Potential for the Production of Carotenoids of Interest in the Polar Diatom Fragilariopsis cylindrus
Abstract
:1. Introduction
2. Results
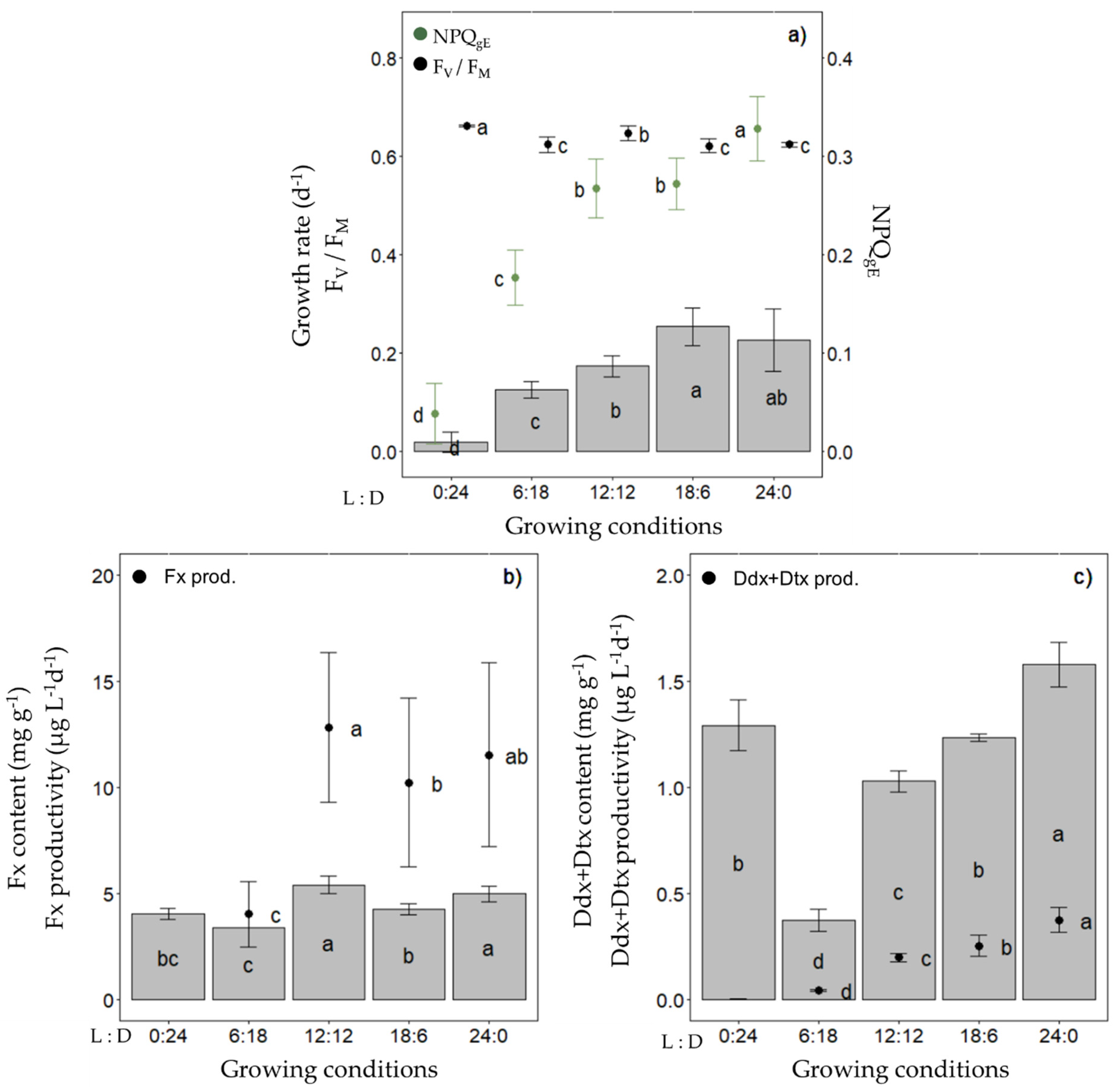
2.1. Production of Fucoxanthin (Fx) and Diadinoxanthin+Diatoxanthin (Ddx+Dtx) in Fragilariopsis cylindrus under a Range of Photoperiods
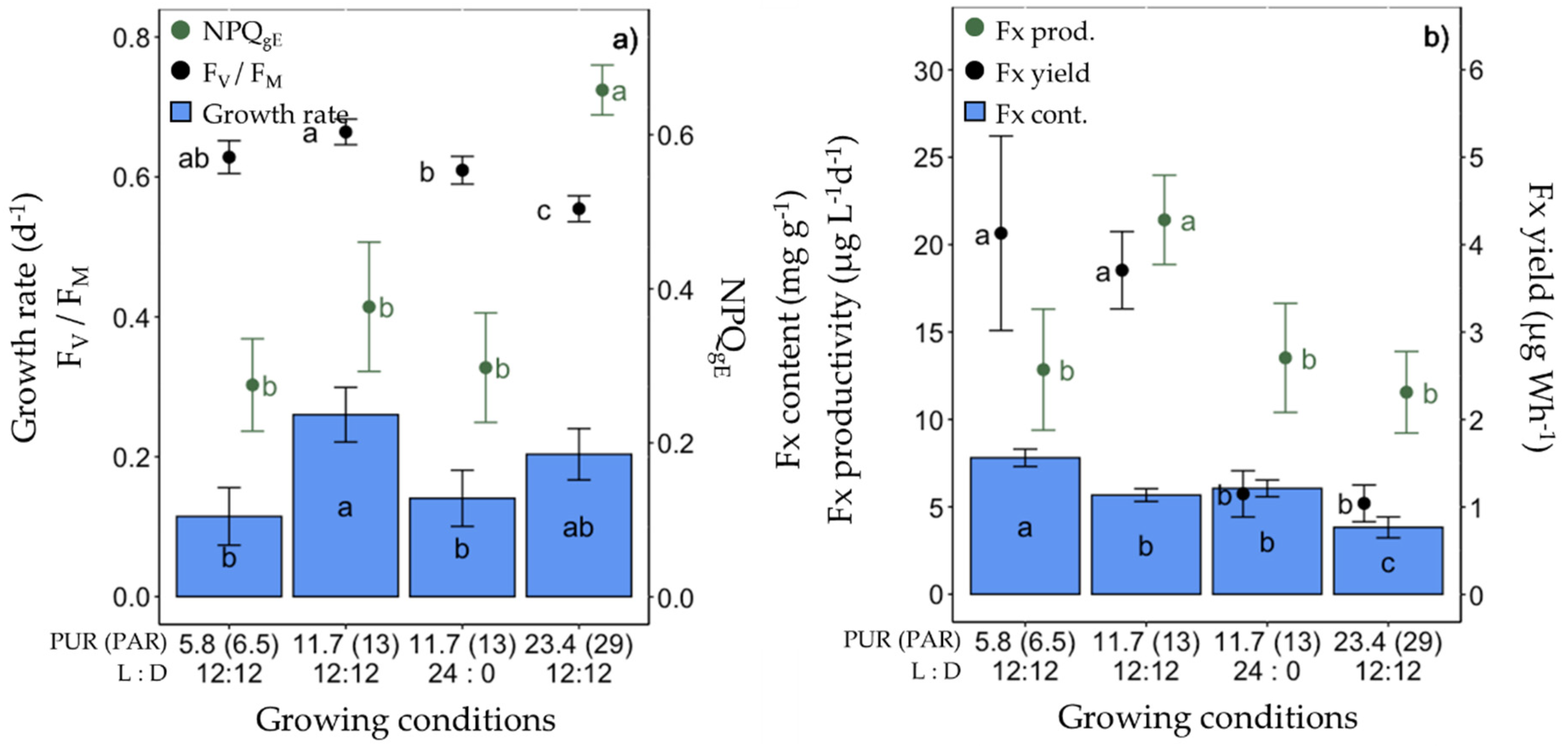
2.2. Production of Fx in F. cylindrus under Different Light Spectra
2.3. Production of Fx in F. cylindrus under Different Light Intensities
2.4. Production of Fx in F. cylindrus under Blue Light of Different Doses
2.5. Production of Fx in F. cylindrus under Different Temperatures
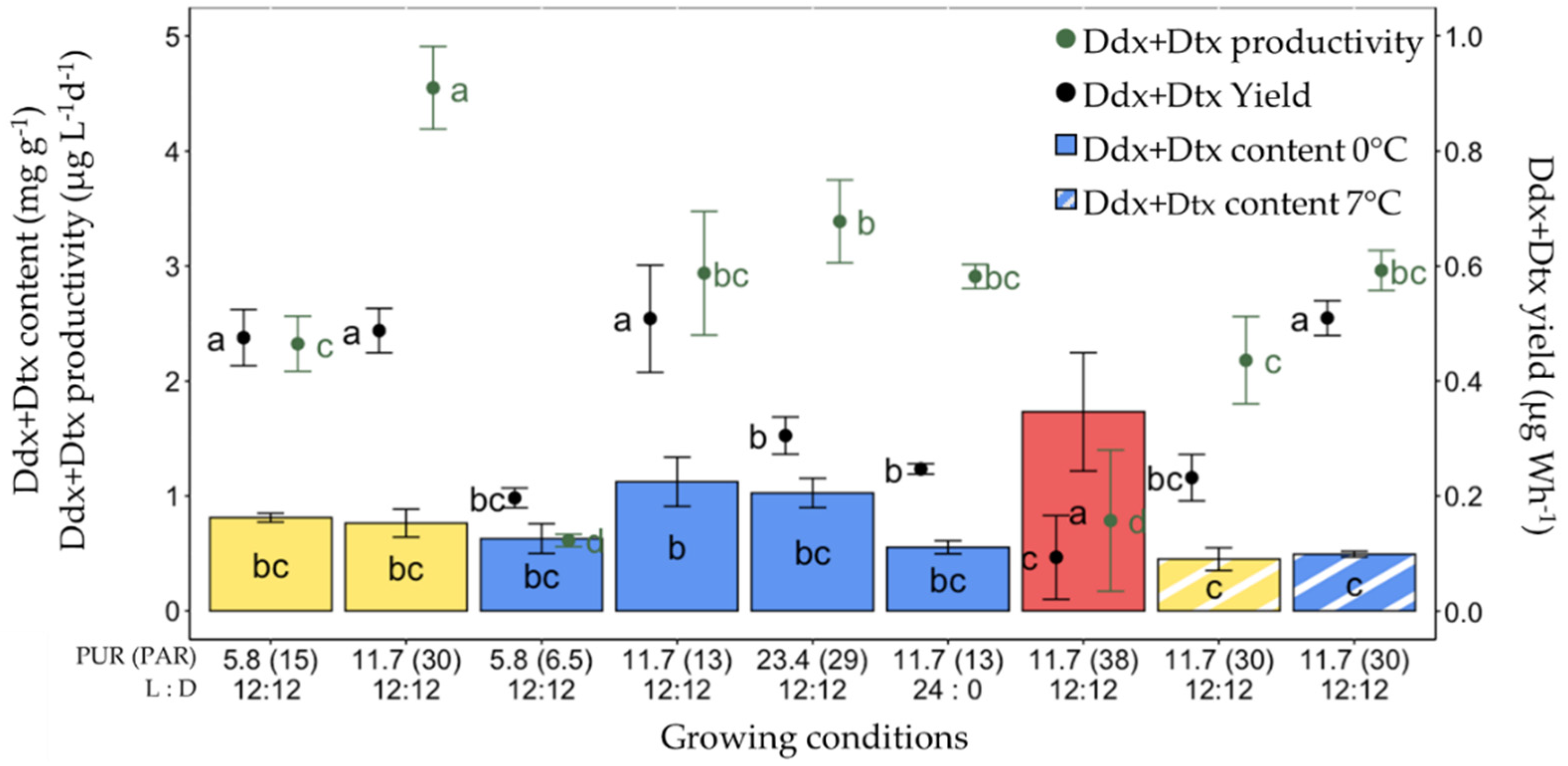
2.6. Production of Ddx+Dtx in F. cylindrus
2.7. Synthesis of All Conditions Tested for the Production of Fx and Ddx+Dtx in F. cylindrus
2.8. Fx producion in F. cylindrus Compared with Temperate Counterparts
3. Discussion
3.1. Acclimation of Fragilariopsis cylindrus to Different ‘White’ and Blue Light Photoperiods, and Intensities, and Effect of the Temperature
3.2. The Unique Response of F. cylindrus to Red Light
3.3. Fucoxanthin Production in F. cylindrus
3.4. Diadinoxanthin–Diatoxanthin Production in F. cylindrus
3.5. Comparison of F. cylindrus Fucoxanthin Production with Temperate Counterparts: Maximization through Growing Conditions
4. Materials and Methods
4.1. Culturing Conditions
4.2. Experimental Conditions and Sampling Plan
4.2.1. Optimization of Fucoxanthin (Fx) Productivity
4.2.2. Comparison of F. cylindrus Fx Productivity with Temperate Counterparts
4.3. PAR, PUR and Determination of Energy Consumption
4.4. Cell Concentration and Growth Rate
4.5. Particulate Organic Carbon and Nitrogen Determination, and Algal Biomass Dry Weight
4.6. Pigment Extraction and Quantification
4.7. Photosynthetic Performances
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2018, 31, 281–299. [Google Scholar] [CrossRef]
- Novoveska, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.; Philippidis, G.P. Fucoxanthin Production from Diatoms: Current Advances and Challenges. In Algae: Multifarious Applications for a Sustainable World; Springer: Singapore, 2021; pp. 227–242. [Google Scholar]
- Seth, K.; Kumar, A.; Rastogi, R.P.; Meena, M.; Vinayak, V. Harish, Bioprospecting of fucoxanthin from, 102475.diatoms—Challenges and perspectives. Algal Res. 2021, 60, 102475. [Google Scholar] [CrossRef]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Sansone, C.; Smerilli, A.; Festa, M.; Noonan, D.M.; Albini, A.; Brunet, C. MMP-9 and IL-1beta as Targets for Diatoxanthin and Related Microalgal Pigments: Potential Chemopreventive and Photoprotective Agents. Mar. Drugs 2021, 19, 354. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R. The Critical Studies of Fucoxanthin Research Trends from 1928 to June 2021: A Bibliometric Review. Mar. Drugs 2021, 19, 606. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M. Isolation and purification of all-trans diadinoxanthin and all-trans diatoxanthin from diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2017, 29, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef]
- Benoiston, A.S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The evolution of diatoms and their biogeochemical functions. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372, 20160397. [Google Scholar] [CrossRef] [Green Version]
- Dautermann, O.; Lyska, D.; Andersen-Ranberg, J.; Becker, M.; Frohlich-Nowoisky, J.; Gartmann, H.; Kramer, L.C.; Mayr, K.; Pieper, D.; Rij, L.M.; et al. An algal enzyme required for biosynthesis of the most abundant marine carotenoids. Sci. Adv. 2020, 6, eaaw9183. [Google Scholar] [CrossRef] [Green Version]
- Lacour, T.; Babin, M.; Lavaud, J. Diversity in Xanthophyll Cycle Pigments Content and Related Nonphotochemical Quenching (NPQ) Among Microalgae: Implications for Growth Strategy and Ecology. J. Phycol. 2020, 56, 245–263. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.Y.; Varjani, S.; Chang, J.S. Producing fucoxanthin from algae—Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2021, 344, 126170. [Google Scholar] [CrossRef]
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef]
- Manfellotto, F.; Stella, G.R.; Falciatore, A.; Brunet, C.; Ferrante, M.I. Engineering the Unicellular Alga Phaeodactylum tricornutum for Enhancing Carotenoid Production. Antioxidants 2020, 9, 757. [Google Scholar] [CrossRef]
- Hao, T.B.; Lu, Y.; Zhang, Z.H.; Liu, S.F.; Wang, X.; Yang, W.D.; Balamurugan, S.; Li, H.Y. Hyperaccumulation of fucoxanthin by enhancing methylerythritol phosphate pathway in Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2021, 105, 8783–8793. [Google Scholar] [CrossRef]
- Velmurugan, A.; Muthukaliannan, G.K. Genetic manipulation for carotenoid production in microalgae an overview. Curr. Res. Biotechnol. 2022, 4, 221–228. [Google Scholar] [CrossRef]
- Cen, S.-Y.; Li, D.-W.; Huang, X.-L.; Huang, D.; Balamurugan, S.; Liu, W.-J.; Zheng, J.-W.; Yang, W.-D.; Li, H.-Y. Crucial carotenogenic genes elevate hyperaccumulation of both fucoxanthin and β-carotene in Phaeodactylum tricornutum. Algal Res. 2022, 64, 102691. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Kvíderová, J.; Shukla, S.P.; Pushparaj, B.; Elster, J. Perspectives of Low-Temperature Biomass Production of Polar Microalgae and Biotechnology Expansion into High Latitudes. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Cham, Switzerland, 2017; pp. 585–600. [Google Scholar]
- Cheregi, O.; Ekendahl, S.; Engelbrektsson, J.; Stromberg, N.; Godhe, A.; Spetea, C. Microalgae biotechnology in Nordic countries—The potential of local strains. Physiol. Plant 2019, 166, 438–450. [Google Scholar] [CrossRef]
- Chauton, M.S.; Forbord, S.; Makinen, S.; Sarno, A.; Slizyte, R.; Mozuraityte, R.; Standal, I.B.; Skjermo, J. Sustainable resource production for manufacturing bioactives from micro- and macroalgae: Examples from harvesting and cultivation in the Nordic region. Physiol. Plant 2021, 173, 495–506. [Google Scholar] [CrossRef]
- Funk, C.; Jensen, P.E.; Skjermo, J. Blue economy in the North: Scandinavian algal biotechnology to the rescue. Physiol. Plant 2021, 173, 479–482. [Google Scholar] [CrossRef]
- Hopes, A.; Thomas, D.N.; Mock, T. Polar Microalgae: Functional Genomics, Physiology, and the Environment. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Cham, Switzerland, 2017; pp. 305–344. [Google Scholar]
- Huseby, S.; Degerlund, M.; Eriksen, G.K.; Ingebrigtsen, R.A.; Eilertsen, H.C.; Hansen, E. Chemical diversity as a function of temperature in six northern diatom species. Mar. Drugs 2013, 11, 4232–4245. [Google Scholar] [CrossRef] [Green Version]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef] [Green Version]
- Lacour, T.; Larivière, J.; Ferland, J.; Bruyant, F.; Lavaud, J.; Babin, M. The Role of Sustained Photoprotective Non-photochemical Quenching in Low Temperature and High Light Acclimation in the Bloom-Forming Arctic Diatom Thalassiosira gravida. Front. Mar. Sci. 2018, 5, 354. [Google Scholar] [CrossRef] [Green Version]
- Ferro, L.; Gentili, F.G.; Funk, C. Isolation and characterization of microalgal strains for biomass production and wastewater reclamation in Northern Sweden. Algal Res. 2018, 32, 44–53. [Google Scholar] [CrossRef]
- Cheregi, O.; Engelbrektsson, J.; Andersson, M.X.; Stromberg, N.; Ekendahl, S.; Godhe, A.; Spetea, C. Marine microalgae for outdoor biomass production-A laboratory study simulating seasonal light and temperature for the west coast of Sweden. Physiol. Plant 2021, 173, 543–554. [Google Scholar] [CrossRef]
- Salazar, J.; Valev, D.; Näkkilä, J.; Tyystjärvi, E.; Sirin, S.; Allahverdiyeva, Y. Nutrient removal from hydroponic effluent by Nordic microalgae: From screening to a greenhouse photobioreactor operation. Algal Res. 2021, 55, 102247. [Google Scholar] [CrossRef]
- Wurtzel, E.T. Changing Form and Function through Carotenoids and Synthetic Biology. Plant Physiol. 2019, 179, 830–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Wang, S.; Verma, S.K.; Hakeem Said, I.; Thomsen, L.; Ullrich, M.S.; Kuhnert, N. Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb. Cell Fact. 2018, 17, 110. [Google Scholar] [CrossRef]
- Archer, L.; McGee, D.; Parkes, R.; Paskuliakova, A.; McCoy, G.R.; Adamo, G.; Cusimano, A.; Bongiovanni, A.; Gillespie, E.; Touzet, N. Antioxidant Bioprospecting in Microalgae: Characterisation of the Potential of Two Marine Heterokonts from Irish Waters. Appl. Biochem. Biotechnol. 2021, 193, 981–997. [Google Scholar] [CrossRef]
- McGee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of spectral intensity and quality of LED lighting on photoacclimation, carbon allocation and high-value pigments in microalgae. Photosynth. Res. 2020, 143, 67–80. [Google Scholar] [CrossRef]
- Brunet, C.; Chandrasekaran, R.; Barra, L.; Giovagnetti, V.; Corato, F.; Ruban, A.V. Spectral radiation dependent photoprotective mechanism in the diatom Pseudo-nitzschia multistriata. PLoS ONE 2014, 9, e87015. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Barra, L.; Carillo, S.; Caruso, T.; Corsaro, M.M.; Dal Piaz, F.; Graziani, G.; Corato, F.; Pepe, D.; Manfredonia, A.; et al. Light modulation of biomass and macromolecular composition of the diatom Skeletonema marinoi. J. Biotechnol. 2014, 192 Pt A, 114–122. [Google Scholar] [CrossRef]
- Smerilli, A.; Orefice, I.; Corato, F.; Gavalas Olea, A.; Ruban, A.V.; Brunet, C. Photoprotective and antioxidant responses to light spectrum and intensity variations in the coastal diatom Skeletonema marinoi. Environ. Microbiol. 2017, 19, 611–627. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Wei, D. Improving Fucoxanthin Production in Mixotrophic Culture of Marine Diatom Phaeodactylum tricornutum by LED Light Shift and Nitrogen Supplementation. Front. Bioeng. Biotechnol. 2020, 8, 820. [Google Scholar] [CrossRef]
- Ehn, J.K.; Papakyriakou, T.N.; Barber, D.G. Inference of optical properties from radiation profiles within melting landfast sea ice. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Nicolaus, M.; Gerland, S.; Hudson, S.R.; Hanson, S.; Haapala, J.; Perovich, D.K. Seasonality of spectral albedo and transmittance as observed in the Arctic Transpolar Drift in 2007. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Mock, T.; Valentin, K. Photosynthesis and Cold Acclimation: Molecular Evidence from a Polar Diatom1. J. Phycol. 2004, 40, 732–741. [Google Scholar] [CrossRef]
- Yan, D.; Endo, H.; Suzuki, K. Increased temperature benefits growth and photosynthetic performance of the sea ice diatom Nitzschia cf. neglecta (Bacillariophyceae) isolated from saroma lagoon, Hokkaido, Japan. J. Phycol. 2019, 55, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Mock, T.; Otillar, R.P.; Strauss, J.; McMullan, M.; Paajanen, P.; Schmutz, J.; Salamov, A.; Sanges, R.; Toseland, A.; Ward, B.J.; et al. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 2017, 541, 536–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavoie, M.; Saint-Beat, B.; Strauss, J.; Guerin, S.; Allard, A.; S, V.H.; Falciatore, A.; Lavaud, J. Genome-Scale Metabolic Reconstruction and In Silico Perturbation Analysis of the Polar Diatom Fragilariopsis cylindrus Predicts High Metabolic Robustness. Biology 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Mock, T.; Hoch, N. Long-term temperature acclimation of photosynthesis in steady-state cultures of the polar diatom Fragilariopsis cylindrus. Photosynth. Res. 2005, 85, 307–317. [Google Scholar] [CrossRef]
- Petrou, K.; Kranz, S.A.; Trimborn, S.; Hassler, C.S.; Ameijeiras, S.B.; Sackett, O.; Ralph, P.J.; Davidson, A.T. Southern Ocean phytoplankton physiology in a changing climate. J. Plant Physiol. 2016, 203, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Croteau, D.; Guérin, S.; Bruyant, F.; Ferland, J.; Campbell, D.A.; Babin, M.; Lavaud, J. Contrasting nonphotochemical quenching patterns under high light and darkness aligns with light niche occupancy in Arctic diatoms. Limnol. Oceanogr. 2021, 66, S231–S245. [Google Scholar] [CrossRef]
- Morin, P.I.; Lacour, T.; Grondin, P.L.; Bruyant, F.; Ferland, J.; Forget, M.H.; Massicotte, P.; Donaher, N.; Campbell, D.A.; Lavaud, J.; et al. Response of the sea-ice diatom Fragilariopsis cylindrus to simulated polar night darkness and return to light. Limnol. Oceanogr. 2020, 65, 1041–1060. [Google Scholar] [CrossRef]
- Croteau, D.; Lacour, T.; Schiffrine, N.; Morin, P.-I.; Forget, M.-H.; Bruyant, F.; Ferland, J.; Lafond, A.; Campbell, D.A.; Tremblay, J.É.; et al. Shifts in growth light optima among diatom species support their succession during the spring bloom in the Arctic. J. Ecol. 2022, 110, 1356–1375. [Google Scholar] [CrossRef]
- Ni, G.; Zimbalatti, G.; Murphy, C.D.; Barnett, A.B.; Arsenault, C.M.; Li, G.; Cockshutt, A.M.; Campbell, D.A. Arctic Micromonas uses protein pools and non-photochemical quenching to cope with temperature restrictions on Photosystem II protein turnover. Photosynth. Res. 2017, 131, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Campbell, D.A.; Serôdio, J. Photoinhibition of Photosystem II in Phytoplankton: Processes and Patterns. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Springer: New York, NY, USA, 2020; pp. 329–365. [Google Scholar]
- Li, G.; Woroch, A.D.; Donaher, N.A.; Cockshutt, A.M.; Campbell, D.A. A Hard Day’s Night: Diatoms Continue Recycling Photosystem II in the Dark. Front. Mar. Sci. 2016, 3, 218. [Google Scholar] [CrossRef] [Green Version]
- Lavaud, J.; Six, C.; Campbell, D.A. Photosystem II repair in marine diatoms with contrasting photophysiologies. Photosynth. Res. 2016, 127, 189–199. [Google Scholar] [CrossRef]
- Lavaud, J.; Goss, R. The Peculiar Features of Non-Photochemical Fluorescence Quenching in Diatoms and Brown Algae. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Dordrecht, The Netherlands, 2014; pp. 421–443. [Google Scholar]
- Buck, J.M.; Sherman, J.; Bartulos, C.R.; Serif, M.; Halder, M.; Henkel, J.; Falciatore, A.; Lavaud, J.; Gorbunov, M.Y.; Kroth, P.G.; et al. Lhcx proteins provide photoprotection via thermal dissipation of absorbed light in the diatom Phaeodactylum tricornutum. Nat. Commun. 2019, 10, 4167. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Mills, M.M.; Kropuenske, L.R.; van Dijken, G.L.; Alderkamp, A.C.; Robinson, D.H. Photophysiology in two major southern ocean phytoplankton taxa: Photosynthesis and growth of Phaeocystis antarctica and Fragilariopsis cylindrus under different irradiance levels. Integr. Comp. Biol. 2010, 50, 950–966. [Google Scholar] [CrossRef] [Green Version]
- Lacour, T.; Larivière, J.; Babin, M. Growth, Chl a content, photosynthesis, and elemental composition in polar and temperate microalgae. Limnol. Oceanogr. 2017, 62, 43–58. [Google Scholar] [CrossRef]
- Kvernvik, A.C.; Rokitta, S.D.; Leu, E.; Harms, L.; Gabrielsen, T.M.; Rost, B.; Hoppe, C.J.M. Higher sensitivity towards light stress and ocean acidification in an Arctic sea-ice-associated diatom compared to a pelagic diatom. New Phytol. 2020, 226, 1708–1724. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.S.; Jungandreas, A.; Jakob, T.; Weisheit, W.; Mittag, M.; Wilhelm, C. Blue light is essential for high light acclimation and photoprotection in the diatom Phaeodactylum tricornutum. J. Exp. Bot. 2013, 64, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Schellenberger Costa, B.; Sachse, M.; Jungandreas, A.; Bartulos, C.R.; Gruber, A.; Jakob, T.; Kroth, P.G.; Wilhelm, C. Aureochrome 1a is involved in the photoacclimation of the diatom Phaeodactylum tricornutum. PLoS ONE 2013, 8, e74451. [Google Scholar] [CrossRef]
- Mann, M.; Serif, M.; Wrobel, T.; Eisenhut, M.; Madhuri, S.; Flachbart, S.; Weber, A.P.M.; Lepetit, B.; Wilhelm, C.; Kroth, P.G. The Aureochrome Photoreceptor PtAUREO1a Is a Highly Effective Blue Light Switch in Diatoms. iScience 2020, 23, 101730. [Google Scholar] [CrossRef]
- Bilcke, G.; Van Craenenbroeck, L.; Castagna, A.; Osuna-Cruz, C.M.; Vandepoele, K.; Sabbe, K.; De Veylder, L.; Vyverman, W. Light intensity and spectral composition drive reproductive success in the marine benthic diatom Seminavis robusta. Sci. Rep. 2021, 11, 17560. [Google Scholar] [CrossRef]
- Petrou, K.; Kranz, S.A.; Doblin, M.A.; Ralph, P.J. Photophysiological Responses of Fragilariopsis Cylindrus (Bacillariophyceae) to Nitrogen Depletion at Two Temperatures(1). J. Phycol. 2012, 48, 127–136. [Google Scholar] [CrossRef]
- Jabre, L.; Bertrand, E.M. Interactive effects of iron and temperature on the growth of Fragilariopsis cylindrus. Limnol. Oceanogr. Let. 2020, 5, 363–370. [Google Scholar] [CrossRef]
- Wilhelm, C.; Jungandreas, A.; Jakob, T.; Goss, R. Light acclimation in diatoms: From phenomenology to mechanisms. Mar. Genom. 2014, 16, 5–15. [Google Scholar] [CrossRef]
- Huysman, M.J.; Fortunato, A.E.; Matthijs, M.; Costa, B.S.; Vanderhaeghen, R.; Van den Daele, H.; Sachse, M.; Inze, D.; Bowler, C.; Kroth, P.G.; et al. AUREOCHROME1a-mediated induction of the diatom-specific cyclin dsCYC2 controls the onset of cell division in diatoms (Phaeodactylum tricornutum). Plant Cell 2013, 25, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Mouget, J.L.; Rosa, P.; Tremblin, G. Acclimation of Haslea ostrearia to light of different spectral qualities—Confirmation of ‘chromatic adaptation’ in diatoms. J. Photochem. Photobiol. B 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Valle, K.C.; Nymark, M.; Aamot, I.; Hancke, K.; Winge, P.; Andresen, K.; Johnsen, G.; Brembu, T.; Bones, A.M. System responses to equal doses of photosynthetically usable radiation of blue, green, and red light in the marine diatom Phaeodactylum tricornutum. PLoS ONE 2014, 9, e114211. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, A.E.; Jaubert, M.; Enomoto, G.; Bouly, J.P.; Raniello, R.; Thaler, M.; Malviya, S.; Bernardes, J.S.; Rappaport, F.; Gentili, B.; et al. Diatom Phytochromes Reveal the Existence of Far-Red-Light-Based Sensing in the Ocean. Plant Cell 2016, 28, 616–628. [Google Scholar] [CrossRef]
- Jaubert, M.; Duchêne, C.; Kroth, P.G.; Rogato, A.; Bouly, J.P.; Falciatore, A. Sensing and signalling in diatom responses to abiotic cues. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Furtauer, L.; Weiszmann, J.; Weckwerth, W.; Nagele, T. Dynamics of Plant Metabolism during Cold Acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [Green Version]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Vuong, T.T.; Choi, J.; Lee, T.S.; Um, J.-I.; Koo, S.Y.; Hwang, K.T.; Kim, S.M. Fucoxanthin biosynthesis has a positive correlation with the specific growth rate in the culture of microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2021, 33, 1473–1485. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, P.; Cai, Q.; Zhang, C.; Gao, B. Maximizing fucoxanthin production in Odontella aurita by optimizing the ratio of red and blue light-emitting diodes in an auto-controlled internally illuminated photobioreactor. Bioresour. Technol. 2021, 344, 126260. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2015, 28, 849–860. [Google Scholar] [CrossRef]
- Lavaud, J.; Rousseau, B.; van Gorkom, H.J.; Etienne, A.L. Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiol. 2002, 129, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Lavaud, J.; Rousseau, B.; Etienne, A.-L. Enrichment of the light-harvesting complex in diadinoxanthin and implications for the nonphotochemical fluorescence quenching in diatoms. Biochemistry 2003, 42, 5802–5808. [Google Scholar] [CrossRef] [Green Version]
- Barnett, A.; Meleder, V.; Blommaert, L.; Lepetit, B.; Gaudin, P.; Vyverman, W.; Sabbe, K.; Dupuy, C.; Lavaud, J. Growth form defines physiological photoprotective capacity in intertidal benthic diatoms. ISME J. 2015, 9, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Nymark, M.; Valle, K.C.; Brembu, T.; Hancke, K.; Winge, P.; Andresen, K.; Johnsen, G.; Bones, A.M. An integrated analysis of molecular acclimation to high light in the marine diatom Phaeodactylum tricornutum. PLoS ONE 2009, 4, e7743. [Google Scholar] [CrossRef] [Green Version]
- Su, Y. The effect of different light regimes on pigments in Coscinodiscus granii. Photosynth Res. 2019, 140, 301–310. [Google Scholar] [CrossRef]
- Arsalane, W.; Rousseau, B.; Duval, J.-C. Influence of the pool size of the xanthophyll cycle on the effects of light stress in a diatom: Competition between photoprotection and phoptoinhibition. J. Photochem. Photobiol. C 1994, 60, 237–243. [Google Scholar] [CrossRef]
- Conceição, D.; Lopes, R.G.; Derner, R.B.; Cella, H.; do Carmo, A.P.B.; Montes D’Oca, M.G.; Petersen, R.; Passos, M.F.; Vargas, J.V.C.; Galli-Terasawa, L.V.; et al. The effect of light intensity on the production and accumulation of pigments and fatty acids in Phaeodactylum tricornutum. J. Appl. Phycol. 2020, 32, 1017–1025. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Comparison of different photobioreactor configurations and empirical computational fluid dynamics simulation for fucoxanthin production. Algal Res. 2019, 37, 195–204. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A review on the progress, challenges and prospects in commercializing microalgal fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Transition from start-up to scale-up for fucoxanthin production in flat plate photobioreactor. J. Appl. Phycol. 2019, 31, 1525–1533. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Hulatt, C.J.; Morales-Sánchez, D.; Wijffels, R.H.; Kiron, V. Fatty acids and proteins from marine cold adapted microalgae for biotechnology. Algal Res. 2019, 42, 101604. [Google Scholar] [CrossRef]
- Svenning, J.B.; Dalheim, L.; Eilertsen, H.C.; Vasskog, T. Temperature dependent growth rate, lipid content and fatty acid composition of the marine cold-water diatom Porosira glacialis. Algal Res. 2019, 37, 11–16. [Google Scholar] [CrossRef]
- Steinrucken, P.; Prestegard, S.K.; de Vree, J.H.; Storesund, J.E.; Pree, B.; Mjos, S.A.; Erga, S.R. Comparing EPA production and fatty acid profiles of three Phaeodactylum tricornutum strains under western Norwegian climate conditions. Algal Res. 2018, 30, 11–22. [Google Scholar] [CrossRef]
- Afonso, C.; Bragança, A.R.; Rebelo, B.A.; Serra, T.S.; Abranches, R. Optimal Nitrate Supplementation in Phaeodactylum tricornutum Culture Medium Increases Biomass and Fucoxanthin Production. Foods 2022, 11, 568. [Google Scholar] [CrossRef]
- Hao, T.-B.; Yang, Y.-F.; Balamurugan, S.; Li, D.-W.; Yang, W.-D.; Li, H.-Y. Enrichment of f/2 medium hyperaccumulates biomass and bioactive compounds in the diatom Phaeodactylum tricornutum. Algal Res. 2020, 47, 101872. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Cherek, P.; Nelson, D.R.; Lin, J.; Rolfsson, O.; Wu, H.; Salehi-Ashtiani, K.; Brynjolfsson, S.; Fu, W. Combined artificial high-silicate medium and LED illumination promote carotenoid accumulation in the marine diatom Phaeodactylum tricornutum. Microb. Cell Fact. 2019, 18, 209. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef]
- Sahin, M.S.; Khazi, M.I.; Demirel, Z.; Dalay, M.C. Variation in growth, fucoxanthin, fatty acids profile and lipid content of marine diatoms Nitzschia sp. and Nanofrustulum shiloi in response to nitrogen and iron. Biocatal. Agric. Biotechnol. 2019, 17, 390–398. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, X.; Wang, X.; Zhu, B.; Pan, K.; Li, Y. Effect of Nitrogen Concentration on the Alkalophilic Microalga Nitzschia sp. NW129-a Promising Feedstock for the Integrated Production of Lipids and Fucoxanthin in Biorefinery. Front. Mar. Sci. 2022, 8. [Google Scholar] [CrossRef]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Fu, W.; Wichuk, K.; Brynjolfsson, S. Developing diatoms for value-added products: Challenges and opportunities. New Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal Physiology and Large-Scale Outdoor Cultures of Microalgae. In The Physiology of Microalgae; Springer: Cham, Switzerland, 2016; pp. 601–652. [Google Scholar]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Bozzato, D.; Jakob, T.; Wilhelm, C.; Trimborn, S. Effects of iron limitation on carbon balance and photophysiology of the Antarctic diatom Chaetoceros cf. simplex. Polar Biol. 2021, 44, 275–287. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Babin, M.; Stramski, D. Light absorption by aquatic particles in the near-infrared spectral region. Limnol. Oceanogr. 2002, 47, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Morel, A. Available, usable, and stored radiant energy in relation to marine photosynthesis. Deep Sea Res. 1978, 25, 673–688. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier/Academic Press: Burlington, VT, USA, 2005. [Google Scholar]
- Ras, J.; Claustre, H.; Uitz, J. Spatial variability of phytoplankton pigment distributions in the Subtropical South Pacific Ocean: Comparison between in situ and predicted data. Biogeosciences 2008, 5, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res. 2004, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lavaud, J.; Perkins, R.; Austen, E.; Bonnanfant, M.; Campbell, D.A. Phytoplankton σPSII and Excitation Dissipation; Implications for Estimates of Primary Productivity. Front. Mar. Sci. 2019, 5, 281. [Google Scholar] [CrossRef] [Green Version]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Pistelli, L.; Mondo, A.D.; Smerilli, A.; Corato, F.; Piscitelli, C.; Pellone, P.; Carbone, D.A.; Sansone, C.; Brunet, C. Microalgal Co-Cultivation Prospecting to Modulate Vitamin and Bioactive Compounds Production. Antioxidants 2021, 10, 1360. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Gao, B.; Huang, L.; Zhang, C. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. 2018, 32, 193–200. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenco-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Pajot, A.; Lavaud, J.; Carrier, G.; Garnier, M.; Saint-Jean, B.; Rabilloud, N.; Baroukh, C.; Bérard, J.-B.; Bernard, O.; Marchal, L.; et al. The Fucoxanthin Chlorophyll a/c-Binding Protein in Tisochrysis lutea: Influence of Nitrogen and Light on Fucoxanthin and Chlorophyll a/c-Binding Protein Gene Expression and Fucoxanthin Synthesis. Front. Plant Sci. 2022, 13, 830069. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
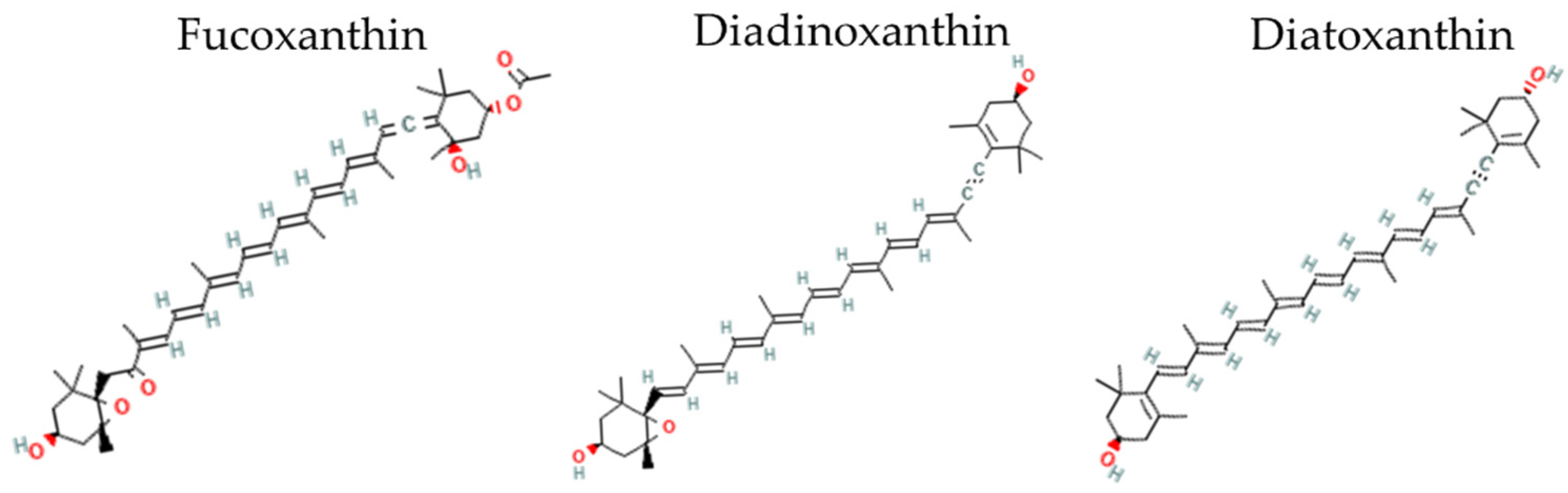




| Temperature (°C) | Photoperiod (Light:Dark) | Spectrum | PUR (µmol Photons m−2 s −1) | PAR (µmol Photons m−2 s −1) | Daily Energy Consumption (for 2.7 L Reactors) |
|---|---|---|---|---|---|
| 0 | 12 L:12 D | ‘White’ | 11.7 | 30 | 25.2 W |
| 0 | 12:12 | ‘White’ | 5.8 | 15 | 13.2 W |
| 7 | 12:12 | ‘White’ | 11.7 | 30 | 25.4 W |
| 0 | 12 L:12 D | Blue (445 nm) | 11.7 | 13 | 15.6 W |
| 0 | 12:12 | Blue | 5.8 | 6.5 | 8.4 W |
| 0 | 24 L:00 D | Blue | 11.7 | 13 | 31.8 W |
| 0 | 12:12 | Blue | 23.4 | 29 | 30 W |
| 7 | 12:12 | Blue | 11.7 | 13 | 15.7 W |
| 0 | 12 L:12 D | Red (660 nm) | 11.7 | 38 | 22.8 W |
| Parameter | Unit | Definition | Meaning | Measurement |
|---|---|---|---|---|
| F0 | No units | Minimum PSII Chl fluorescence yield | Used to calculate Fv/Fm | Rapid Light Curves-RLCs, after 30 min of dark acclimation |
| FM | No units | Maximum PSII Chl fluorescence yield | Used to calculate Fv/Fm, NPQ, YNPQ, YNO | RLCs, during a saturating pulse after 30 min of dark acclimation |
| F′ | No units | F for illuminated cells | Used to compute rETR | RLCs, after 30 s of illumination at specific light intensity-E |
| FM′ | No units | FM for illuminated cells | Used to compute NPQ and rETR | RLCs, during a saturating pulse after 30 s of illumination at specific E |
| FgE | No units | F for cells illuminated with the growing light gE | Used to calculate YPSII, YNPQ and YNO | RLCs, after 30 s of illumination at E the closest to the growing light gE |
| FMgE | No units | FM for cells illuminated with growing light gE | Used to compute YPSII and YNPQ. | RLCs, during a saturating pulse after 30 s of illumination at E the closest to the growing light gE |
| FV/FM | No units | Maximum photosynthetic efficiency of PSII; FV = FM – F0 | The dark-acclimated photochemical efficiency of photosystem II | / |
| rETR | μmol electrons m−2 s−1 | Relative photosynthetic electron transport rate | Effective quantum yield of photochemistry vs. E | RLCs |
| NPQ | rel. unit. | Non-photochemical quenching | Estimates the photoprotective dissipation of excess light energy | RLCs |
| rETRmax | μmol electrons m−2 s−1 | rETR-E curve asymptote | Maximum relative photosynthetic electron transport rate | Derived from fitted rETR-E curves measured with RLCs |
| NPQmax | rel. unit. | NPQ-E curve asymptote | Maximum non-photochemical quenching | RLCs |
| NPQgE | rel. unit. | Non-photochemical quenching | Estimates of the photoprotective dissipation of excess energy under the growing light intensity gE | RLCs |
| YPSII | rel. unit. | Quantum yield of photochemical energy conversion in PSII = () | Estimates the fraction of energy photochemically converted through PSII | RLCs |
| YNPQ | rel. unit. | Quantum yield of regulated non-photochemical energy loss in PSII = () | Estimates the fraction of energy dissipated as heat via the regulated NPQ | RLCs |
| YNO | rel. unit. | Quantum yield of non-regulated non-photochemical energy loss in PSII = () | Estimates the fraction of energy that is passively dissipated as heat and fluorescence | RLCs |
| Parameter | Unit | Definition | Meaning | Method |
| Chl a | mg L−1 | Volumetric chlorophyll a concentration | Chl a concentration | HPLC pigments quantification |
| Fx | mol 100 mol−1 | Fucoxanthin | Fx for 100 mol of Chl a | HPLC pigments quantification |
| Ddx | mol 100 mol−1 | Diadinoxanthin | Ddx for 100 mol of Chl a | HPLC pigments quantification |
| Dtx | mol 100 mol−1 | Diatoxanthin | Dtx for 100 mol of Chl a | HPLC pigments quantification |
| Ddx+Dtx | mol 100 mol−1 | Xanthophyll pool | Ddx+Dtx for 100 mol of Chl a | HPLC pigments quantification |
| Cells | cells mL−1 | Algae cellular density | Count of cells per volume of culture | Particle sizer and counter |
| μ | d−1 | Growth rate | Population division rate per day | Calculated every 24 h |
| P | Wh | Power consumption | Power consumption of the lightning source for a culture of 2.7 L. | Consumption measured at the outlet for a 24 h period |
| C | mg L1 | Total particulate carbon | Carbon content of the particulate fraction of the culture | CHN analyser |
| N | mg L−1 | Total particulate nitrogen content | Nitrogen content of the particulate fraction of the culture | CHN analyser |
| DW | mg L−1 | Dry weight | Dry weight of the particulate fraction of the culture | Gravimetry |
| C/N | g g−1 | Carbon:nitrogen ratio | / | / |
| Cellular C | pg cell−1 | Intracellular carbon content | / | / |
| Cellular N | pg cell−1 | Intracellular nitrogen content | / | / |
| Cellular Chl a | pg cell−1 | Intracellular chlorophyll a content | / | / |
| Fx cont. | mg g−1 | Fucoxanthin content | Fucoxanthin content per dry weight of algae cells | / |
| Ddx+Dtx cont. | mg g−1 | Diadinoxanthin+diatoxanthin content | Diadinoxanthin+diatoxanthin content per unit of dry weight of algae cells | / |
| Fx prod. | µg L−1 day−1 | Fucoxanthin productivity | Fucoxanthin produced per day in culturing conditions | / |
| Ddx+Dtx prod. | µg L−1 day−1 | Diadinoxanthin+diatoxanthin productivity | Diadinoxanthin+diatoxanthin produced per day in culturing conditions | / |
| Fx yield | µg Wh | Fucoxanthin production | Fucoxanthin produced per unit of energy consumed | / |
| Ddx+tx yield | µg Wh | Diadinoxanthin+diatoxanthin production | Diadinoxanthin+diatoxanthin produced per unit of energy consumed | / |
| PAR (µmol Photons m−2 s−1) | Growth Rate (Day−1) | Fucoxanthin Productivity (µg L−1 Day−1) | Ddx+Dtx Productivity (µg L−1 Day−1) | |
|---|---|---|---|---|
| Nitzschia frigida | 15 | 0.17 ± 0.01 | 32.4 ± 0.9 | 2.82 ± 0.08 |
| 50 | 0.12 ± 0.01 | 10.5 ± 0.9 | 1.72 ± 0.15 | |
| Fragilariopsis cylindrus | 15 | 0.1 ± 0.03 | 4.89 ± 2.21 | 2.34 ± 0.78 |
| 50 | 0.25 ± 0.05 | 11.93 ± 3.39 | 5.45 ± 1.55 | |
| Thalassiosira gravida | 10 | 0.21 ± 0.03 | 32.5 ± 5.5 | 1.12 ± 0.19 |
| 50 | 0.32 ± 0.01 | 112.6 ± 15.5 | 8.86 ± 1.22 | |
| Chaetoceros neogracilis | 15 | 0.55 ± 0.01 | 200.4 ± 8.6 | 4.65 ± 0.13 |
| 50 | 0.62 ± 0.03 | 261.7 ± 15.7 | 32.38 ± 1.39 | |
| Chaetoceros gelidus | 15 | 0.20 ± 0.07 | 35.4 ± 9.3 | 2.94 ± 0.77 |
| 50 | 0.33 ± 0.03 | 62.7 ± 4.5 | 13.24 ± 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guérin, S.; Raguénès, L.; Croteau, D.; Babin, M.; Lavaud, J. Potential for the Production of Carotenoids of Interest in the Polar Diatom Fragilariopsis cylindrus. Mar. Drugs 2022, 20, 491. https://doi.org/10.3390/md20080491
Guérin S, Raguénès L, Croteau D, Babin M, Lavaud J. Potential for the Production of Carotenoids of Interest in the Polar Diatom Fragilariopsis cylindrus. Marine Drugs. 2022; 20(8):491. https://doi.org/10.3390/md20080491
Chicago/Turabian StyleGuérin, Sébastien, Laura Raguénès, Dany Croteau, Marcel Babin, and Johann Lavaud. 2022. "Potential for the Production of Carotenoids of Interest in the Polar Diatom Fragilariopsis cylindrus" Marine Drugs 20, no. 8: 491. https://doi.org/10.3390/md20080491
APA StyleGuérin, S., Raguénès, L., Croteau, D., Babin, M., & Lavaud, J. (2022). Potential for the Production of Carotenoids of Interest in the Polar Diatom Fragilariopsis cylindrus. Marine Drugs, 20(8), 491. https://doi.org/10.3390/md20080491






