Scholar] [CrossRef]
- Polito, L.; Cravini, M.; Poletti, L.; Lay, L. Simple Synthesis of Versatile Coumarin Scaffolds. Synth. Commun. 2006, 36, 2203–2209. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Kumar, H.V. An Expeditious Coumarin Synthesis via a “Pseudocycloaddition” Between Salicylaldehydes and Ketene. Synth. Commun. 2015, 45, 232–235. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Zhang, H.; Yang, Y.; Wang, F. Synthesis of 2H-Chromenones from Salicylaldehydes and Arylacetonitriles. Molecules 2017, 22, 1197. [Google Scholar] [CrossRef]
- Sharma, U.; Naveen, T.; Maji, A.; Manna, S.; Maiti, D. Palladium-Catalyzed Synthesis of Benzofurans and Coumarins from Phenols and Olefins. Angew. Chem. Int. Ed. 2013, 52, 12669–12673. [Google Scholar] [CrossRef]
- Wienhold, M.; Molloy, J.J.; Daniliuc, C.G.; Gilmour, R. Coumarins by Direct Annulation: β-Borylacrylates as Ambiphilic C3-Synthons. Angew. Chem. Int. Ed. 2021, 133, 695–699. [Google Scholar] [CrossRef]
- Gadakh, S.K.; Dey, S.; Sudalai, A. Rh-Catalyzed Synthesis of Coumarin Derivatives from Phenolic Acetates and Acrylates via C–H Bond Activation. J. Org. Chem. 2015, 80, 11544–11550. [Google Scholar] [CrossRef] [PubMed]
- Kutubi, M.S.; Hashimoto, T.; Kitamura, T. Improved Synthesis of Coumarins by Iron(III)-Catalyzed Cascade Reaction of Propiolic Acids and Phenols. Synthesis 2011, 2011, 1283–1289. [Google Scholar] [CrossRef]
- Liu, X.-G.; Zhang, S.-S.; Jiang, C.-Y.; Wu, J.-Q.; Li, Q.; Wang, H. Cp*Co(III)-Catalyzed Annulations of 2-Alkenylphenols with CO: Mild Access to Coumarin Derivatives. Org. Lett. 2015, 17, 5404–5407. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Taddeo, V.A.; Genovese, S. Ytterbium triflate promoted coupling of phenols and propiolic acids: Synthesis of coumarins. Tetrahedron Lett. 2016, 57, 2939–2942. [Google Scholar] [CrossRef]
- Konrádová, D.; Kozubíková, H.; Doležal, K.; Pospíšil, J. Microwave-Assisted Synthesis of Phenylpropanoids and Coumarins: Total Synthesis of Osthol. Eur. J. Org. Chem. 2017, 2017, 5204–5213. [Google Scholar] [CrossRef]
- Li, X.; Chen, A.; Zhou, Y.; Huang, L.; Fang, Z.; Gan, H.; Guo, K. Two-stage flow synthesis of coumarin via O-acetylation of salicylaldehyde. J. Flow Chem. 2015, 5, 82–86. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, B. Visible-light promoted oxidative cyclization of cinnamic acid derivatives using xanthone as the photocatalyst. Org. Biomol. Chem. 2021, 19, 568–573. [Google Scholar] [CrossRef]
- Valizadeh, H.; Vaghefi, S. One-Pot Wittig and Knoevenagel Reactions in Ionic Liquid as Convenient Methods for the Synthesis of Coumarin Derivatives. Synth. Commun. 2009, 39, 1666–1678. [Google Scholar] [CrossRef]
- Prabhala, P.; Savanur, H.M.; Sutar, S.M.; Malunavar, S.S.; Kalkhambkar, R.G.; Laali, K.K. Facile one-pot synthetic access to libraries of diversely substituted 3-aryl (Alkyl)-coumarins using ionic liquid (IL) or conventional base/solvent, and an IL-mediated approach to novel coumarin-bearing diaryl-ethynes. Tetrahedron Lett. 2020, 61, 151854. [Google Scholar] [CrossRef]
- Wei, J.; Wang, P.; Jia, Q.; Huang, J.; Du, Z.; Zhang, K.; Wang, J. Amine-Catalyzed Cascade Synthesis of 3,4-Diunsubstituted Coumarins. Eur. J. Org. Chem. 2013, 2013, 4499–4502. [Google Scholar] [CrossRef]
- Zhan, K.; Li, Y. Visible-Light photocatalytic E to Z isomerization of activated olefins and its application for the syntheses of coumarins. Catalysts 2017, 7, 337. [Google Scholar] [CrossRef]
- Shu, P.; Xu, H.; Zhang, L.; Li, J.; Liu, H.; Luo, Y.; Yang, X.; Ju, Z.; Xu, Z. Synthesis of (Z)-Cinnamate Derivatives via Visible-Light-Driven E-to-Z Isomerization. SynOpen 2019, 03, 103–107. [Google Scholar] [CrossRef]
- Cervi, A.; Vo, Y.; Chai, C.L.L.; Banwell, M.G.; Lan, P.; Willis, A.C. Gold(I)-Catalyzed Intramolecular Hydroarylation of Phenol-Derived Propiolates and Certain Related Ethers as a Route to Selectively Functionalized Coumarins and 2H-Chromenes. J. Org. Chem. 2021, 86, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, J.; Lee, K. Metal-free, Brønsted acid-mediated synthesis of coumarin derivatives from phenols and propiolic acids. Tetrahedron Lett. 2016, 57, 3600–3603. [Google Scholar] [CrossRef]
- Tao, L.-y.; Zhang, J.-y.; Liang, Y.-j.; Chen, L.-m.; Zheng, L.-s.; Wang, F.; Mi, Y.-j.; She, Z.-g.; To, K.K.W.; Lin, Y.-c.; et al. Anticancer Effect and Structure-Activity Analysis of Marine Products Isolated from Metabolites of Mangrove Fungi in the South China Sea. Mar. Drugs 2010, 8, 1094–1105. [Google Scholar] [CrossRef]
- Garo, E.; Starks, C.M.; Jensen, P.R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, Cytotoxic Modified Dipeptides from the Marine-Derived Fungus Trichoderma virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, Q.-Q.; Zhu, W.-M.; Cui, C.-B.; Fan, G.-T. Trichodermamide A and aspergillazine A, two cytotoxic modified dipeptides from a marine-derived fungus Spicaria elegans. Arch. Pharmacal. Res. 2005, 28, 1042–1046. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Sun, K.; Zhu, W. Effects of High Salt Stress on Secondary Metabolite Production in the Marine-Derived Fungus Spicaria elegans. Mar. Drugs 2011, 9, 535–542. [Google Scholar] [CrossRef]
- Capon, R.J.; Ratnayake, R.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Aspergillazines A–E: Novel heterocyclic dipeptides from an Australian strain of Aspergillus unilateralis. Org. Biomol. Chem. 2005, 3, 123–129. [Google Scholar] [CrossRef]
- Jans, P.E.; Mfuh, A.M.; Arman, H.D.; Shaffer, C.V.; Larionov, O.V.; Mooberry, S.L. Cytotoxicity and Mechanism of Action of the Marine-Derived Fungal Metabolite Trichodermamide B and Synthetic Analogues. J. Nat. Prod. 2017, 80, 676–683. [Google Scholar] [CrossRef]
- Yamazaki, H.; Rotinsulu, H.; Takahashi, O.; Kirikoshi, R.; Namikoshi, M. Induced production of a new dipeptide with a disulfide bridge by long-term fermentation of marine-derived Trichoderma cf. brevicompactum. Tetrahedron Lett. 2016, 57, 5764–5767. [Google Scholar] [CrossRef]
- Matsuo, H.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Higo, M.; Nonaka, K.; Nagano, Y.; Takahashi, Y.; Ōmura, S.; Nakashima, T. Hatsusamides A and B: Two New Metabolites Produced by the Deep-Sea-Derived Fungal Strain Penicillium steckii FKJ-0213. Mar. Drugs 2020, 18, 513. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Longden, J.; Avery, V.M.; Healy, P.C. The isolation, structure determination and cytotoxicity of the new fungal metabolite, trichodermamide C. Bioorg. Med. Chem. Lett. 2008, 18, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Joullié, M.M. Enantioselective Total Syntheses of Trichodermamides A and B. J. Am. Chem. Soc. 2008, 130, 17236–17237. [Google Scholar] [CrossRef]
- Mfuh, A.M.; Zhang, Y.; Stephens, D.E.; Vo, A.X.T.; Arman, H.D.; Larionov, O.V. Concise Total Synthesis of Trichodermamides A, B, and C Enabled by an Efficient Construction of the 1,2-Oxazadecaline Core. J. Am. Chem. Soc. 2015, 137, 8050–8053. [Google Scholar] [CrossRef]
- Reich, H.J. Organoselenium chemistry. Synthetic transformations based on allyl selenide anions. J. Org. Chem. 1975, 40, 2570–2572. [Google Scholar] [CrossRef]
- Lu, C.-D.; Zakarian, A. Total Synthesis of (±)-Trichodermamide B and of a Putative Biosynthetic Precursor to Aspergillazine A Using an Oxaza-Cope Rearrangement. Angew. Chem. Int. Ed. 2008, 47, 6829–6831. [Google Scholar] [CrossRef]
- Santana, L.; Uriarte, E.; Roleira, F.; Milhazes, N.; Borges, F. Furocoumarins in medicinal chemistry. Synthesis, natural occurrence and biological activity. Curr. Med. Chem. 2004, 11, 3239–3261. [Google Scholar] [CrossRef] [PubMed]
- Uwaifo, A.O.; Billings, P.C.; Heidelberger, C. Mutation of Chinese Hamster V79 Cells and Transformation and Mutation of Mouse Fibroblast C3H/10T½ Clone 8 Cells by Aflatoxin B1 and Four Other Furocoumarins Isolated from Two Nigerian Medicinal Plants. Cancer Res. 1983, 43, 1054–1058. [Google Scholar] [PubMed]
- Cadierno, V. Metal-catalyzed routes for the synthesis of furocoumarins and coumestans. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 53–96. [Google Scholar]
- Rao, M.L.; Nand, S.; Murty, V.N. Metal-Catalyzed Divergent Synthetic Methods for Pyrrolocoumarins and Furocoumarins. Asian J. Org. Chem. 2022, 11, e202100604. [Google Scholar] [CrossRef]
- Cai, X.; Ji, D.; Liu, J.; Hu, M.; Jin, Z. A New Approach to the Synthesis of Bergapten. Chem. Res. Chin. Univ. 2022, 31, 1–5. [Google Scholar] [CrossRef]
- Tan, N.; Tao, Y.; Pan, J.; Wang, S.; Xu, F.; She, Z.; Lin, Y.; Gareth Jones, E. Isolation, structure elucidation, and mutagenicity of four alternariol derivatives produced by the mangrove endophytic fungus No. 2240. Chem. Nat. Compd. 2008, 44, 296–300. [Google Scholar] [CrossRef]
- Jasim, S.; Mustafa, Y. A Review of Classical and Advanced Methodologies for Benzocoumarin Synthesis. J. Med. Chem. Sci. 2022, 5, 676–694. [Google Scholar] [CrossRef]
- Koch, K.; Podlech, J.; Pfeiffer, E.; Metzler, M. Total synthesis of alternariol. J. Org. Chem. 2005, 70, 3275–3276. [Google Scholar] [CrossRef]
- Won, M.; Kwon, S.; Kim, T.-H. An efficient synthesis of alternariol. J. Korean Chem. Soc. 2015, 59, 471–474. [Google Scholar] [CrossRef][Green Version]
- Gallardo-Donaire, J.; Martin, R. Cu-catalyzed mild C (sp2)–H functionalization assisted by carboxylic acids en route to hydroxylated arenes. J. Am. Chem. Soc. 2013, 135, 9350–9353. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Vakuliuk, O.; Gryko, D.T. Color-tunable fluorescent dyes based on benzo [c] coumarin. Eur. J. Org. Chem. 2013, 2013, 5631–5644. [Google Scholar] [CrossRef]
- Pottie, I.R.; Nandaluru, P.R.; Benoit, W.L.; Miller, D.O.; Dawe, L.N.; Bodwell, G.J. Synthesis of 6 H-Dibenzo [b,d] pyran-6-ones Using the Inverse Electron Demand Diels–Alder Reaction. J. Org. Chem. 2011, 76, 9015–9030. [Google Scholar] [CrossRef]
- Nandaluru, P.R.; Bodwell, G.J. Multicomponent Synthesis of 6 H-Dibenzo [b, d] pyran-6-ones and a Total Synthesis of Cannabinol. Org. Lett. 2012, 14, 310–313. [Google Scholar] [CrossRef]
- Poudel, T.N.; Lee, Y.R. An advanced and novel one-pot synthetic method for diverse benzo [c] chromen-6-ones by transition-metal free mild base-promoted domino reactions of substituted 2-hydroxychalcones with β-ketoesters and its application to polysubstituted terphenyls. Org. Biomol. Chem. 2014, 12, 919–930. [Google Scholar] [CrossRef]
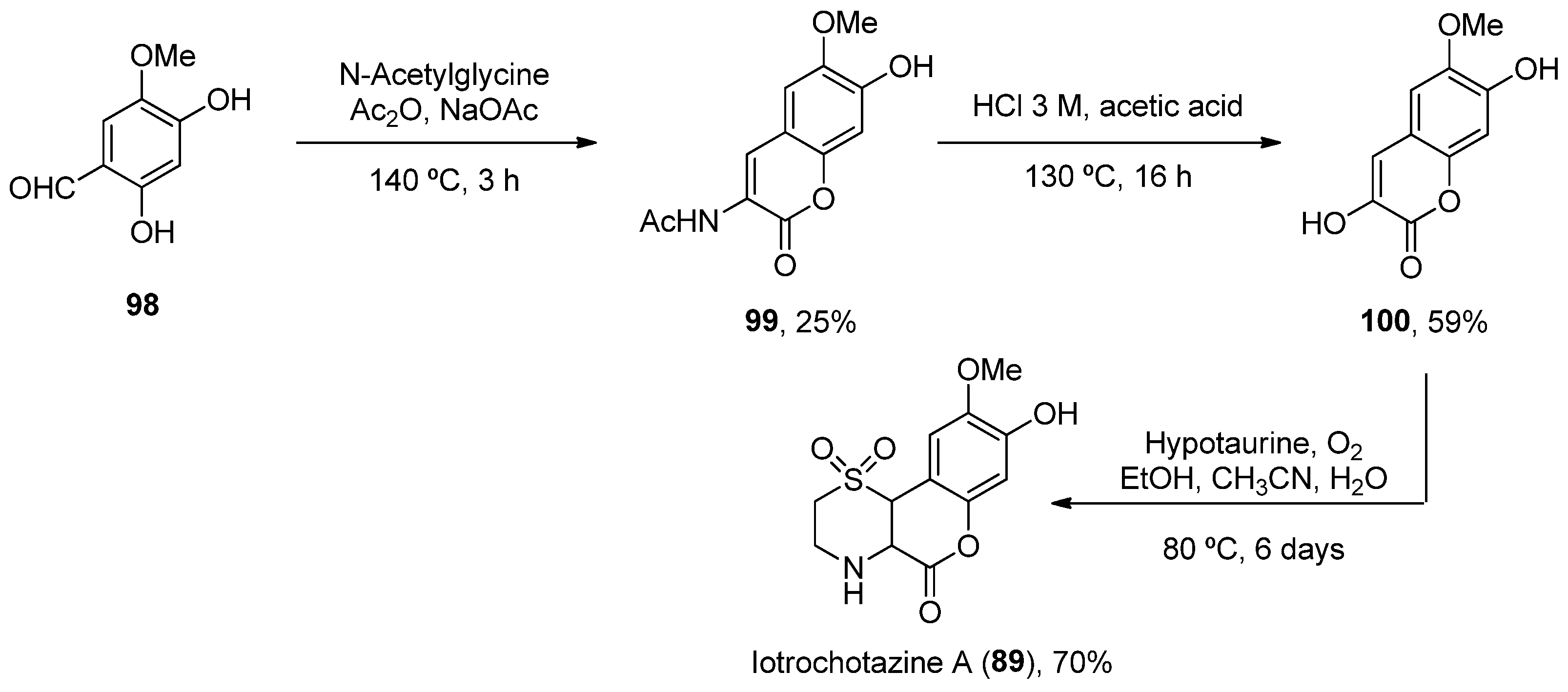
- Grkovic, T.; Pouwer, R.H.; Vial, M.L.; Gambini, L.; Noël, A.; Hooper, J.N.; Wood, S.A.; Mellick, G.D.; Quinn, R.J. NMR Fingerprints of the Drug-like Natural-Product Space Identify Iotrochotazine A: A Chemical Probe to Study Parkinson’s Disease. Angew. Chem. Int. Ed. 2014, 53, 6070–6074. [Google Scholar] [CrossRef]
- Takara, K.; Kuniyoshi, A.; Wada, K.; Kinjyo, K.; Iwasaki, H. Antioxidative flavan-3-ol glycosides from stems of Rhizophora stylosa. Biosci. Biotechnol. Biochem. 2008, 72, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Awale, S.; Tezuka, Y.; Wang, S.; Kadota, S. Facile and regioselective synthesis of phenylpropanoid-substituted flavan-3-ols. Org. Lett. 2002, 4, 1707–1709. [Google Scholar] [CrossRef]
- Lin, A.-Q.; Du, L.; Fang, Y.-C.; Wang, F.-Z.; Zhu, T.-J.; Gu, Q.-Q.; Zhu, W.-M. iso-α-Cyclopiazonic acid, a new natural product isolated from the marine-derived fungus Aspergillus flavus CF-3. Chem. Nat. Compd. 2009, 45, 677–680. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Qu, H.-J.; Liu, P.; Miao, C.; Zhu, T.; Li, J.; Hong, K.; Zhu, W. Antimicrobial aflatoxins from the marine-derived fungus Aspergillus flavus 092008. Arch. Pharmacal. Res. 2012, 35, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.A. Magical Mushrooms, Mischievous Molds: The Remarkable Story of the Fungus Kingdom and Its Impact on Human Affairs. Am. Sci. 1998, 86, 578–579. [Google Scholar]
- Wang, Z.; Yang, L. Advances in the Total Synthesis of Aflatoxins. Front. Chem. 2021, 30, 1024. [Google Scholar] [CrossRef]
- Buechi, G.; Foulkes, D.; Kurono, M.; Mitchell, G.F.; Schneider, R.S. Total synthesis of racemic aflatoxin B1. J. Am. Chem. Soc. 1967, 89, 6745–6753. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D. Palladium Catalyzed Kinetic and Dynamic Kinetic Asymmetric Transformations of γ-Acyloxybutenolides. Enantioselective Total Synthesis of (+)-Aflatoxin B1 and B2a. J. Am. Chem. Soc. 2003, 125, 3090–3100. [Google Scholar] [CrossRef]
- Pla, D.; Albericio, F.; Álvarez, M. Recent advances in lamellarin alkaloids: Isolation, synthesis and activity. Anticancer Agents Med. Chem. 2008, 8, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ishibashi, F.; Iwao, M. Lamellarin alkaloids: Isolation, synthesis, and biological activity. Alkaloids Chem. Biol. 2020, 83, 1–112. [Google Scholar] [CrossRef] [PubMed]
- Facompré, M.; Tardy, C.; Bal-Mahieu, C.; Colson, P.; Perez, C.; Manzanares, I.; Cuevas, C.; Bailly, C. Lamellarin D: A novel potent inhibitor of topoisomerase I. Cancer Res. 2003, 63, 7392–7399. [Google Scholar]
- Reddy, S.M.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New potent cytotoxic lamellarin alkaloids from Indian ascidian Didemnum obscurum. Tetrahedron 2005, 61, 9242–9247. [Google Scholar] [CrossRef]
- Plisson, F.; Huang, X.C.; Zhang, H.; Khalil, Z.; Capon, R.J. Lamellarins as Inhibitors of P-Glycoprotein-Mediated Multidrug Resistance in a Human Colon Cancer Cell Line. Chem. Asian J. 2012, 7, 1616–1623. [Google Scholar] [CrossRef]
- Pla, D.; Albericio, F.; Álvarez, M. Progress on lamellarins. MedChemComm 2011, 2, 689–697. [Google Scholar] [CrossRef]
- Silyanova, E.; Samet, A.; Semenov, V. A Two-Step Approach to a Hexacyclic Lamellarin Core via 1, 3-Dipolar Cycloaddition of Isoquinolinium Ylides to Nitrostilbenes. J. Org. Chem. 2022, 87, 6444–6453. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Yang, S.; Miao, X.; Li, D.; Wang, D. Titanium-Mediated aza-Nazarov Annulation for the Synthesis of N-Fused Tricycles: A General Method to Access Lamellarin Analogues. J. Org. Chem. 2022, 87, 10319–10332. [Google Scholar] [CrossRef]
- Manjappa, B.K.; Lin, J.-M.; Yang, D.-Y. Construction of pentacyclic lamellarin skeleton via Grob reaction: Application to total synthesis of lamellarins H and D. J. Org. Chem. 2017, 82, 7648–7656. [Google Scholar] [CrossRef]
- Morikawa, D.; Morii, K.; Yasuda, Y.; Mori, A.; Okano, K. Convergent total synthesis of lamellarins and their congeners. J. Org. Chem. 2020, 85, 8603–8617. [Google Scholar] [CrossRef]
- Morii, K.; Yasuda, Y.; Morikawa, D.; Mori, A.; Okano, K. Total Synthesis of Lamellarins G, J, L, and Z Using One-Pot Halogen Dance/Negishi Coupling. J. Org. Chem. 2021, 86, 13388–13401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, T.; Liu, Q.; Xu, Y.; Xie, G.; Lv, X.; Ding, S.; Wang, X.; Li, C. External oxidant-free oxidation/[3+ 2] cycloaddition/aromatization cascade: Electrochemical synthesis of polycyclic N-heterocycles. Chem. Commun. 2019, 55, 8398–8401. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, Y.; Che, C.-M. Tungsten catalysed decarboxylative [3+ 2] cycloaddition aromatization: One-pot synthesis of trifluoromethyl-pyrrolo [2, 1-a] isoquinolines with visible light irradiation. Org. Chem. Front. 2022, 9, 2779–2785. [Google Scholar] [CrossRef]
- Kumar, V.; Salam, A.; Kumar, D.; Khan, T. Concise and Scalable Total Syntheses of Lamellarin Z and other Natural Lamellarins. ChemistrySelect 2020, 5, 14510–14514. [Google Scholar] [CrossRef]
- Plisson, F.; Conte, M.; Khalil, Z.; Huang, X.C.; Piggott, A.M.; Capon, R.J. Kinase inhibitor scaffolds against neurodegenerative diseases from a Southern Australian ascidian, Didemnum sp. ChemMedChem 2012, 7, 983–990. [Google Scholar] [CrossRef]
- Fan, G.; Li, Z.; Shen, S.; Zeng, Y.; Yang, Y.; Xu, M.; Bruhn, T.; Bruhn, H.; Morschhäuser, J.; Bringmann, G. Baculiferins A–O, O-sulfated pyrrole alkaloids with anti-HIV-1 activity, from the Chinese marine sponge Iotrochota baculifera. Biorg. Med. Chem. 2010, 18, 5466–5474. [Google Scholar] [CrossRef]
- Ting-Chao, C. Immunosuppressive Ningalin Compounds. WO2006026496A2, 2004. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2006026496 (accessed on 2 November 2022).
- Wu, C.-K.; Weng, Z.; Yang, D.-Y. One-pot construction of 1-phenylchromeno [3, 4-b] pyrrol-4 (3 H)-one: Application to total synthesis of ningalin B and a pyrrolocoumarin-based electrochromic switch. Org. Lett. 2019, 21, 5225–5228. [Google Scholar] [CrossRef]
- Du, L.; Zhu, T.; Liu, H.; Fang, Y.; Zhu, W.; Gu, Q. Cytotoxic polyketides from a marine-derived fungus Aspergillus glaucus. J. Nat. Prod. 2008, 71, 1837–1842. [Google Scholar] [CrossRef]
- Ovenden, S.P.; Sberna, G.; Tait, R.M.; Wildman, H.G.; Patel, R.; Li, B.; Steffy, K.; Nguyen, N.; Meurer-Grimes, B.M. A Diketopiperazine Dimer from a Marine-Derived Isolate of Aspergillus niger. J. Nat. Prod. 2004, 67, 2093–2095. [Google Scholar] [CrossRef]
- Hiort, J.; Maksimenka, K.; Reichert, M.; Perović-Ottstadt, S.; Lin, W.; Wray, V.; Steube, K.; Schaumann, K.; Weber, H.; Proksch, P. New natural products from the sponge-derived fungus Aspergillus niger. J. Nat. Prod. 2004, 67, 1532–1543. [Google Scholar] [CrossRef]
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Duan, Z.; Chen, Y.; Luan, Y.; Gu, Q.; Liu, Y.-K.; Li, D. Aspergiolides A and B: Core Structural Establishment and Synthesis of Structural Analogues. J. Org. Chem. 2019, 84, 4451–4457. [Google Scholar] [CrossRef] [PubMed]








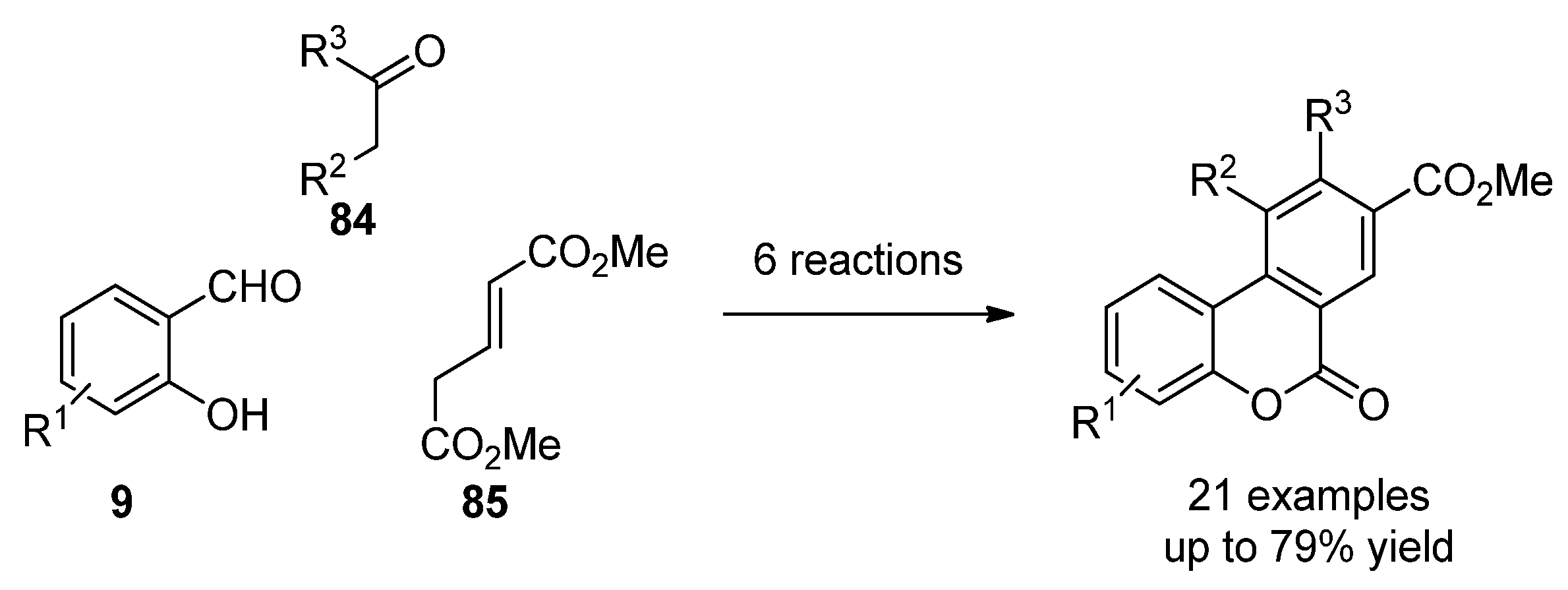







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Peña, L.; Matos, M.J.; López, E. Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation. Mar. Drugs 2023, 21, 37. https://doi.org/10.3390/md21010037
Fernández-Peña L, Matos MJ, López E. Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation. Marine Drugs. 2023; 21(1):37. https://doi.org/10.3390/md21010037
Chicago/Turabian StyleFernández-Peña, Laura, Maria João Matos, and Enol López. 2023. "Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation" Marine Drugs 21, no. 1: 37. https://doi.org/10.3390/md21010037
APA StyleFernández-Peña, L., Matos, M. J., & López, E. (2023). Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation. Marine Drugs, 21(1), 37. https://doi.org/10.3390/md21010037