Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health
Abstract
1. Introduction
2. Bioactive Compounds of Sea Cucumbers and Their Antioxidant Activity
2.1. Antioxidant Potential of Sea Cucumber Phenolics and Their Beneficial Effects on Human Health
| Species | Body Parts | TPC (mg GAE/g) | TFC (mg RE/g) | Antioxidant Assays | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH (%) | ABTS (mg TE/g) | HRSA (mg TE/g) | MCA (mg EDTAE/g) | ORAC (mmol TE/g) | |||||
| Holothuria forskali | Dried sea cucumber (extract) | 3.19–5.21 | NA | NA | NA | NA | NA | NA | [34] |
| Holothuria forskali | Dried sea cucumber (hydroethanolic and aqueous extracts) | 0.48 | NA | 1.06 f | 18.83 b | NA | NA | NA | [35] |
| Holothuriaarguinensis | Dried sea cucumber (hydroethanolic and aqueous extracts) | 0.84 | NA | 0.13 f | 22.34 b | NA | NA | NA | [35] |
| Holothuriamammata | Dried sea cucumber (hydroethanolic and aqueous extracts) | 0.79 | NA | 0.31 f | 30.89 b | NA | NA | NA | [35] |
| Holothuria atra | Body wall (phosphate buffer extract) | NA | NA | 82 to 95 | NA | NA | NA | NA | [31] |
| Holothuria atra | Dried sea cucumber (extract) | Detected | Detected | NA | NA | NA | NA | NA | [36] |
| Holothuria arenicola | Body wall (phosphate buffer extract) | NA | NA | 82–95 | NA | NA | NA | NA | [32] |
| Holothuria scabra | Dried sea cucumber (hexanes, ethyl acetate, and n-butanol extracts) | 20–46.54 | NA | NA | NA | NA | NA | NA | [37] |
| Holothuria scabra | Dried sea cucumber (methanol extract) | 30.52 | NA | 33.77 c | NA | NA | NA | NA | [22] |
| Holothuria scabra | Sea cucumber without viscera (aqueous and organic extracts) | 1.53–4.85 | NA | NA | NA | NA | NA | NA | [21] |
| Holothuria scabra | Dried sea cucumber (extracts) | 2.02–2.86 | 0.35–2.49 e | [23] | |||||
| Holothuria leucospilota | Dried body wall (methanol, acetone, and water extracts) | 4.58 | 0.84 | NA | NA | NA | NA | [20] | |
| Holothuria leucospilota | Sea cucumber without viscera (aqueous and organic extracts) | 2.91–9.7 | NA | 3.91–5.44 | NA | NA | NA | NA | [21] |
| Cucumaria frondosa | Body wall (acetone extract) | 3.05–3.98 | 1.22–1.55 | 4.98–5.04 d | 7.51–8.01 | 10.47–10.65 | 0.41–0.53 | NA | [5] |
| Cucumaria frondosa | Viscera (acetone extract) | 2.32–3.02 | 1.01–1.24 a | 4.37–4.62 d | 7.36–7.87 | 9.57–9.85 | 0.29–0.44 | NA | [24] |
| Cucumaria frondosa | Tentacles/flowers (acetone extract) | 3.09 | 1.61 | 6.67 d | NA | NA | 0.55 | NA | [38] |
| Cucumaria frondosa | Fresh and dried sea cucumber with/without viscera (methanol extract) | 0.88–1.08 | NA | 4.51–7.48 b | NA | NA | NA | 2.09–2.6 | [19] |
| Cucumaria frondosa | Dried digestive tract, gonads, muscles, and respiratory apparatus (extract) | 0.22–2.36 | 0.029–0.59 | NA | NA | NA | 140–800 b | [25] | |
| Stichopus variegatus | Dried sea cucumber without viscera (aqueous extract) | 10.55–10.9 | NA | 1.67–2.3 c | NA | NA | NA | NA | [27] |
| Apostichopus japonicus | Dried internal organs (extract) | 13.6–116.90 | NA | NA | NA | NA | NA | NA | [39] |
| Apostichopus japonicus | Dried body wall (water and ethanol extracts) | 18.65–40.99 | 5.92–30.38 | 3.2–16.37 b | 0.83–1.5 b | NA | NA | NA | [28] |
| Apostichopus japonicus | Dried sea cucumber (methanol extract) | 3.53–20.37 | NA | NA | NA | NA | NA | NA | [40] |
| Stichopus chloronotus | Sea cucumber without viscera (aqueous and organic extracts) | 1.66–8.27 | NA | 2.13 c | NA | NA | NA | NA | [21] |
| Species | Body Parts | Identified Compounds (mg/100 g) | References |
|---|---|---|---|
| Cucumaria frondosa | Body wall | Protocatechuic acid (8.86), gallic acid (7.34),catechin (5.19), p-coumaric acid (5.11), epigallocatechin gallate (4.87), ellagic acid (4.55), hydroxygallic acid (3.9), p-hydroxybenzoic acid (3.66), p-coumaroyl glycolic acid (3.56), isoferulic acid (3.4), quercetin (3.35), p-hydroxybenzaldehyde (3.12), vanillic acid (3.04), cinnamic acid (2.9), syringic acid (2.51), myricetin (1.04), phlorizin (0.96), sinapinic acid (0.94), p-hydroxycoumarin (0.93), and caffeic acid (0.7) | [5] |
| Cucumaria frondosa | Viscera | Catechin (9.33), p-coumaric acid (7.15), protocatechuic acid (7.13), hydroxygallic acid (6.2), quercetin (5.48), gallic acid (5.66), chlorogenic acid (5.53), cinnamic acid (4.78), ellagic acid (4.33), syringic acid (4.23), p-hydroxybenzaldehyde (3.34), sinapinic acid (3.07), vanillic acid (3.05), caffeoyl glucoside (2.47), p-hydroxybenzoic acid (1.89), scopoletin (1.56), homovanillic acid (1.03), caffeic acid (1.01), p-coumaroyl glycolic acid (0.91), p-hydroxycoumarin (0.8), isoferulic acid (0.76), chicoric acid (0.73), and leachianol F (0.63) | [24] |
| Cucumaria frondosa | Tentacles/flower | Protocatechuic acid (6.91), catechin (6.32), gallic acid (6.14), p-coumaric acid (4.9), gallic acid monohydrate (4.46), quercetin (4.07), ellagic acid (3.86), cinnamic acid (3.35), sinapinic acid (2.56), syringic acid (2.51), p-hydroxybenzaldehyde (2.41), vanillic acid (2.4), chicoric acid (2.32), chlorogenic acid (2.25), caffeic acid (1.91), isoferulic acid (1.74), fraxin (1.64), kaempferol 3-O-glucoside (1.46), p-hydroxybenzoic acid (1.42), quercetin-3-O-arabinose (1.34), caffeoyl glucoside (1.05), epigallocatechin gallate (1.05), rosmarinic acid (1.05), scopoletin (1.04), homoveratric acid (1), ferulic acid (0.97), sinapine (0.97), homovanillic acid (0.97), p-coumaroyl glycolic acid (0.73), ferulic acid hexoside (0.72), and myricetin (0.2) | [38] |
| Holothuria atra | Body wall | Chlorogenic acid (80.34%), coumaric acid (2.43), pyrogallol (2.25%), and rutin (0.82) | [31] |
| Holothuria atra | Body wall | Chlorogenic acid (92.86%), pyrogallol (2.99%), rutin (1.83%), coumaric acid (1.55%), and catechin (0.51) | [30] |
| Holothuria arenicola | Body wall | Chlorogenic acid (89.66%), pyrogallol (1.88%), coumaric acid (1.23%), and rutin (1.06%) | [32] |
| Holothuria scabra | Dried sea cucumber | 3-Hydroxybenzaldehyde and 4-hydroxybenzaldehyde | [22] |
| Holothuria leucospilota | Body wall | 2,4-bis(1,1-dimethylethyl)-phenol | [20] |
| Holothuria tubulosa | Body wall | Epicatechin (790 µg/g), 2,5-dihydroxybenzoic acid (130.54–158.89 µg/g), ellagic acid (109.25–558.67 µg/g), gallic acid (133.16–205.87 µg/g)), chlorogenic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, cinnamic acid, rutin, naringin, and quercetin | [33] |
| Holothuria forskali | Digestive tract, muscle, body wall, gonad, and respiratory tree | Quinic acid (0.39–0.47 μg/mL), salvianolic acid (0.039–0.057 μg/mL), caffeoylquinic acid (0.13–0.14 μg/mL), caffeic acid, syringic acid, trans ferulic acid, o-coumaric acid, rosmarinic acid, and gallic acid | [29] |
2.2. Antioxidant Potential of Protein Hydrolysates and Peptides and Their Health Benefits
| Species | Body Parts | Protein Hydrolysates/Collagens/Peptides | Antioxidant Assays | References | ||||
|---|---|---|---|---|---|---|---|---|
| DPPH (%) | ABTS (µmol TE/g) | HRSA (%) | MCA (µmol EDTAE/g) | ORAC (µmol TE/g) | ||||
| Cucumaria frondosa | Body wall, tentacles, and internal organs | Protein hydrolysates using Alcalase, Corolase, and Flavourzyme | 7–14 a | 17.79–79.08 | NA | 16.5–37.43 | NA | [43] |
| Cucumaria frondosa | Viscera | Protein hydrolysates using Alcalase, Neutrase, trypsin, papain, bromelain, and Flavourzyme | 14.42 | NA | 27.04 | NA | NA | [46] |
| Cucumaria frondosa | Viscera | Protein hydrolysates using Alcalase | NA | NA | NA | NA | 421 | [48] |
| Isostichopus fuscus | Body wall | Protein hydrolysates and peptides using proteases | NA | NA | NA | NA | 0.00072 | [60] |
| Holothuria parvula | Dried sea cucumber | Protein hydrolysates using Neutrase | 5.25 b | NA | NA | NA | NA | [59] |
| Holothuria leucospilat | Whole animal | Protein hydrolysates using Alcalase and Flavourzyme | 35.3–68.27 | NA | NA | NA | NA | [41] |
| Holothuria scabra | Dried sea cucumber | Protein hydrolysates using papain, Alcalase, and Flavourzyme | 0.34–3.82 b | 1.28–1.65 b | NA | NA | NA | [57] |
| Acaudina molpadioides | Body wall | Protein hydrolysates using papain, pepsin, trypsin, and Neutrase | ~32 | NA | NA | NA | NA | [45] |
| Apostichopus japonicus | Egg | Protein hydrolysates using papain and Flavourzyme | NA | NA | 37–89.82 | NA | NA | [55] |
| Apostichopus japonicus | Body wall | Collagen using pepsin | 45.58 | NA | ~90 | NA | NA | [53] |
| Apostichopus japonicus | Body wall | Protein hydrolysates Flavourzyme | NA | NA | 0.28 b | NA | NA | [54] |
2.3. Antioxidant Potential of Sea Cucumber Polysaccharides
2.4. Antioxidant Potential of Carotenoids and Physiological Effects of PUFAs
2.5. Antioxidant Potential of Other Bioactive Compounds of Sea Cucumber
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, Z.; Palmer, C.H.; Dave, D.; Jiao, G.; Zhao, Y.; Tan, Z.; Chisholm, J.; Zhang, J.; Stefanova, R.; Hossain, A.; et al. Arsenic speciation in sea cucumbers: Identification and quantitation of water-extractable species. Environ. Pollut. 2020, 266, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Effect of high-pressure processing (HPP) on phenolics of North Atlantic sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2022, 70, 3489–3501. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ho, C.T. Antioxidant measurement and applications: An overview. In Antioxidant Measurement and Applications; American Chemical Society: Washington, DC, USA, 2007; pp. 2–7. [Google Scholar]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 66–105. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products : Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidants: Principles and applications. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–14. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 55, 1188–1192. [Google Scholar] [CrossRef]
- Ceesay, A.; Nor Shamsudin, M.; Aliyu-Paiko, M.; Ismail, I.S.; Nazarudin, M.F.; Mohamed Alipiah, N. Extraction and characterization of organ components of the Malaysian sea cucumber Holothuria leucospilota yielded bioactives exhibiting diverse properties. BioMed Res. Int. 2019, 2019, 2640684. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Hashim, R.B.; Taher, M.; Daud, J.M.; Ikeda, M.-A.; Zali, B.I. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009, 37, 376–387. [Google Scholar]
- Nobsathian, S.; Tuchinda, P.; Sobhon, P.; Tinikul, Y.; Poljaroen, J.; Tinikul, R.; Sroyraya, M.; Poomton, T.; Chaichotranunt, S. An antioxidant activity of the whole body of Holothuria scabra. Chem. Biol. Technol. Agric. 2017, 4, 17–21. [Google Scholar] [CrossRef]
- Wulandari, D.A.; Gustini, N.; Murniasih, T.; Bayu, A.; Sari, M.; Syahputra, G.; Harahap, I.A.; Rasyid, A.; Moria, S.B.; Rahmawati, S.I.; et al. Nutritional value and biological activities of sea cucumber Holothuria scabra cultured in the open pond system. J. Aquat. Food Prod. Technol. 2022, 31, 599–614. [Google Scholar] [CrossRef]
- Hossain, A.; Yeo, J.D.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP). Antioxidants 2022, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, É. Producing high antioxidant activity extracts from echinoderm by products by using pressured liquid extraction. Biotechnology 2010, 9, 523–528. [Google Scholar] [CrossRef][Green Version]
- Ridhowati, S.; Zakaria, F.R.; Syah, D.; Chasanah, E. Anticancer and antioxidant activities from sea cucumber (Stichopus variegatus) flour dried vacuum oven. Pertanika J. Trop. Agric. Sci. 2018, 41, 1125–1138. [Google Scholar]
- Husni, A.; Shin, I.S.; You, S.G.; Chung, D. Antioxidant properties of water and aqueous ethanol extracts and their crude saponin fractions from a far-eastern sea cucumber, Stichopus japonicus. Food Sci. Biotechnol. 2009, 18, 419–424. [Google Scholar]
- Telahigue, K.; Ghali, R.; Nouiri, E.; Labidi, A.; Hajji, T. Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J. Mar. Biol. Assoc. U. K. 2020, 100, 229–237. [Google Scholar] [CrossRef]
- Esmat, A.Y.; Said, M.M.; Soliman, A.A.; El-Masry, K.S.H.; Badiea, E.A. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition 2013, 29, 258–267. [Google Scholar] [CrossRef]
- Dakrory, A.I.; Fahmy, S.R.; Soliman, A.M.; Mohamed, A.S.; Amer, S.A.M. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced Hepatorenal diseases in rats. BioMed Res. Int. 2015, 2015, 563652. [Google Scholar] [CrossRef]
- Fahmy, S.R. Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats. BMC Complement. Altern. Med. 2015, 15, 14. [Google Scholar] [CrossRef]
- Alper, M.; Günes, M. Evaluation of cytotoxic, apoptotic effects and phenolic compounds of sea cucumber Holothuria tubulosa (Gmelin, 1791) extracts. Turkish J. Vet. Anim. Sci. 2020, 44, 641–655. [Google Scholar] [CrossRef]
- García, J.; Méndez, D.; Álvarez, M.; Sanmartin, B.; Vazquez Sobrado, R.; Regueiro, L.; Atanassova, M. Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly. LWT Food Sci. Technol. 2019, 109, 55–62. [Google Scholar] [CrossRef]
- Carletti, A.; Cardoso, C.; Lobo-Arteaga, J.; Sales, S.; Juliao, D.; Ferreira, I.; Chainho, P.; Dionísio, M.A.; Gaudêncio, M.J.; Afonso, C.; et al. Antioxidant and anti-inflammatory extracts from sea cucumbers and tunicates induce a pro-osteogenic effect in zebrafish larvae. Front. Nutr. 2022, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Sukmiwati, M.; Ilza, M.; Putri, A.E.; Sidauruk, S.W. Antibacterial activity of sea cucumber (Holothuria atra) against Pseudomonas aeruginosa. IOP Conf. Ser. Earth Environ. Sci. 2019, 404, 012047. [Google Scholar] [CrossRef]
- Pranweerapaiboon, K.; Apisawetakan, S.; Nobsathian, S.; Itharat, A.; Sobhon, P.; Chaithirayanon, K. An ethyl-acetate fraction of Holothuria scabra modulates inflammation in vitro through inhibiting the production of nitric oxide and pro-inflammatory cytokines via NF-κB and JNK pathways. Inflammopharmacology 2020, 28, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Senadheera, R.L.T.; Dave, D.; Shahidi, F. Phenolic profiles of Atlantic sea cucumber tentacles and their biological properties. Food Res. Int. 2022. under revision. [Google Scholar]
- Nguyen, T.H.; Kim, S.M. α-Glucosidase inhibitory activities of fatty acids purified from the internal organ of sea cucumber Stichopus japonicas. J. Food Sci. 2015, 80, H841–H847. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ryu, B.M.; Qian, Z.J.; Kim, S.K. Sea cucumber, Stichopus japonicus ethyl acetate fraction modulates the lipopolysaccharide induced iNOS and COX-2 via MAPK signaling pathway in murine macrophages. Environ. Toxicol. Pharmacol. 2010, 30, 68–75. [Google Scholar] [CrossRef]
- Safari, R.; Yaghoubzadeh, Z. Antioxidant activity of bioactive peptides extracted from sea cucumber (Holothuria leucospilata). Int. J. Pept. Res. Ther. 2020, 26, 2393–2398. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea cucumber derived type I collagen: A comprehensive review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Antioxidant potential and physicochemical properties of protein hydrolysates from body parts of North Atlantic sea cucumber (Cucumaria frondosa). Food Prod. Process. Nutr. 2021, 3, 3. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-X.; Xu, H.-P.; Li, Y.; Zhang, Q.-W.; Xie, H. Preparation and evaluation of peptides with potential antioxidant activity by microwave assisted enzymatic hydrolysis of collagen from sea cucumber Acaudina molpadioides obtained from Zhejiang province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Tao, H.; Qin, S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. Technol. 2016, 25, 940–952. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2010, 45, 147–154. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, L.; Wang, S.; Zhao, M.; Liu, X. Anti-diabetic and anti-hyperlipidemic effects of sea cucumber (Cucumaria frondosa) gonad hydrolysates in type II diabetic rats. Food Sci. Hum. Wellness 2022, 11, 1614–1622. [Google Scholar] [CrossRef]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In vitro antiviral activities of enzymatic hydrolysates extracted from byproducts of the Atlantic holothurian Cucumaria frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- Lin, L.; Yang, K.; Zheng, L.; Zhao, M.; Sun, W.; Zhu, Q.; Liu, S. Anti-aging effect of sea cucumber (Cucumaria frondosa) hydrolysate on fruit flies and d-galactose-induced aging mice. J. Funct. Foods 2018, 47, 11–18. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant peptides isolated from sea cucumber Stichopus japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Zhu, B.-W.; Dong, X.-P.; Zhou, D.-Y.; Gao, Y.; Yang, J.-F.; Li, D.-M.; Zhao, X.-K.; Ren, T.-T.; Ye, W.-X.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tang, Q.; Wang, Y.; Yaoguang, C.; Qin, Z.; Changhu, X. Antioxidation Activities of Low-Molecular-Weight Gelatin Hydrolysate Isolated from the Sea Cucumber Stichopus japonicus. J. Ocean Univ. China 2010, 9, 94–98. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhang, Y.; Lu, Y.; Wang, M.; Wang, G.; Liu, X. Purification and antioxidant ability of peptide from egg in sea cucumber Apostichopus japonicus. Int. J. Food Prop. 2017, 20, 306–317. [Google Scholar] [CrossRef]
- Guo, K.; Su, L.; Wang, Y.; Liu, H.; Lin, J.; Cheng, P.; Yin, X.; Liang, M.; Wang, Q.; Huang, Z. Antioxidant and anti-aging effects of a sea cucumber protein hydrolyzate and bioinformatic characterization of its composing peptides. Food Funct. 2020, 11, 5004–5016. [Google Scholar] [CrossRef] [PubMed]
- Doungapai, C.; Siriwoharn, T.; Malila, Y.; Autsavapromporn, N.; Makkhun, S.; Yarnpakdee, S.; Jantanasakulwong, K.; Regenstein, J.M.; Wangtueai, S.; Brück, W.; et al. UV-B Protective and Antioxidant Activities of Protein Hydrolysate from Sea Cucumber (Holothuria scabra) Using Enzymatic Hydrolysis. Front. Mar. Sci. 2022, 9, 892255. [Google Scholar] [CrossRef]
- Rathnayake, A.U.; Abuine, R.; Palanisamy, S.; Lee, J.K.; Byun, H.G. Characterization and purification of β−secretase inhibitory peptides fraction from sea cucumber (Holothuria spinifera) enzymatic hydrolysates. Process Biochem. 2021, 111, 86–96. [Google Scholar] [CrossRef]
- Fawzya, Y.N.; Putra, N.A.; Witarto, A.B.; Patantis, G. Golden sea cucumber: Identification and the antioxidant activity of its collagen hydrolysates. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2020, 15, 119–129. [Google Scholar] [CrossRef]
- Hernndez-Smano, A.C.; Hernndez-Ledesma, B. Release of antioxidant peptides from the body wall proteins of the sea cucumber Isostichopus fuscus. Nat. Prod. Commun. 2015, 10, 1427–1430. [Google Scholar]
- Liu, X.; Sun, Z.; Zhang, M.; Meng, X.; Xia, X.; Yuan, W.; Xue, F.; Liu, C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 2012, 90, 1664–1670. [Google Scholar] [CrossRef]
- Zhu, B.W.; Zhou, D.Y.; Li, T.; Yan, S.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis Discus Hannai Ino). Food Chem. 2010, 121, 712–718. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, Q.; Zhang, Y.; Li, J.; Zhu, X.; Wen, J.; Liu, J.; Zhao, L.; Zhao, J.; Zhao, L. Enzyme-assisted extraction optimization, characterization and antioxidant activity of polysaccharides from sea cucumber Phyllophorus proteus. Molecules 2018, 23, 590. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Simal-Gandara, J.; Gao, L.; Xu, C.; Tao, X.; Zuo, Z.; Ning, Z.; Wang, L.; Gao, N.; Zhao, J. Structure elucidation of fucan sulfate from sea cucumber Holothuria fuscopunctata through a bottom-up strategy and the antioxidant activity analysis. Int. J. Mol. Sci. 2022, 23, 4488. [Google Scholar]
- Li, Q.; Jiang, S.; Shi, W.; Qi, X.; Song, W.; Mou, J.; Yang, J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020, 231, 115767. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ye, X.; Sun, Y.; Wu, D.; Wu, N.; Hu, Y.; Chen, S. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef]
- Li, R.; Huahua, Y.U.; Yang, Y.; Song, L.; Rong’e, X.; Xiaolin, C.; Li, P. Sulfated polysaccharides with antioxidant and anticoagulant activity from the sea cucumber Holothuria fuscogliva. Chin. J. Oceanol. Limnol. 2017, 35, 763–769. [Google Scholar] [CrossRef]
- Mou, J.; Li, Q.; Qi, X.; Yang, J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym. 2018, 185, 41–47. [Google Scholar] [CrossRef]
- Yu, L.; Xue, C.; Chang, Y.; Xu, X.; Ge, L.; Liu, G.; Wang, Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014, 146, 113–119. [Google Scholar] [CrossRef]
- Zou, S.; Pan, R.; Dong, X.; He, M.; Wang, C. Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2016, 51, 650–658. [Google Scholar] [CrossRef]
- Wang, J.; Shi, S.; Li, F.; Du, X.; Kong, B.; Wang, H.; Xia, X. Physicochemical properties and antioxidant activity of polysaccharides obtained from sea cucumber gonads via ultrasound-assisted enzymatic techniques. LWT Food Sci. Technol. 2022, 160, 113307. [Google Scholar] [CrossRef]
- Qi, H.; Ji, X.; Liu, S.; Feng, D.; Dong, X.; He, B.; Srinivas, J.; Yu, C. Antioxidant and anti-dyslipidemic effects of polysaccharidic extract from sea cucumber processing liquor. Electron. J. Biotechnol. 2017, 28, 1–6. [Google Scholar] [CrossRef]
- Matsuno, T.; Tsushima, M. Comparative biochemical studies of carotenoids in sea cucumber. Comp. Biochem. Physiol. 1995, 111, 597–605. [Google Scholar] [CrossRef]
- Tsushima, M.; Fujiwara, Y.; Matsuno, T. Novel marine di-Z-carotenoids: Cucumariaxanthins A, B, and C from the sea cucumber Cucumaria japonica. J. Nat. Prod. 1996, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Nakachi, S.; Kobayashi, R.; Mori, M.; Sakagami, Y. A new carotenoid, 9Z,9′Z-tetrahydroastaxanthin, from the sea cucumber Plesiocolochirus minutus. Tetrahedron Lett. 2015, 56, 5954–5955. [Google Scholar] [CrossRef]
- Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Thuy Hang, C.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and advantages of supercritical CO2 extraction of sea cucumber Cucumaria frondosa japonica semper, 1868. Molecules 2020, 25, 4088. [Google Scholar] [CrossRef]
- Chasanah, E.; Fawzya, Y.N.; Tarman, K.; Januar, H.I.; Nursid, M. Fatty acid profile, carotenoid content, and in vitro anticancer Activity of Karimunjawa and Lampung sea cucumber. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016, 11, 117. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef]
- Wang, X.; Cong, P.; Chen, Q.; Li, Z.; Xu, J.; Xue, C. Characterizing the phospholipid composition of six edible sea cucumbers by NPLC-Triple TOF-MS/MS. J. Food Compos. Anal. 2020, 94, 103626. [Google Scholar] [CrossRef]
- Liu, Y.; Dave, D.; Trenholm, S.; Ramakrishnan, V.V.; Murphy, W. Effect of drying on nutritional composition of atlantic sea cucumber (Cucumaria frondosa) viscera derived from Newfoundland fisheries. Processes 2021, 9, 703. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.H.; Wang, Y.M.; Xue, C.H.; Kurihara, H.; Takahashi, K. Antitumour activity of EPA-enriched phospholipids liposomes against S180 ascitic tumour-bearing mice. J. Funct. Foods 2015, 19, 970–982. [Google Scholar] [CrossRef]
- Wu, F.J.; Xue, Y.; Liu, X.F.; Xue, C.H.; Wang, J.F.; Du, L.; Takahashi, K.; Wang, Y.M. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem. Int. 2014, 64, 9–17. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Wen, M.; Du, L.; Xue, C.; Wang, J.; Xu, J.; Wang, Y. Rapid modulation of lipid metabolism in C57BL/6J mice induced by eicosapentaenoic acid-enriched phospholipid from Cucumaria frondosa. J. Funct. Foods 2017, 28, 28–35. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Shi, D.; Wang, J.; Wang, Y.; Lou, Q.; Xue, C. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic effects via activating phosphoinositide 3-kinase/protein kinase B signal pathway. J. Biosci. Bioeng. 2014, 117, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hu, S.; Xu, H.; Wang, J.; Xue, C.; Wang, Y. Long-chain bases from Cucumaria frondosa inhibit adipogenesis and regulate lipid metabolism in 3T3-L1 adipocytes. Food Sci. Biotechnol. 2016, 25, 1753–1760. [Google Scholar] [CrossRef]
- Jia, Z.; Song, Y.; Tao, S.; Cong, P.; Wang, X.; Xue, C.; Xu, J. Structure of sphingolipids from sea cucumber Cucumaria frondosa and structure-specific cytotoxicity against human hepg2 cells. Lipids 2016, 51, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Althunibat, O.; Ridzwan, B.; Taher, M.; Daud, J.; Jauhari Arief Ichwan, S.; Qaralleh, H. Antioxidant and cytotoxic properties of two sea cucumbers, Holothuria edulis Lesson and Stichopus horrens Selenka. Acta Biol. Hung. 2013, 64, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M.; Ghandourah, M.; Al-Farawati, R. Sulfated triterpene glycosides from the Saudi red sea cucumber Holothuria atra with antioxidant and cytotoxic activities. Thalassas 2021, 37, 817–824. [Google Scholar] [CrossRef]
- Ansharullah; Tamrin; Patadjai, A.B.; Asranuddin. Powder production of sea cucumber (Holothuria scabra): Effect of processing methods on the antioxidant activities and physico-chemical characteristics. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 331–334. [Google Scholar] [CrossRef]
- Nugroho, A.; Harahap, I.A.; Ardiansyah, A.; Bayu, A.; Rasyid, A.; Murniasih, T.; Setyastuti, A.; Putra, M.Y. Antioxidant and antibacterial activities in 21 species of Indonesian sea cucumbers. J. Food Sci. Technol. 2022, 59, 239–248. [Google Scholar] [CrossRef]
- Rasyid, A.; Putra, M.Y.; Yasman. Free radical scavenging activity of five selected sea cucumbers collected from Lampung waters, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 890, 012014. [Google Scholar] [CrossRef]
- Wulandari, D.A.; Murniasih, T.; Sari, M.; Syahputra, G.; Rasyid, A.; Septiana, E.; Untari, F.; Harahap, I.A.; Ardiansyah, A.; Gustini, N.; et al. Characterization, antioxidant and antibacterial activity of cultivated sea cucumbers from Bali, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 744, 012102. [Google Scholar] [CrossRef]
- Wu, F.-J.; Xue, Y.; Tang, Q.-J.; Xu, J.; Du, L.; Xue, C.-H.; Takahashi, K.; Wang, Y.-M. The protective effects of cerebrosides from sea cucumber and starfish on the oxidative damage in PC12 cells. J. Oleo Sci. 2013, 62, 717–727. [Google Scholar] [CrossRef] [PubMed]
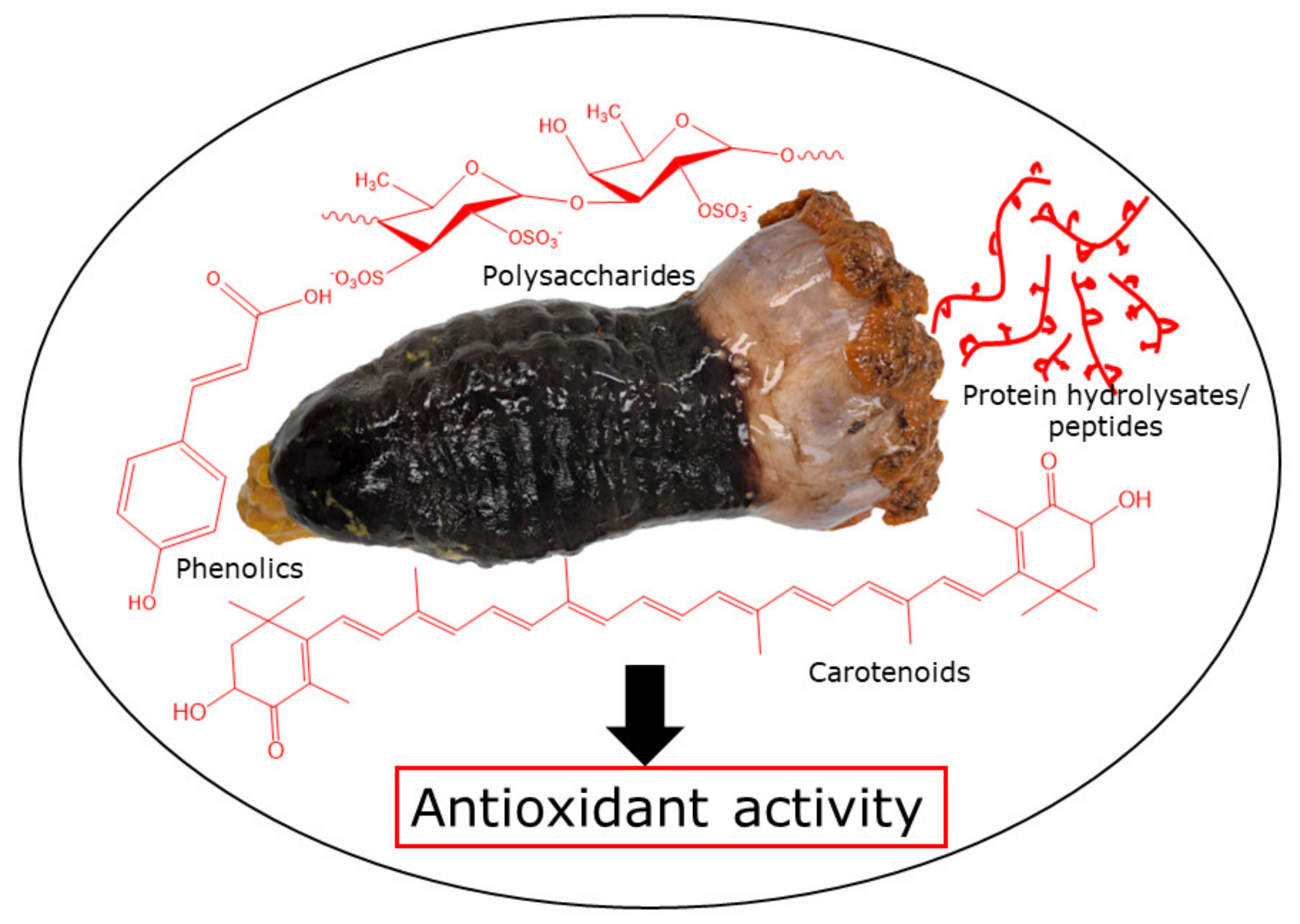
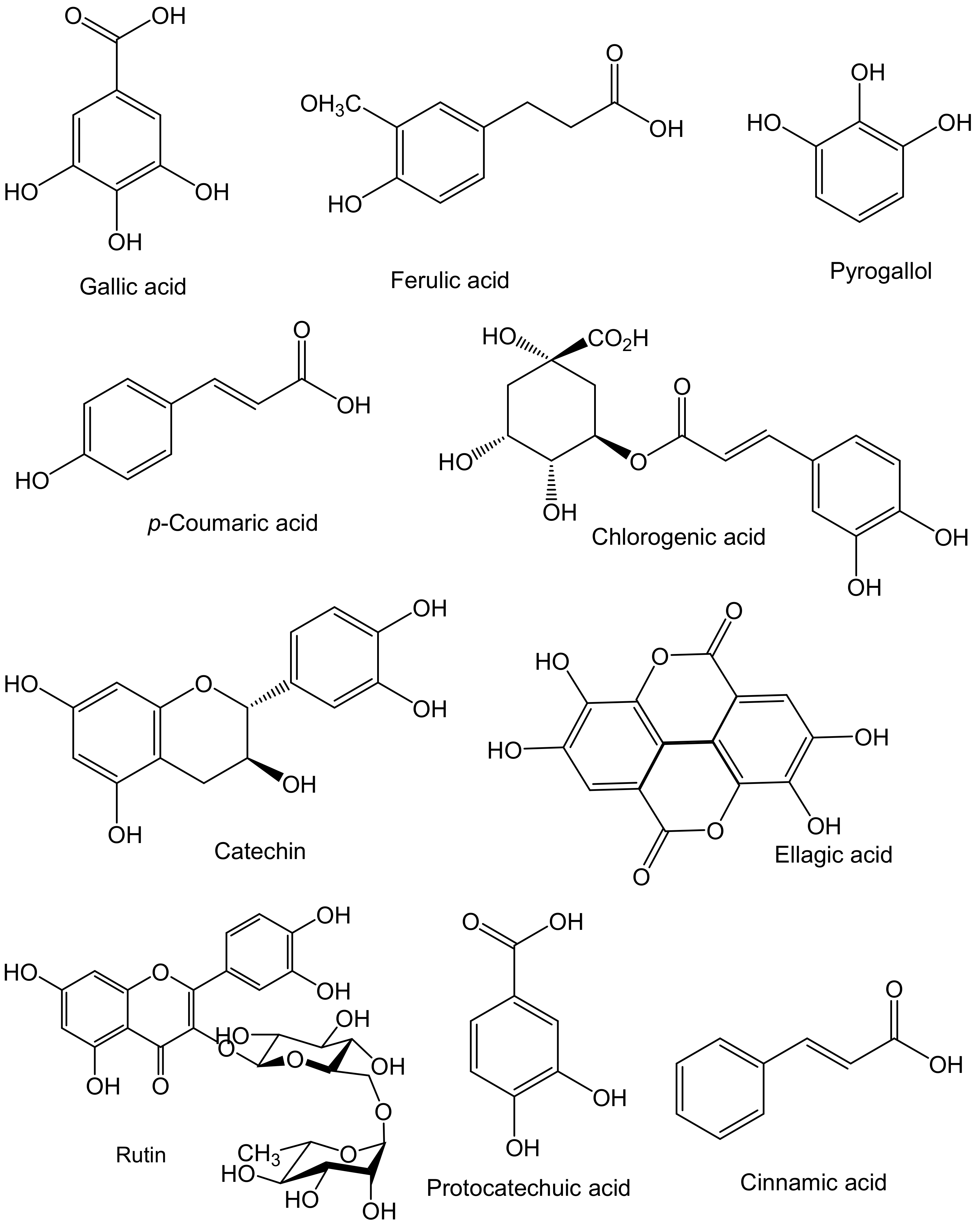
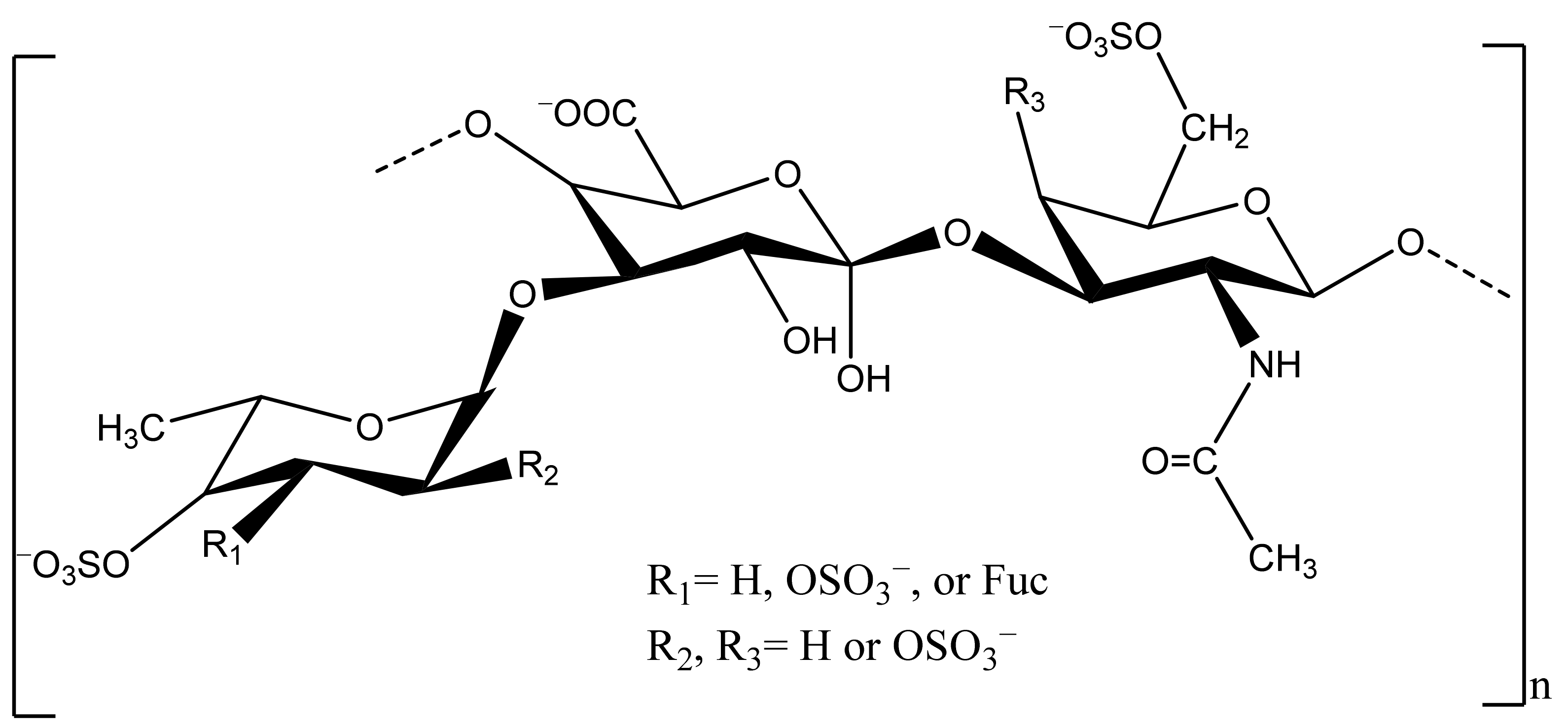
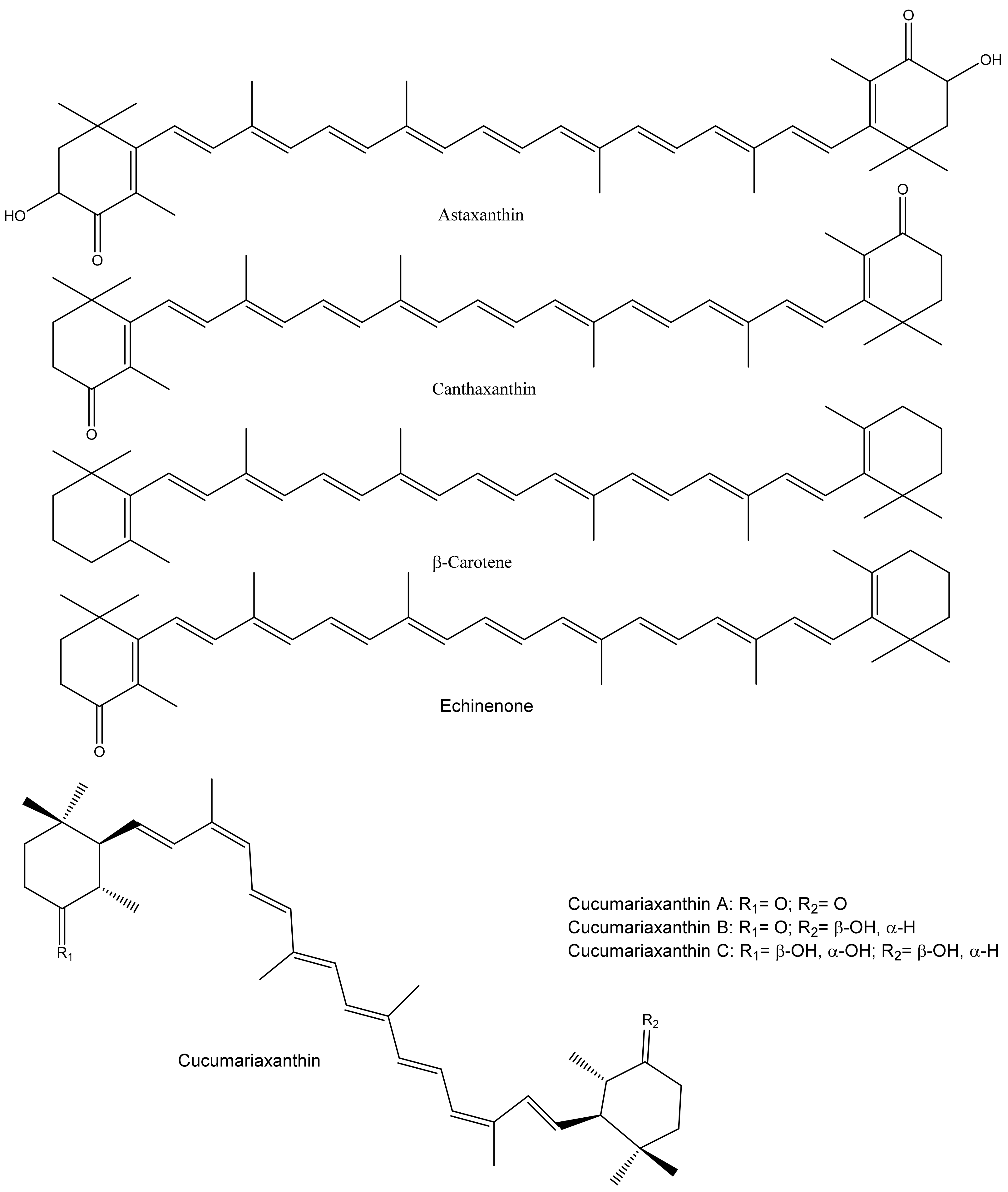
| Health Effects | Species | Body Parts | Responsible Compounds/Extracts | Results/Mechanisms | References |
|---|---|---|---|---|---|
| Anticancer | Holothuria tubulosa | Body wall | Aqueous and methanolic extracts rich in epicatechin and ellagic acid | Inhibited the growth of cancer cell lines and induced apoptosis in A549 (human non-small lung carcinoma) and HeLa (cervix adenocarcinoma) cells | [33] |
| Holothuria scabra, Holothuria leucospilota, and Stichopus chloronotus | Sea cucumber without viscera | Aqueous extracts | Inhibited the growth of C33A (human cervical cancer) and A549 cancer cells | [21] | |
| Stichopus variegatus | Dried sea cucumber | Aqueous extracts | Possessed cytotoxicity on colon cancer cells WiDr, breast cancer cells T47D, and normal cells Vero | [27] | |
| Holothuria scabra | Dried sea cucumber | Extracts | Exhibited cytotoxic activity against human breast cancer cells (MDA-MB 231) | [23] | |
| DNA oxidation inhibition | Cucumaria frondosa | Dried body wall and internal organs | Acetone extracts rich in phenolic acids and flavonoids | Inhibited hydroxyl and peroxyl radical-induced DNA oxidation | [5,24] |
| Anti-inflammatory | Apostichopus japonicus | Fresh sea cucumber | Ethyl acetate extract. | Inhibited the productions of NO (nitric oxide) and PGE2 (prostaglandin E2) by inhibiting iNOS (inducible nitric oxide synthase) and COX-2 (cycloxygenase-2) | [40] |
| Holothuria scabra | Dried sea cucumber | Hexanes, ethyl acetate, and n-butanol extracts | Inhibited pro-inflammatory cytokine synthesis | [37] | |
| LDL oxidation inhibition | Cucumaria frondosa | Dried body wall and internal organs | Acetone extracts rich in phenolic acids and flavonoids | Inhibited primary oxidation products, conjugated dienes (CD) | [5,24] |
| Hepatoprotective and curative | Holothuria atra | Body wall | Phosphate buffer extracts rich in chlorogenic acid | Alleviated the hepatorenal toxicity resulting from DMBA (7,12-dimethylbenz[a]anthracene) hydrocarbon exposure | [31] |
| Holothuria atra | Body wall | Organic and aqueous extracts rich in chlorogenic acid | Exhibited hepatoprotective activity against thioacetamide-induced liver fibrosis in a rat model | [30] | |
| Anti-cholestatic | Holothuria arenicola | Body wall | Phosphate buffer extracts rich in chlorogenic acid | Prevented liver damage following cholestasis | [32] |
| Antibacterial | Holothuria atra | Dried sea cucumber | Hexane, ethyl acetate, and butanol extracts | Showed inhibitory activity against Pseudomonas aeruginosa | [36] |
| Holothuria forskali | Digestive tract, muscle, body wall, gonad, and respiratory tree | Ethyl-acetate extracts rich in quinic acid | Escherichia coli and Bacillus subtilis were inhibited | [29] | |
| α-Glucosidase inhibition | Apostichopus japonicus | Dried internal organs | Organic extracts | Showed potential to inhibit α-glucosidase enzyme | [39] |
| Cucumaria frondosa | Body wall | Acetone extracts rich in phenolic acids and flavonoids | Slowed down the activity of α-glucosidase enzyme | [5] | |
| Antiglycation | Cucumaria frondosa | Dried body wall and internal organs | Acetone extracts rich in phenolic acids and flavonoids | Controlled the formation of advanced glycation end products(AGEs) | [5,24] |
| Anti-tyrosinase | Cucumaria frondosa | Dried internal organs | Acetone extracts rich in phenolic acids and flavonoids | Inhibited tyrosinase enzyme | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.; Dave, D.; Shahidi, F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Mar. Drugs 2022, 20, 521. https://doi.org/10.3390/md20080521
Hossain A, Dave D, Shahidi F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Marine Drugs. 2022; 20(8):521. https://doi.org/10.3390/md20080521
Chicago/Turabian StyleHossain, Abul, Deepika Dave, and Fereidoon Shahidi. 2022. "Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health" Marine Drugs 20, no. 8: 521. https://doi.org/10.3390/md20080521
APA StyleHossain, A., Dave, D., & Shahidi, F. (2022). Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Marine Drugs, 20(8), 521. https://doi.org/10.3390/md20080521








