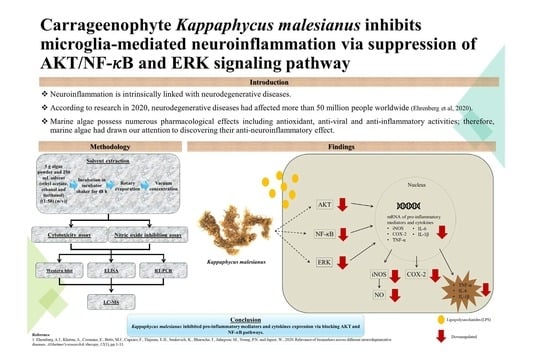
Carrageenophyte Kappaphycus malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-κB and ERK Signaling Pathways
Abstract
1. Introduction
2. Results
2.1. Effect of K. malesianus Extracts on the Viability of BV2 Microglia
2.2. Effect of K. malesianus Extracts on NO Production in LPS-Stimulated BV2 Microglia
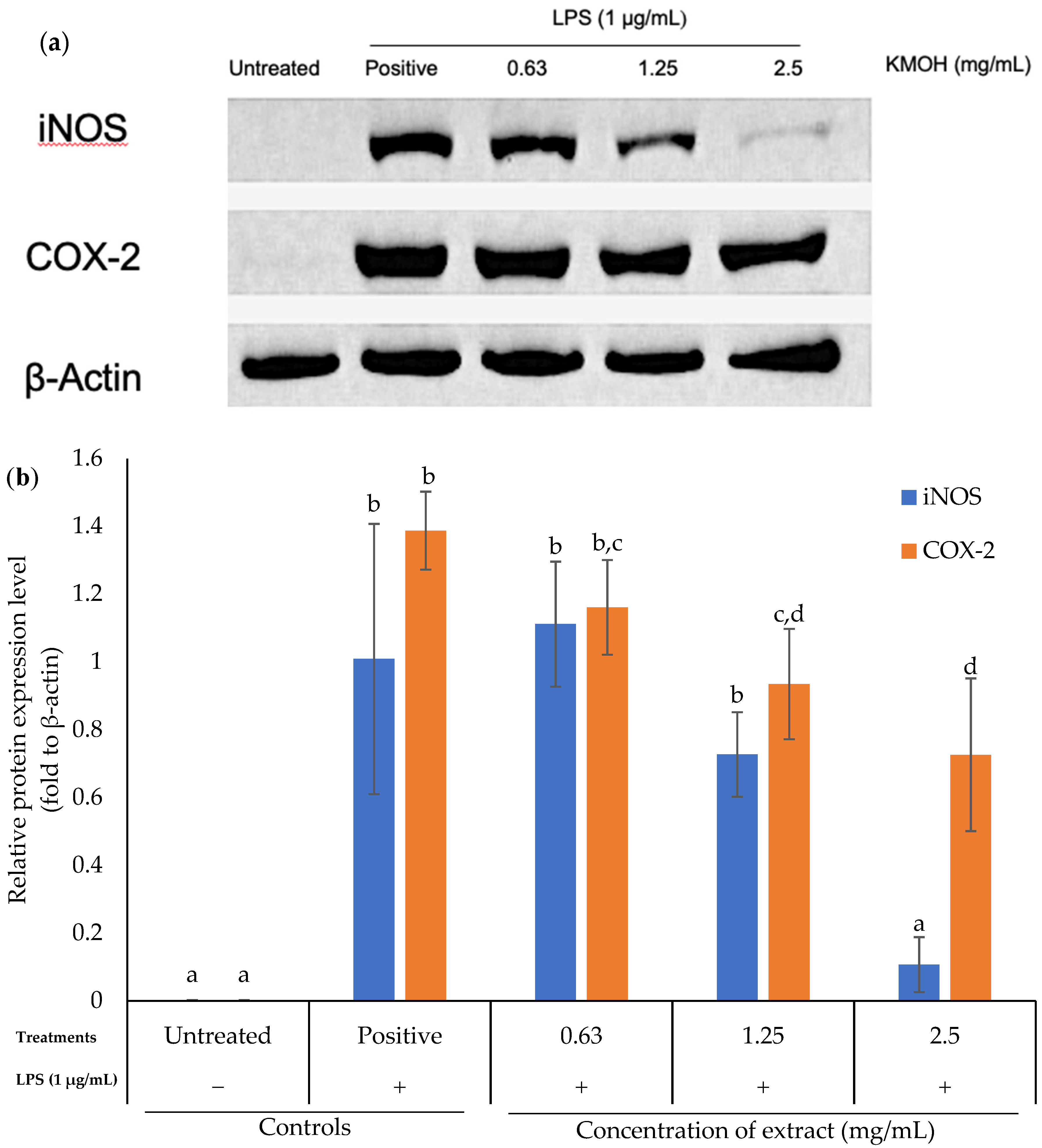
2.3. Effects of K. malesianus Methanol Extract on iNOS and COX-2 Protein Expression in LPS-Stimulated BV2 Microglia
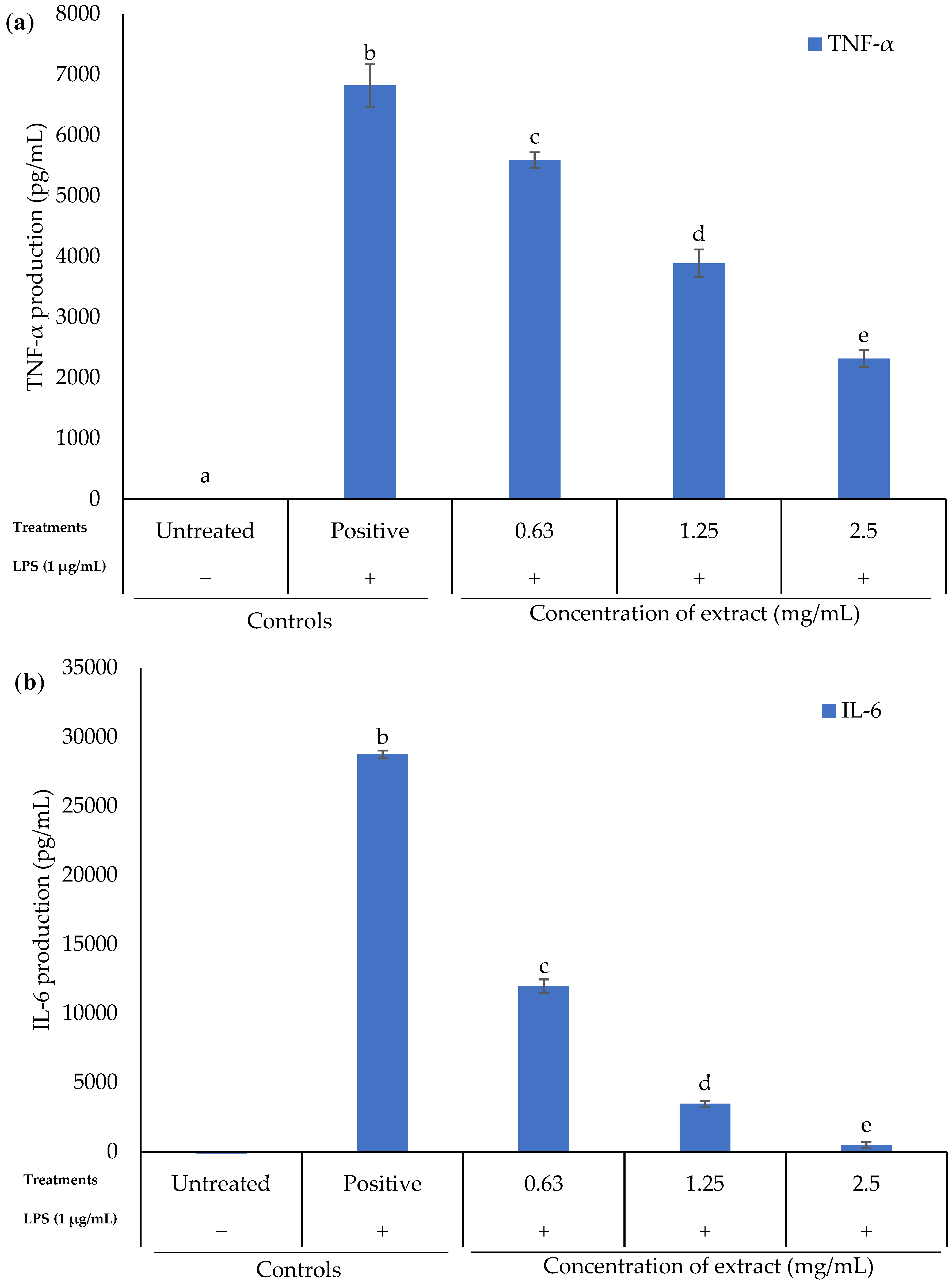
2.4. Effect of K. malesianus Methanol Extract on Proinflammatory Cytokines Expression in LPS-Stimulated BV2 Microglia
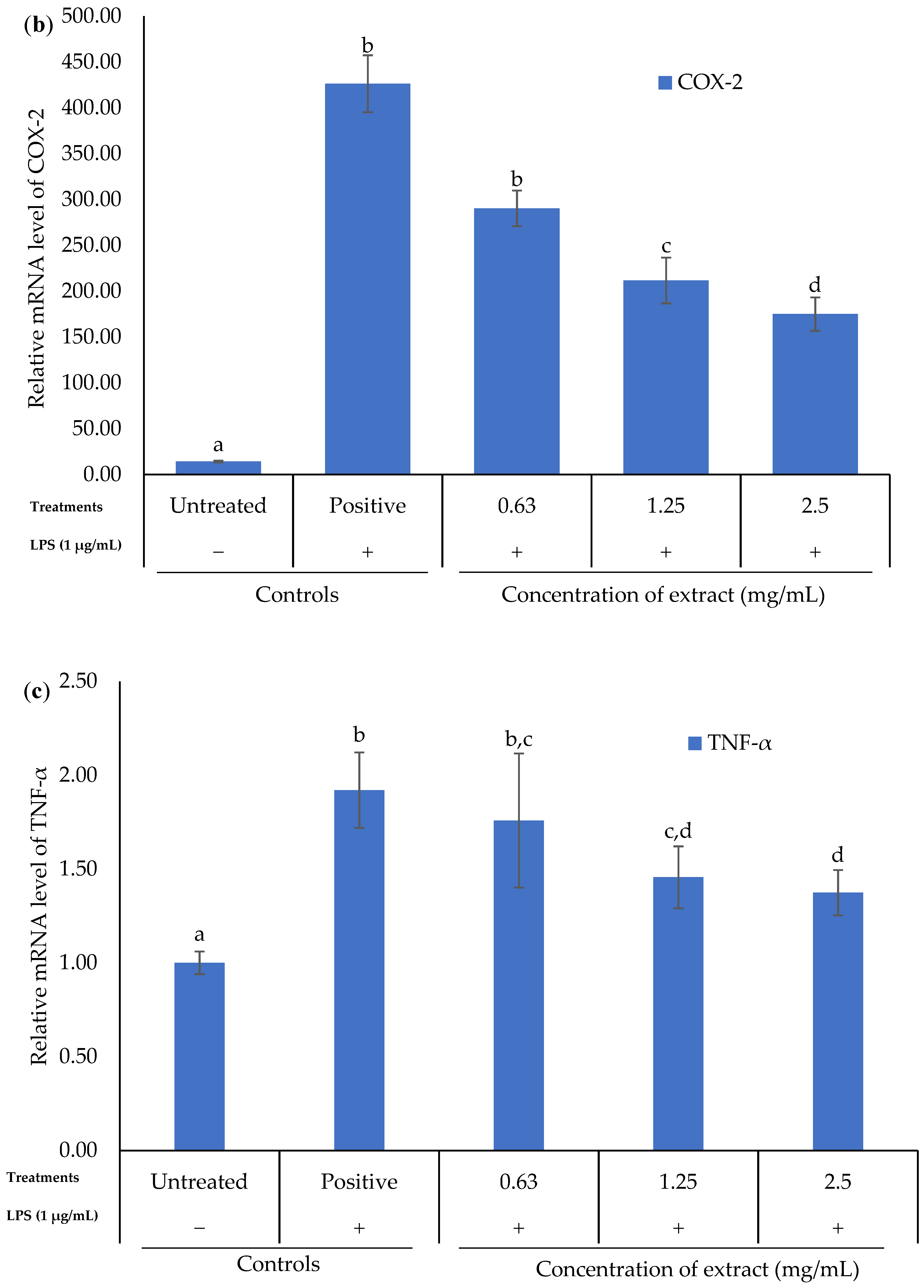
2.5. Effect of K. malesianus Methanol Extract on Proinflammatory Mediators in LPS-Stimulated BV2 Microglia Using RT-PCR
2.6. Effect of K. malesianus Methanol Extract on the AKT and ERK Signaling Pathway in LPS-Stimulated BV2 Microglia
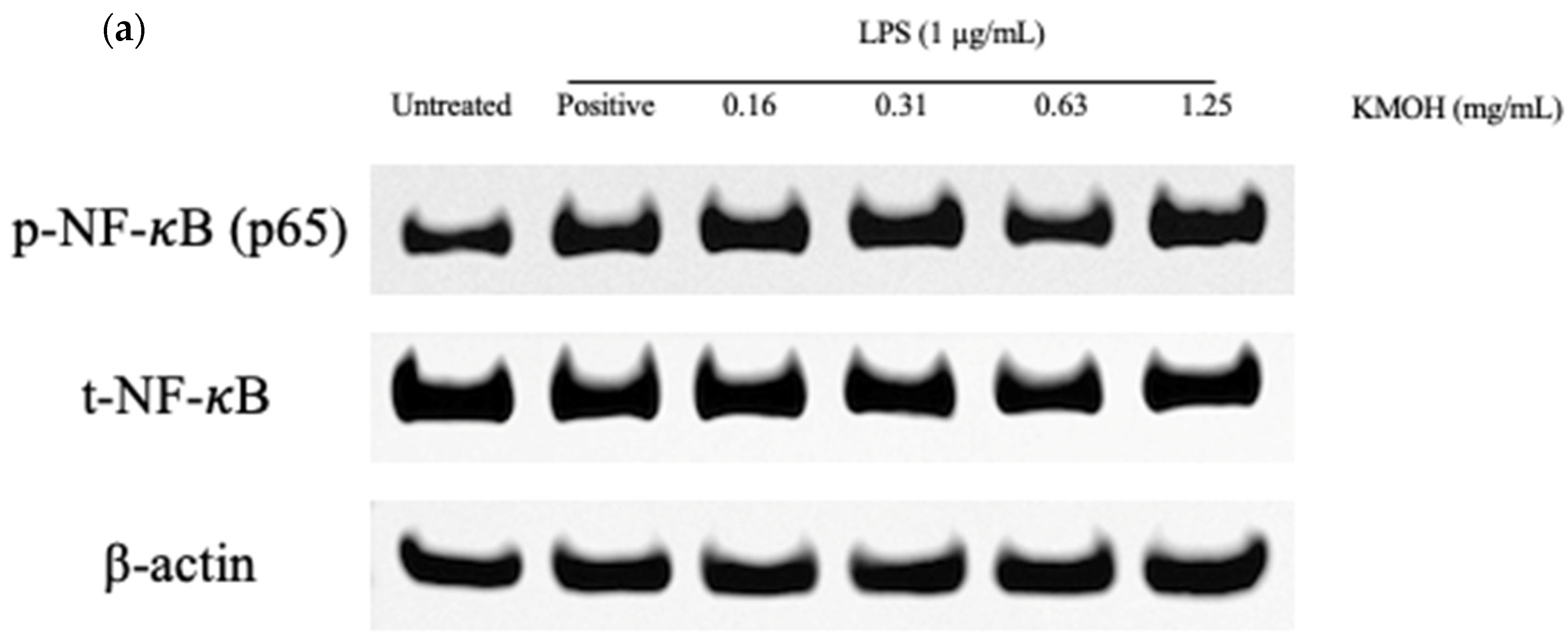
2.7. Effect of K. malesianus Methanol Extract on the NF-κB Signaling Pathway in LPS-Stimulated BV2 Microglia
2.8. Proposed Bioactive Compounds Present in K. malesianus Methanol Extract
3. Discussion
4. Materials and Methods
4.1. Seaweed Collection and Extract Preparation
4.2. Cell Culture
4.3. 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Cell Viability Assay
4.4. Measurement of Nitric Oxide
4.5. Western Blot Analysis
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.8. Separation and Analysis of Major Compound(s) Using Liquid Chromatography-Mass Spectrometry (LC-MS)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Age Structure. 2019. Available online: https://ourworldindata.org/age-structure#citation (accessed on 29 April 2020).
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A.; Bernal, L.; Surguchev, A.A. Phytochemicals as regulators of genes involved in synucleinopathies. Biomolecules 2021, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Hauss-Wegrzyniak, B.; Dobrzanski, P.; Stoehr, J.D.; Wenk, G.L. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998, 780, 294–303. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 1995, 63, 289–302. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Chu, E.; Mychasiuk, R.; Hibbs, M.L.; Semple, B.D. Dysregulated phosphoinositide 3-kinase signaling in microglia: Shaping chronic neuroinflammation. J. Neuroinflamm. 2021, 18, 1–17. [Google Scholar] [CrossRef]
- Emamian, E.S. AKT/GSK3 signaling pathway and schizophrenia. Front. Mol. Neurosci. 2012, 5, 33. [Google Scholar] [CrossRef]
- Risso, G.; Blaustein, M.; Pozzi, B.; Mammi, P.; Srebrow, A. Akt/PKB: One kinase, many modifications. Biochem. J. 2015, 468, 203–214. [Google Scholar] [CrossRef]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signaling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappaB in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- D’Acquisto, F.; Iuvone, T.; Rombolà, L.; Sautebin, L.; Di Rosa, M.; Carnuccio, R. Involvement of NF-KB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997, 418, 175–178. [Google Scholar] [CrossRef]
- Qiu, Z.; Lu, P.; Wang, K.; Zhao, X.; Li, Q.; Wen, J.; Zhang, H.; Li, R.; Wei, H.; Lv, Y.; et al. Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem. Res. 2020, 45, 345–353. [Google Scholar] [CrossRef]
- Abdelrhman, A.M.; Ashour, M.; Al-Zahaby, M.A.; Sharawy, Z.Z.; Nazmi, H.; Zaki, M.A.; Ahmed, N.H.; Ahmed, S.R.; El-Haroun, E.; Van Doan, H.; et al. Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquac. Rep. 2022, 25, 101212. [Google Scholar] [CrossRef]
- Ashour, M.; Mabrouk, M.M.; Abo-Taleb, H.A.; Sharawy, Z.Z.; Ayoub, H.F.; Van Doan, H.; Davies, S.J.; El-Haroun, E.; Goda, A.M.A. A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture 2021, 543, 736915. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- El-Shenody, R.A.; Ashour, M.; Ghobara, M.M.E. Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Braz. J. Food Technol. 2019, 22, e2018203. [Google Scholar] [CrossRef]
- Elshobary, M.E.; El-Shenody, R.A.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci. 2020, 35, 100567. [Google Scholar] [CrossRef]
- De Araújo, I.W.F.; Rodrigues, J.A.G.; Quinderé, A.L.G.; Silva, J.D.; de Freitas Marciel, G.; Ribeiro, N.A.; Vanderlei, E.D.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.; et al. Analgesic and anti-inflammatory actions on bradykinin route of a polysulfated fraction from alga Ulva lactuca. Int. J. Biol. Macromol. 2016, 92, 820–830. [Google Scholar] [CrossRef]
- Berri, M.; Slugocki, C.; Olivier, M.; Helloin, E.; Jacques, I.; Salmon, H.; Demais, H.; Le Goff, M.; Collen, P.N. Marine-sulfated polysaccharides extract of Ulva armoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. J. Appl. Phycol. 2016, 28, 2999–3008. [Google Scholar] [CrossRef]
- Khan, S.A.; Abid, M.; Hussain, F. Antifungal activity of aqueous and methanolic extracts of some seaweeds against common soil-borne plant pathogenic fungi. Pak. J. Bot. 2017, 49, 1211–1216. [Google Scholar]
- Sornsiri, J.; Srisook, K.; Pornngam, P.; Sootanan, P. Prediction of biochemical mechanism of anti-inflammation explained from two marine-derived bioactive compounds. Agric. Nat. Resour. 2018, 52, 588–595. [Google Scholar] [CrossRef]
- Choi, H.; Mascuch, S.J.; Villa, F.A.; Byrum, T.; Teasdale, M.E.; Smith, J.E.; Preskitt, L.B.; Rowley, D.C.; Gerwick, L.; Gerwick, W.H. Honaucins A-C, potent inhibitors of inflammation and bacterial quorum sensing: Synthetic derivatives and structure-activity relationships. Chem. Biol. 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Hashim, I.I.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; et al. Anti-inflammatory effects of novel polysaccharide sacran extracted from cyanobacterium Aphanothece sacrum in various inflammatory animal models. Biol. Pharm. Bull. 2016, 39, 1172–1178. [Google Scholar] [CrossRef]
- Tan, J.; Lim, P.E.; Phang, S.M.; Rahiman, A.; Nikmatullah, A.; Sunarpi, H.; Hurtado, A.Q. Kappaphycus malesianus sp. nov.: A new species of Kappaphycus (Gigartinales, Rhodophyta) from Southeast Asia. J. Appl. Phycol. 2014, 26, 1273–1285. [Google Scholar] [CrossRef]
- Ranganayaki, P.; Susmitha, S.; Vijayaraghavan, R. Study on metabolic compounds of Kappaphycus alvarezii and its in vitro analysis of anti-inflammatory activity. Int. J. Curr. Res. Acad. Rev. 2014, 2, 157–166. [Google Scholar]
- Tirtawijaya, G.; Haque, M.N.; Choi, J.S.; Moon, I.S.; Meinita, M.D.; Choi, J.S.; Hong, Y.K. Spinogenesis and synaptogenesis effects of the red seaweed Kappaphycus alvarezii and its isolated cholesterol on hippocampal neuron cultures. Prev. Nutr. Food Sci. 2019, 24, 418. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Wang, L.; Jing, H. Characterization of key aroma-active compounds in black garlic by sensory-directed flavor analysis. J. Agric. Food Chem. 2019, 67, 7926–7934. [Google Scholar] [CrossRef]
- Demange, L.; Dugave, C. Synthesis of phosphinic alanyl-proline surrogates Alaψ(PO2R-CH) Pro as potential inhibitors of the human cyclophilin hCyp-18. Tetrahedron Lett. 2001, 42, 6295–6297. [Google Scholar] [CrossRef]
- Chen, B.S.; Yang, L.H.; Ye, J.L.; Huang, T.; Ruan, Y.P.; Fu, J.; Huang, P.Q. Diastereoselective synthesis and bioactivity of long-chain anti-2-amino-3-alkanols. Eur. J. Med. Chem. 2011, 46, 5480–5486. [Google Scholar] [CrossRef]
- Jiménez, C.; Crews, P. Novel marine sponge amino acids, 10.1 xestoaminols from Xestospongia sp. J. Nat. Prod. 1990, 53, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Dorta, G.; Martín, V.S.; Padrón, J.M. Synthesis and antiproliferative activity of glutamic acid-based dipeptides. Amino Acids 2015, 47, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.I.; Nigawara, Y.; Nishino, E.; Takagi, I.; Maeda, K.; Tadano, K.I.; Ogawa, S. Stereoselective total syntheses of (−)-desoxoprosopinine and (−)-desoxoprosophylline: Palladium(O)-catalyzed intramolecular N-alkylation for the key piperidine ring formation. Tetrahedron 1994, 50, 5681–5704. [Google Scholar] [CrossRef]
- Ayanwuyi, L.O.; Yaro, A.H.; Abodunde, O.M. Analgesic and anti-inflammatory effects of the methanol stem bark extract of Prosopis africana. Pharm. Biol. 2010, 48, 296–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konishi, T.; Satsu, H.; Hatsugai, Y.; Aizawa, K.; Inakuma, T.; Nagata, S.; Sakuda, S.H.; Nagasawa, H.; Shimizu, M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. Br. J. Pharmacol. 2004, 143, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Liaw, C.C.; Lin, M.K.; Chen, C.J.; Chao, C.L.; Chao, C.H.; Kuo, Y.H.; Chiu, Y.P.; Peng, Y.S.; Huang, H.C. Anti-influenza virus activity and chemical components from the parasitic plant Cuscuta japonica choisy on Dimocarpus longans Lour. Molecules 2020, 25, 4427. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Reichek, N.; Willenbrock, R.; Zannad, F.; Phillips, R.A.; Roniker, B.; Kleiman, J.; Krause, S.; Burns, D.; Williams, G.H. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: The 4E-left ventricular hypertrophy study. Circulation 2003, 108, 1831–1838. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Brown, N.J. Eplerenone: Cardiovascular protection. Circulation 2003, 107, 2512–2518. [Google Scholar] [CrossRef]
- Xiao, J.; Shimada, M.; Liu, W.; Hu, D.; Matsumori, A. Anti-inflammatory effects of eplerenone on viral myocarditis. Eur. J. Heart Fail. 2009, 11, 349–353. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Poloniae Pharmaceutica. Drug Res. 2010, 67, 3–12. [Google Scholar]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lameula-Raventos, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Nagarani, N.; Kumaraguru, A.K. Chemical characterization, temperature stability, and enzymatic studies on edible marine algae Kappaphycus alvarezii (Doty). J. Aquat. Food Prod. Technol. 2012, 21, 480–492. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.P.T.; Kang, C.H.; Park, S.Y.; Choi, Y.H.; Moon, D.O.; Kim, G.Y. Methanol extract of Polyopes lancifolius inhibits the expression of pro-inflammatory mediators in LPS-stimulated BV2 microglia cells via downregulation of the NF-κB pathway. Trop. J. Pharm. Res. 2012, 11, 43–50. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Kang, M.C.; Lee, W.W.; Lee, H.S.; Kamada, T.; Vairappan, C.S.; Jeon, Y.J. 5β-Hydroxypalisadin B isolated from red alga Laurencia snackeyi attenuates inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Algae 2014, 29, 333–341. [Google Scholar] [CrossRef]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Anti-inflammatory effect of Petalonia binghamiae in LPS-induced macrophages is mediated by suppression of iNOS and COX-2. Int. J. Agric. Biol. 2010, 12, 754–758. [Google Scholar]
- Sumayya, S.; Lubaina, A.; Murugan, K. Suppression of pro-inflammatory cytokines and mediators via the inhibition of the NF-κB in LPS-induced RAW 264.7 macrophage cells by purified terpenoid extract from Hypnea musciformis (Wulfen) J V Lamouroux. J. Adv. Sci. Res. 2020, 11, 84–91. [Google Scholar]
- Ryan, S.; O’Gorman, D.M.; Nolan, Y.M. Evidence that the marine-derived multi-mineral aquamin has anti-inflammatory effects on cortical glial-enriched cultures. Phytother. Res. 2011, 25, 765–767. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Jang, B.C.; Paik, J.H.; Kim, S.P.; Shin, D.H.; Song, D.K.; Park, J.G.; Suh, M.H.; Park, J.W.; Suh, S.I. Catalase induced expression of inflammatory mediators via activation of NF-κB, PI3K/AKT, p70S6K, and JNKs in BV2 microglia. Cell. Signal. 2005, 17, 25–633. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.W.; Jayawardena, T.U.; Cha, S.-H.; Jeon, Y.J. Dieckol, an algae-derived phenolic compound, suppresses airborne particulate matter-induced skin aging by inhibiting the expressions of pro-inflammatory cytokines and matrix metalloproteinases through regulating NF-κB, AP-1, and MAPKs signaling pathways. Food Chem. Toxicol. 2020, 146, 111823. [Google Scholar] [CrossRef]
- Lee, A.K.; Sung, S.H.; Kim, Y.C.; Kim, S.G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-a and COX-2 expression by sauchinone effects on I-kBα, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003, 139, 11–20. [Google Scholar] [CrossRef]
- Jing, H. Black garlic: Processing, composition change, and bioactivity. eFood 2020, 1, 242–246. [Google Scholar] [CrossRef]
- Jha, V.; Kauloorkar, S.V.; Pradeep, K. Stereoselective approach to 2,6-disubstituted piperidin-3-ol: Synthesis of (–)-deoxoprosopinine and (+)-deoxoprosophylline. Eur. J. Org. Chem. 2014, 2014, 4897–4902. [Google Scholar] [CrossRef]
- Sasmita, A.O.; Ling, A.P.K.; Voon, K.G.L.; Koh, R.Y.; Wong, Y.P. Madecassoside activates anti-neuroinflammatory mechanisms by inhibiting lipopolysaccharide-induced microglial inflammation. Int. J. Mol. Med. 2018, 41, 3033–3040. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.W.; Chen, F. Fucoxanthinol from the diatom Nitzschia laevis ameliorates neuroinflammatory responses in lipopolysaccharide-stimulated BV-2 microglia. Mar. Drugs 2020, 18, 116. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, Y.H.; Kim, Y.R.; Choi, J.J.; Yang, C.; Jang, I.S.; Lee, M.Y. Antineuroinflammatory and neuroprotective effects of Gyejibokryeong-Hwan in lipopolysaccharide-stimulated BV2 microglia. Evid. Based Complement. Altern. Med. 2019, 2019, 7585896. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y.T. Erythronium japonicum alleviates inflammatory pain by inhibiting MAPK activation and by suppressing NF-kB activation via ERK/Nrf2/HO-1 signaling pathway. Antioxidants 2020, 9, 626. [Google Scholar] [CrossRef] [PubMed]







| No | Compound Name | Formula | Chemical Structure | m/z | Mass | Bioactivity | References |
|---|---|---|---|---|---|---|---|
| 1. | 2,6-Nonadien-1-ol | C9H16O | 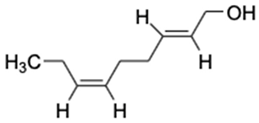 | 158.154 | 140.1201 | Key aroma-active compound that contributes fresh flavors to black garlic | [29] |
| 2. | Alanyl-Proline | C8H14N2O3 | 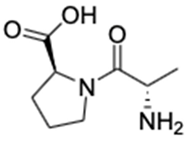 | 187.1075 | 186.1003 | Inhibitor of human cyclophilin hCyp-18 | [30] |
| 3. | Xestoaminol C | C14H31NO |  | 230.2478 | 229.2405 | Antitumor activity, antimicrobial activity and antiparasitic activity | [31,32] |
| 4. | Glutamyl-Proline | C10H16N2O5 | 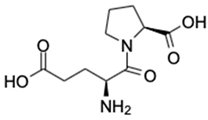 | 245.1133 | 244.1059 | Antitumor activity | [33] |
| 5. | Prosopinine | C16H33NO3 |  | 288.2535 | 287.2463 | Anaesthetic activity; antibiotic, analgesic and anti-inflammatory activity | [34,35] |
| 6. | 1-Monopalmitin | C19H38O4 | 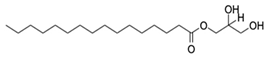 | 331.284 | 330.2768 | Antitumor activity, antiviral activity | [36,37] |
| 7. | Eplerenone | C24H30O6 | 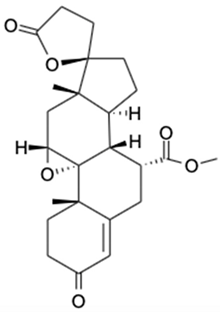 | 415.2124 | 414.2048 | Reduced mortality and morbidity in patients with acute myocardial infarction; reduced blood pressure; antiinflammatory activity | [38,39,40,41] |
| mRNA Species | Primer Sequence | Reference |
|---|---|---|
| iNOS | 5′-TTGCCACGGACGAGACGGATAGG-3′ 5′-GGGCACATGCAAGGAAGGGAACTC-3′ | [60] |
| COX-2 | 5′-TGCTGGTGGAAAAACCTCGT-3′ 5′-GGTGCTCGGCTTCCAGTATT-3′ | [60] |
| TNF-α | 5′-GAAAAGCAAGCAGCCAACCA-3′ 5′-CGGATCATGCTTTCTGTGCTC-3′ | [61] |
| IL-1β | 5′-GCTGAAAGCTCTCCACCTCA-3′ 5′-AGGCCACAGGTATTTTGTCG-3′ | [62] |
| IL-6 | 5′-GAGGATACCACTCCCAACAGACC-3′ 5′-AAGTGCATCATCGTTGTTCATACA-3′ | [62] |
| GAPDH | 5′-GGAGCGAGACCCCACTAACAT-3′ 5′-GTGAGTTGTCATATTTCTCGTGG-3′ | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, N.J.-Y.; Ngu, E.-L.; Pang, J.-R.; Wong, K.-H.; Ardianto, C.; Ming, L.C.; Lim, S.-H.; Walvekar, S.G.; Anwar, A.; Yow, Y.-Y. Carrageenophyte Kappaphycus malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-κB and ERK Signaling Pathways. Mar. Drugs 2022, 20, 534. https://doi.org/10.3390/md20080534
Lai NJ-Y, Ngu E-L, Pang J-R, Wong K-H, Ardianto C, Ming LC, Lim S-H, Walvekar SG, Anwar A, Yow Y-Y. Carrageenophyte Kappaphycus malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-κB and ERK Signaling Pathways. Marine Drugs. 2022; 20(8):534. https://doi.org/10.3390/md20080534
Chicago/Turabian StyleLai, Nicole Jean-Yean, Ee-Ling Ngu, Jun-Rui Pang, Kah-Hui Wong, Chrismawan Ardianto, Long Chiau Ming, Siew-Huah Lim, Shweta Gangasa Walvekar, Ayaz Anwar, and Yoon-Yen Yow. 2022. "Carrageenophyte Kappaphycus malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-κB and ERK Signaling Pathways" Marine Drugs 20, no. 8: 534. https://doi.org/10.3390/md20080534
APA StyleLai, N. J.-Y., Ngu, E.-L., Pang, J.-R., Wong, K.-H., Ardianto, C., Ming, L. C., Lim, S.-H., Walvekar, S. G., Anwar, A., & Yow, Y.-Y. (2022). Carrageenophyte Kappaphycus malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-κB and ERK Signaling Pathways. Marine Drugs, 20(8), 534. https://doi.org/10.3390/md20080534