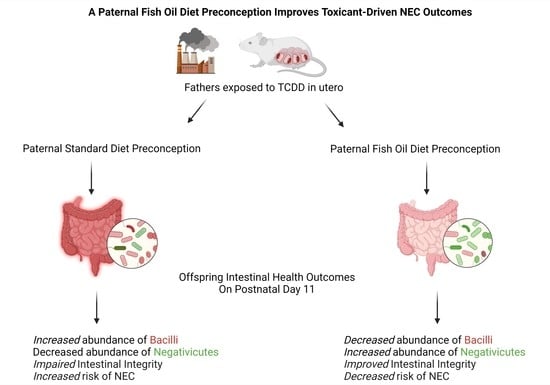
A Paternal Fish Oil Diet Preconception Modulates the Gut Microbiome and Attenuates Necrotizing Enterocolitis in Neonatal Mice
Abstract
:1. Introduction
2. Results
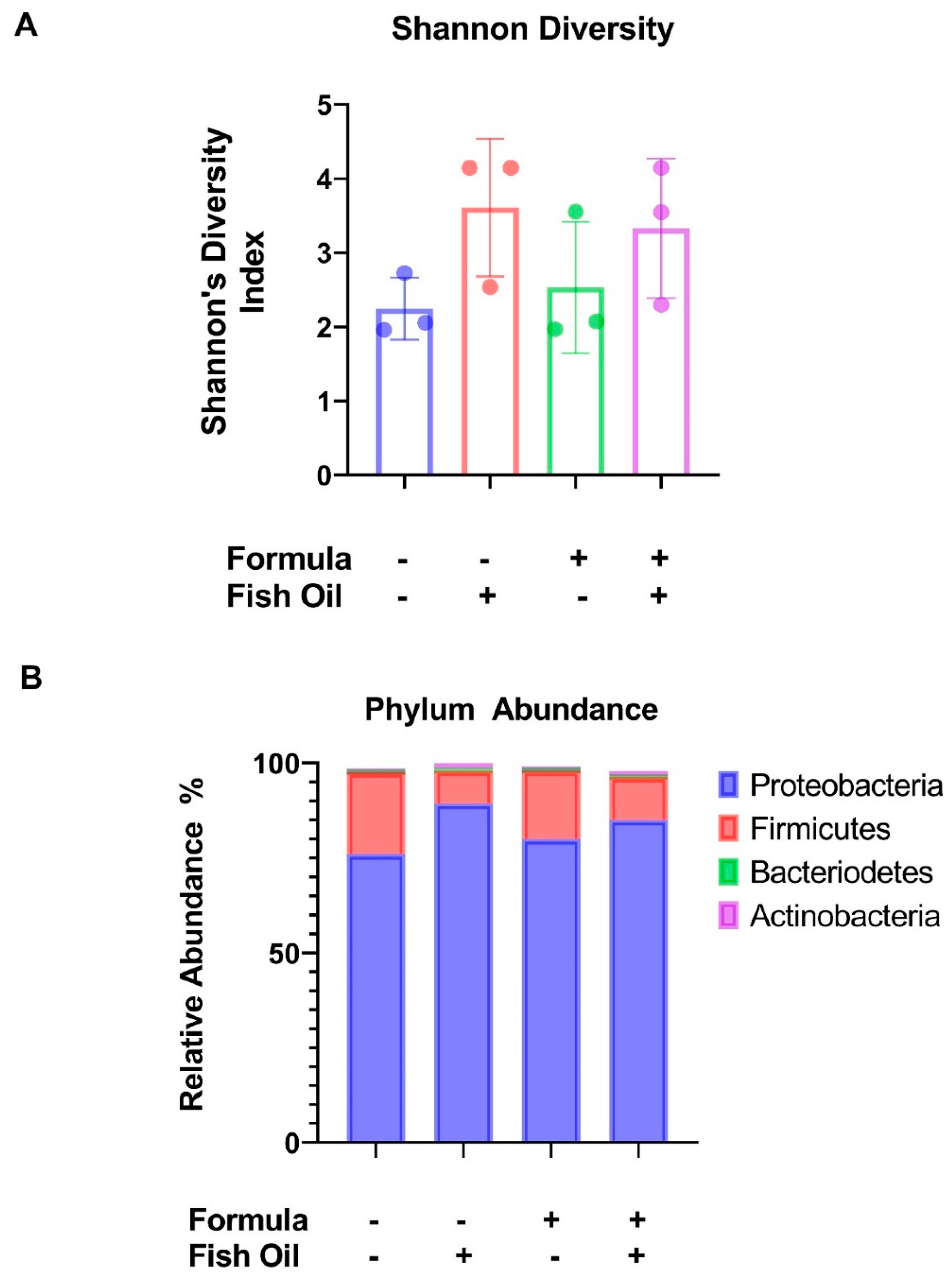
2.1. Paternal Preconception Diet Influences His Offspring’s Gut Microbial Diversity at the Phylum Level in F2CT Pups
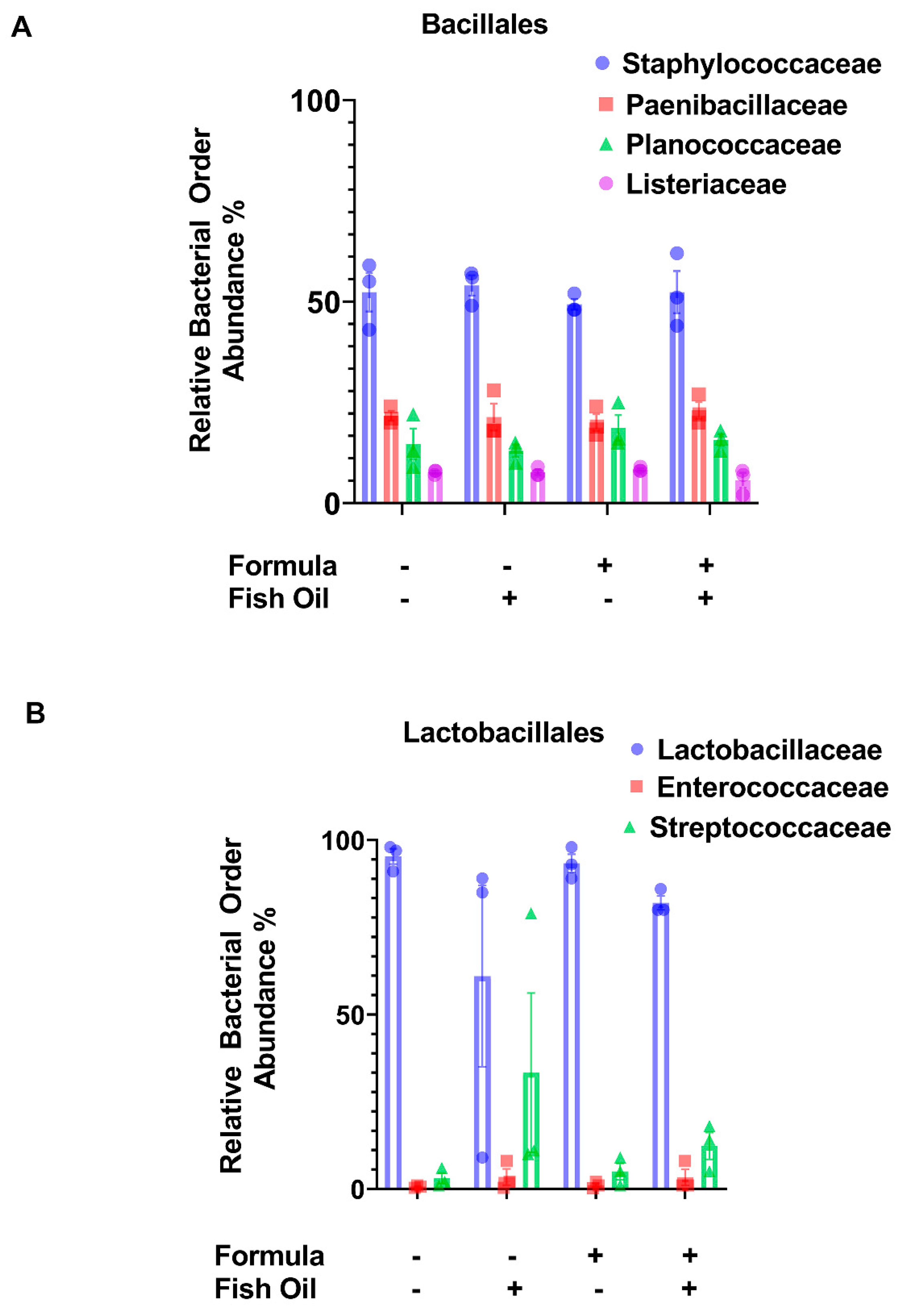
2.2. Paternal Diet Preconception Influences the Relative Abundance of Firmicute Classes in F2CT Pups
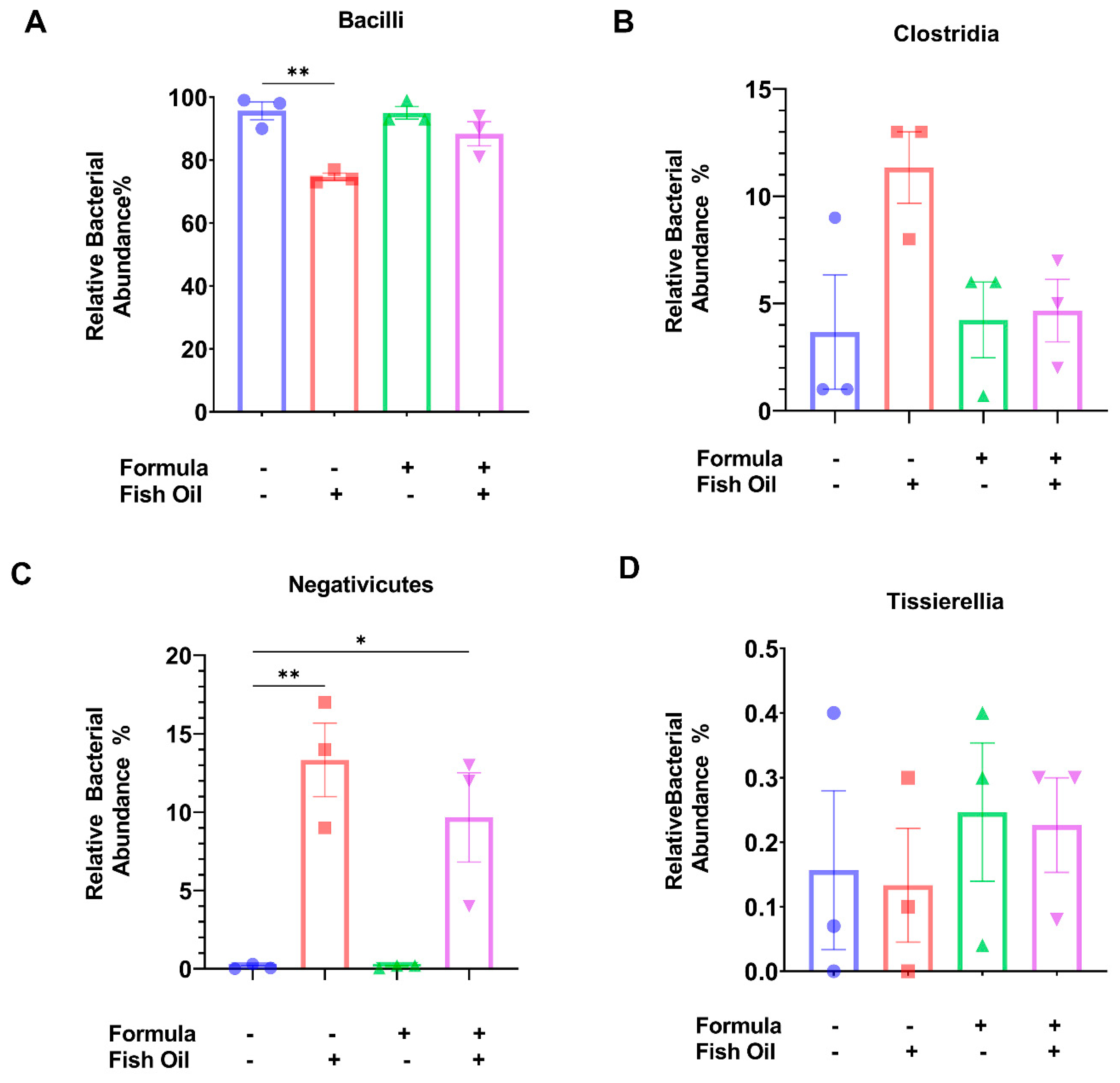
2.3. Paternal Diet Preconception Influences the Relative Abundance of Bacilli and Negativicutes Orders in F2CT Pups
2.4. Paternal Diet Preconception Alters the Firmicute Abundance in F2TCDD Pups
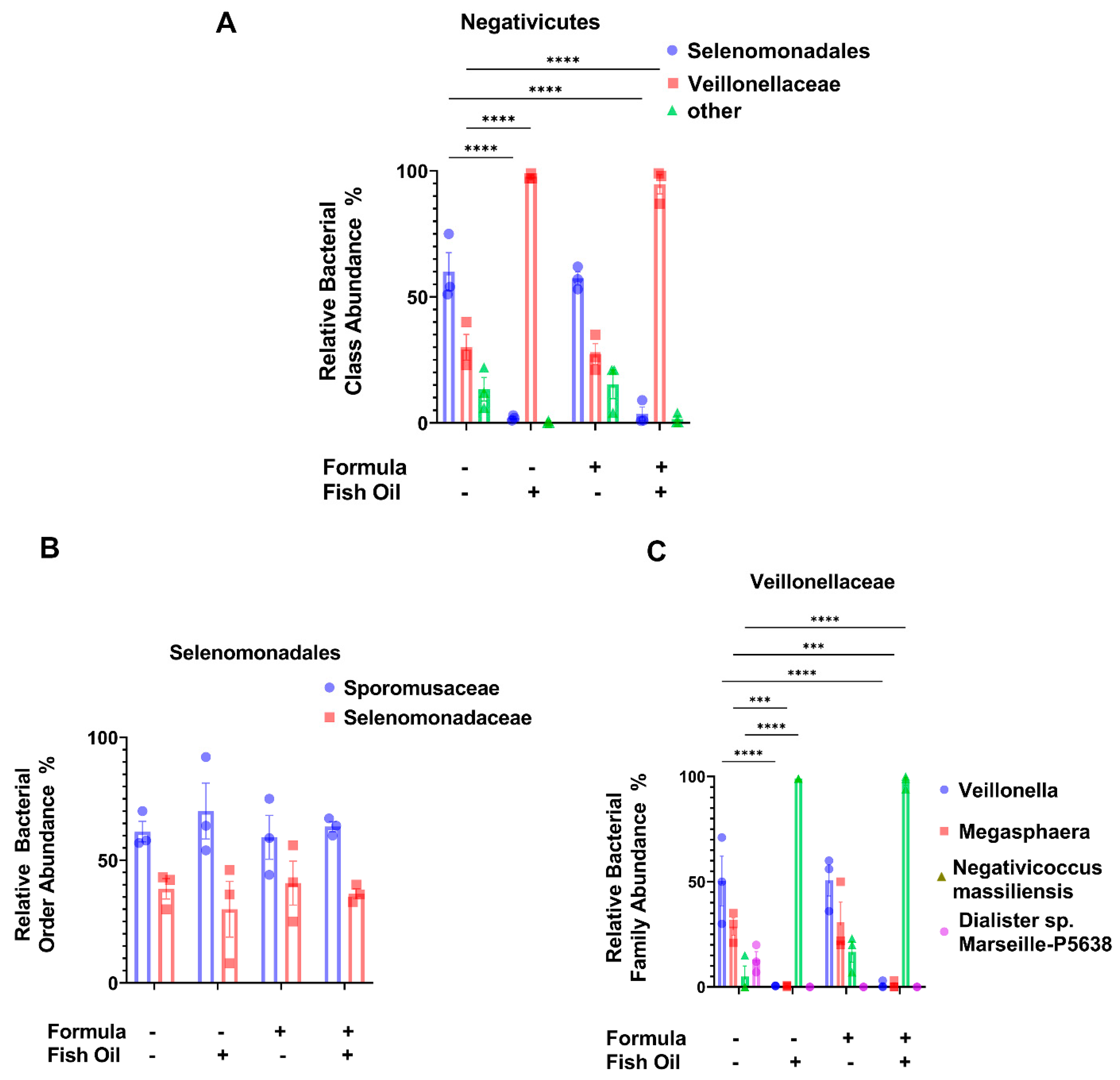
2.5. Paternal Fish Oil Consumption Alters the Abundance of Negativicutes in F2TCDD Pups
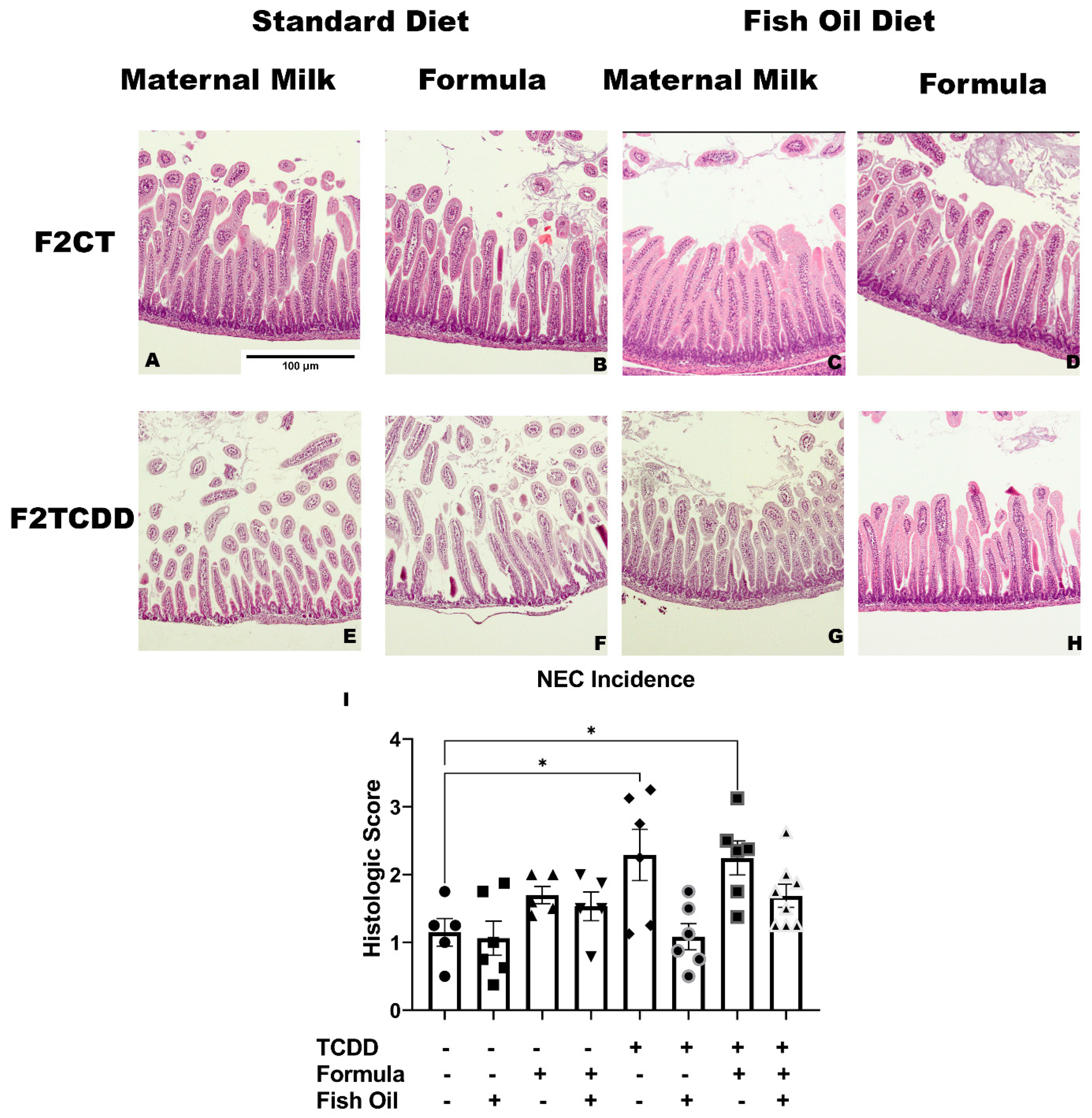
2.6. A Paternal Fish Oil Diet Preconception Attenuates Susceptibility to NEC
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Mating, Exposure, and Diet Scheme
4.3.1. TCDD Exposure
4.3.2. Diet and Mating Scheme for the F1 Generation
4.4. Formula Feeding
4.5. Euthanasia and Sample Collection
4.6. Bacterial Isolation and Next-Generation Sequencing
4.7. Assessment of Necrotizing Enterocolitis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenny, L.C.; Kell, D.B. Immunological Tolerance, Pregnancy, and Preeclampsia: The Roles of Semen Microbes and the Father. Front. Med. 2017, 4, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nõlvak, H.; Preem, J.K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Tan, G.C.; Wong, K.K.; Anushia, S.; Cheah, F.C. Gardnerella vaginalis in perinatology: An overview of the clinicopathological correlation. Malays. J. Pathol. 2018, 40, 267–286. [Google Scholar] [PubMed]
- Schoenmakers, S.; Steegers-Theunissen, R.; Faas, M. The matter of the reproductive microbiome. Obstet. Med. 2019, 12, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Mokshagundam, S.; Rinaudo, P.F.; Osteen, K.G.; Bruner-Tran, K.L. Paternal developmental toxicant exposure is associated with epigenetic modulation of sperm and placental Pgr and Igf2 in a mouse model. Biol. Reprod. 2018, 99, 864–876. [Google Scholar] [CrossRef] [Green Version]
- Fundora, J.B.; Guha, P.; Shores, D.R.; Pammi, M.; Maheshwari, A. Intestinal dysbiosis and necrotizing enterocolitis: Assessment for causality using Bradford Hill criteria. Pediatr. Res. 2020, 87, 235–248. [Google Scholar] [CrossRef]
- Morrow, A.L.; Lagomarcino, A.J.; Schibler, K.R.; Taft, D.H.; Yu, Z.; Wang, B.; Altaye, M.; Wagner, M.; Gevers, D.; Ward, D.V.; et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.A.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: A prospective case-control study. Lancet 2016, 387, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1322. [Google Scholar] [CrossRef]
- Ficara, M.; Pietrella, E.; Spada, C.; Della Casa Muttini, E.; Lucaccioni, L.; Iughetti, L.; Berardi, A. Changes of intestinal microbiota in early life. J. Matern. Fetal Neonatal Med. 2020, 33, 1036–1043. [Google Scholar] [CrossRef]
- Shulhan, J.; Dicken, B.; Hartling, L.; Larsen, B.M. Current Knowledge of Necrotizing Enterocolitis in Preterm Infants and the Impact of Different Types of Enteral Nutrition Products. Adv. Nutr. 2017, 8, 80–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConaha, M.E.; Ding, T.; Lucas, J.A.; Arosh, J.A.; Osteen, K.G.; Bruner-Tran, K.L. Preconception omega-3 fatty acid supplementation of adult male mice with a history of developmental 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure prevents preterm birth in unexposed female partners. Reproduction 2011, 142, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Rumph, J.T.; Rayford, K.J.; Stephens, V.R.; Ameli, S.; Nde, P.N.; Osteen, K.G.; Bruner-Tran, K.L. A Preconception Paternal Fish Oil Diet Prevents Toxicant-Driven New Bronchopulmonary Dysplasia in Neonatal Mice. Toxics 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.-F.; Hern Tan, L.T.; Ramadas, A.; Ab Mutalib, N.-S.; Lee, L.-H. Exploring the Role of Gut Bacteria in Health and Disease in Preterm Neonates. Int. J. Environ. Res. Public Health 2020, 17, 6963. [Google Scholar] [CrossRef]
- McMurtry, V.E.; Gupta, R.W.; Tran, L.; Blanchard, E.E.t.; Penn, D.; Taylor, C.M.; Ferris, M.J. Bacterial diversity and Clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Dobbler, P.T.; Procianoy, R.S.; Mai, V.; Silveira, R.C.; Corso, A.L.; Rojas, B.S.; Roesch, L.F.W. Low Microbial Diversity and Abnormal Microbial Succession Is Associated with Necrotizing Enterocolitis in Preterm Infants. Front. Microbiol. 2017, 8, 2243. [Google Scholar] [CrossRef] [Green Version]
- Mokshagundam, S.; Ding, T.; Rumph, J.T.; Dallas, M.; Stephens, V.R.; Osteen, K.G.; Bruner-Tran, K.L. Developmental 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure of either parent enhances the risk of necrotizing enterocolitis in neonatal mice. Birth Defects Res. 2020, 112, 1209–1223. [Google Scholar] [CrossRef]
- Ostlie, D.J.; Spilde, T.L.; St Peter, S.D.; Sexton, N.; Miller, K.A.; Sharp, R.J.; Gittes, G.K.; Snyder, C.L. Necrotizing enterocolitis in full-term infants. J. Pediatr. Surg. 2003, 38, 1039–1042. [Google Scholar] [CrossRef]
- Abbo, O.; Harper, L.; Michel, J.L.; Ramful, D.; Breden, A.; Sauvat, F. Necrotizing enterocolitis in full term neonates: Is there always an underlying cause? J. Neonatal Surg. 2013, 2, 29. [Google Scholar] [CrossRef]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The Maternal Infant Microbiome: Considerations for Labor and Birth. MCN Am. J. Matern. Child Nurs. 2017, 42, 318–325. [Google Scholar] [CrossRef]
- Yu, H.N.; Zhu, J.; Pan, W.S.; Shen, S.R.; Shan, W.G.; Das, U.N. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef]
- Younge, N.; Yang, Q.; Seed, P.C. Enteral High Fat-Polyunsaturated Fatty Acid Blend Alters the Pathogen Composition of the Intestinal Microbiome in Premature Infants with an Enterostomy. J. Pediatr. 2017, 181, 93–101.e6. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Saiman, L.; Zhou, J.; Della-Latta, P.; Jia, H.; Graham, P.L., 3rd. Concordance of Gastrointestinal Tract Colonization and Subsequent Bloodstream Infections With Gram-negative Bacilli in Very Low Birth Weight Infants in the Neonatal Intensive Care Unit. Pediatr. Infect. Dis. J. 2010, 29, 831–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botsford, K.B.; Weinstein, R.A.; Boyer, K.M.; Nathan, C.; Carman, M.; Paton, J.B. Gram-negative bacilli in human milk feedings: Quantitation and clinical consequences for premature infants. J. Pediatr. 1986, 109, 707–710. [Google Scholar] [CrossRef]
- Durrani, M.; Nazli, R.; Fatima, S.; Abubakr, M. Impact Of Feeding Practice On Diversity Pattern Of The Gut Microbiome in Infants. J. Ayub Med. Coll. Abbottabad 2020, 32, 551–557. [Google Scholar]
- Hosomi, R.; Matsudo, A.; Sugimoto, K.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Eicosapentaenoic Acid and Docosahexaenoic Acid Ethyl Esters Influence the Gut Microbiota and Bacterial Metabolites in Rats. J. Oleo Sci. 2021, 70, 1469–1480. [Google Scholar] [CrossRef]
- Duan, M.; Han, Z.; Huang, N. Changes of intestinal microflora in neonatal necrotizing enterocolitis: A single-center study. J. Int. Med. Res. 2020, 48, 300060520957804. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kwok, L.-Y.; Xi, X.; Zhong, Z.; Ma, T.; Xu, H.; Meng, H.; Zhao, F.; Zhang, H. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 2020, 12, 1794266. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Cortez, Y.; Vera, N.; Villena, G.K.; Gutiérrez-Correa, M. Metagenomic analysis of microbial community of an Amazonian geothermal spring in Peru. Genome Data 2016, 9, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Gupta, G.; Sharma, A.; Kaur, B.; El-Sheikh, M.A.; Alyemeni, M.N. Metagenomic analysis exploring taxonomic and functional diversity of bacterial communities of a Himalayan urban fresh water lake. PLoS ONE 2021, 16, e0248116. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [Green Version]


| F2 Generation Nomenclature | Was the Pup’s Father (F1 Generation) Exposed to TCDD in Utero? | Did the Pup’s Father (F1 Generation) Receive a Fish Oil Preconception Diet? | Did the Pup (F2 Generation) Receive Postnatal Formula Supplementation? |
|---|---|---|---|
| F2CT | No | No | No |
| F2CT/Fish | No | Yes | No |
| F2CT/Form | No | No | Yes |
| F2CT/Fish/Form | No | Yes | Yes |
| F2TCDD | Yes | No | No |
| F2TCDD/Fish | Yes | Yes | No |
| F2TCDD/Form | Yes | No | Yes |
| F2TCDD/Fish/Form | Yes | Yes | Yes |
| Pup Group | Average NEC Score | Overall NEC Incidence |
|---|---|---|
| F2CT | 1.15 | 0/5 = 0% |
| F2CT/Fish | 0.90 | 0/6 = 0% |
| F2CT/Form | 1.7 | 2/5 = 40% |
| F2CT/Fish/Form | 1.5 | 1/5 = 20% |
| F2TCDD | 2.29 | 4/6 = 66% |
| F2TCDD/Fish | 1.1 | 0/6 = 0% |
| F2TCDD/Form | 2.22 | 5/6 = 83% |
| F2TCDD/Fish/Form | 1.68 | 2/8 = 25% |
| Paternal Standard Diet | Paternal Fish Oil Diet |
|---|---|
| ↑ Bacilli | ↓ Bacilli |
| ↓ Negativicutes | ↑ Negativicutes |
| ↑ Selenomondales | ↓ Selenomondales |
| ↑ Veillonellaceae | ↓Veillonellaceae |
| ↓ Negativicoccus | ↑ Negativicoccus |
| ↑ Megasphaera | ↓ Megasphaera |
| ↑ Veillonella | ↓ Veillonella |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumph, J.T.; Stephens, V.R.; Ameli, S.; Gaines, P.N.; Osteen, K.G.; Bruner-Tran, K.L.; Nde, P.N. A Paternal Fish Oil Diet Preconception Modulates the Gut Microbiome and Attenuates Necrotizing Enterocolitis in Neonatal Mice. Mar. Drugs 2022, 20, 390. https://doi.org/10.3390/md20060390
Rumph JT, Stephens VR, Ameli S, Gaines PN, Osteen KG, Bruner-Tran KL, Nde PN. A Paternal Fish Oil Diet Preconception Modulates the Gut Microbiome and Attenuates Necrotizing Enterocolitis in Neonatal Mice. Marine Drugs. 2022; 20(6):390. https://doi.org/10.3390/md20060390
Chicago/Turabian StyleRumph, Jelonia T., Victoria R. Stephens, Sharareh Ameli, Philip N. Gaines, Kevin G. Osteen, Kaylon L. Bruner-Tran, and Pius N. Nde. 2022. "A Paternal Fish Oil Diet Preconception Modulates the Gut Microbiome and Attenuates Necrotizing Enterocolitis in Neonatal Mice" Marine Drugs 20, no. 6: 390. https://doi.org/10.3390/md20060390
APA StyleRumph, J. T., Stephens, V. R., Ameli, S., Gaines, P. N., Osteen, K. G., Bruner-Tran, K. L., & Nde, P. N. (2022). A Paternal Fish Oil Diet Preconception Modulates the Gut Microbiome and Attenuates Necrotizing Enterocolitis in Neonatal Mice. Marine Drugs, 20(6), 390. https://doi.org/10.3390/md20060390