New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia laevis
Abstract
1. Introduction
2. Results and Discussion
2.1. Glucose Supplementation Enhances Biomass Accumulation
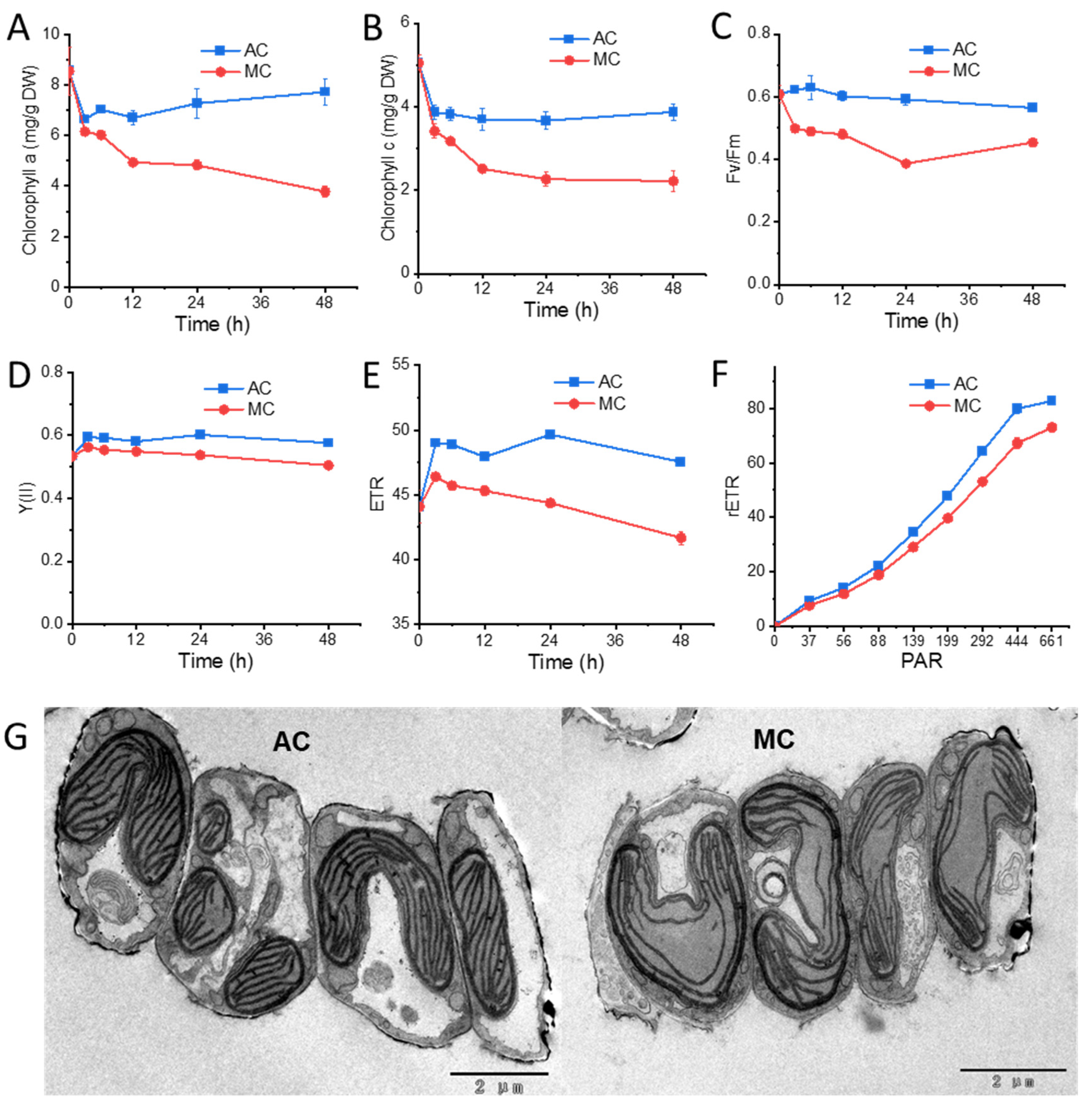
2.2. Cells under Glucose Supplementation Exhibit Reduced Photosynthesis Activity
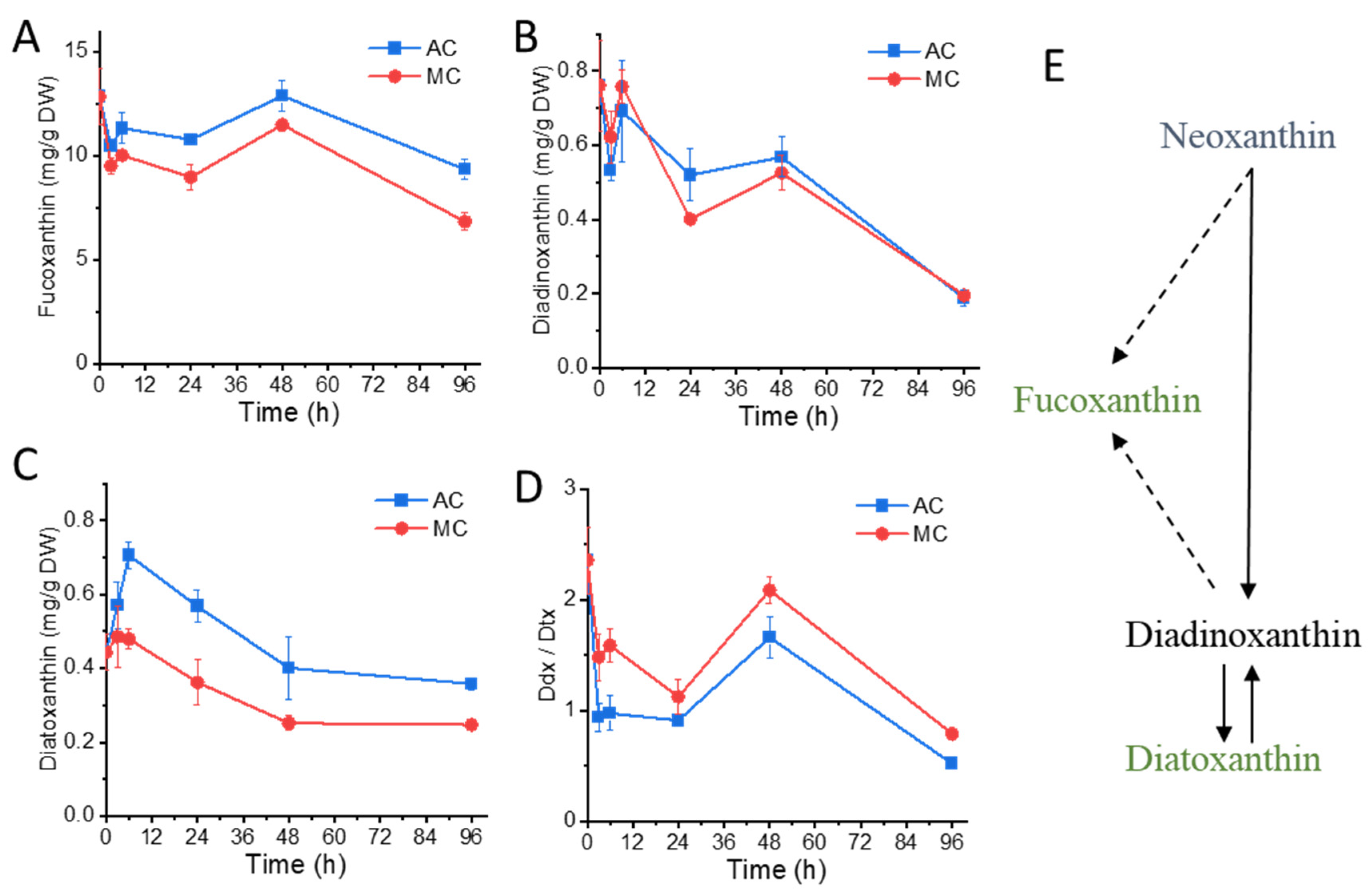
2.3. Glucose Supplementation Alters Xanthophylls Profiles
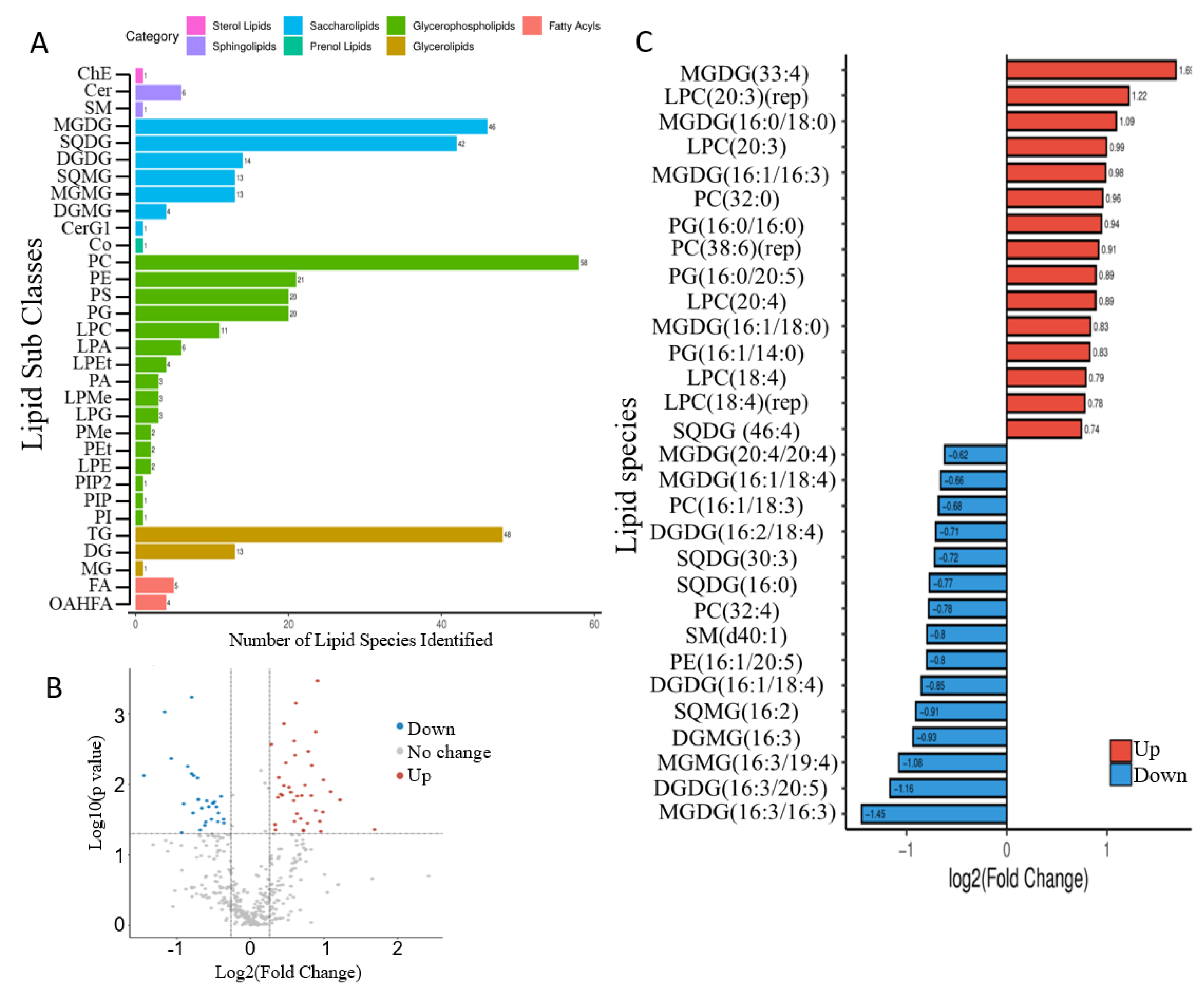
2.4. Mixotrophic Cultivation Improves Fatty Acid Productivity
2.5. Glucose Sensing Is Involved in the Regulation of Pigments and Lipids
3. Materials and Methods
3.1. Algal Strain and Cultivation Conditions
3.2. Biomass and Parameters for Photosynthesis System
3.3. Analysis of Xanthophylls
3.4. Transmission Electron Microscopy (TEM)
3.5. Lipidomic Analysis
3.6. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, B.; Liu, B.; Wei, H.; Cheng, K.W.; Chen, F. Extract of the Microalga Nitzschia laevis Prevents High-Fat-Diet-Induced Obesity in Mice by Modulating the Composition of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1800808. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Liu, Y. Anti-Inflammatory and Apoptotic Signaling Effect of Fucoxanthin on Benzo(A)Pyrene-Induced Lung Cancer in Mice. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Jacobson, T.A. Effects of Eicosapentaenoic Acid Versus Docosahexaenoic Acid on Serum Lipids: A Systematic Review and Meta-Analysis. Curr. Atheroscler. Rep. 2012, 14, 93. [Google Scholar] [CrossRef][Green Version]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arter. Throm. Vas. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Lu, X.; Liu, B.; He, Y.; Guo, B.; Sun, H.; Chen, F. Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresour. Technol. 2019, 294, 122145. [Google Scholar] [CrossRef]
- Nagao, R.; Kato, K.; Suzuki, T.; Ifuku, K.; Uchiyama, I.; Kashino, Y.; Dohmae, N.; Akimoto, S.; Shen, J.R.; Miyazaki, N.; et al. Structural basis for energy harvesting and dissipation in a diatom PSII-FCPII supercomplex. Nat. Plants 2019, 5, 890–901. [Google Scholar] [CrossRef]
- Wang, W.; Yu, L.J.; Xu, C.; Tomizaki, T.; Zhao, S.; Umena, Y.; Chen, X.; Qin, X.; Xin, Y.; Suga, M.; et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science 2019, 363, eaav0365. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic Pathway and Health Benefits of Fucoxanthin, an Algae-Specific Xanthophyll in Brown Seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Muizelaar, W.; Boelen, P.; Buma, A.G.J. Environmental and nutrient conditions influence fucoxanthin productivity of the marine diatom Phaeodactylum tricornutum grown on palm oil mill effluent. J. Appl. Phycol. 2019, 31, 111–122. [Google Scholar] [CrossRef]
- Dias, E.; Oliveira, M.; Manageiro, V.; Vasconcelos, V.; Canica, M. Deciphering the role of cyanobacteria in water resistome: Hypothesis justifying the antibiotic resistance (phenotype and genotype) in Planktothrix genus. Sci. Total Environ. 2019, 652, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, K.; Wan, L.L.; Li, A.F.; Hu, Q.; Zhang, C.W. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Muhlroth, A.; Li, K.; Rokke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar. Drugs 2013, 11, 4662–4697. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Y.; Jiang, J.P.; Wang, H.T.; Cao, X.P.; Xue, S.; Yang, Q.; Wang, W.L. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Jiang, Y.; Chen, F. salt-induced alterations in lipid composition of diatom Nitzschia laevis (bacillariophyceae) under heterotrophic culture condition. J. Phycol. 2008, 44, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.S.; Gallaher, S.D.; Westcott, D.J.; Iwai, M.; Louie, K.B.; Mueller, M.; Walter, A.; Foflonker, F.; Bowen, B.P.; Ataii, N.N.; et al. Regulation of Oxygenic Photosynthesis during Trophic Transitions in the Green Alga Chromochloris zofingiensis. Plant Cell 2019, 31, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Heterotrophic production of eicosapentaenoid acid by the diatom Nitzschia laevis: Effects of silicate and glucose. J. Ind. Microbiol. Biotechnol. 2000, 25, 218–224. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F. Effects of medium glucose concentration and pH on docosahexaenoic acid content of heterotrophic Crypthecodinium cohnii. Process Biochem. 2000, 35, 1205–1209. [Google Scholar] [CrossRef]
- Chen, G.Q.; Chen, F. Growing phototrophic cells without light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Jiang, Y.; Chen, F. High cell density culture of the diatom Nitzschia laevis for eicosapentaenoic acid production: Fed-batch development. Process Biochem. 2002, 37, 1447–1453. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, C.; Schmalzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, D.Z.; Zhang, Y.; Chen, F. Glucose triggers cell structure changes and regulates astaxanthin biosynthesis in Chromochloris zofingiensis. Algal Res. 2019, 39, 101455. [Google Scholar] [CrossRef]
- Beer, A.; Gundermann, K.; Beckmann, J.; Buchel, C. Subunit composition and pigmentation of fucoxanthin-chlorophyll proteins in diatoms: Evidence for a subunit involved in diadinoxanthin and diatoxanthin binding. Biochemistry 2006, 45, 13046–13053. [Google Scholar] [CrossRef]
- Mondal, M.; Ghosh, A.; Tiwari, O.N.; Gayen, K.; Das, P.; Mandal, M.K.; Halder, G. Influence of carbon sources and light intensity on biomass and lipid production of Chlorella sorokiniana BTA 9031 isolated from coalfield under various nutritional modes. Energy Convers. Manag. 2017, 145, 247–254. [Google Scholar] [CrossRef]
- Ren, X.J.; Chen, J.K.; Deschenes, J.S.; Tremblay, R.; Jolicoeur, M. Glucose feeding recalibrates carbon flux distribution and favours lipid accumulation in Chlorella protothecoides through cell energetic management. Algal Res. 2016, 14, 83–91. [Google Scholar] [CrossRef]
- Li, T.T.; Kirchhoff, H.; Gargouri, M.; Feng, J.; Cousins, A.B.; Pienkos, P.T.; Gang, D.R.; Chen, S.L. Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res. 2016, 19, 30–38. [Google Scholar] [CrossRef]
- Deng, X.Y.; Xue, C.Y.; Chen, B.; Amoah, P.K.; Li, D.; Hu, X.L.; Gao, K. Glucose addition-induced changes in the growth and chemical compositions of a freshwater microalga Chlorella kessleri. J. Chem. Technol. Biot. 2019, 94, 1202–1209. [Google Scholar] [CrossRef]
- Sun, P.; Wong, C.-C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 2019, 277, 566–572. [Google Scholar] [CrossRef]
- Pi, X.; Zhao, S.H.; Wang, W.D.; Liu, D.S.; Xu, C.Z.; Han, G.Y.; Kuang, T.Y.; Sui, S.F.; Shen, J.R. The pigment-protein network of a diatom photosystem II-light-harvesting antenna supercomplex. Science 2019, 365, 463. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Nowicka, B.; Jakubowska, A.; Strzalka, W.; Burda, K.; Strzalka, K. The xanthophyll cycle in diatom Phaeodactylum tricornutum in response to light stress. Plant Physiol. Biochem. 2020, 152, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Buchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Wilhelm, C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc. Natl. Acad. Sci. USA 1999, 96, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Production potential of eicosapentaenoic acid by the diatom Nitzschia Laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- Goss, R.; Latowski, D. Lipid Dependence of Xanthophyll Cycling in Higher Plants and Algae. Front. Plant Sci. 2020, 11, 455. [Google Scholar] [CrossRef]
- Schmid-Siegert, E.; Stepushenko, O.; Glauser, G.; Farmer, E.E. Membranes as structural antioxidants recycling of malondialdehyde to its source in oxidation-sensitive chloroplast fatty acids. J. Biol. Chem. 2016, 291, 13005–13013. [Google Scholar] [CrossRef]
- Roth, M.S.; Westcott, D.J.; Iwai, M.; Niyogi, K.K. Hexokinase is necessary for glucose-mediated photosynthesis repression and lipid accumulation in a green alga. Commun. Biol. 2019, 2, 347. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Sandmann, G.; Chen, F. Glucose sensing and the mitochondrial alternative pathway are involved in the regulation of astaxanthin biosynthesis in the dark-grown Chlorella zofingiensis (Chlorophyceae). Planta 2008, 228, 735–743. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, B, C1 and C2 in Higher-Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pfl. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Mao, X.; Lao, Y.; Sun, H.; Li, X.; Yu, J.; Chen, F. Time-resolved transcriptome analysis during transitions of sulfur nutritional status provides insight into triacylglycerol (TAG) and astaxanthin accumulation in the green alga Chromochloris zofingiensis. Biotechnol. Biofuels 2020, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.L.; Zeng, C.W.; Liu, S.Q. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acids | AC Group 1 | MC Group 2 |
|---|---|---|
| TFA content (% DW) | 8.53 ± 0.54 3 | 9.42 ± 0.07 |
| TFA yield (mg/L) | 65.70 ± 0.82 | 125.66 ± 3.50 |
| EPA (% DW) | 1.31 ± 0.11 | 1.52 ± 0.06 |
| EPA yield (mg/L) | 10.15 ± 0.88 | 20.36 ± 1.22 |
| C14:0 (% TFA) | 3.05 ± 0.02 | 3.77 ± 0.06 |
| C16:0 (% TFA) | 16.78 ± 0.26 | 19.42 ± 0.34 |
| C16:1 (% TFA) | 27.72 ± 0.51 | 26.74 ± 0.68 |
| C16:2 (% TFA) | 9.95 ± 0.19 | 9 ± 0.52 |
| C16:3 (% TFA) | 4.68 ± 0.1 | 2.38 ± 0.11 |
| C18:0 (% TFA) | 0.65 ± 0.04 | 0.57 ± 0.05 |
| C18:1 (% TFA) | 0.84 ± 0.05 | 1.65 ± 0.09 |
| C18:2 (% TFA) | 0.48 ± 0.02 | 0.94 ± 0.05 |
| C18:3 (% TFA) | 2.38 ± 0.04 | 2.27 ± 0.08 |
| C18:4 (% TFA) | 2.18 ± 0.06 | 1.03 ± 0.04 |
| C20:4 (% TFA) | 14.51 ± 0.18 | 14.47 ± 0.38 |
| C20:5 (% TFA) | 15.43 ± 0.37 | 16.2 ± 0.6 |
| C24:0 (% TFA) | 1.33 ± 0.08 | 1.55 ± 0.01 |
| SFA (% TFA) 4 | 21.82 ± 0.32 | 25.31 ± 0.38 |
| MFA (% TFA) 5 | 28.56 ± 0.52 | 28.39 ± 0.74 |
| PUFA (% TFA) 6 | 49.63 ± 0.71 | 46.3 ± 0.98 |
| ∆/mol 7 | 2.15 ± 0.03 | 2.05 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Wang, X.; Ge, M.; Chen, F.; Liu, B. New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia laevis. Mar. Drugs 2022, 20, 456. https://doi.org/10.3390/md20070456
Mao X, Wang X, Ge M, Chen F, Liu B. New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia laevis. Marine Drugs. 2022; 20(7):456. https://doi.org/10.3390/md20070456
Chicago/Turabian StyleMao, Xuemei, Xia Wang, Mengdie Ge, Feng Chen, and Bin Liu. 2022. "New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia laevis" Marine Drugs 20, no. 7: 456. https://doi.org/10.3390/md20070456
APA StyleMao, X., Wang, X., Ge, M., Chen, F., & Liu, B. (2022). New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia laevis. Marine Drugs, 20(7), 456. https://doi.org/10.3390/md20070456







