Amino Acid-Coupled Bromophenols and a Sulfated Dimethylsulfonium Lanosol from the Red Alga Vertebrata lanosa
Abstract
1. Introduction
2. Results
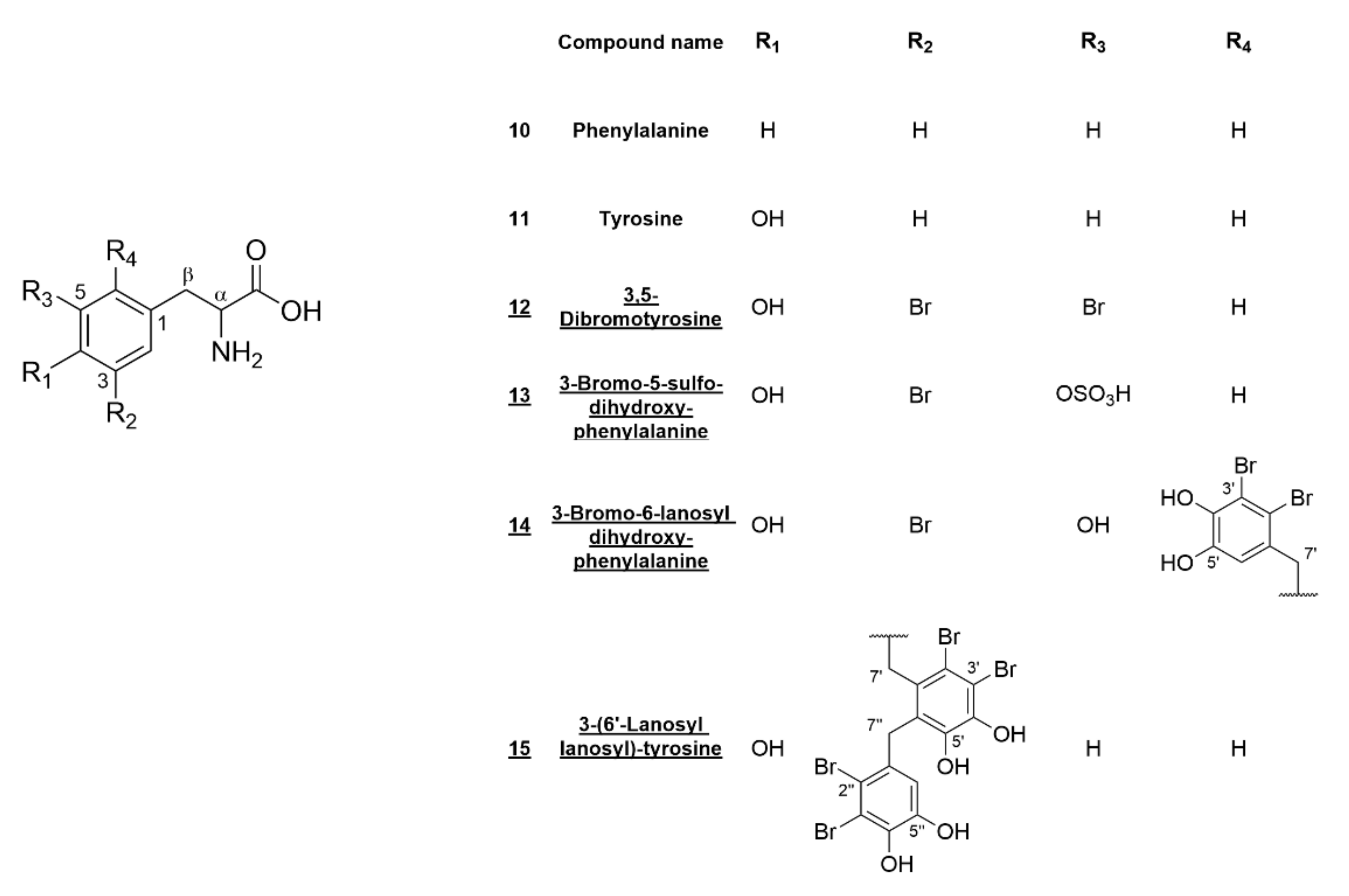
2.1. Sulfated Bromophenols and Amino Acid Derivatives
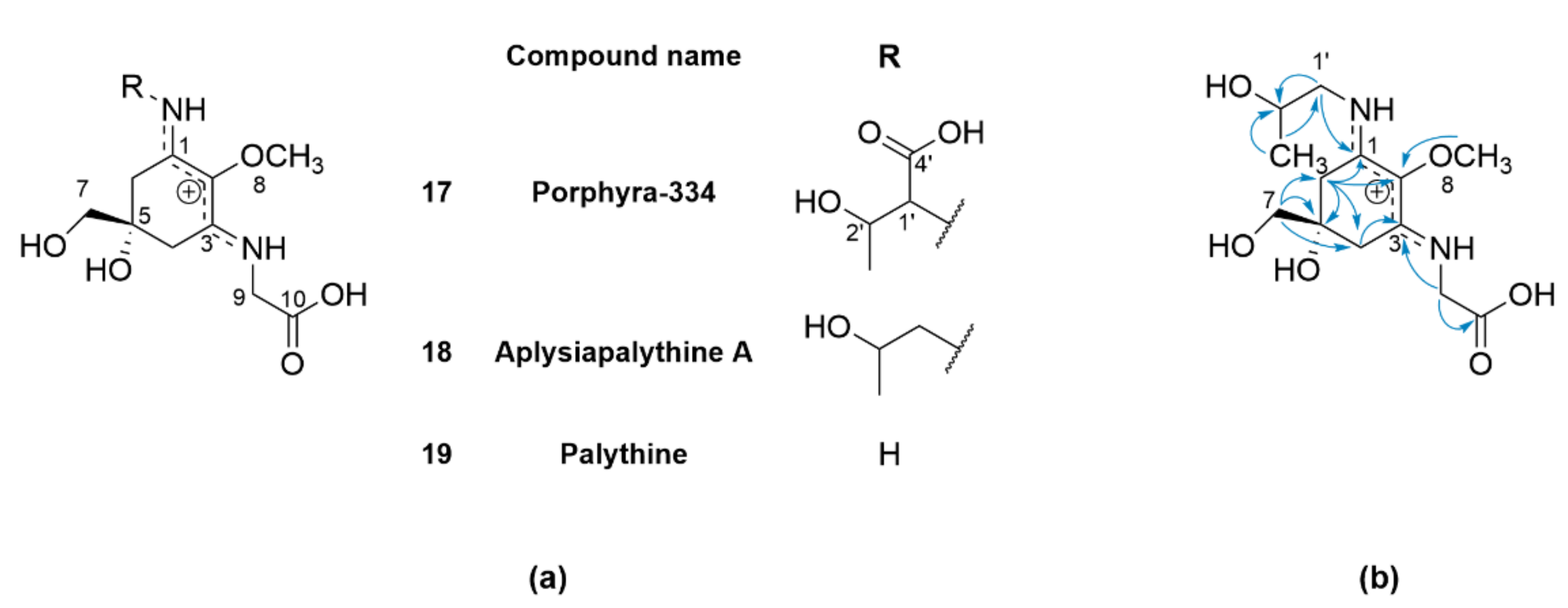
2.2. Mycosporine-Like Amino Acids
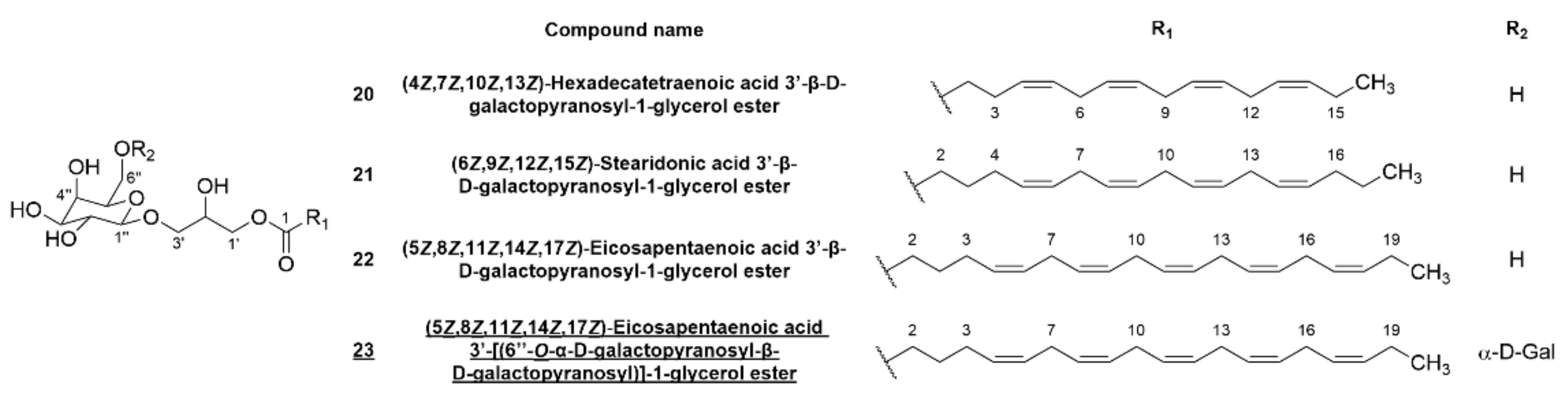
2.3. Bromophenols and Glycerogalactolipids
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. General Analytical Methods
4.3. Hydrolysis of Glycosides
4.4. Capillary Zone Electrophoresis (CZE)
4.5. Extraction and Fractionation
4.5.1. Solvent Partitioning of the Extracts
4.5.2. Fast Centrifugal Partition Chromatography (FCPC)
4.5.3. Medium-Pressure Liquid Chromatography (MPLC)
4.5.4. Sugar Precipitation and Ion Exchange Chromatography
4.5.5. Preparative HPLC
4.6. Structure Elucidation of Isolated Compounds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guiry, M.D. AlgaeBase: World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 3 May 2022).
- Bjordal, M.V.; Jensen, K.H.; Sjøtun, K. A field study of the edible red alga Vertebrata lanosa (Rhodophyta). J. Appl. Phycol. 2020, 32, 671–681. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Stoffelen, H.; Glombitza, K.W.; Murawski, U.; Bielaczek, J.; Egge, H. Bromphenole aus Polysiphonia lanosa (L.) Tandy. Planta Med. 1972, 22, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.-W.; Stoffelen, H.; Murawski, U.; Bielaczek, J.; Egge, H. Antibiotica aus Algen. Planta Med. 1974, 25, 105–114. [Google Scholar] [CrossRef]
- Hofer, S.; Hartmann, A.; Orfanoudaki, M.; Ngoc, H.N.; Nagl, M.; Karsten, U.; Heesch, S.; Ganzera, M. Development and Validation of an HPLC Method for the Quantitative Analysis of Bromophenolic Compounds in the Red Alga Vertebrata lanosa. Mar. Drugs 2019, 17, 675. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.K.; Hansen, E.; Isaksson, J.; Andersen, J.H. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar. Drugs 2013, 11, 2769–2784. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.W.; Sukopp, I.; Wiedenfeld, H. Antibiotics from Algae XXXVII. Rhodomelol and Methylrhodomelol from Polysiphonia lanosa. Planta Med. 1985, 51, 437–440. [Google Scholar] [CrossRef]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wheelhouse, R.T.; Wright, C.W. In-vitro cytotoxic activities of the major bromophenols of the red alga Polysiphonia lanosa and some novel synthetic isomers. J. Nat. Prod. 2004, 67, 1445–1449. [Google Scholar] [CrossRef]
- Ragan, M.A.; Mackinnon, M.D. Paired-ion reversed-phase high-performance liquid chromatography of phenol sulfates in synthetic mixtures, algas extracts and urine. J. Chromatogr. A 1979, 178, 505–513. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Stoffelen, H. 2,3-Dibrom-5-hydroxybenzyl-1′,4-disulfat (dikaliumsalz) aus Rhodomelaceen. Planta Med. 1972, 22, 391–395. [Google Scholar] [CrossRef]
- Hodgkin, J.H.; Craigie, J.S.; McInnes, A.G. the occurrence of 2,3-dibromobenzyl alcohol 4,5-disulfate, dipotassium salt, in Polysiphonia lanosa. Can. J. Chem. 1966, 44, 74–78. [Google Scholar] [CrossRef]
- Batey, J.F.; Turvey, J.R. The galactan sulphate of the red alga Polysiphonia lanosa. Carbohydr. Res. 1975, 43, 133–143. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wright, C.W. Seasonal Variation in Bromophenol Content of Polysiphonia lanosa. Nat. Prod. Comm. 2006, 1, 1934578X0600100109. [Google Scholar] [CrossRef]
- De Carvalho, L.R.; Guimarães, S.M.d.B.; Roque, N.F. Sulfated bromophenols from Osmundaria obtusiloba (C. Agardh) R. E. Norris (Rhodophyta, Ceramiales). Rev. Bras. Bot. 2006, 29, 453–459. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujimaki, K.; Sampei, O.; Murai, A. Gonyol: Methionine-induced sulfonium accumulation in a dinoflagellate Gonyaulax polyedra. Tetrahedron Lett. 1993, 34, 8481–8484. [Google Scholar] [CrossRef]
- Phillips, R.S.; Busby, S.; Edenfield, L.; Wickware, K. Preparation of 3-bromo-L-tyrosine and 3,5-dibromo-L-tyrosine. Amino Acids 2013, 44, 529–532. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Wu, S.; Wei, Y.; Liu, Q.; Liu, J.; Jiao, Q. Two-step enzymatic synthesis of tyramine from raw pyruvate fermentation broth. J. Mol. Catal. B Enzym. 2016, 124, 38–44. [Google Scholar] [CrossRef]
- Manley, S.L.; Chapman, D.J. Formation of 3-bromo-4-hydroxybenzaldehyde from L-tyrosine in cell-free homogenates of Odonthalia floccosa (Rhodophyceae): A proposed biosynthetic pathway for brominated phenols. FEBS Lett. 1978, 93, 97–101. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef]
- Nishida, Y.; Saburi, W.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2022, 20, 184. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. Microbial Transformation of Dihydroxyphenylacetic Acid by the Marine-Derived Bacterium Stappia sp. Bull. Korean Chem. Soc. 2014, 35, 2870–2872. [Google Scholar] [CrossRef]
- Popplewell, W.L.; Northcote, P.T. Colensolide A: A new nitrogenous bromophenol from the New Zealand marine red alga Osmundaria colensoi. Tetrahedron Lett. 2009, 50, 6814–6817. [Google Scholar] [CrossRef]
- Rho, M.-C.; Yasuda, K.; Matsunaga, K.; Ohizumi, Y. A monogalactopyranosyl acylglycerol from Oltmannsiellopsis unicellularis (NIES-359). Phytochemistry 1997, 44, 1507–1509. [Google Scholar] [CrossRef]
- Hiraga, Y.; Shikano, T.; Widianti, T.; Ohkata, K. Three new glycolipids with cytolytic activity from cultured marine dinoflagellate Heterocapsa circularisquama. Nat. Prod. Res. 2008, 22, 649–657. [Google Scholar] [CrossRef]
- Fu, M.; Koulman, A.; van Rijssel, M.; Lützen, A.; de Boer, M.K.; Tyl, M.R.; Liebezeit, G. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon 2004, 43, 355–363. [Google Scholar] [CrossRef]
- Borisova, A.S.; Ivanen, D.R.; Bobrov, K.S.; Eneyskaya, E.V.; Rychkov, G.N.; Sandgren, M.; Kulminskaya, A.A.; Sinnott, M.L.; Shabalin, K.A. α-Galactobiosyl units: Thermodynamics and kinetics of their formation by transglycosylations catalysed by the GH36 α-galactosidase from Thermotoga maritima. Carbohydr. Res. 2015, 401, 115–121. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Song, J.-H.; Kim, T.; Shin, W.-S.; Park, G.M.; Lee, S.; Kim, Y.-J.; Choi, P.; Kim, H.; Kim, H.-S.; et al. Anti-human rhinoviral activity of polybromocatechol compounds isolated from the rhodophyta, Neorhodomela aculeata. Mar. Drugs 2012, 10, 2222–2233. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.-M.; Gloer, J.B.; Wang, B.-G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two new bromophenols from the red alga Odonthalia corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Li, K.; Li, X.-M.; Gloer, J.B.; Wang, B.-G. Isolation, characterization, and antioxidant activity of bromophenols of the marine red alga Rhodomela confervoides. J. Agric. Food Chem. 2011, 59, 9916–9921. [Google Scholar] [CrossRef]
- Lever, J.; Curtis, G.; Brkljača, R.; Urban, S. Bromophenolics from the Red Alga Polysiphonia decipiens. Mar. Drugs 2019, 17, 497. [Google Scholar] [CrossRef]
- Han, L.; Xu, N.; Shi, J.; Yan, X.; Zeng, C. Isolation and pharmacological activities of bromophenols from Rhodomela confervoides. Chin. J. Oceanol. Limnol. 2005, 23, 226–229. [Google Scholar]
- Dong, H.; Dong, S.; Erik Hansen, P.; Stagos, D.; Lin, X.; Liu, M. Progress of Bromophenols in Marine Algae from 2011 to 2020: Structure, Bioactivities, and Applications. Mar. Drugs 2020, 18, 411. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- Choi, Y.K.; Ye, B.-R.; Kim, E.-A.; Kim, J.; Kim, M.-S.; Lee, W.W.; Ahn, G.-N.; Kang, N.; Jung, W.-K.; Heo, S.-J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef]
- Mikami, D.; Kurihara, H.; Kim, S.M.; Takahashi, K. Red algal bromophenols as glucose 6-phosphate dehydrogenase inhibitors. Mar. Drugs 2013, 11, 4050–4057. [Google Scholar] [CrossRef]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J.; Fan, X.; He, L. Bromophenols coupled with methyl gamma-ureidobutyrate and bromophenol sulfates from the red alga Rhodomela confervoides. J. Nat. Prod. 2006, 69, 206–210. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Wang, S.; Li, S.; Cao, P.; Yang, Y.; Lü, Y.; Shi, J.; Xu, N.; Fan, X.; et al. Bromophenols coupled with derivatives of amino acids and nucleosides from the red alga Rhodomela confervoides. J. Nat. Prod. 2005, 68, 691–694. [Google Scholar] [CrossRef]
- Flodin, C.; Whitfield, F.B. Biosynthesis of bromophenols in marine algae. Water Sci. Technol. 1999, 40, 53–58. [Google Scholar] [CrossRef]
- Li, K.; Li, X.-M.; Ji, N.-Y.; Wang, B.-G. Natural bromophenols from the marine red alga Polysiphonia urceolata (Rhodomelaceae): Structural elucidation and DPPH radical-scavenging activity. Bioorganic Med. Chem. 2007, 15, 6627–6631. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.R.; Oh, M.-J.; Jung, S.-J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Weinstein, B.; Rold, T.L.; Harrell, C.E., Jr.; Burns, M.W.; Waaland, J.R., III. Reexamination of the Bromophenols in the Red Alga Rhodomela larix. Phytochemistry 1979, 14, 2667–2670. [Google Scholar] [CrossRef]
- Barreto, M.; Meyer, J. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (Rhodophyta) and a visual exploration of its biofilm covering. S. Afr. J. Bot. 2006, 72, 521–528. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Takashima, K.; Hayashi, I.; Suzuki, M. Feeding-deterrent Bromophenols from Odonthalia corymbifera. Phytochemistry 1997, 45, 485–487. [Google Scholar] [CrossRef]
- Ito, T.; Asano, Y.; Tanaka, Y.; Takabe, T. Regulation of biosynthesis of dimethylsulfoniopropionate and its uptake in sterile mutant of Ulva pertusa (Chlorophyta). J. Phycol. 2011, 47, 517–523. [Google Scholar] [CrossRef]
- Challenger, F.; Simpson, M.I. 320. Studies on biological methylation. Part XII. A precursor of the dimethyl sulphide evolved by Polysiphonia fastigiata. Dimethyl-2-carboxyethylsulphonium hydroxide and its salts. J. Chem. Soc. 1948, 1591–1597. [Google Scholar] [CrossRef]
- Cantoni, G.L.; Anderson, D.G.; Rosenthal, E. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J. Biol. Chem. 1956, 222, 171–177. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef]
- Edwards, D.M.; Reed, R.H.; Stewart, W.D.P. Osmoacclimation in Enteromorpha intestinalis: Long-term effects of osmotic stress on organic solute accumulation. Mar. Biol. 1988, 98, 467–476. [Google Scholar] [CrossRef]
- Gage, D.A.; Rhodes, D.; Nolte, K.D.; Hicks, W.A.; Leustek, T.; Cooper, A.J.; Hanson, A.D. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature 1997, 387, 891–894. [Google Scholar] [CrossRef]
- Ravanel, S.; Gakière, B.; Job, D.; Douce, R. The specific features of methionine biosynthesis and metabolism in plants. Proc. Natl. Acad. Sci. USA 1998, 95, 7805–7812. [Google Scholar] [CrossRef]
- Lee, P.A.; de Mora, S.J. Intracellular Dimethylsulfoxide (DMSO) in Unicellular Marine Algae: Speculations on its Origin and Possible Biological Role. J. Phycol. 1999, 35, 8–18. [Google Scholar] [CrossRef]
- Thume, K.; Gebser, B.; Chen, L.; Meyer, N.; Kieber, D.J.; Pohnert, G. The metabolite dimethylsulfoxonium propionate extends the marine organosulfur cycle. Nature 2018, 563, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Hayashi, K.; Kobayashi, M. Heterosigma-glycolipids I and II, two new Galactolipids containing Octadecatetraenoyl and Eicosapentaenoyl Residues from Dinoflagellate Heterosigma sp. Chem. Pharm. Bull. 1989, 37, 849–851. [Google Scholar] [CrossRef][Green Version]
- Hölzl, G.; Dörmann, P. Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Gerwick, W.H. Galactolipids from the temperate red marine alga Gracilariopsis lemaneiformis. Phytochemistry 1990, 29, 1433–1440. [Google Scholar] [CrossRef]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef]
- Albersheim, P.; Nevins, D.J.; English, P.D.; Karr, A. A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 1967, 5, 340–345. [Google Scholar] [CrossRef]
- Noe, C.R.; Freissmuth, J. Capillary zone electrophoresis of aldose enantiomers: Separation after derivatization with S-(-)-1-phenylethylamine. J. Chromatogr. A 1995, 704, 503–512. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- Alamsjah, M.A.; Hirao, S.; Ishibashi, F.; Fujita, Y. Isolation and structure determination of algicidal compounds from Ulva fasciata. Biosci. Biotechnol. Biochem. 2005, 69, 2186–2192. [Google Scholar] [CrossRef]


| 7 | 16 | |||
|---|---|---|---|---|
| Pos. | 13C | 1H | 13C | 1H |
| 1 | 127.78 (C) | - | 131.44 a (C) | - |
| 2 | 118.86 (C) | - | 114.34 a (C) | - |
| 3 | 125.05 (C) | - | 112.70 a (C) | - |
| 4 | 141.40 (C) | - | 145.62 a (C) | - |
| 5 | 151.20 (C) | - | 146.80 a (C) | - |
| 6 | 121.76 (CH) | 7.25 (s) | 115.86 (CH) | 6.93 (s) |
| 7 | 50.16 (CH) | 4.80 (s) | 75.85 (CH2) | 4.43 (s) |
| 8 | 25.28 (2 CH3) | 2.97 (s) | 162.73 a (CO) | - |
| 1′ | 172.22 a (COOH) | - | ||
| 2′ | 53.63 (CH) | 3.98 (t, 6.4) | ||
| 3′ | 28.78 (CH2) | 2.00 (d, 4.1) 1.90–1.85 (m) | ||
| 4′ | 27.07 (CH2) | 1.66 (m) | ||
| 5′ | 39.63 b (CH2) | 3.20–3.17 (m) 3.16–3.11 (m) | ||
| 12 in D2O | 13 in D2O | 14 in CD3OD | 15 in D2O/CD3OD 1:1 (v/v) | |||||
|---|---|---|---|---|---|---|---|---|
| Pos. | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H |
| COOH | 172.64 (COOH) | - | 172.18 (COOH) | - | 172.02 a (COOH) | - | 173.95 (COOH) | - |
| α | 55.36 (CH) | 4.19 (dd, 7.6, 5.7) | 54.95 (CH) | 4.30 (ddd, 7.9, 5.4, 1.0) | 55.36 (CH) | 3.80 (dd, 8.9, 6.4) | 58.32 (CH) | 3.71 (d, 4.5) |
| β | 35.25 (CH2) | 3.23 (dd, 14.7, 5.6) 3.11 (dd, 14.8, 7.6) | 35.37 (CH2) | 3.29 (dd, 15.0, 5.6) 3.13 (dd, 15.0, 7.9) | 34.70 (CH2) | 3.08 (dd, 14.7, 6.2) 2.79 (dd, 14.7, 8.8) | 36.85 (CH2) | 2.70 (dd, 14.8, 9.0) 3.01 (dd, 14.7, 4.8) |
| 1 | 130.25 (C) | - | 128.05 (C) | - | 126.35 (C) | - | 126.97 (C) | - |
| 2 | 134.10 (CH) | 7.49 (s) | 132.12 (CH) | 7.40 (dd, 2.2, 1.1) | 125.50 (CH) | 7.01 (s) | 129.49 (CH) | 6.24 (d, 2.2) |
| 3 | 112.20 (C) | - | 111.84 (C) | - | 109.97 (C) | - | 126.47 (C) | - |
| 4 | 150.14 (C) | - | 146.47 (C) | - | 143.67 (C) | - | 154.23 (C) | - |
| 5 | 112.20 (C) | 140.47 (C) | - | 147.44 (C) | - | 115.88 (CH) | 6.74 (d, 8.2) | |
| 6 | 134.10 (CH) | 7.49 (s) | 123.92 (CH) | 7.29 (dd, 2.2, 1.1) | 128.62 (C) | - | 128.51 (CH) | 6.89 (d, 8.1) |
| 1′ | 132.49 (C) | - | 133.16 (C) | - | ||||
| 2′ | 116.62 (C) | - | 119.29 (C) | - | ||||
| 3′ | 114.45 (C) | - | 113.94 (C) | - | ||||
| 4′ | 143.93 (C) | - | 143.33 (C) | - | ||||
| 5′ | 146.29 (C) | - | 145.39 (C) | - | ||||
| 6′ | 115.01 (CH) | 6.14 (s) | 128.45 (C) | - | ||||
| 7′ | 34.17 (CH2) | 4.10 (d, 17.6) 4.01 (d, 17.5) | 34.83 (CH2) | 4.01 (s) | ||||
| 1′’ | 132.74 (C) | - | ||||||
| 2′’ | 116.63 (C) | - | ||||||
| 3′’ | 114.25 (C) | - | ||||||
| 4′’ | 145.28 (C) | - | ||||||
| 5′’ | 142.37 (C) | - | ||||||
| 6′’ | 115.59 (CH) | 6.17 (s) | ||||||
| 7′’ | 34.92 (CH2) | 3.96 (s) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobtorweihen, J.; Schmitt, M.; Spiegler, V. Amino Acid-Coupled Bromophenols and a Sulfated Dimethylsulfonium Lanosol from the Red Alga Vertebrata lanosa. Mar. Drugs 2022, 20, 420. https://doi.org/10.3390/md20070420
Jacobtorweihen J, Schmitt M, Spiegler V. Amino Acid-Coupled Bromophenols and a Sulfated Dimethylsulfonium Lanosol from the Red Alga Vertebrata lanosa. Marine Drugs. 2022; 20(7):420. https://doi.org/10.3390/md20070420
Chicago/Turabian StyleJacobtorweihen, Joshua, Marthe Schmitt, and Verena Spiegler. 2022. "Amino Acid-Coupled Bromophenols and a Sulfated Dimethylsulfonium Lanosol from the Red Alga Vertebrata lanosa" Marine Drugs 20, no. 7: 420. https://doi.org/10.3390/md20070420
APA StyleJacobtorweihen, J., Schmitt, M., & Spiegler, V. (2022). Amino Acid-Coupled Bromophenols and a Sulfated Dimethylsulfonium Lanosol from the Red Alga Vertebrata lanosa. Marine Drugs, 20(7), 420. https://doi.org/10.3390/md20070420







