Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential
Abstract
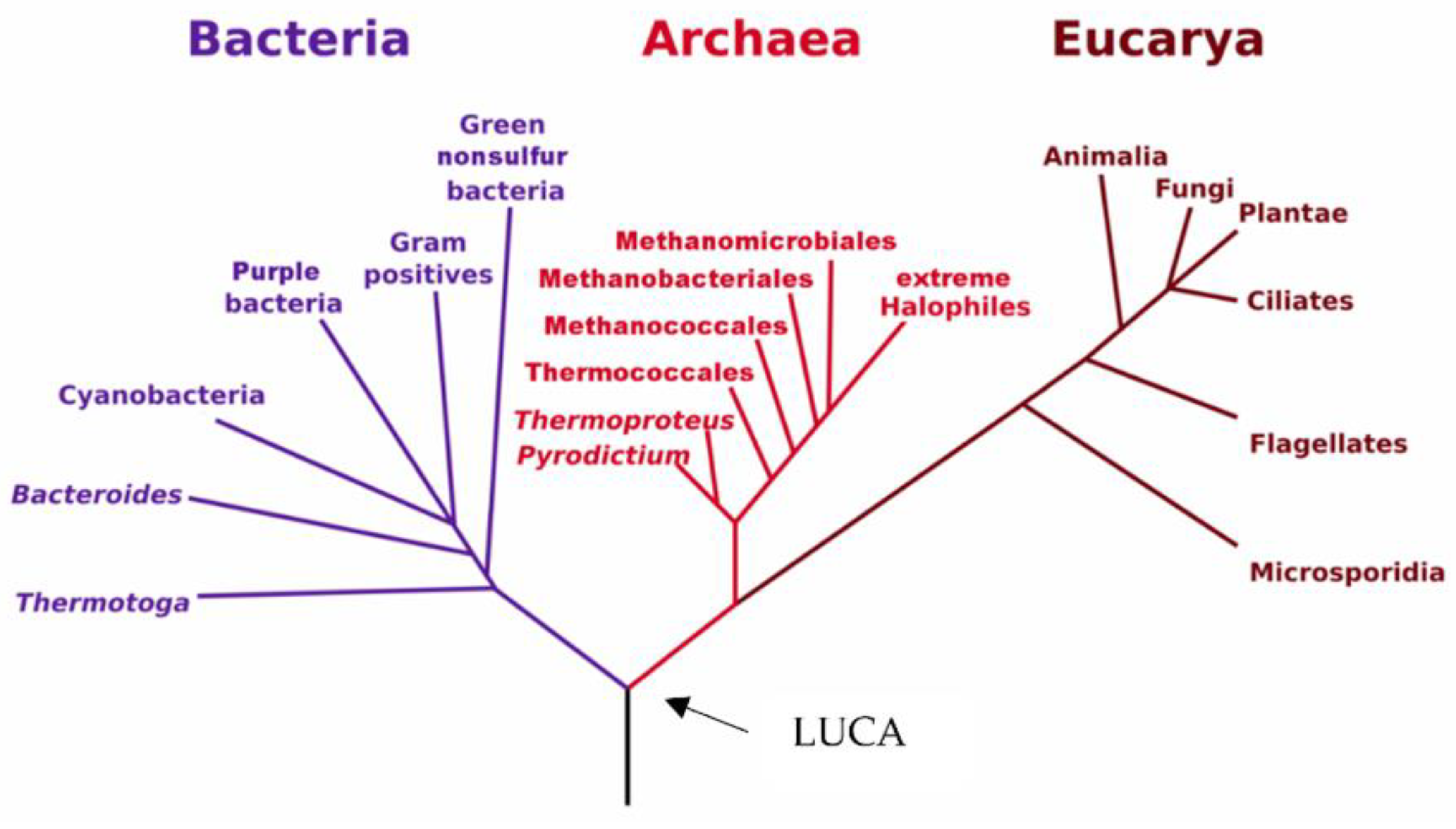
1. Discovery of Archaea
2. Ecology and Classification of Archaea
3. Cell Biology and Biochemistry of Archaea
4. Archaea Pigments: Bacteriorhodopsin and Carotenoids
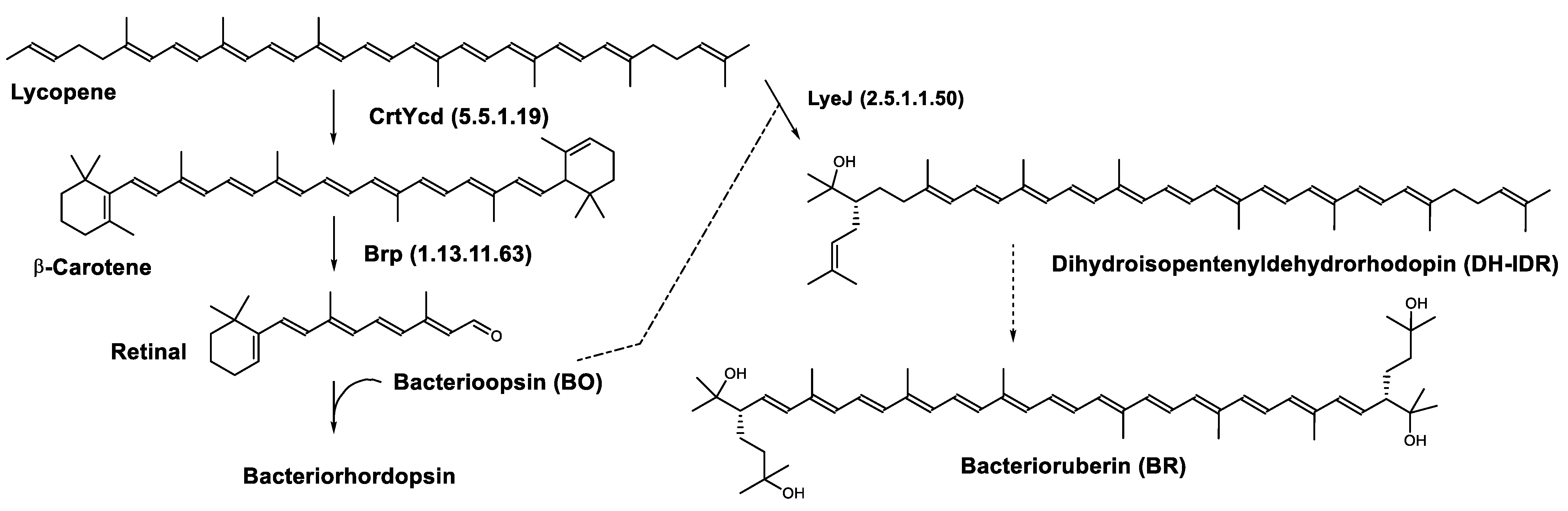
4.1. Bacteriorhodopsin
4.2. Archaea Carotenoids
4.2.1. Overview of the Structure and Function of Carotenoids
4.2.2. Ecophysiological Function of Carotenoids in Archaea
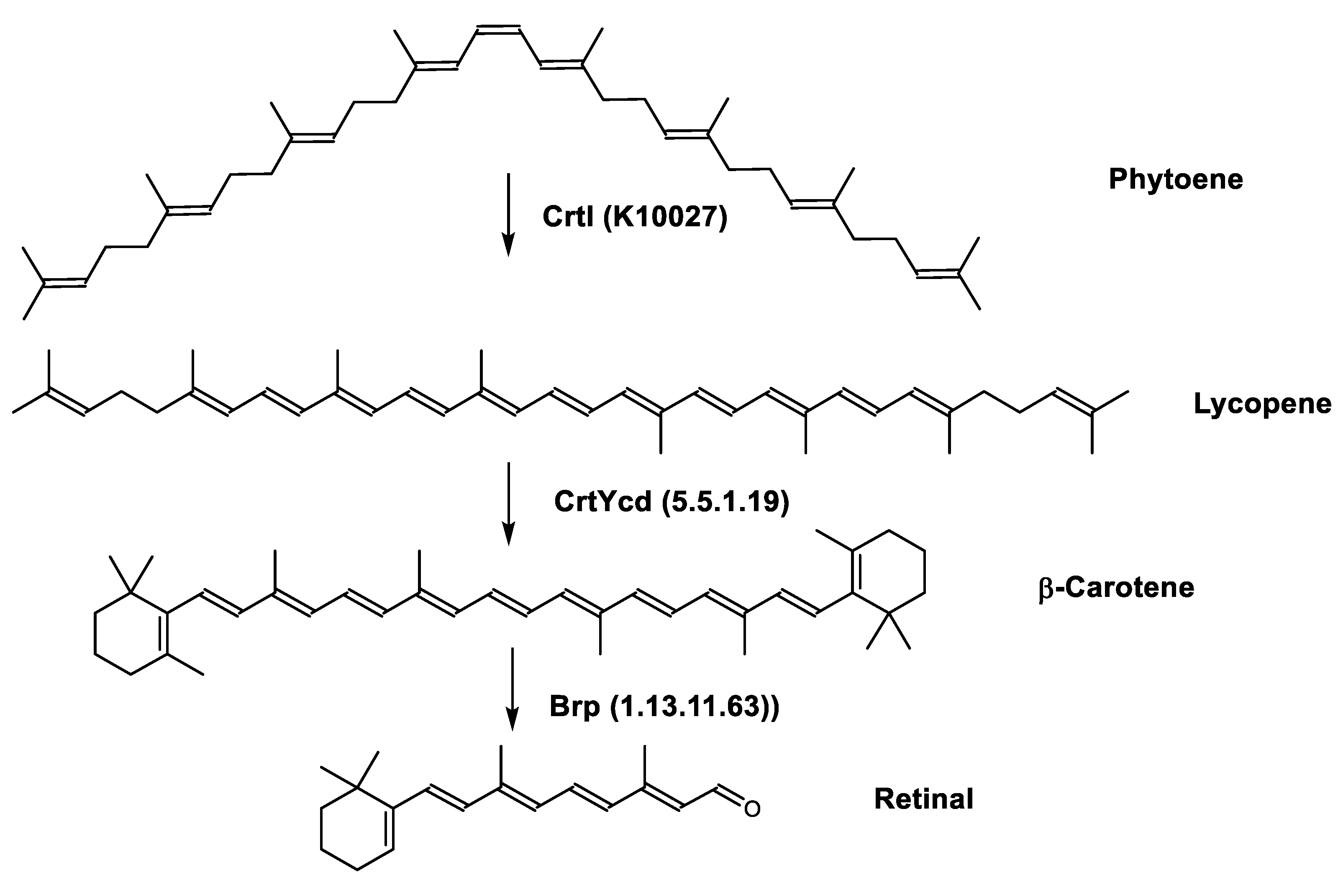
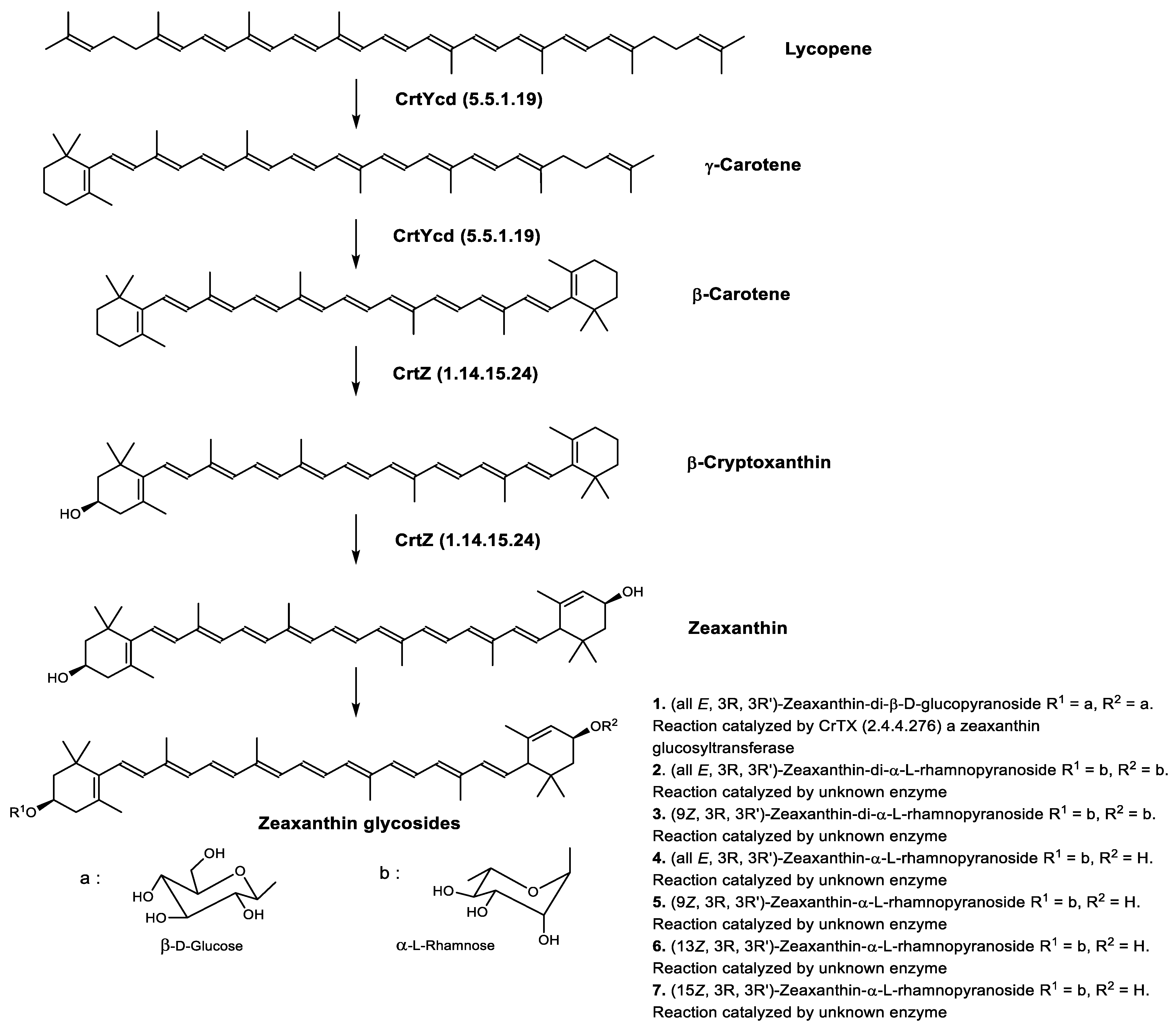
4.2.3. Focus on Archaea Carotenoids Structures, Biosynthesis Pathways and Archaea Producing Species
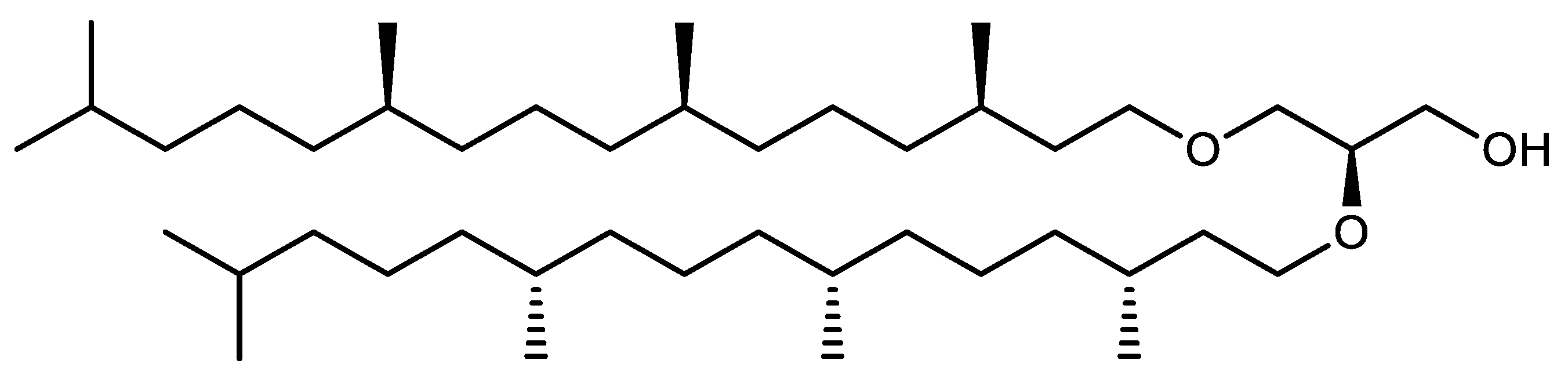
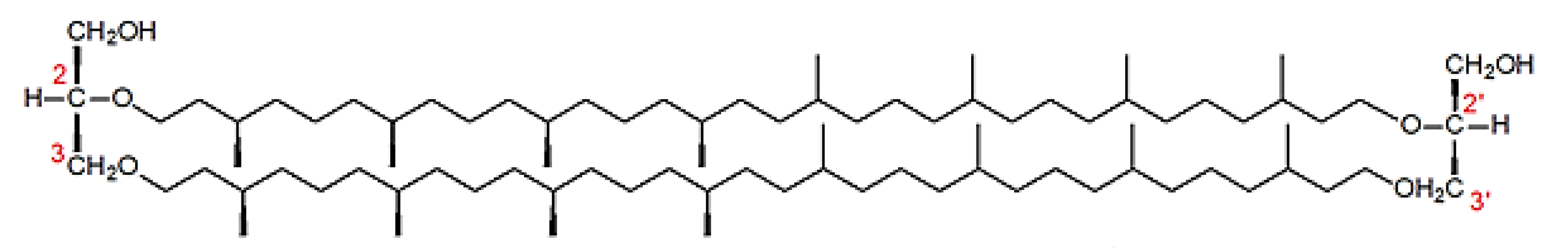
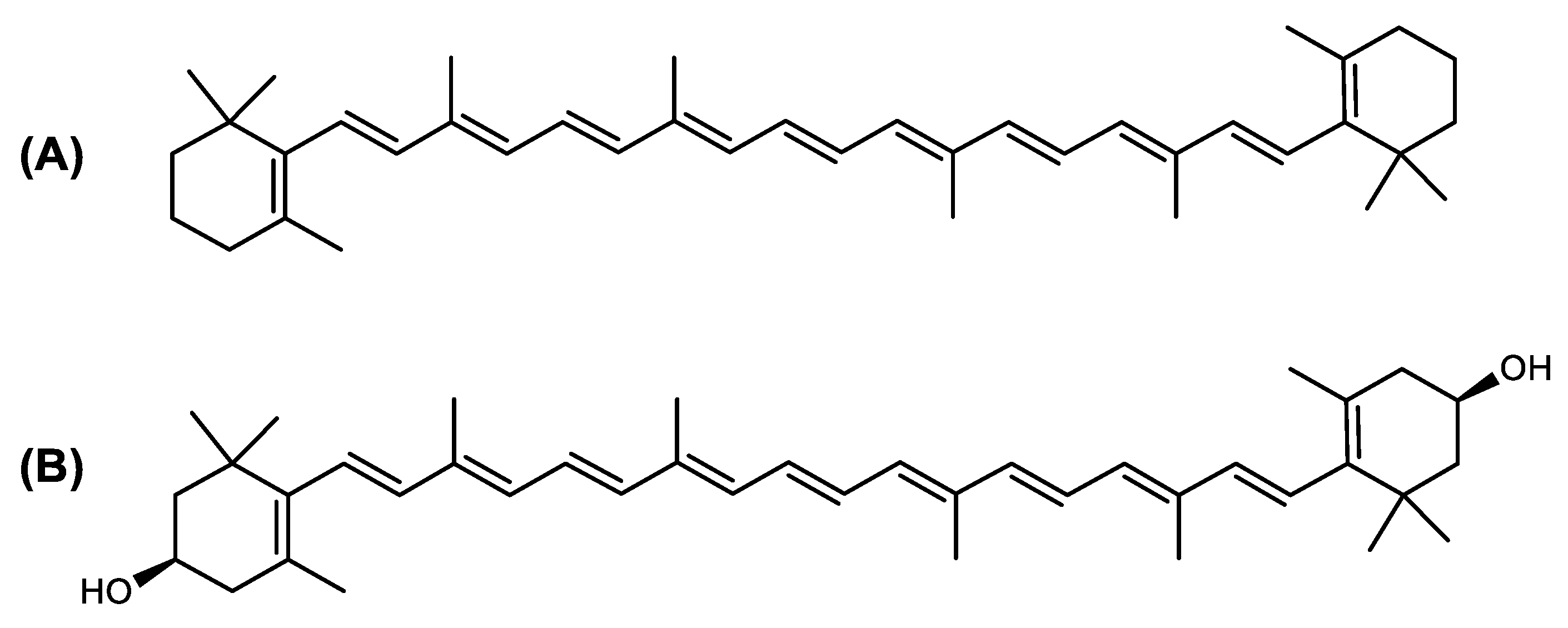
Structure of Archaea Carotenoids
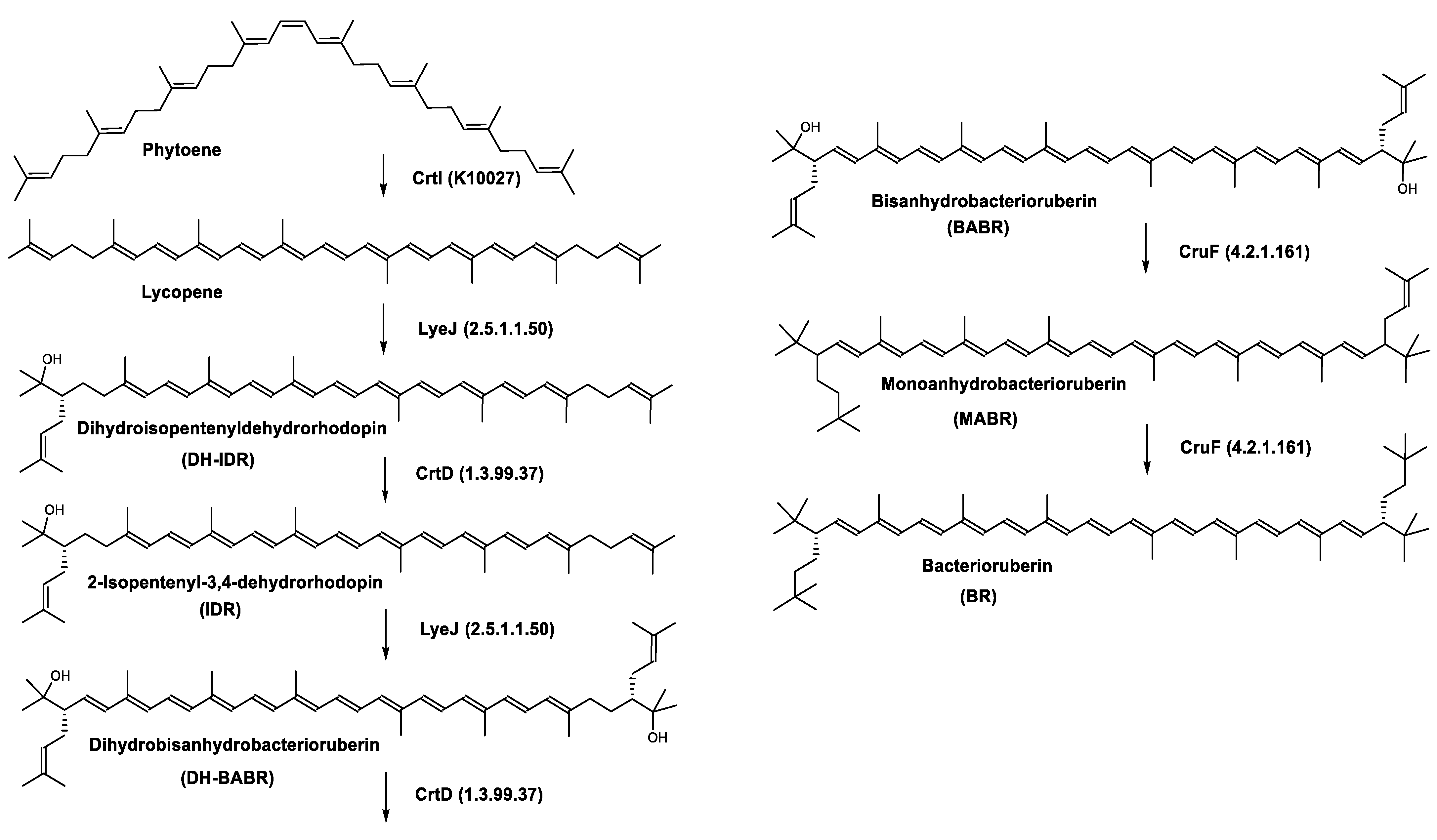
Biosynthesis of Carotenoids in Archaea
Carotenoids Biosynthesis in Haloarcula japonica
Regulation of the Bacterioruberin Synthesis
Carotenoids Biosynthesis in Sulfolobus shibatae
4.2.4. Biological Activities of Archaea Carotenoids
4.2.5. Biotechnological Considerations for the Production of Archaea Carotenoids
4.3. Focus on Bacterioruberin Biological Activities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a Natural System of Organisms: Proposal for the Domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.M.; Zolghadr, B.; Driessen, A.J.M.; Albers, S.-V.; Jarrell, K.F. Cell Surface Structures of Archaea. J. Bacteriol. 2008, 190, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- De Lourdes Moreno, M.; Sánchez-Porro, C.; García, M.T.; Mellado, E. Carotenoids’ Production from Halophilic Bacteria. In Microbial Carotenoids from Bacteria and Microalgae; Barredo, J.-L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 892, pp. 207–217. ISBN 978-1-61779-878-8. [Google Scholar]
- Alvares, J.J.; Furtado, I.J. Characterization of Multicomponent Antioxidants from Haloferax Alexandrinus GUSF-1 (KF796625). 3 Biotech. 2021, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E. A Square Bacterium. Nature 1980, 283, 69–71. [Google Scholar] [CrossRef]
- Oren, A. Life at High Salt Concentrations. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 421–440. ISBN 978-3-642-30122-3. [Google Scholar]
- Cavicchioli, R. Cold-Adapted Archaea. Nat. Rev. Microbiol. 2006, 4, 331–343. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Siddiqui, K.S. Cold-Adapted Enzymes. In Enzyme Technology; Pandey, A., Webb, C., Soccol, C.R., Larroche, C., Eds.; Springer: New York, NY, USA, 2006; pp. 615–638. ISBN 978-1-4419-2124-6. [Google Scholar]
- Makarova, K.S.; Yutin, N.; Bell, S.D.; Koonin, E.V. Evolution of Diverse Cell Division and Vesicle Formation Systems in Archaea. Nat. Rev. Microbiol. 2010, 8, 731–741. [Google Scholar] [CrossRef]
- Rosenshine, I.; Mevarech, M. Isolation and Partial Characterization of Plasmids Found in Three Halobacterium Volcanii Isolates. Can. J. Microbiol. 1989, 35, 92–95. [Google Scholar] [CrossRef]
- Forterre, P. The Origin of DNA Genomes and DNA Replication Proteins. Curr. Opin. Microbiol. 2002, 5, 525–532. [Google Scholar] [CrossRef]
- Huang, L. Unveiling the Beauty of Archaea. Sci. China Life Sci. 2012, 55, 375–376. [Google Scholar] [CrossRef][Green Version]
- Lattuati, A.; Guezennec, J.; Metzger, P.; Largeau, C. Lipids of Thermococcus Hydrothermalis, an Archaea Isolated from a Deep-Sea Hydrothermal Vent. Lipids 1998, 33, 319–326. [Google Scholar] [CrossRef]
- Pond, J.L.; Langworthy, T.A.; Holzer, G. Long-Chain Diols: A New Class of Membrane Lipids from a Thermophilic Bacterium. Science 1986, 231, 1134–1136. [Google Scholar] [CrossRef]
- Kornprobst, J.-M. Substances Naturelles D’origine Marine: Chimiodiversité, Pharmacodiversité, Biotechnologie; Lavoisier: Paris, France, 2005; ISBN 978-2-7430-0721-8. [Google Scholar]
- Danon, A.; Stoeckenius, W. Photophosphorylation in Halobacterium halobium. Proc. Natl. Acad. Sci. USA 1974, 71, 1234–1238. [Google Scholar] [CrossRef]
- Koch, M.K.; Oesterhelt, D. MpcT Is the Transducer for Membrane Potential Changes in Halobacterium Salinarum: MpcT Is the Transducer for ΔΨ Changes in Halobacterium salinarum. Mol. Microbiol. 2005, 55, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like Protein from the Purple Membrane of Halobacterium Halobium. Nat. New Biol. 1971, 233, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kandori, H. Ion-Pumping Microbial Rhodopsins. Front. Mol. Biosci. 2015, 2, 52. [Google Scholar] [CrossRef]
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and Animal Rhodopsins: Structures, Functions, and Molecular Mechanisms. Chem. Rev. 2014, 114, 126–163. [Google Scholar] [CrossRef]
- Spudich, J.L.; Yang, C.-S.; Jung, K.-H.; Spudich, E.N. Retinylidene Proteins: Structures and Functions from Archaea to Humans. Annu. Rev. Cell Dev. Biol. 2000, 16, 365–392. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chang, H.N.; Um, Y.S.; Hong, S.H. Bacteriorhodopsin Production by Cell Recycle Culture of Halobacterium Halobium. Biotechnol. Lett. 1998, 20, 763–765. [Google Scholar] [CrossRef]
- Oren, A. Molecular Ecology of Extremely Halophilic Archaea and Bacteria. FEMS Microbiol. Ecol. 2002, 39, 1–7. [Google Scholar] [CrossRef]
- Wagner, N.L.; Greco, J.A.; Ranaghan, M.J.; Birge, R.R. Directed Evolution of Bacteriorhodopsin for Applications in Bioelectronics. J. R. Soc. Interface 2013, 10, 20130197. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Yin, J.-J.; Subczynski, W.K.; Hyde, J.S.; Kusumi, A. Pulse EPR Detection of Lipid Exchange between Protein-Rich Raft and Bulk Domains in the Membrane: Methodology Development and Its Application to Studies of Influenza Viral Membrane. Biophys. J. 2001, 80, 738–748. [Google Scholar] [CrossRef]
- Hampp, N.; Oesterhelt, D. Bacteriorhodopsin and Its Potential in Technical Applications. In Nanobiotechnology; Niemeyer, C.M., Mirkin, C.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 146–167. ISBN 978-3-527-60245-2. [Google Scholar]
- Puthenveetil, R.; Vinogradova, O. Optimization of the Design and Preparation of Nanoscale Phospholipid Bilayers for Its Application to Solution NMR: Nanodiscs and NMR. Proteins 2013, 81, 1222–1231. [Google Scholar] [CrossRef]
- Knoblauch, C.; Griep, M.; Friedrich, C. Recent Advances in the Field of Bionanotechnology: An Insight into Optoelectric Bacteriorhodopsin, Quantum Dots, and Noble Metal Nanoclusters. Sensors 2014, 14, 19731–19766. [Google Scholar] [CrossRef] [PubMed]
- Grout, M.J. Application of Bacteriorhodopsin for Optical Limiting Eye Protection Filters. Opt. Mater. 2000, 14, 155–160. [Google Scholar] [CrossRef]
- Kahya, N.; Brown, D.A.; Schwille, P. Raft Partitioning and Dynamic Behavior of Human Placental Alkaline Phosphatase in Giant Unilamellar Vesicles. Biochemistry 2005, 44, 7479–7489. [Google Scholar] [CrossRef]
- Dummer, A.M.; Bonsall, J.C.; Cihla, J.B.; Lawry, S.M.; Johnson, G.C.; Peck, R.F. Bacterioopsin-Mediated Regulation of Bacterioruberin Biosynthesis in Halobacterium Salinarum. J. Bacteriol. 2011, 193, 5658–5667. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.V.; Premaraban, T.; Berthoumieu, O.; Watts, A.; Davis, J.J. Engineered Bacteriorhodopsin: A Molecular Scale Potential Switch. Chem. Eur. J. 2012, 18, 5632–5636. [Google Scholar] [CrossRef]
- Gagez, A.-L.; Thiery, V.; Pasquet, V.; Cadoret, J.-P.; Picot, L. Epoxycarotenoids and Cancer. Review. Bioact. Compd. 2012, 8, 109–141. [Google Scholar] [CrossRef]
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef]
- de Oliveira-Júnior, R.G.; Grougnet, R.; Bodet, P.-E.; Bonnet, A.; Nicolau, E.; Jebali, A.; Rumin, J.; Picot, L. Updated Pigment Composition of Tisochrysis Lutea and Purification of Fucoxanthin Using Centrifugal Partition Chromatography Coupled to Flash Chromatography for the Chemosensitization of Melanoma Cells. Algal Res. 2020, 51, 102035. [Google Scholar] [CrossRef]
- De Oliveira, R.G., Jr.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; da Silva Almeida, J.R.G.; Ferraz, C.A.A.; Picot, L. Bixin, an Apocarotenoid Isolated from Bixa Orellana, L., Sensitizes Human Melanoma Cells to Dacarbazine-Induced Apoptosis through ROS-Mediated Cytotoxicity. Food Chem. Toxicol. 2019, 125, 549–561. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.G., Jr.; Adrielly, A.F.C.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of Tumor Cells to Chemotherapy by Natural Products: A Systematic Review of Preclinical Data and Molecular Mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Juin, C.; Oliveira, R.G.D., Jr.; Fleury, A.; Oudinet, C.; Pytowski, L.; Bérard, J.-B.; Nicolau, E.; Thiéry, V.; Lanneluc, I.; Beaugeard, L.; et al. Zeaxanthin from Porphyridium Purpureum Induces Apoptosis in Human Melanoma Cells Expressing the Oncogenic BRAF V600E Mutation and Sensitizes Them to the BRAF Inhibitor Vemurafenib. Rev. Bras. Farmacogn. 2018, 28, 457–467. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella Tertiolecta Extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef]
- Haguet, Q.; Bonnet, A.; Bérard, J.-B.; Goldberg, J.; Joguet, N.; Fleury, A.; Thiéry, V.; Picot, L. Antimelanoma Activity of Heterocapsa Triquetra Pigments. Algal Res. 2017, 25, 207–215. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.-Y.; Kwan, H.-Y. Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer. Mar. Drugs 2022, 20, 370. [Google Scholar] [CrossRef]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, Metabolite of Fucoxanthin, Improves Obesity-Induced Inflammation in Adipocyte Cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef]
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of Carotenoids from the Extremely Halophilic Archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 100. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gemello, E.; Gammone, M.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Gammone, M.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Martin, L. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids of Biotechnological Importance. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Germany, 2014; Volume 148, pp. 449–467. ISBN 978-3-319-20106-1. [Google Scholar]
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Rao, A.; Rao, L. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.M.; Canela-Garayoa, R. Analytical Tools for the Analysis of Carotenoids in Diverse Materials. J. Chromatogr. A 2012, 1224, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jehlička, J.; Oren, A. Raman Spectroscopy in Halophile Research. Front. Microbiol. 2013, 4, 380. [Google Scholar] [CrossRef]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of Carotenoids with High Antioxidant Capacity Produced by Extremophile Microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Lutnaes, B.F.; Oren, A.; Liaaen-Jensen, S. New C(40)-Carotenoid Acyl Glycoside as Principal Carotenoid in Salinibacter Ruber, an Extremely Halophilic Eubacterium. J. Nat. Prod. 2002, 65, 1340–1343. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Oren, A. Bacterioruberin and Salinixanthin Carotenoids of Extremely Halophilic Archaea and Bacteria: A Raman Spectroscopic Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 99–103. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment: Carotenoids: Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H.; Truscott, T.G. The Interaction of Dietary Carotenoids with Radical Species. Arch. Biochem. Biophys. 2001, 385, 13–19. [Google Scholar] [CrossRef]
- Bayr, H. Reactive Oxygen Species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in Cancer Apoptosis—The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. The Microbiology of Red Brines. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 113, pp. 57–110. ISBN 978-0-12-820709-3. [Google Scholar]
- Wang, X.-D.; Russell, R.M. Procarcinogenic and Anticarcinogenic Effects of β-Carotene. Nutr. Rev. 2009, 57, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and Pro-Oxidant Activities of Carotenoids and Their Oxidation Products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From Molecule to Function. Mol. Nutr. Food Res. 2017, 61, 1600469. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and Carotenoid Conversion Products in Adipose Tissue Biology and Obesity: Pre-Clinical and Human Studies. Biochim. Biophys. Acta Mol. Cell Biol Lipids 2020, 1865, 158676. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Baños, M.; Montero, Z.; Torregrosa-Crespo, J.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaea: A Promising Biosource for Carotenoid Production. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; Volume 1261, pp. 165–174. ISBN 9789811573590. [Google Scholar]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.; Martínez-Espinosa, R. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Ramos-Merchante, A.; Fuentes, J.; Sayago, A.; Fernández-Recamales, Á.; Martínez-Espinosa, R.; Vega, J.; Vílchez, C.; Garbayo, I. Optimization of Growth and Carotenoid Production by Haloferax Mediterranei Using Response Surface Methodology. Mar. Drugs 2018, 16, 372. [Google Scholar] [CrossRef]
- Kelly, M.; Jensen, S.L.; Theander, O.; Cyvin, S.J.; Hagen, G. Bacterial Carotenoids. XXVI. C50-Carotenoids. 2. Bacterioruberin. Acta Chem. Scand. 1967, 21, 2578–2580. [Google Scholar] [CrossRef]
- Lazrak, T.; Wolff, G.; Albrecht, A.-M.; Nakatani, Y.; Ourisson, G.; Kates, M. Bacterioruberins Reinforce Reconstituted Halobacterium Lipid Membranes. Biochim. Biophys. Acta Biomembr. 1988, 939, 160–162. [Google Scholar] [CrossRef]
- Shahmohammadi, H.R.; Asgarani, E.; Terato, H.; Saito, T.; Ohyama, Y.; Gekko, K.; Yamamoto, O.; Ide, H. Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium Salinarium against DNA-Damaging Agents. J. Radiat. Res. 1998, 39, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-J.; Ku, K.-L.; Lee, M.-H.; Su, N.-W. Influence of Nutritive Factors on C50 Carotenoids Production by Haloferax Mediterranei ATCC 33500 with Two-Stage Cultivation. Bioresour. Technol. 2010, 101, 6487–6493. [Google Scholar] [CrossRef]
- Will Chen, C.; Hsu, S.; Lin, M.-T.; Hsu, Y. Mass Production of C50 Carotenoids by Haloferax Mediterranei in Using Extruded Rice Bran and Starch under Optimal Conductivity of Brined Medium. Bioprocess. Biosyst. Eng. 2015, 38, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a Protection Mechanism against Oxidative Stress in Haloferax Mediterranei. Antioxidants 2020, 9, 1060. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as Modulators of Lipid Membrane Physical Properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Yoshimura, K.; Kouyama, T. Structural Role of Bacterioruberin in the Trimeric Structure of Archaerhodopsin-2. J. Mol. Biol. 2008, 375, 1267–1281. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Pugh, E.L.; Kramer, J.K.; Kates, M. Isolation and Identification of Dehydrosqualene and C 40 -Carotenoid Pigments in Halobacterium Cutirubrum. Biochim. Biophys. Acta 1972, 260, 492–506. [Google Scholar] [CrossRef]
- Yang, Y.; Yatsunami, R.; Ando, A.; Miyoko, N.; Fukui, T.; Takaichi, S.; Nakamura, S. Complete Biosynthetic Pathway of the C50 Carotenoid Bacterioruberin from Lycopene in the Extremely Halophilic Archaeon Haloarcula japonica. J. Bacteriol. 2015, 197, 1614–1623. [Google Scholar] [CrossRef]
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Maleki Zanjani, B.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of Carotenoid Production by Halorubrum sp. TBZ126; an Extremely Halophilic Archeon from Urmia Lake. Adv. Pharm. Bull. 2014, 4, 61–67. [Google Scholar] [CrossRef]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the Extreme Halophilic Archaeon Haloterrigena Turkmenica: Identification and Antioxidant Activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Kull, D.R.; Pfander, H. Isolation and Structure Elucidation of Carotenoid Glycosides from the Thermoacidophilic Archaea Sulfolobus shibatae. J. Nat. Prod. 1997, 60, 371–374. [Google Scholar] [CrossRef]
- Sui, L.; Liu, L.; Deng, Y. Characterization of Halophilic C50 Carotenoid-Producing Archaea Isolated from Solar Saltworks in Bohai Bay, China. Chin. J. Ocean. Limnol. 2014, 32, 1280–1287. [Google Scholar] [CrossRef]
- Strand, A.; Shivaji, S.; Liaaen-Jensen, S. Bacterial Carotenoids 55. C50-Carotenoids 25.† Revised Structures of Carotenoids Associated with Membranes in Psychrotrophic Micrococcus Roseus. Biochem. Syst. Ecol. 1997, 25, 547–552. [Google Scholar] [CrossRef]
- Arpin, N.; Fiasson, J.-L.; Norgård, S.; Borch, G.; Liaaen-Jensen, S. Bacterial Carotenoids. XLVI. C50-Carotenoids. 14. C50-Carotenoids from Arthrobacter Glacialis. Acta Chem. Scand. 1975, 29b, 921–926. [Google Scholar] [CrossRef]
- El-Sayed, W.S.M.; Takaichi, S.; Saida, H.; Kamekura, M.; Abu-Shady, M.; Seki, H.; Kuwabara, T. Effects of Light and Low Oxygen Tension on Pigment Biosynthesis in Halobacterium Salinarum, Revealed by a Novel Method to Quantify Both Retinal and Carotenoids. Plant. Cell Physiol. 2002, 43, 379–383. [Google Scholar] [CrossRef]
- Ronnekleiv, M. Bacterial Carotenoids 53∗ C50-Carotenoids 23; Carotenoids of Haloferax Volcanii versus Other Halophilic Bacteria. Biochem. Syst. Ecol. 1995, 23, 627–634. [Google Scholar] [CrossRef]
- Bidle, K.A.; Hanson, T.E.; Howell, K.; Nannen, J. HMG-CoA Reductase Is Regulated by Salinity at the Level of Transcription in Haloferaxvolcanii. Extremophiles 2007, 11, 49–55. [Google Scholar] [CrossRef]
- Horikoshi, K.; Aono, R.; Nakamura, S. The Triangular Halophilic ArchaebacteriumHaloarcula Japonica Strain TR-1. Experientia 1993, 49, 497–502. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant Activities of Carotenes and Xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Peck, R.F.; Johnson, E.A.; Krebs, M.P. Identification of a Lycopene β-Cyclase Required for Bacteriorhodopsin Biogenesis in the Archaeon Halobacterium salinarum. J. Bacteriol. 2002, 184, 2889–2897. [Google Scholar] [CrossRef]
- Peck, R.F.; Echavarri-Erasun, C.; Johnson, E.A.; Ng, W.V.; Kennedy, S.P.; Hood, L.; DasSarma, S.; Krebs, M.P. Brp and Blh Are Required for Synthesis of the Retinal Cofactor of Bacteriorhodopsin in Halobacterium Salinarum. J. Biol. Chem. 2001, 276, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Peck, R.F.; Pleşa, A.M.; Graham, S.M.; Angelini, D.R.; Shaw, E.L. Opsin-Mediated Inhibition of Bacterioruberin Synthesis in Halophilic Archaea. J. Bacteriol. 2017, 199, e00303-17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shand, R.F.; Betlach, M.C. Expression of the Bop Gene Cluster of Halobacterium Halobium Is Induced by Low Oxygen Tension and by Light. J. Bacteriol. 1991, 173, 4692–4699. [Google Scholar] [CrossRef]
- Yang, C.F.; DasSarma, S. Transcriptional Induction of Purple Membrane and Gas Vesicle Synthesis in the Archaebacterium Halobacterium Halobium Is Blocked by a DNA Gyrase Inhibitor. J. Bacteriol 1990, 172, 4118–4121. [Google Scholar] [CrossRef]
- Baliga, N.S.; Kennedy, S.P.; Ng, W.V.; Hood, L.; DasSarma, S. Genomic and Genetic Dissection of an Archaeal Regulon. Proc. Natl. Acad. Sci. USA 2001, 98, 2521–2525. [Google Scholar] [CrossRef]
- Tarasov, V.Y.; Besir, H.; Schwaiger, R.; Klee, K.; Furtwängler, K.; Pfeiffer, F.; Oesterhelt, D. A Small Protein from the Bop-Brp Intergenic Region of Halobacterium Salinarum Contains a Zinc Finger Motif and Regulates Bop and CrtB1 Transcription: Zinc Finger Regulator of Bop and CrtB1 Expression. Mol. Microbiol. 2008, 67, 772–780. [Google Scholar] [CrossRef]
- Ourisson, G.; Nakatani, Y. Bacterial Carotenoids as Membrane Reinforcers: A General Role for Polyterpenoids: Membrane Stabilization. In Carotenoids Chemistry and Biology; Krinsky, N.I., Mathews-Roth, M.M., Taylor, R.F., Eds.; Springer: Boston, MA, USA, 1989; pp. 237–246. [Google Scholar] [CrossRef]
- Yasushi, K. New Trends in Photobiology. J. Photochem. Photobiol. B Biol. 1991, 9, 265–280. [Google Scholar] [CrossRef]
- de Las Rivas, J.; Abadía, A.; Abadía, J. A New Reversed Phase-HPLC Method Resolving All Major Higher Plant Photosynthetic Pigments. Plant. Physiol. 1989, 91, 190–192. [Google Scholar] [CrossRef]
- Moshell, A.N.; Bjornson, L. Photoprotection in Erythropoietic Protoporphyria: Mechanism of Photoprotection by Beta Carotene. J. Investig. Dermatol. 1977, 68, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Iwasaki-Kino, Y.; Aizawa, K.; Terao, J.; Mukai, K. Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method Using a Microplate Reader. J. AOAC Int. 2016, 99, 193–197. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a Green Algal Carotenoid, as a Novel Functional Compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, K.; Tsimilli-Michael, M.; Papageorgiou, G.C. On the Question of the Light-Harvesting Role of β-Carotene in Photosystem II and Photosystem I Core Complexes. Plant. Physiol. Biochem. 2014, 81, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological Activity of Lycopene Metabolites: Implications for Cancer Prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Mukai, K. Antioxidant Activity of Foods: Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Nutr. Sci. Vitam. 2019, 65, 285–302. [Google Scholar] [CrossRef]
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.; Mukai, K. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Carotenoids and Food Extracts in Solution. Development of a Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Agric. Food Chem. 2010, 58, 9967–9978. [Google Scholar] [CrossRef]
- Aizawa, K.; Iwasaki, Y.; Ouchi, A.; Inakuma, T.; Nagaoka, S.; Terao, J.; Mukai, K. Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method. 2. Measurements of the SOAC Values for Carotenoids and Food Extracts. J. Agric. Food Chem. 2011, 59, 3717–3729. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Murakosh, M.; Ii, T.; Takemura, M.; Kuchide, M.; Kanazawa, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; et al. Carotenoids in Cancer Chemoprevention. Cancer Metastasis Rev. 2002, 21, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory Effect of Natural Carotenoids on Epstein-Barr Virus Activation Activity of a Tumor Promoter in Raji Cells. A Screening Study for Anti-Tumor Promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Cooney, R.V.; Bertram, J.S. Carotenoids Up-Regulate Connexin43 Gene Expression Independent of Their Provitamin A or Antioxidant Properties. Cancer Res. 1992, 52, 5707–5712. [Google Scholar] [PubMed]
- Chew, B.P.; Park, J.S. Carotenoid Action on the Immune Response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Gohto, Y.; Gellermann, W.; Ermakov, I.V.; Sasano, H.; Seto, T.; Bernstein, P.S. Skin Carotenoid Index in a Large Japanese Population Sample. Sci. Rep. 2019, 9, 9318. [Google Scholar] [CrossRef]
- Nagao, A.; Olson, J.A. Enzymatic Formation of 9-Cis, 13-Cis, and All-Trans Retinals from Isomers of Beta-Carotene. FASEB J. 1994, 8, 968–973. [Google Scholar] [CrossRef]
- Eroglu, A.; Harrison, E.H. Carotenoid Metabolism in Mammals, Including Man: Formation, Occurrence, and Function of Apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730. [Google Scholar] [CrossRef]
- Srinivasan, M.; Devipriya, N.; Kalpana, K.B.; Menon, V.P. Lycopene: An Antioxidant and Radioprotector against γ-Radiation-Induced Cellular Damages in Cultured Human Lymphocytes. Toxicology 2009, 262, 43–49. [Google Scholar] [CrossRef]
- Méndez-Robles, M.D.; Permady, H.H.; Jaramillo-Flores, M.E.; Lugo-Cervantes, E.C.; Cardador-Martínez, A.; Canales-Aguirre, A.A.; López-Dellamary, F.; Cerda-García-Rojas, C.M.; Tamariz, J. C-26 and C-30 Apocarotenoids from Seeds of Ditaxis Heterantha with Antioxidant Activity and Protection against DNA Oxidative Damage. J. Nat. Prod. 2006, 69, 1140–1144. [Google Scholar] [CrossRef]
- Ford, N.; Elsen, A.; Zuniga, K.; Lindshield, B.; Erdman, J. Lycopene and Apo-12′-Lycopenal Reduce Cell Proliferation and Alter Cell Cycle Progression in Human Prostate Cancer Cells. Nutr. Cancer 2011, 63, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Lu, I.-H.; Chen, H.-Y.; Hu, M.-L. Lycopene Inhibits the Proliferation of Androgen-Dependent Human Prostate Tumor Cells through Activation of PPARγ-LXRα-ABCA1 Pathway. J. Nutr. Biochem. 2012, 23, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, I.-S.; Lee, D.G. Damage to the Cytoplasmic Membrane and Cell Death Caused by Lycopene in Candida Albicans. J. Microbiol. Biotechnol. 2007, 17, 1797–1804. [Google Scholar]
- Saito, T.; Miyabe, Y.; Ide, H.; Yamamoto, O. Hydroxyl Radical Scavenging Ability of Bacterioruberin. Radiat. Phys. Chem. 1997, 50, 267–269. [Google Scholar] [CrossRef]
- Kottemann, M.; Kish, A.; Iloanusi, C.; Bjork, S.; DiRuggiero, J. Physiological Responses of the Halophilic Archaeon Halobacterium sp. Strain NRC1 to Desiccation and Gamma Irradiation. Extremophiles 2005, 9, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.; Binder, M.; von Lintig, J.; Wiens, M.; Schröder, H.C. Differential Expression of the Demosponge (Suberites Domuncula) Carotenoid Oxygenases in Response to Light: Protection Mechanism Against the Self-Produced Toxic Protein (Suberitine). Mar. Drugs 2012, 10, 177–199. [Google Scholar] [CrossRef]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.; Ohta, Y. Lipids of Haloferax Alexandrinus Strain TMT: An Extremely Halophilic Canthaxanthin-Producing Archaeon. J. Biosci. Bioeng. 2002, 93, 37–43. [Google Scholar] [CrossRef]
- Gambacorta, A.; Gliozzi, A.; De Rosa, M. Archaeal Lipids and Their Biotechnological Applications. World J. Microbiol. Biotechnol. 1995, 11, 115–131. [Google Scholar] [CrossRef]
- Nicolaus, B.; Moriello, V.S.; Lama, L.; Poli, A.; Gambacorta, A. Polysaccharides from Extremophilic Microorganisms. Orig. Life Evol. Biosph. 2004, 34, 159–169. [Google Scholar] [CrossRef]
- D’Souza, S.E.; Altekar, W.; D’Souza, S.F. Adaptive Response of Haloferax Mediterranei to Low Concentrations of NaCl (<20%) in the Growth Medium. Arch. Microbiol. 1997, 168, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M. Optimization of Total Carotenoid Production by Halorubrum sp. TBZ126 Using Response Surface Methodology. Microb. Biochem. Technol. 2014, 06, 286–294. [Google Scholar] [CrossRef]
- Van der Oost, J.; Ciaramella, M.; Moracci, M.; Pisani, F.M.; Rossi, M.; de Vos, W.M. Molecular Biology of Hyperthermophilic Archaea. In Biotechnology of Extremophiles; Antranikian, G., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1998; Volume 61, pp. 87–115. ISBN 978-3-540-63817-9. [Google Scholar]
- Patel, G.B.; Sprott, G.D. Archaeobacterial Ether Lipid Liposomes (Archaeosomes) as Novel Vaccine and Drug Delivery Systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef] [PubMed]
- Quillaguamán, J.; Guzmán, H.; Van-Thuoc, D.; Hatti-Kaul, R. Synthesis and Production of Polyhydroxyalkanoates by Halophiles: Current Potential and Future Prospects. Appl. Microbiol. Biotechnol. 2010, 85, 1687–1696. [Google Scholar] [CrossRef]
- Chen, G.-Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Patel, M.K. Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef]
- Kirk, R.G.; Ginzburg, M. Ultrastructure of Two Species of Halobacterium. J. Ultrastruct. Res. 1972, 41, 80–94. [Google Scholar] [CrossRef]
- Legault, B.A.; Lopez-Lopez, A.; Alba-Casado, J.C.; Doolittle, W.F.; Bolhuis, H.; Rodriguez-Valera, F.; Papke, R.T. Environmental Genomics of “Haloquadratum Walsbyi” in a Saltern Crystallizer Indicates a Large Pool of Accessory Genes in an Otherwise Coherent Species. BMC Genom. 2006, 7, 171. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of Various Archae- and Eubacterial Strains as Industrial Polyhydroxyalkanoate Producers from Whey. Macromol. Biosci. 2007, 7, 218–226. [Google Scholar] [CrossRef]
- Han, J.; Lu, Q.; Zhou, L.; Zhou, J.; Xiang, H. Molecular Characterization of the PhaEC Hm Genes, Required for Biosynthesis of Poly(3-Hydroxybutyrate) in the Extremely Halophilic Archaeon Haloarcula marismortui. Appl. Environ. Microbiol. 2007, 73, 6058–6065. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Q.; Zhao, W.; Wang, H.; Xu, D. Newly Isolated Archaerhodopsin from a Strain of Chinese Halobacteria and Its Proton Pumping Behavior. Biochim. Biophys. Acta Biomembr. 2000, 1466, 260–266. [Google Scholar] [CrossRef]
- Feng, J.; Liu, H.-C.; Chu, J.-F.; Zhou, P.-J.; Tang, J.-A.; Liu, S.-J. Genetic Cloning and Functional Expression in Escherichia Coli of an Archaerhodopsin Gene from Halorubrum Xinjiangense. Extremophiles 2006, 10, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ding, X.; Peng, B.; Zhao, Y.; Ding, J.; Watts, A.; Zhao, X. Novel Expression and Characterization of a Light Driven Proton Pump Archaerhodopsin 4 in a Halobacterium Salinarum Strain. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological Properties of Carotenoids Extracted from Halobacterium Halobium Isolated from a Tunisian Solar Saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hou, J.; Cui, H.-L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as Precision Weapons in War against Cancer Chemotherapy Induced Toxicity—Exploring the Armoury of Obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Zalazar, L.; Pagola, P.; Miró, M.V.; Churio, M.S.; Cerletti, M.; Martínez, C.; Iniesta-Cuerda, M.; Soler, A.J.; Cesari, A.; De Castro, R. Bacterioruberin Extracts from a Genetically Modified Hyperpigmented Haloferax Volcanii Strain: Antioxidant Activity and Bioactive Properties on Sperm Cells. J. Appl. Microbiol. 2019, 126, 796–810. [Google Scholar] [CrossRef]
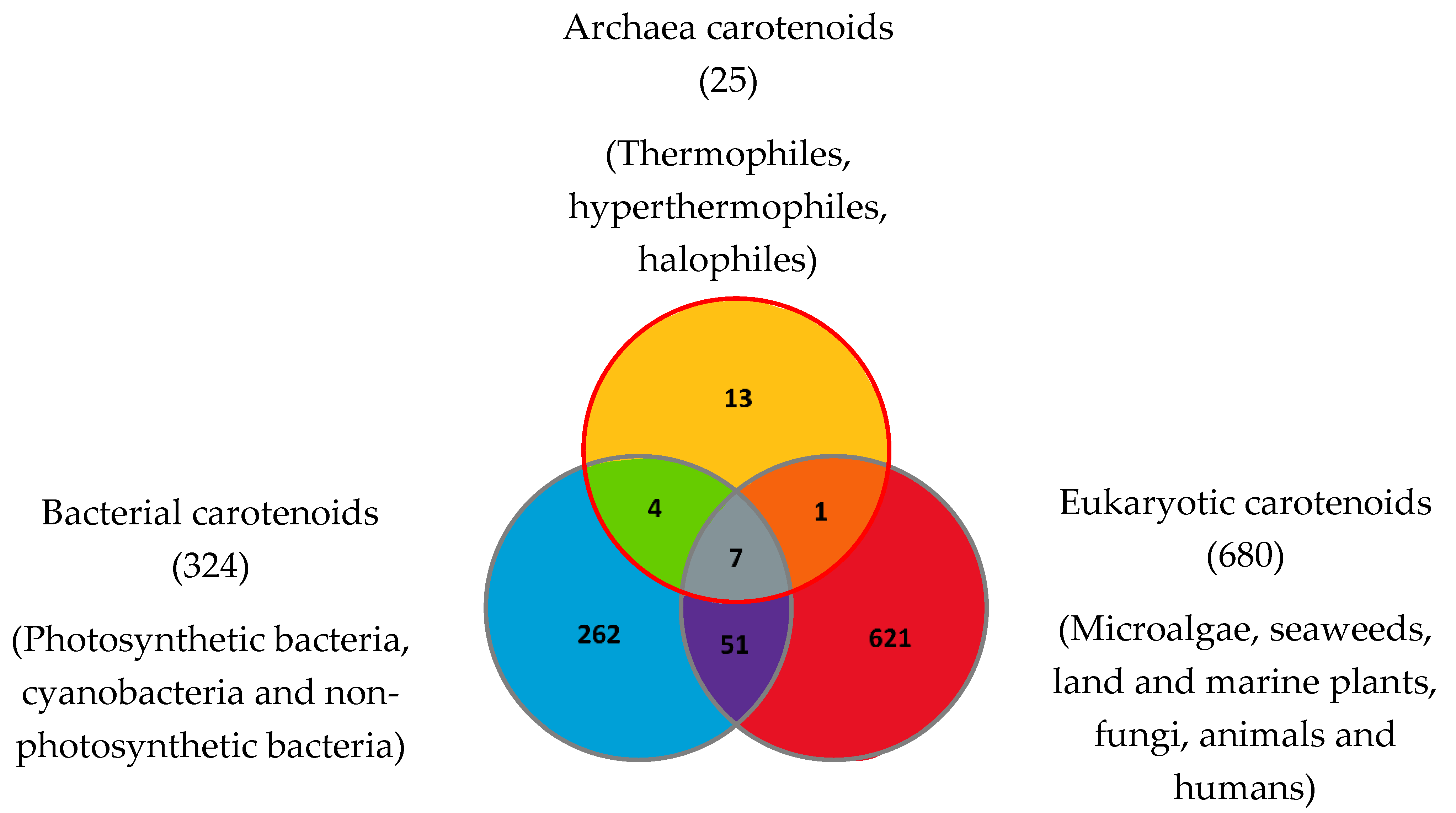
| Carotenoids or Apocarotenoids Present in the Three Domains of Life | Carotenoids Common to Archaea and Eukaryotes | Carotenoids Common to Archaea and Bacteria | Archaea-Specific Carotenoids |
|---|---|---|---|
| β-Carotene | (13Z)-β-Carotene (can also be found in bacteria from the photoisomerization of (all-E) -β-Carotene). | Bacterioruberin (BR) | Dihydrobisanhydrobacterioruberin |
| Dihydroisopentenyldehydrorhodopin | |||
| Lycopene or (all-E)-Lycopene | 3′,4′-Dihydromonoanhydrobacterioruberin | ||
| Bisanhydrobacterioruberin (BABR) | 1′,2′-Epoxy-2′-(2,3-epoxy-3-methylbutyl)-2-(3-hydroxy-3-methylbutyl)-3′,4′-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-caroten-1-ol | ||
| Phytoene or (15Z)-Phytoene | 2-Isopentenyl-3,4-dehydrorhodopin | ||
| 3,4,3′,4′-Tetrahydrobisanhydrobacterioruberin | |||
| Phytofluene or (15Z)-Phytofluene | Trisanhydrobacterioruberin | ||
| Monoanhydrobacterioruberin (MABR) | (9Z)-Zeaxanthin-3′-rhamnoside | ||
| (13Z)-Zeaxanthin-3′-rhamnoside | |||
| (15Z)-Zeaxanthin-3′-rhamnoside | |||
| (all-E)-Phytofluene | Zeaxanthin diglucoside | Zeaxanthin dirhamnoside | |
| (9Z)-Zeaxanthin dirhamnoside | |||
| Retinal or Vitamin A aldehyde (apocarotenoid) | Zeaxanthin monorhamnoside |
| Carotenoid Common Name | Chemical Name and Raw Formula | Structure | Archaea-Producing Species |
|---|---|---|---|
| C40 Hydrocarbons | |||
| Astaxanthin | 3,3′-Dihydroxy-β,β-carotene-4,4′-dione C40H52O4 | 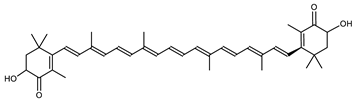 | Haloferax alexandrinus–Archaea: Euryarchaeota [4] |
| β-Carotene | β,β-carotene C40H56 | 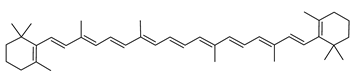 | Haloarcula japonica–Archaea: Euryarchaeota [87] Halobacterium cutirubrum–Archaea: Euryarchaeota [86] Halorubrum chaoviator Halo-G–Archaea: Euryarchaeota [88] |
| (13Z)-β-Carotene | (13Z)-β,β-carotene C40H56 | 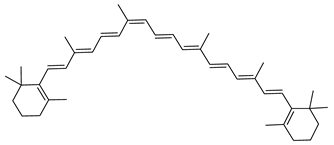 | Halobacterium cutirubrum–Archaea: Euryarchaeota [86] |
| Canthaxanthin | β,β-Carotene-4,4′-dione C40H52O2 |  | Haloferax alexandrinus–Archaea: Euryarchaeota [4] |
| Lycopene or (all-E)-Lycopene | ψ,ψ-carotene C40H56 |  | Haloarcula japonica–Archaea: Euryarchaeota [87] Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Halorubrum chaoviator Halo-G–Archaea: Euryarchaeota [88] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] |
| Phytoene or (15Z)-Phytoene | (15Z)-7,8,11,12,7′,8′,11′,12′-octahydro-ψ,ψ-carotene C40H64 | 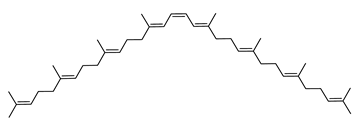 | Halobacterium cutirubrum–Archaea: Euryarchaeota [86] Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] |
| Phytofluene or (15Z)-Phytofluene | (15Z)-7,8,11,12,7′,8′-hexahydro-ψ,ψ-carotene C40H62 |  | Halobacterium cutirubrum–Archaea: Euryarchaeota [86] Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] |
| (all-E)-Phytofluene | 7,8,11,12,7′,8′-hexahydro-ψ,ψ-carotene C40H62 | 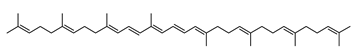 | Halobacterium cutirubrum–Archaea: Euryarchaeota [86] |
| Zeaxanthin diglucoside | (3R,3′R)-3,3′-di(β-d-glucopyranosyloxy)-β,β-carotene C52H76O10 | 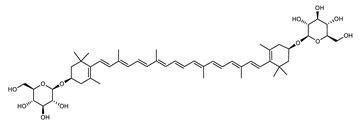 | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| (9Z)-Zeaxanthin-3′-Rhamnoside | (9Z,3R,3′R)-3′-(α-l-rhamnopyranosyloxy)-β,β-caroten-3-ol C46H66O6 |  | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| (13Z)-Zeaxanthin-3′-Rhamnoside | (13Z,3R,3′R)-3′-(α-l-rhamnopyranosyloxy)-β,β-caroten-3-ol C46H66O6 | 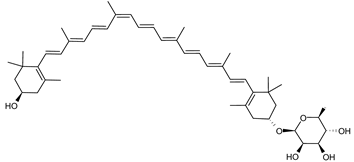 | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| (15Z)-Zeaxanthin-3′-Rhamnoside | (15Z,3R,3′R)-3′-(α-l-rhamnopyranosyloxy)-β,β-caroten-3-ol C46H66O6 | 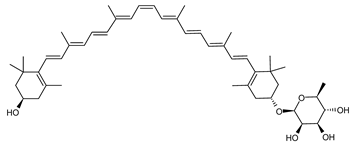 | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| Zeaxanthin dirhamnoside | (3R,3′R)-3,3′-di-(α-l-rhamnopyranosyloxy)-β,β-carotene C52H76O10 | 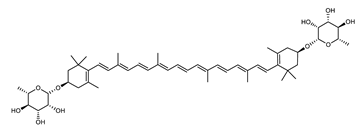 | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| (9Z)-Zeaxanthin dirhamnoside | (9Z,3R,3′R)-3,3′-di-(α-l-rhamnopyranosyloxy)-β,β-carotene C52H76O10 |  | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| Zeaxanthin monorhamnoside | (3R,3′R)-3′-(α-l-rhamnopyranosyloxy)-β,β-caroten-3-ol C46H66O6 |  | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| C45 Hydroxycarotenoids | |||
| Dihydroisopentenyldehydrorhodopin | (2S)-2-(3-methylbut-2-enyl)-1,2-dihydro-ψ,ψ-caroten-1-ol C45H66O |  | Haloarcula japonica–Archaea: Euryarchaeota [87] |
| 2-Isopentenyl-3,4-dehydrorhodopin | (2S)-2-(3-methylbut-2-enyl)-3,4-didehydro-1,2-dihydro-ψ,ψ-caroten-1-ol C45H64O | 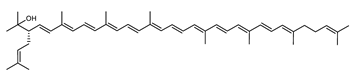 | Haloarcula japonica–Archaea: Euryarchaeota [87] |
| C50 Hydroxycarotenoids | |||
| Bacterioruberin (BR) | (2S,2′S)-2,2′-bis-(3-hydroxy-3-methylbutyl)-3,4,3′,4′-tetrahydro-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H76O4 | 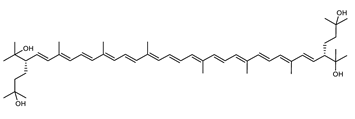 | Haloarcula japonica–Archaea: Euryarchaeota [87] Halobacterium salinarium–Archaea: Euryarchaeota [61] Halobacterium strain SP-2–Archaea: Euryarchaeota [91] Halococcus morrhuae–Archaea: Euryarchaeota [61] Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Halorubrum chaoviator Halo-G–Archaea: Euryarchaeota [88] Halorubrum strain SP-4–Archaea: Euryarchaeota [91] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] also present in Micrococcus roseus [92] |
| Bisanhydrobacterioruberin (BABR) | (2S,2′S)-2,2′-bis-(3-methylbut-2-enyl)-3,4,3′,4′-tetradehydro-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H72O2 | 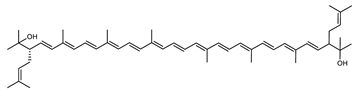 | Haloarcula japonica–Archaea: Euryarchaeota [87] Halobacterium salinarium–Archaea: Euryarchaeota [61] Halococcus morrhuae–Archaea: Euryarchaeota [61] Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] also present in Micrococcus roseus [92] and Arthrobacter glacialis [93] |
| Dihydrobisanhydrobacterioruberin (DH-BABR) | (2S,2′S)-2,2′-bis-(3-methylbut-2-enyl)-3,4-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H74O2 | 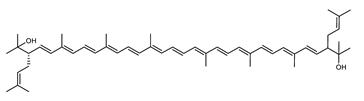 | Haloarcula japonica–Archaea: Euryarchaeota [87] |
| 3′,4′-Dihydromonoanhydrobacterioruberin | (2S,2′R)-2-(3-hydroxy-3-methylbutyl)-2′-(3-methylbut-2-enyl)-3,4-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H76O3 | 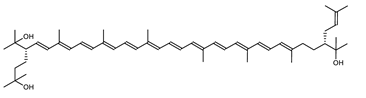 | Haloarcula japonica–Archaea: Euryarchaeota [87] |
| 1′,2′-Epoxy-2′-(2,3-epoxy-3-methylbutyl)-2-(3-hydroxy-3-methylbutyl)-3′,4′-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-caroten-1-ol | 1′,2′-epoxy-2′-(2,3-epoxy-3-methylbutyl)-2-(3-hydroxy-3-methylbutyl)-3′,4′-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-caroten-1-ol C50H74O4 |  | Halobacterium sp.–Archaea: Euryarchaeota |
| Haloxanthin | (2R,2′R)-2′-(3-Methylbut-2-enyl)-2-(3-methyl-1,3-peroxybutyl)-3,4-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H74O4 | 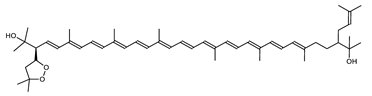 | Haloferax alexandrinus- Archaea: Euryarchaeota [4] |
| Monoanhydrobacterioruberin (MABR) | 2-(3-hydroxy-3-methylbutyl)-2′-(3-methylbut-2-enyl)-3,4,3′,4′-tetradehydro-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H74O3 |  | Haloarcula japonica–Archaea: Euryarchaeota [87] Halobacterium sp.–Archaea: Euryarchaeota Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] also present in Micrococcus roseus [92] |
| 3,4,3′,4′-Tetrahydrobisanhydrobacterioruberin | (2R,2′R)-bis-(3-methylbut-2-enyl)-1,2,1′,2′-tetrahydro-ψ,ψ-carotene-1,1′-diol C50H76O2 |  | Haloarcula japonica–Archaea: Euryarchaeota [87] |
| Trisanhydrobacterioruberin | 2,2′-bis(3-methylbut-2-enyl)-3,4,3′,4′-tetradehydro-1,2-dihydro-ψ, ψ-caroten-1-ol C50H70O | 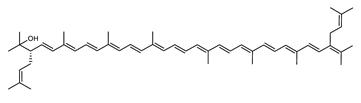 | Halobacterium salinarium–Archaea: Euryarchaeota [61] Halobacterium sp.–Archaea: Euryarchaeota Halococcus morrhuae–Archaea: Euryarchaeota [61] |
| Apocarotenoids | |||
| Retinal or Vitamin A aldehyde | 15-apo-β-caroten-15-al C20H28O | 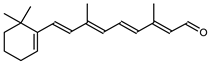 | Haloarcula japonica–Archaea: Euryarchaeota [87] Halobacterium salinarum–Archaea: Euryarchaeota [94] |
| Archaea Classification | Carotenoid Composition | Species or Genus Studied |
|---|---|---|
| Thermophiles and hyperthermophiles | β-Carotene Lycopene or (all-E)-Lycopene Phytoene or (15Z)-Phytoene (9Z)-Zeaxanthin-3′-Rhamnoside (13Z)-Zeaxanthin-3′-Rhamnoside (15Z)-Zeaxanthin-3′-Rhamnoside Zeaxanthin diglucoside (9Z)-Zeaxanthin dirhamnoside Zeaxanthin dirhamnoside Zeaxanthin monorhamnoside | Sulfolobus shibatae–Archaea: Crenarchaeota [90] |
| Halophiles | Astaxanthin Bacterioruberin (BR) β-Carotene (13Z)-β-Carotene Bisanhydrobacterioruberin (BABR) Canthaxanthin (all-E)-Phytofluene Dihydrobisanhydrobacterioruberin (DH-BABR) Dihydrobisanhydrobacterioruberin Dihydroisopentenyldehydrorhodopin 3′,4′-Dihydromonoanhydrobacterioruberin 1′,2′-Epoxy-2′-(2,3-epoxy-3-methylbutyl)-2-(3-hydroxy-3-methylbutyl)-3′,4′-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-caroten-1-ol Haloxanthin 3-Hydroxy-echinenone 2-Isopentenyl-3,4-dehydrorhodopin Lycopene or (all-E)-Lycopene Monoanhydrobacterioruberin (MABR) Phytoene or (15Z)-Phytoene Phytofluene or (15Z)-Phytofluene Retinal (apocarotenoid) 3,4,3′,4′-Tetrahydrobisanhydrobacterioruberin Trisanhydrobacterioruberin | Halobacterium cutirubrum–Archaea: Euryarchaeota [86] Haloarcula japonica–Archaea: Euryarchaeota [45,87] Haloferax alexandrinus–Archaea: Euryarchaeota [4] Halorubrum chaoviator Halo-G–Archaea: Euryarchaeota [88] Halococcus morrhuae–Archaea: Euryarchaeota [86] Haloferax alexandrinus GUSF-1–Archaea: Euryarchaeota [4] Haloterrigena turkmenica–Archaea: Euryarchaeota [89] Described in Haloferax volcanii [76,95,96] |
| Methanogens | Unknown | Methanogens are unstudied for their carotenoid and apocarotenoids composition. The group contains various genera such as Methanopyrus (both methanogenic and thermophilic), Methanosarcina, Methanomicrococcus and Methanosaeta |
| Psychrophiles | Unknown | Psychrophiles are unstudied for their carotenoid and apocarotenoids composition. Some examples of psychrophilic species are Methanococcoides burtonii and Methanogenium frigidum |
| Carotenogenesis Pathway after Phytoene Formation | Gene | Enzyme |
|---|---|---|
| Bacterioruberin biosynthetic pathway | CrtD (1.3.99.37) CruF (4.2.1.161) LyeJ (2.5.1.1.50) | 3,4-desaturase 2”,3”-hydratase Bifunctional lycopene elongase and 1,2-hydratase |
| Lycopene biosynthetic pathway | CrtB (2.5.1.32) CrtE (2.5.1.29) CrtI (K10027) FDPS (2.5.1.10) | 15-cis-phytoene synthase Geranylgeranyl diphosphate synthase Phytoene desaturase Farnesyl diphosphate synthase |
| Retinal biosynthetic pathway | Brp (1.13.11.63) CrtYcd (5.5.1.19) | β-carotene dioxygenase Lycopene β-cyclase |
| Zeaxanthin biosynthetic pathway | CrtYcd (5.5.1.19) CrtZ (1.14.15.24) | Lycopene β-cyclase β-carotene 3-hydroxylase |
| Common Name | Biological Activities and Properties |
|---|---|
| C40 Hydrocarbons | |
| β-Carotene | Photosynthetic pigment present in all organisms making oxygenic photosynthesis from cyanobacteria to higher plants [108,109] Photoprotective agent [110] Present in the reaction-center complexes (RC) and the light-harvesting complexes (LHC) of photosystem I (PSI) as well as the RC and the core LHC of photosystem II (PSII) [111,112,113] Provitamin A [114,115] Antioxidant—free radical scavenger/singlet oxygen quencher, 101 times stronger than that of α-tocopherol (SOAC value: 101) [110,111,116,117,118,119] Anti-apoptotic agent (mouse model of traumatic brain injury) preventing loss of Bcl2, preventing accumulation of Bax, and preventing accumulation or activation of Caspase 3 [120] β-carotene-derived retinoid acids bind with retinoid acid receptor (PAR) and retinoid X receptor. Receptors dimerization leads to a functional transcription factor regulating gene expression during neurogenesis. Neuroprotective activity against apoptosis [120] Anticarcinogenic activity [121,122] Cell differentiation and proliferation promoter by upregulating Connexin 43 gene [123] Immune response enhancement in animals and humans [124] Found in human skin throughout the epidermis, dermis and also the subcutaneous [125] |
| (13Z)-β-Carotene | Provitamin A activity (10% of that of all-trans-β-carotene) [126,127] |
| Lycopene or (all-E)-Lycopene | Photoprotection [114] Radioprotection against gamma-radiation-induced cellular damages [128] Strong antioxidant —strong singlet-quenching ability—141 times stronger than that of α-tocopherol (SOAC value: 141) [111,114,116,117,118,119] Protecting mitochondria and mitochondrial DNA by antioxidant properties and treatment with lycopene prevents loss of mitochondrial inner membrane potential during ROS challenge [120] Antiradical activity [129] Found in human skin throughout the epidermis, dermis and also the subcutaneous [125] Anticarcinogenic activity by reducing insulin growth factor 1 (IGF-1) stimulation with an increase in membrane-associated IGF-binding proteins; also slows down IGF-1-stimulated cell cycle progression [114,116,121,122,130] Inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARγ-LXRα-ABCA1 [131] Anti-apoptotic agent—preventing loss of Bcl2 and Bcl-xL, preventing accumulation of Bax, preventing accumulation or release of Cytochrome C, and preventing accumulation or activation of Caspase 3 [120] Anti-inflammation [114] Anti-inflammatory effects of lycopene may help alleviate neuropsychiatric diseases such as post-traumatic stress disorder and depression [120] Antimicrobial activity against S. aureus, and E. coli O-157 [132] Antifungal activity against C. albicans by arresting their cell cycle [132] Cell differentiation and proliferation promoter by upregulating Connexin 43 gene [123] Non-Provitamin A [114] |
| Phytoene | Anticarcinogenic activity [121] |
| Phytofluene | Anticarcinogenic activity—more active than β-carotene [122] |
| (all-E)-Phytofluene | No biological activity reported |
| Zeaxanthin diglucoside | No biological activity reported |
| (9Z)-Zeaxanthin-3′-rhamnoside | No biological activity reported |
| (13Z)-Zeaxanthin-3′-rhamnoside | No biological activity reported |
| (15Z)-Zeaxanthin-3′-rhamnoside | No biological activity reported |
| Zeaxanthin dirhamnoside | No biological activity reported |
| (9Z)-Zeaxanthin dirhamnoside | No biological activity reported |
| Zeaxanthin monorhamnoside | No biological activity reported |
| C45 Hydroxycarotenoids | |
| Dihydroisopentenyldehydrorhodopin (DH-IDR) | No biological activity reported |
| 2-Isopentenyl-3,4-dehydrorhodopin (IPR) | No biological activity reported |
| C50 Hydroxycarotenoids | |
| Bacterioruberin (BR) | Antioxidant activity—much better radical scavenger than that of β-carotene as it contains 13 pairs of conjugated double bonds [45,133] limits oxidation due to H2O2 exposure [80,134] Photoprotective activity—limits oxidative DNA damage from UV irradiation [80,134] Radio protective activity—limits oxidative DNA damage from gamma irradiation [80,134] |
| Bisanhydrobacterioruberin (BABR) | No biological activity reported |
| Dihydrobisanhydrobacterioruberin (DH-BABR) | No biological activity reported |
| 3′,4′-dihydromonoanhydrobacterioruberin | No biological activity reported |
| 1′,2′-epoxy-2′-(2,3-epoxy-3-methylbutyl)-2-(3-hydroxy-3-methylbutyl)-3′,4′-didehydro-1,2,1′,2′-tetrahydro-ψ,ψ-caroten-1-ol | No biological activity reported |
| Monoanhydrobacterioruberin (MABR) | No biological activity reported |
| 3,4,3′,4′-tetrahydrobisanhydrobacterioruberin | No biological activity reported |
| Trisanhydrobacterioruberin | No biological activity reported |
| Apocarotenoids | |
| Retinal or Vitamin A aldehyde | Photoreception in human retina Abolishes the function of the toxin suberitine at a stoichiometric ratio of 1:1 [135] Isomerized to 13Z-retinal under light, and isomerized back to all-trans retinal in the dark |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grivard, A.; Goubet, I.; Duarte Filho, L.M.d.S.; Thiéry, V.; Chevalier, S.; de Oliveira-Junior, R.G.; El Aouad, N.; Guedes da Silva Almeida, J.R.; Sitarek, P.; Quintans-Junior, L.J.; et al. Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Mar. Drugs 2022, 20, 524. https://doi.org/10.3390/md20080524
Grivard A, Goubet I, Duarte Filho LMdS, Thiéry V, Chevalier S, de Oliveira-Junior RG, El Aouad N, Guedes da Silva Almeida JR, Sitarek P, Quintans-Junior LJ, et al. Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Marine Drugs. 2022; 20(8):524. https://doi.org/10.3390/md20080524
Chicago/Turabian StyleGrivard, Antoine, Isabelle Goubet, Luiz Miranda de Souza Duarte Filho, Valérie Thiéry, Sylvie Chevalier, Raimundo Gonçalves de Oliveira-Junior, Noureddine El Aouad, Jackson Roberto Guedes da Silva Almeida, Przemysław Sitarek, Lucindo José Quintans-Junior, and et al. 2022. "Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential" Marine Drugs 20, no. 8: 524. https://doi.org/10.3390/md20080524
APA StyleGrivard, A., Goubet, I., Duarte Filho, L. M. d. S., Thiéry, V., Chevalier, S., de Oliveira-Junior, R. G., El Aouad, N., Guedes da Silva Almeida, J. R., Sitarek, P., Quintans-Junior, L. J., Grougnet, R., Agogué, H., & Picot, L. (2022). Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Marine Drugs, 20(8), 524. https://doi.org/10.3390/md20080524










