High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography
Abstract
:1. Introduction
2. Results
2.1. FX content in the Freeze-Dried I. zhangjiangensis Powder
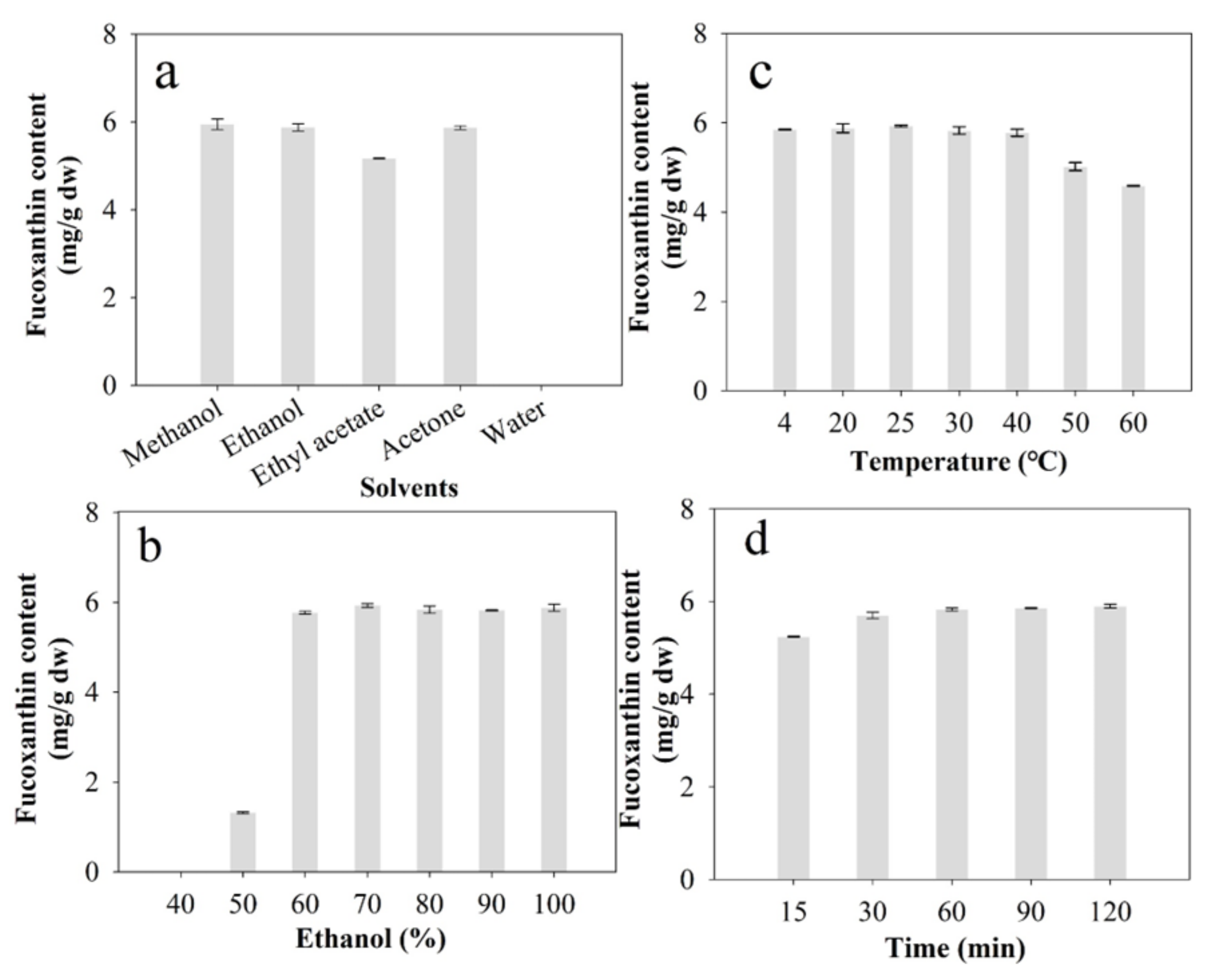
2.2. Influence of Different Extraction Conditions on FX Yield
2.2.1. Influence of Different Solvent Types
2.2.2. Influence of the Ethanol/Water Mixed Solvent
2.2.3. Influence of Temperature
2.2.4. Influence of Extraction Time
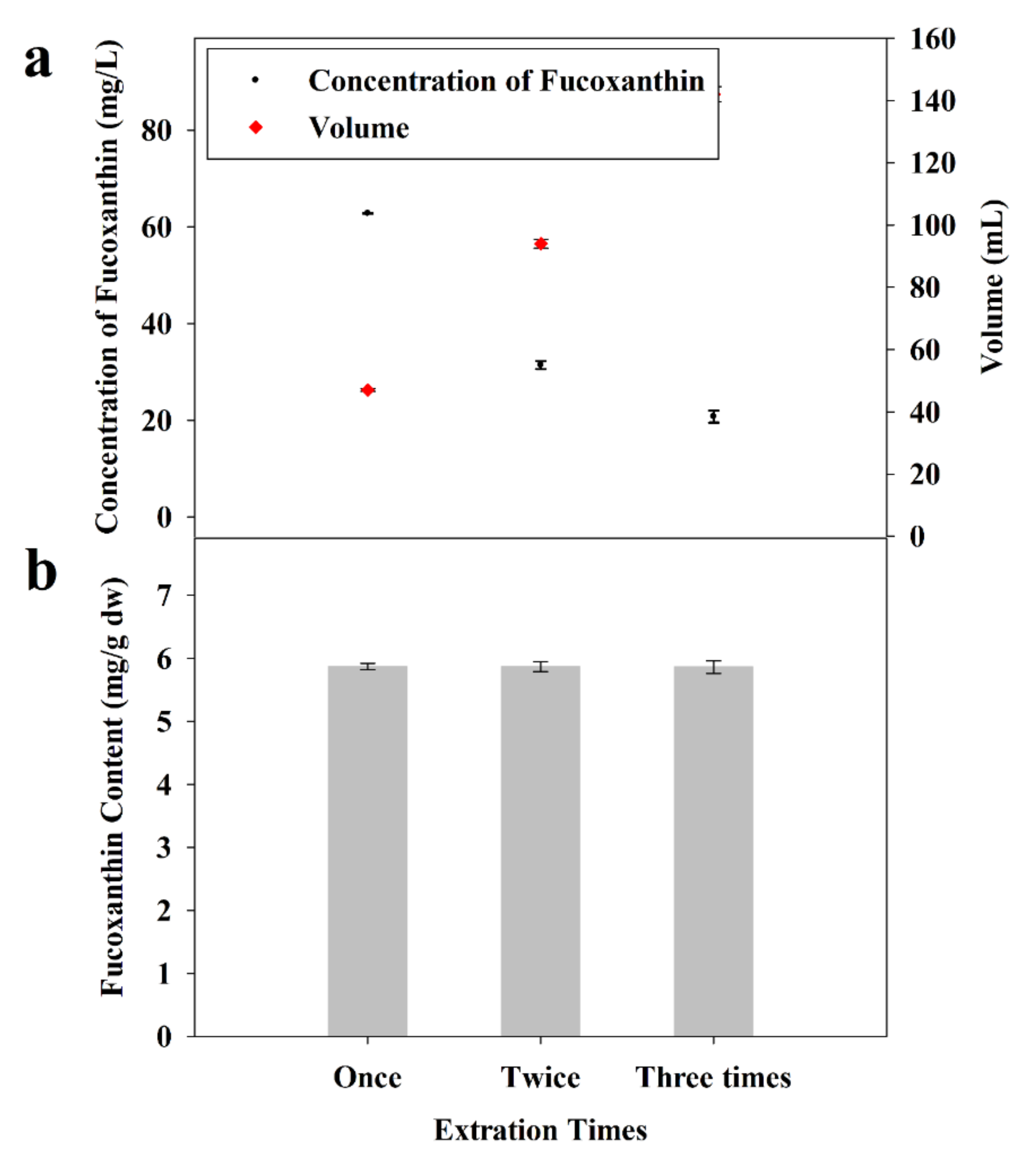
2.2.5. Influence of Number of Extractions
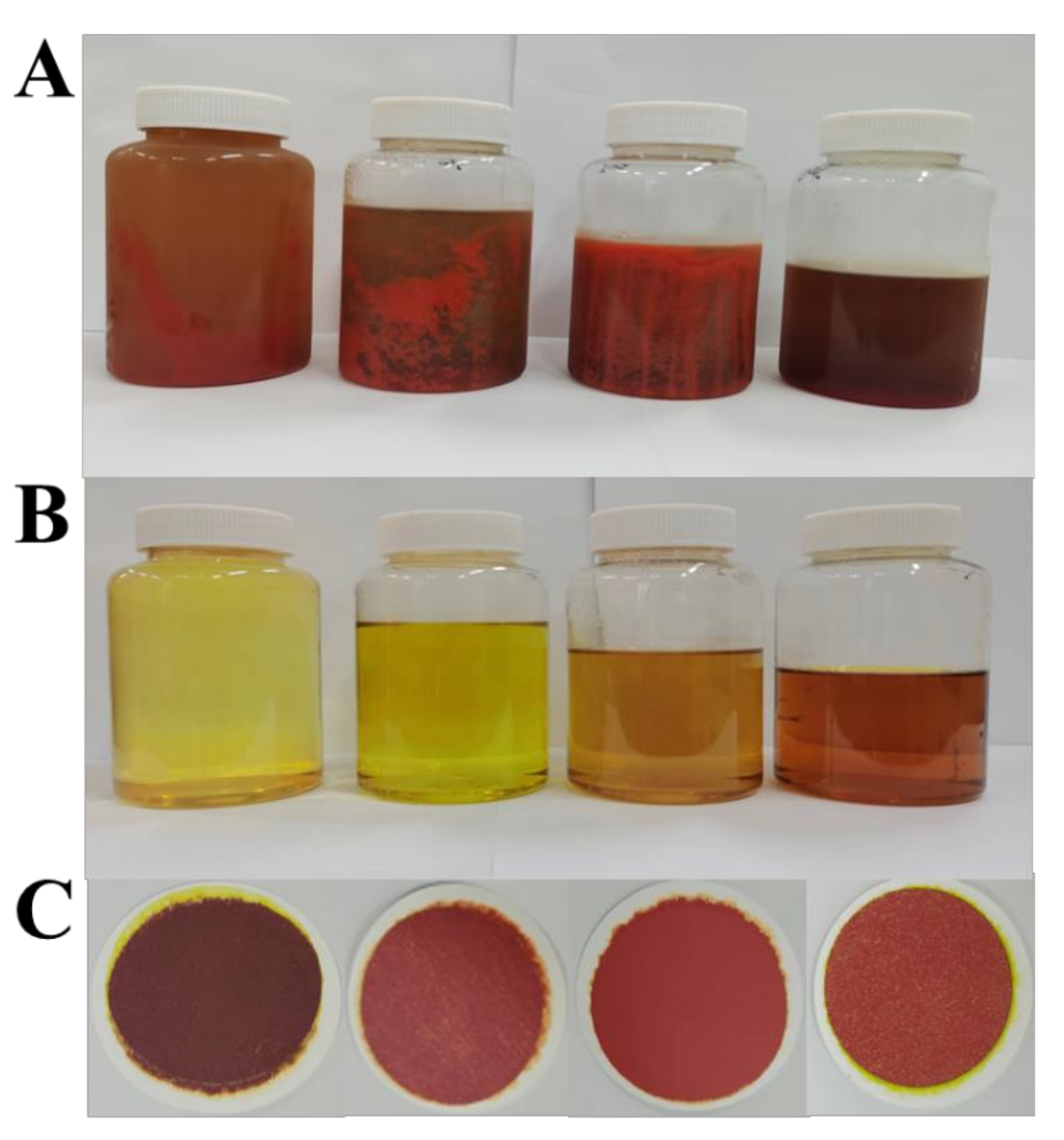
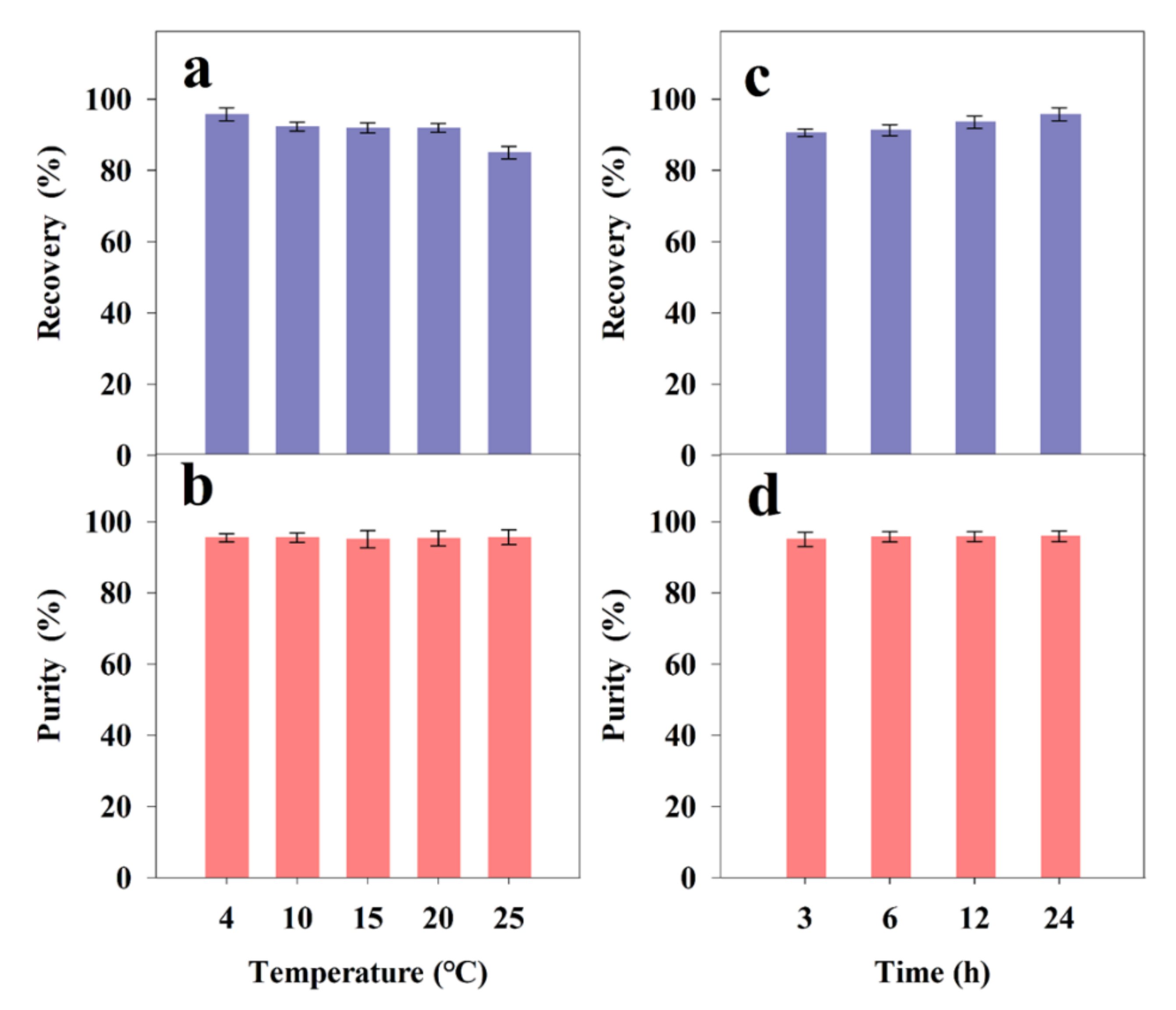
2.3. Separation of FX by ODS Column Chromatography
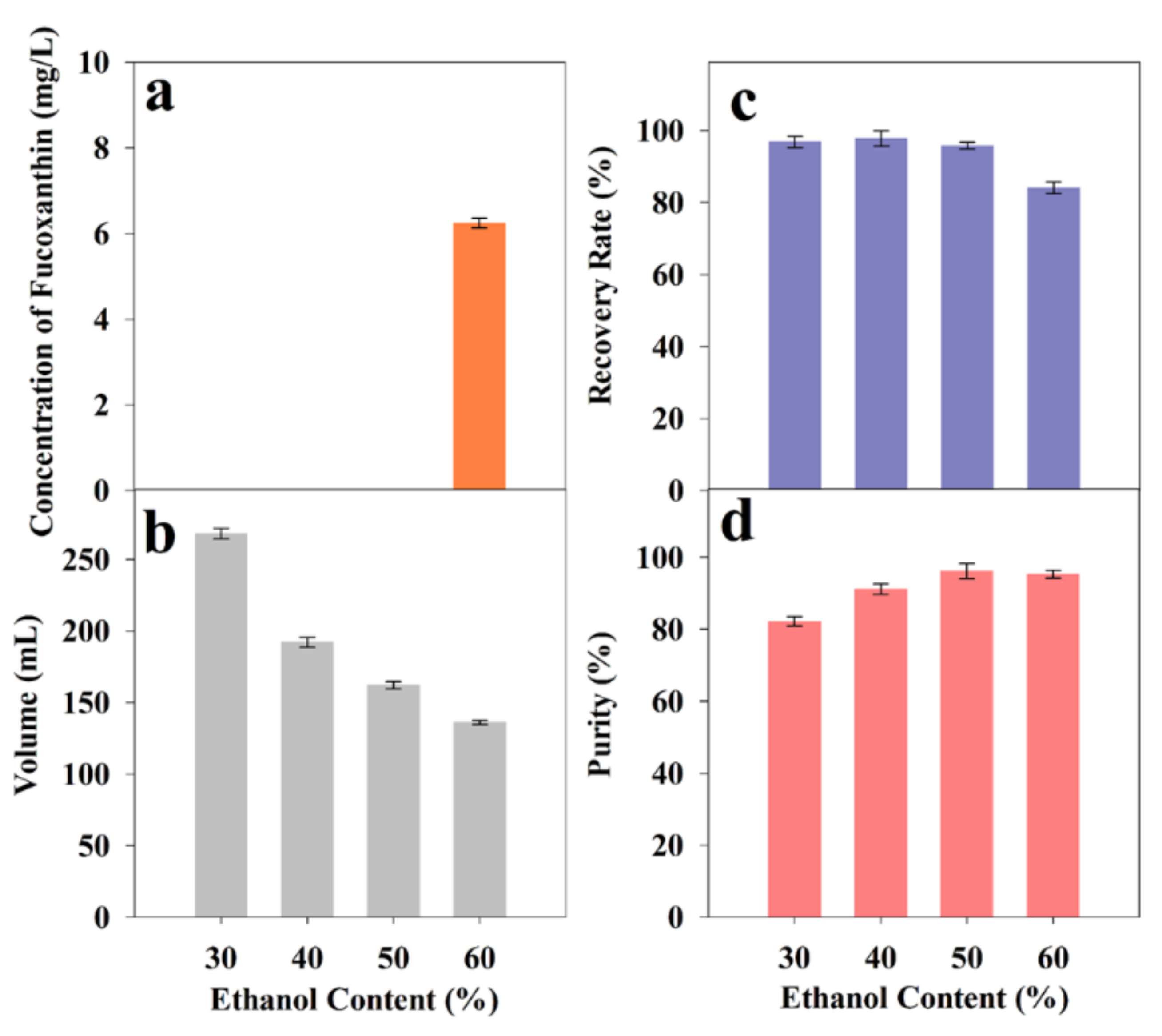
2.4. Optimization of Ethanol Precipitation
2.4.1. Effect of Ethanol Content on Precipitation
2.4.2. Effect of Temperature on Precipitation
2.4.3. Effect of Precipitation Time on Precipitation
2.5. Identification of FX
3. Materials and Methods
3.1. Strains and Cultural Conditions
3.2. Extraction and Quantitative Analysis of FX from I. zhangjiangensis
3.3. Optimization of FX Extraction from I. zhangjiangensis
3.4. ODS Column Chromatographic Experiments
3.5. Optimization of Ethanol Precipitation
3.6. TLC Analysis of FX
3.7. Identification of FX from I. zhangjiangensis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.-H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels—A review. Int. J. Mol. Sci. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Fu, J.; Xiong, J.; Zhang, C. Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J. Biosi. Bioeng. 2018, 126, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Wang, Y.; Yang, S.; Wang, J.; Wu, T.; Lu, X.; Chu, Y.; Chen, F. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresour. Technol. 2022, 347, 126401. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A review on the progress, challenges and prospects in commercializing microalgal fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Acta Mol. Cell Biol. Lipids. 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complementary Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Lourenço-Lopes, C.; Silva, A.; Pereira, A.G.; Fraga-Corral, M.; Zhao, C.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Pigment composition of nine brown algae from the Iberian Northwestern Coastline: Influence of the extraction solvent. Mar. Drugs 2022, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of natural deep futectic solvents for extraction of hydrophilic and lipophilic compounds from fucus vesiculosus. Molecules 2021, 46, 4198. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A.; Shamsollahi, Z. Production of fucoxanthin by the microalga Tisochrysis lutea: A review of recent developments. Aquaculture 2020, 516, 734637. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Volcani, B.E. The polymorphic diatom Phaeodactylum tricornutum: Ultrastructure of its morphotypes. J. Phycol. 1978, 14, 10–21. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A diatom cell factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Sadhasivam, G.; Kandasamy, S.; Poomani, K.; Thayumanavan, P. Purification of fucoxanthin from Sargassum wightii Greville and understanding the inhibition of angiotensin 1-converting enzyme: An in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 148, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Domínguez, M.C.; Salinas, F.; Medina, E.; Rincón, B.; Martín, M.Á.; Gutiérrez, M.C.; Cerezal-Mezquita, P. Supercritical fluid extraction of fucoxanthin from the diatom Phaeodactylum tricornutum and biogas production through anaerobic digestion. Mar. Drugs 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, C.; Cheng, P.; Zhu, J.; Hou, Y.; Li, Y.; Zhang, J. A simple and efficient strategy for fucoxanthin extraction from the microalga Phaeodactylum tricornutum. Algal Res. 2022, 61, 102610. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, S.-M.; Pan, C.-H.; Chung, D. Effects of heating, aerial exposure and illumination on stability of fucoxanthin in canola oil. Food Chem. 2014, 145, 505–513. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Huang, J.J.; Xu, W.W.; Lin, S.L.; Cheung, P.C. Phytochemical profiles of marine phytoplanktons: An evaluation of their in vitro antioxidant and anti-proliferative activities. Food Funct. 2016, 7, 5002–5017. [Google Scholar] [CrossRef]
- Gilbert López, B.; Lmendiola, J.; Broek, L.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef]
- Zin, M.; Bánvölgyi, S. Conventional extraction of betalain compounts from beetroot peels with aqueous ethanol solvent. Acta Aliment. 2020, 49, 163–169. [Google Scholar] [CrossRef]
- Dziuba, J.; Bochenek, A.; Ozimek, L.; Fornal, J. Microstructure of milk proteins coagulated by rennin in the presence of ethanol precipitated whey proteins. Can. Inst. Food Sci. Technol. J. 1988, 21, 300–304. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Yan, A.; Feng, L.; Wan, Y. Fractionation, structure and conformation characterization of polysaccharides from anoectochilus roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, R.; Shimomura, Y. Industrial high-performance liquid chromatography purification of docosahexaenoic acid ethyl ester and docosapentaenoic acid ethyl ester from single-cell oil. J. Am. Oil Chem. Soc. 1997, 74, 1435–1440. [Google Scholar] [CrossRef]
- Tai, Y.; Shen, J.; Luo, Y.; Qu, H.; Gong, X. Research progress on the ethanol precipitation process of traditional Chinese medicine. Chinese Med. 2020, 15, 84. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, J.; Wang, L.; Zhu, J.; Cui, W.; Hou, H.; Zhang, J.; Zhou, C.; Yan, X. Isolation and purification of fucoxanthin from brown seaweed Sargassum horneri using open ODS column chromatography and ethanol precipitation. Molecules 2021, 26, 3777. [Google Scholar] [CrossRef]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a marine-derived Carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Wang, H.-T.; Meng, Y.-Y.; Cao, X.-P.; Ai, J.-N.; Zhou, J.-N.; Xue, S.; Wang, W.-L. Coordinated response of photosynthesis, carbon assimilation, and triacylglycerol accumulation to nitrogen starvation in the marine microalgae Isochrysis zhangjiangensis (Haptophyta). Bioresour. Technol. 2015, 177, 282–288. [Google Scholar] [CrossRef]
- Warkoyo, W.; Saati, E. The solvent effectiveness on extraction process of seaweed pigment. Makara J. Technol. 2011, 15, 5–8. [Google Scholar]
- Rivera, S.; Canela, R. Influence of sample processing on the analysis of carotenoids in maize. Molecules 2012, 17, 11255–11268. [Google Scholar] [CrossRef]
- Magpusao, J.; Giteru, S.; Oey, I.; Kebede, B. Effect of high pressure homogenization on microstructural and rheological properties of A. platensis, Isochrysis, Nannochloropsis and Tetraselmis species. Algal Res. 2021, 56, 102327. [Google Scholar]
- Büchel, C. Fucoxanthin-chlorophyll proteins in diatoms: 18 and 19 kDa subunits assemble into different oligomeric states. Biochemistry 2003, 42, 13027–13034. [Google Scholar] [CrossRef] [PubMed]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A promising green solvent for the deconstruction of lignocellulose. ChemSusChem 2018, 11, 3559–3575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Wu, H.; Lim, S.J.; Wan Mustapha, W.; Maskat, M.; Said, M. Characterisation and stability of pigments extracted from Sargassum binderi obtained from Semporna, Sabah. Sains Malays. 2014, 43, 1345–1354. [Google Scholar]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Wang, G.; Dai, S.; He, H.; Xiang, W. A comparative analysis of fatty acid composition and fucoxanthin content in six Phaeodactylum tricornutum strains from different origins. Chin. J. Oceanol. 2016, 34, 391–398. [Google Scholar] [CrossRef]
- Joy, S.; Rahman, P.; Khare, S.K.; Sharma, S. Production and characterization of glycolipid biosurfactant from Achromobacter sp. (PS1) isolate using one-factor-at-a-time (OFAT) approach with feasible utilization of ammonia-soaked lignocellulosic pretreated residues. Bioprocess Biosyst. Eng. 2019, 42, 1301–1315. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, G.; Ye, Y.; Zhao, J.; Zhou, C.; Zhu, J.; Li, Y.; Zhang, J.; Yan, X. High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography. Mar. Drugs 2022, 20, 510. https://doi.org/10.3390/md20080510
Zhuang G, Ye Y, Zhao J, Zhou C, Zhu J, Li Y, Zhang J, Yan X. High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography. Marine Drugs. 2022; 20(8):510. https://doi.org/10.3390/md20080510
Chicago/Turabian StyleZhuang, Gengjie, Yuemei Ye, Junling Zhao, Chengxu Zhou, Junwang Zhu, Yanrong Li, Jinrong Zhang, and Xiaojun Yan. 2022. "High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography" Marine Drugs 20, no. 8: 510. https://doi.org/10.3390/md20080510
APA StyleZhuang, G., Ye, Y., Zhao, J., Zhou, C., Zhu, J., Li, Y., Zhang, J., & Yan, X. (2022). High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography. Marine Drugs, 20(8), 510. https://doi.org/10.3390/md20080510






