Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and Anticoagulant Activity
Abstract
:1. Introduction
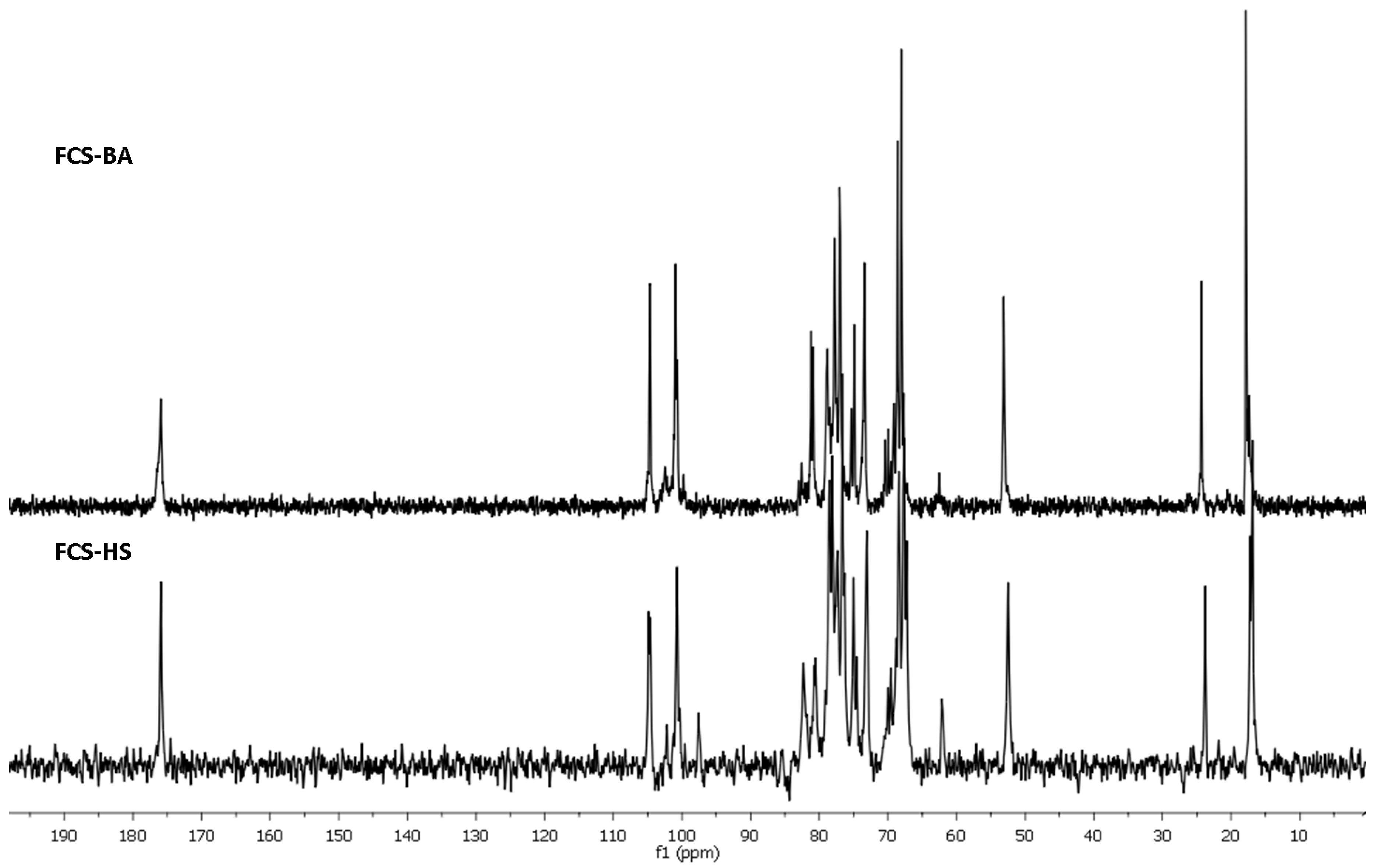
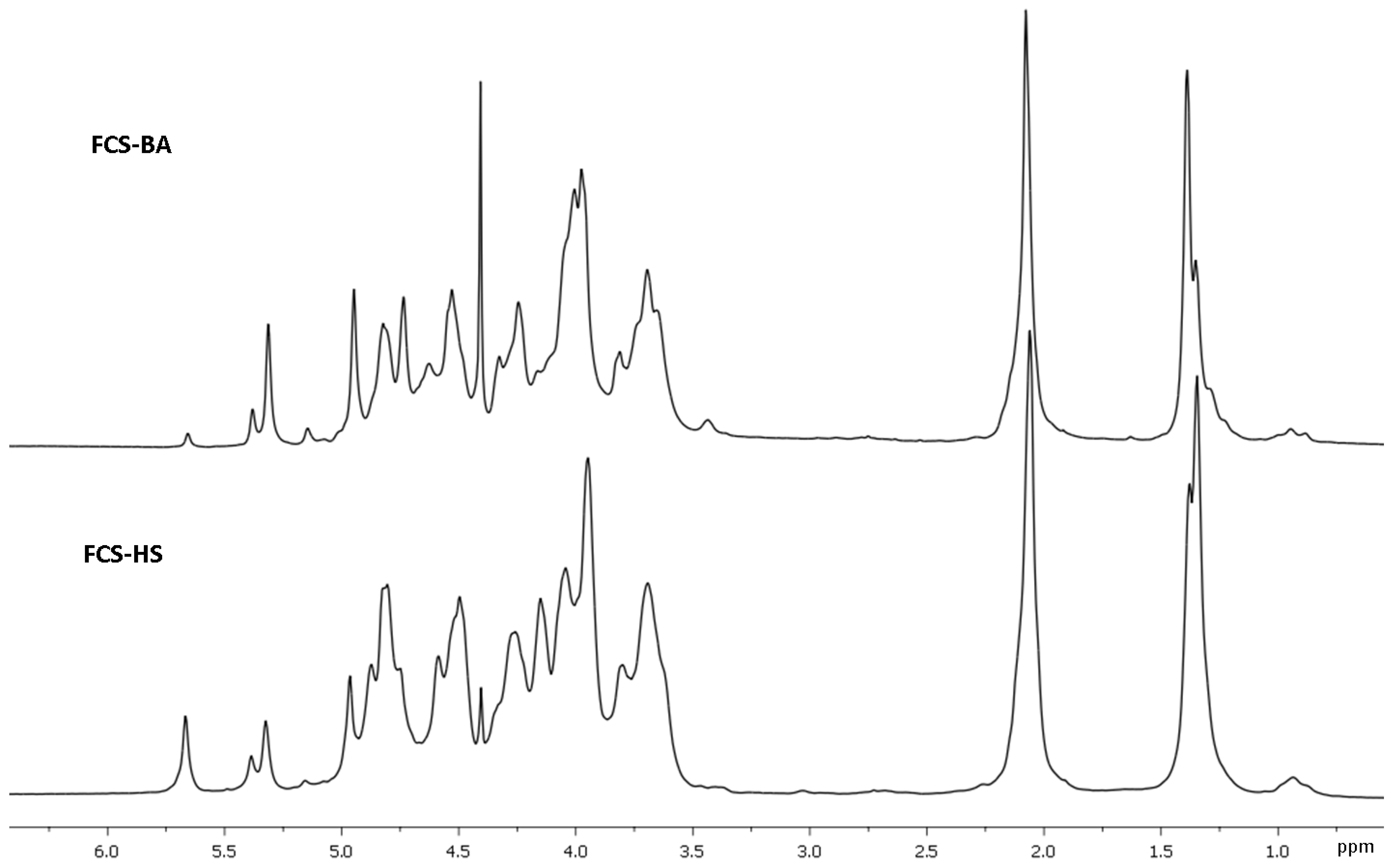
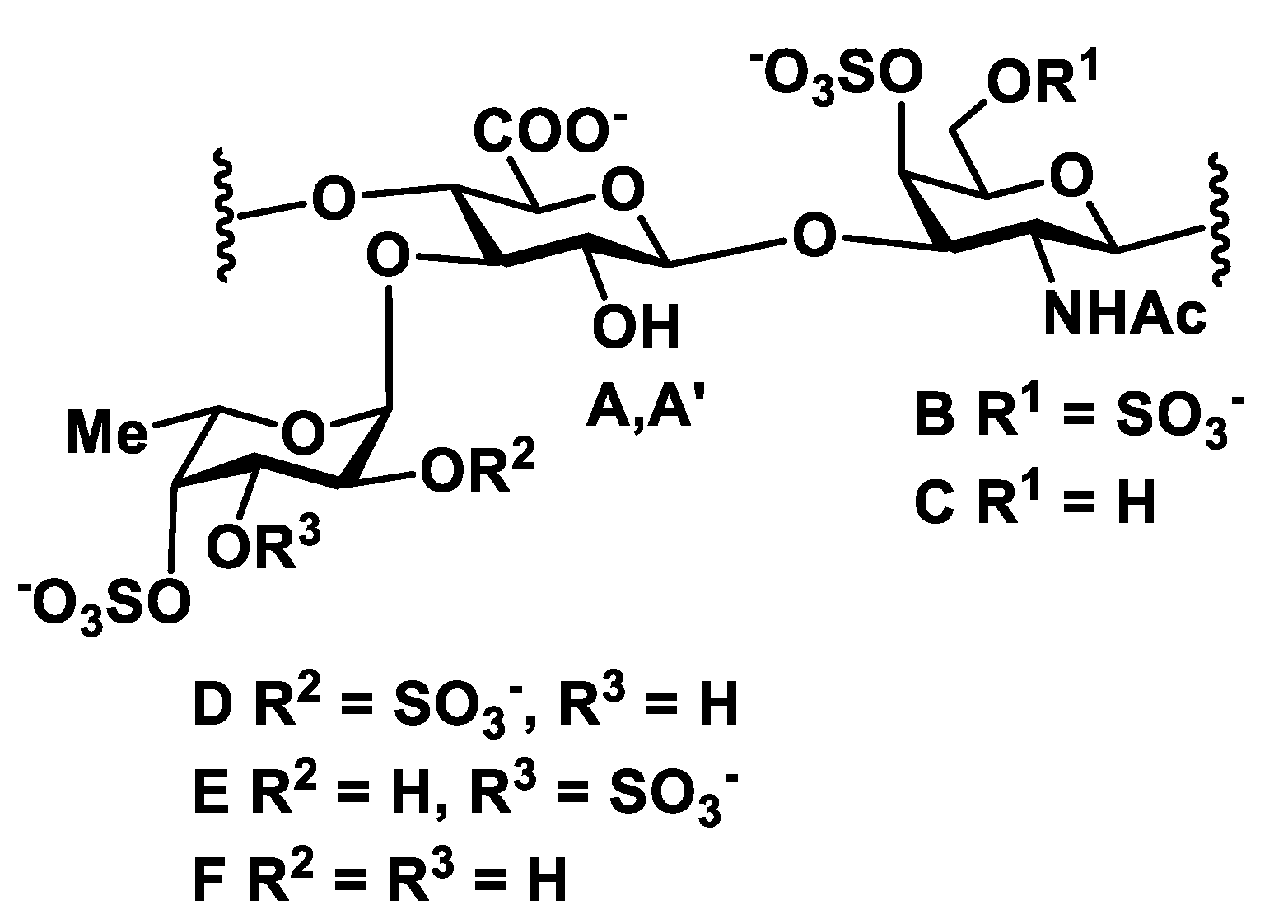
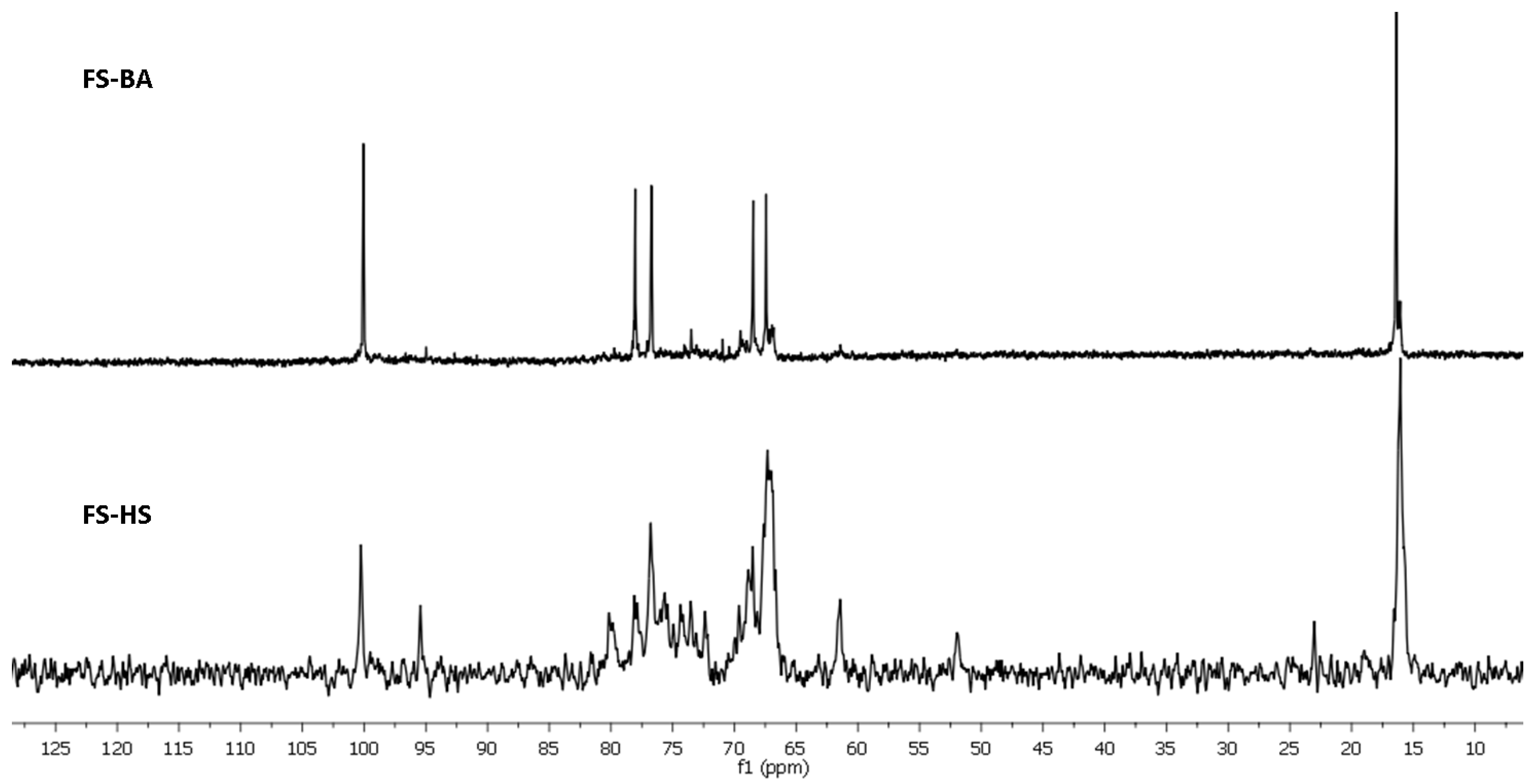
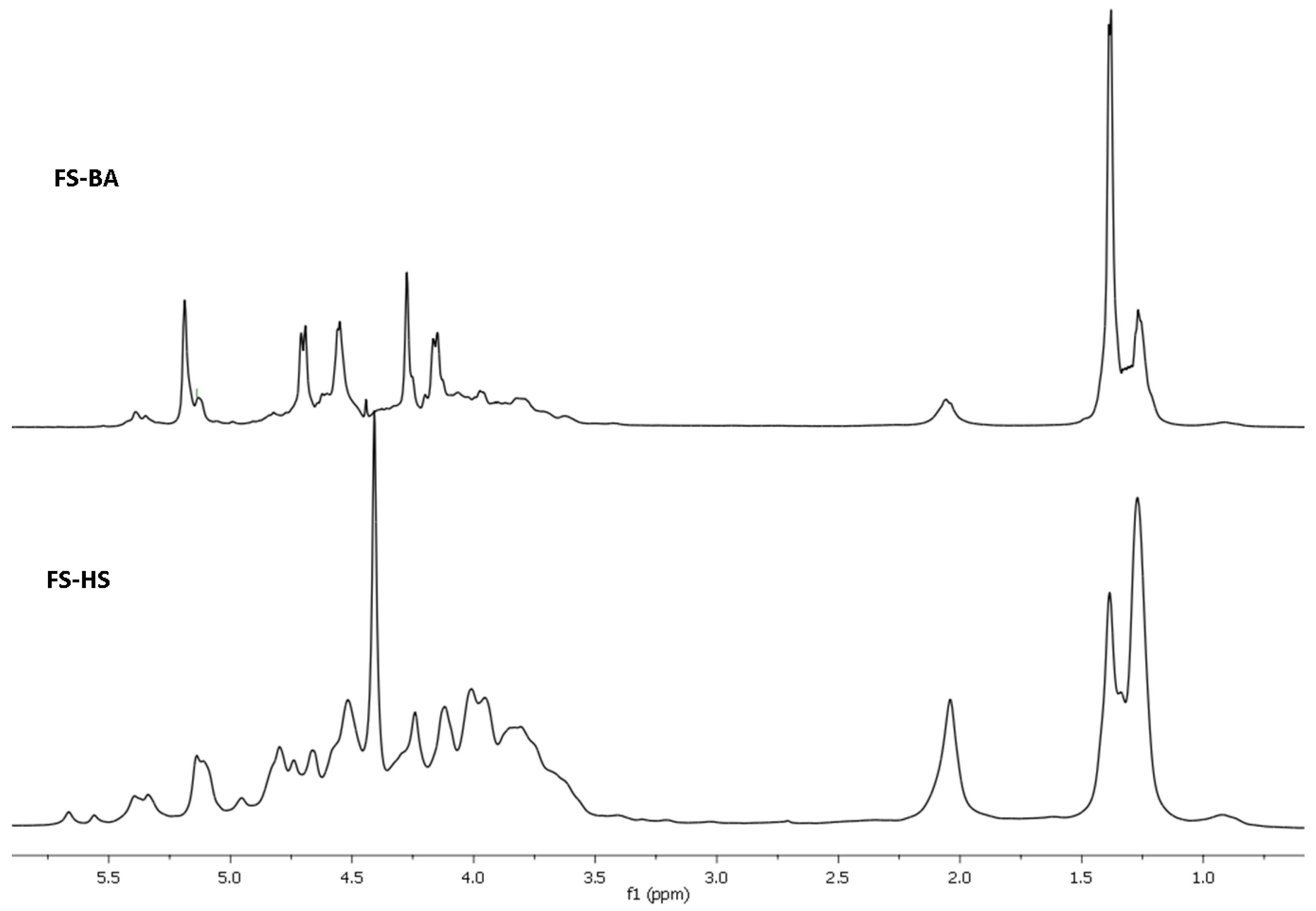
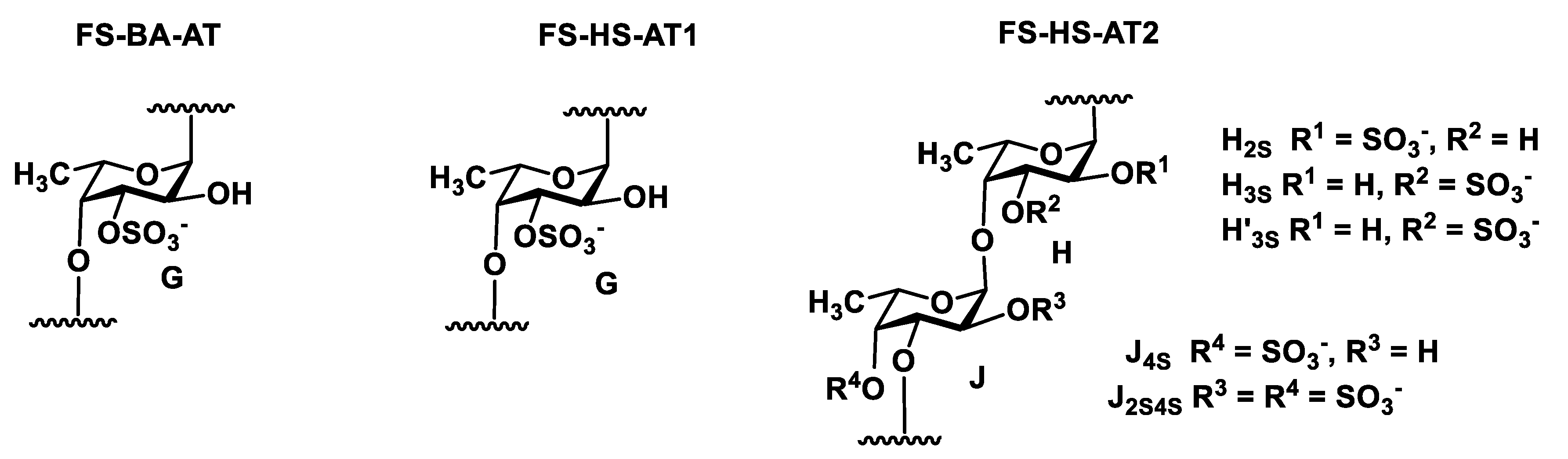
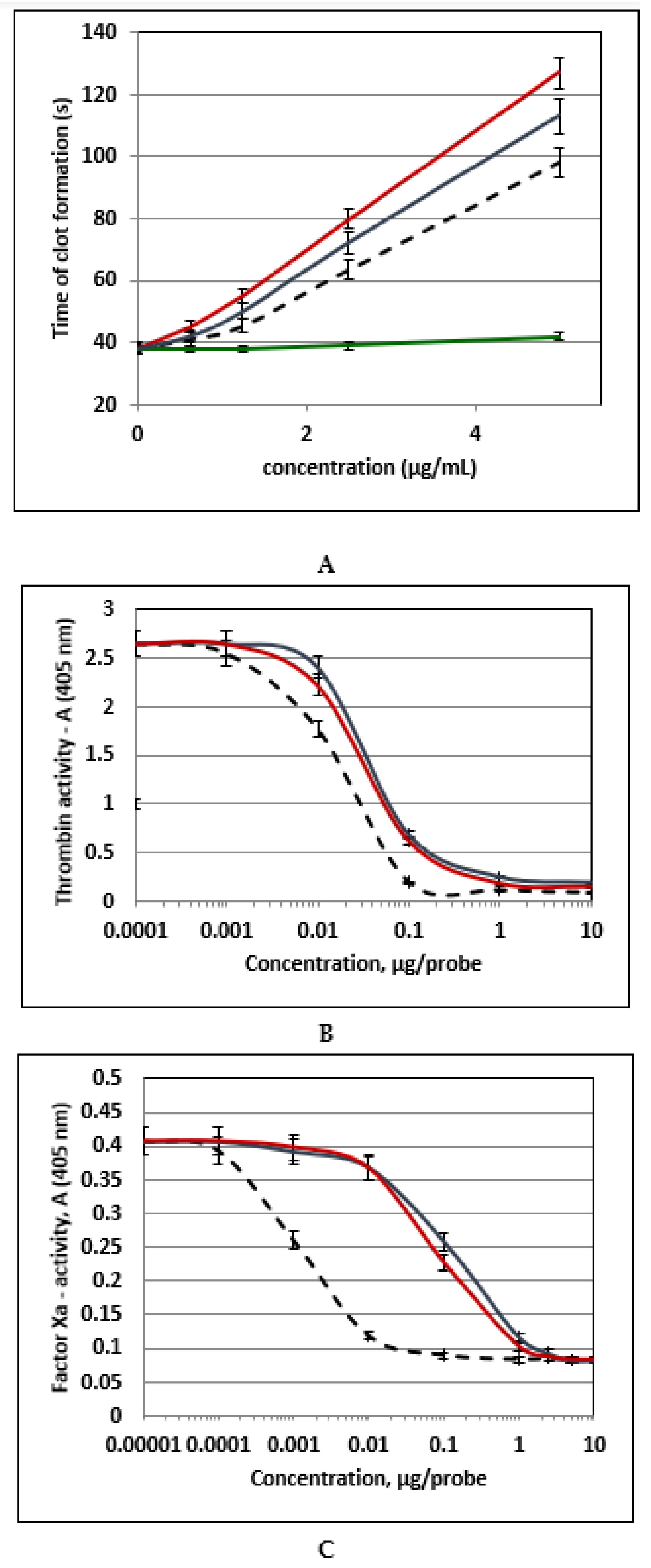
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Isolation of Polysaccharides
3.3. Dilute Acid Treatment of Samples
3.4. Agarose Gel Electrophoresis (PAGE)
3.5. NMR Spectroscopy
3.6. Anticoagulant Activity Measured in Clotting Time Test
3.7. Effect of Polysaccharides on Thrombin or Factor Xa Inactivation by Antithrombin III
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pomin, V.H. Holothurian fucosylated chondroitin sulfates. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. New insight on the structural diversity of holothurian fucosylated chondroitin sulfates. Pure Appl. Chem. 2019, 91, 1065–1071. [Google Scholar] [CrossRef]
- Gong, P.-X.; Li, Q.-Y.; Wu, Y.-C.; Lu, W.-Y.; Zeng, J.; Li, H.-J. Structural elucidation and antidiabetic activity of fucosylated chondroitin sulfate from sea cucumber Stichopus japonicas. Carbohydr. Polym. 2021, 262, 117969. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Kusaykin, M.I.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structure and biological activity of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria japonica. Glycobiology 2016, 26, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Nifantiev, N.E.; Usov, A.I. Two fucosylated chondroitin sulfates from the sea cucumber Eupentacta fraudatrix. Carbohydr. Polym. 2017, 164, 8–12. [Google Scholar] [CrossRef]
- Santos, G.R.C.; Porto, A.C.O.; Soares, P.A.G.; Vilanova, E.; Mourão, P.A.S. Exploring the structure of fucosylated chondroitin sulfate through bottom-up nuclear magnetic resonance and electrospray ionization-high-resolution mass spectrometry approach. Glycobiology 2017, 27, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Vieira, R.P.; Mourão, P.A.S. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A.S. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-β-D-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [CrossRef]
- Soares, P.A.G.; Ribeiro, K.A.; Valente, A.P.; Capillé, N.V.; Oliveira, S.-N.M.C.G.; Tovar, A.M.F.; Pereira, M.S.; Vilanova, E.; Mourão, P.A.S. A unique fucosylated chondroitin sulfate type II with strikingly homogeneous and neatly distributed α-fucose branches. Glycobiology 2018, 28, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhou, L.; Gao, N.; Lin, L.; Sun, H.; Chen, D.; Cai, Y.; Zuo, Z.; Hu, K.; Huang, S.; et al. Unveiling the disaccharide-branched glycosaminoglycan and anticoagulant potential of its derivatives. Biomacromolecules 2021, 22, 1244–1255. [Google Scholar] [CrossRef]
- Mao, H.; Cai, Y.; Li, S.; Sun, H.; Lin, L.; Pan, Y.; Yang, W.; He, Z.; Chen, R.; Zhou, L.; et al. A new fucosylated glycosaminoglycan containing disaccharide branches from Acaudina molpadioides: Unusual structure and anti-intrinsic tenase activity. Carbohydr. Polym. 2020, 245, 116503. [Google Scholar] [CrossRef]
- Li, S.; Zhong, W.; Pan, Y.; Lin, L.; Cai, Y.; Mao, H.; Zhang, T.; Li, S.; Chen, R.; Zhou, L.; et al. Structural characterization and anticoagulant analysis of the novel branched fucosylated glycosaminoglycan from the sea cucumber Holothuria nobilis. Carbohydr. Polym. 2021, 269, 118290. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Jiang, T.; Lv, Z. Novel branch patterns and anticoagulant activity of glycosaminoglycan from sea cucumber Apostichopus japonicus. Int. J. Biol. Macromol. 2015, 72, 911–918. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Silchenko, A.S.; Grebnev, B.B.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Fucosylated chondroitin sulfates from the sea cucumbers Paracaudina chilensis and Holothuria hilla: Structures and anticoagulant activity. Mar. Drugs 2020, 18, 540. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Q.; Lv, K.; Ma, H.; Gao, C.; Liu, Y.; Zhang, S.; Zhao, L. Low-molecular-weight fucosylated glycosaminoglycan and its oligosaccharides from sea cucumber as novel anticoagulants: A review. Carbohydr. Polym. 2021, 251, 117034. [Google Scholar] [CrossRef]
- Lu, W.; Yang, Z.; Chen, J.; Wang, D.; Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021, 272, 118526. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Mourão, P.A.S. Pharmacological activities of sulfated fucose-rich po0lysaccharides after oral administration: Perspectives for the development of new carbohydrate-based drugs. Mar. Drugs 2021, 19, 425. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, Q.; Liu, B.; Chen, F.; Wang, M. Holothurian fucosylated chondroitin sulfates and their potential benefits for human health: Structures and biological activities. Carbohydr. Polym. 2022, 275, 118691. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, D.; Mourão, P.A.S. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Borodina, E.Y.; Nifantiev, N.E.; Usov, A.I. A highly regular fucan sulfate from the sea cucumber Stichopus horrens. Carbohydr. Res. 2018, 456, 5–9. [Google Scholar] [CrossRef]
- Shang, S.; Mou, R.; Zhang, Z.; Gao, N.; Lin, L.; Li, Z.; Wu, M.; Zhao, J. Structural analysis and anticoagulant activities of three highly regular fucan sulfates as novel intrinsic factor Xase inhibitors. Carbohydr. Polym. 2018, 195, 257–266. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Liu, J.; Lin, L.; Sun, H.; Yang, W.; Cai, Y.; Gao, N.; Zhou, L.; Qin, H.; et al. A regular fucan sulfate from Stichopus herrmanni and its peroxide depolymerization: Structure and anticoagulant activity. Carbohydr. Polym. 2021, 256, 117513. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Sun, H.; Su, L.; Zhou, D.; Zhang, X.; Shanggui, D.; Chen, Y. Structure and anticoagulant activity of a sulfated fucan from the sea cucumber Acaudina leucoprocta. Int. J. Biol. Macromol. 2020, 164, 87–94. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Ye, X.; Li, G.; Yu, G.; Xue, C.; Chai, W. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. Acta 2012, 1820, 989–1000. [Google Scholar] [CrossRef]
- Yu, L.; Ge, L.; Xue, C.; Chang, Y.; Zhang, C.; Xu, X.; Wang, Y. Structural study of fucoidan from sea cucumber Acaudina molpadioides: A fucoidan containing novel tetrafucose repeating unit. Food Chem. 2014, 142, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xue, C.; Chang, Y.; Xu, X.; Ge, L.; Liu, G.; Wang, Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014, 146, 113–119. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Li, J.; Ye, X.; Ding, T.; Liu, D.; Chen, J.; Ge, Z.; Chen, S. Identification of a highly sulfated fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated tetrasaccharides units. Carbohydr. Polym. 2015, 134, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hu, Y.; Yu, L.; McClements, D.J.; Xu, X.; Liu, G.; Xue, C. Primary structure and chain conformation of fucoidan extracted from sea cucumber Holothuria tubulosa. Carbohydr. Polym. 2016, 136, 1091–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Niu, Q.; Li, S.; Zhang, X.; Liu, C.; Cai, C.; Li, G.; Yu, G. Fucoidan from sea cucumber Holothuria polii: Structural elucidation and stimulation of hematopoietic activity. Int. J. Biol. Macromol. 2020, 154, 1123–1131. [Google Scholar] [CrossRef]
- Chen, G.; Yu, L.; Zhang, Y.; Chang, Y.; Liu, Y.; Shen, J.; Xue, C. Utilizing heterologously overexpressed endo-1,3-fucanase to investigate the structure of sulfated fucan from sea cucumber (Holothuria hilla). Carbohydr. Polym. 2021, 272, 118480. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, W.; Yin, R.; Zhou, L.; Li, Z.; Wu, M.; Zhao, J. An anticoagulant fucan sulfate with hexasaccharide repeating units from the sea cucumber Holothuria albiventer. Carbohydr. Res. 2018, 464, 12–18. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Zhang, Z.; Zhang, X.; Yang, H.; Li, H.; Liu, M.; Shao, Y.; Zhao, X.; Zhang, H. Oligosaccharide mapping analysis by HILIC-ESI-HCD-MS/MS for structural elucidation of fucoidan from sea cucumber Holothuria floridana. Carbohydr. Polym. 2022, 275, 118694. [Google Scholar] [CrossRef]
- Wu, M.; Xu, L.; Zhao, L.; Xiao, C.; Gao, N.; Luo, L.; Yang, L.; Li, Z.; Chen, L.; Zhao, J. Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Mar. Drugs 2015, 13, 2063–2084. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Xue, C.; Chang, Y.; Hu, Y.; Xu, X.; Ge, L.; Liu, G. Structure and rheological characteristics of fucoidan from sea cucumber Apostichopus japonicus. Food Chem. 2015, 180, 71–76. [Google Scholar] [CrossRef]
- Gao, N.; Chen, R.; Mou, R.; Xiang, J.; Zhou, K.; Li, Z.; Zhao, J. Purification, structural characterization and anticoagulant activities of four sulfated polysaccharides from sea cucumber Holothuria fuscopunctata. Int. J. Biol. Macromol. 2020, 164, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhou, L.; Lin, L.; Cai, Y.; Sun, H.; Zhao, L.; Gao, N.; Yin, R.; Zhao, J. Physicochemical characteristics and anticoagulant activities of the polysaccharides from sea cucumber Pattalus mollis. Mar. Drugs 2019, 17, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Gao, N.; Zuo, Z.; Li, S.; Zheng, W.; Shi, X.; Liu, Q.; Ma, T.; Yin, R.; Li, X.; et al. Five distinct fucan sulfates from sea cucumber Pattalus mollis: Purification, structural characterization and anticoagulant activities. Int. J. Biol. Macromol. 2021, 186, 535–543. [Google Scholar] [CrossRef]
- Pham Duc, T.; Ly, B.M.; Usoltseva, R.V.; Shevchenko, N.M.; Rasin, A.B.; Anastyuk, S.D.; Malyarenko, O.S.; Zvyagintseva, T.N.; San, P.T.; Ermakova, S.P. A novel sulfated fucan from Vietnamese sea cucumber Stichopus variegatus: Isolation, structure and anticancer activity in vitro. Int. J. Biol. Macromol. 2018, 117, 1101–1109. [Google Scholar]
- Yin, R.; Zhou, L.; Gao, N.; Li, Z.; Zhao, L.; Shang, F.; Wu, M.; Zhao, J. Oligosaccharides from depolymerized fucosylated glycosaminoglycan: Structures and minimum size for intrinsic factor Xase complex inhibition. J. Biol. Chem. 2018, 293, 14089–14099. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H. NMR structural determination of unique invertebrate glycosaminoglycans endowed with medical properties. Carbohydr. Res. 2015, 413, 41–50. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. Structural analysis of holothurian fucosylated chondroitin sulfates: Degradation versus non-destructive approach. Carbohydr. Res. 2019, 476, 6–11. [Google Scholar] [CrossRef]
- Mourão, P.A.S. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbst, A.G.; Ustyuzhanina, N.E.; Nifantiev, N.E. Computational study of the possible formation of the ternary complex between thrombin, antithrombin III and fucosylated chondroitin sulfates. Mendeleev Commun. 2015, 25, 420–421. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Zakharova, A.N.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Polysaccharides of algae: 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorg. Chem. 2007, 33, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae: 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]
- Guo, X.; Condra, M.; Kimura, K.; Berth, G.; Dautzenberg, H.; Dubin, P.L. Determination of molecular weight of heparin by size exclusion chromatography with universal calibration. Anal. Biochem. 2003, 312, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers Apostichopus japonicus and Actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Borodina, E.Y.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: Structure and effects on coagulation. Carbohydr. Polym. 2017, 167, 20–26. [Google Scholar] [CrossRef] [PubMed]
| Sample | Fuc | Gal | GlcNAc | GalNAc | UA, Na-salt | SO3Na | Molecular Weight, kDa (Dispercity) |
|---|---|---|---|---|---|---|---|
| SP-BA | 1.00 | 0.14 | 0.07 | 0.30 | 0.30 | 2.05 | |
| FCS-BA | 1.00 | 0.09 | n.d. | 0.78 | 0.83 | 4.04 | 32 (1.55) |
| FS-BA-AT | 1.00 | 0.09 | 0.04 | 0.06 | 0.04 | 1.28 | 55 (1.35) |
| SP-HS | 1.00 | 0.12 | 0.08 | 0.3 | 0.21 | 2.44 | |
| FCS-HS | 1.00 | 0.12 | 0.04 | 0.74 | 1.10 | 3.44 | 30 (1.62) |
| FS-HS-AT | 1.00 | 0.09 | 0.04 | 0.18 | 0.18 | 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Nikogosova, S.P.; Hang, C.T.T.; Thinh, P.D.; Trung, D.T.; Van, T.T.T.; Shashkov, A.S.; et al. Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and Anticoagulant Activity. Mar. Drugs 2022, 20, 380. https://doi.org/10.3390/md20060380
Ustyuzhanina NE, Bilan MI, Dmitrenok AS, Tsvetkova EA, Nikogosova SP, Hang CTT, Thinh PD, Trung DT, Van TTT, Shashkov AS, et al. Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and Anticoagulant Activity. Marine Drugs. 2022; 20(6):380. https://doi.org/10.3390/md20060380
Chicago/Turabian StyleUstyuzhanina, Nadezhda E., Maria I. Bilan, Andrey S. Dmitrenok, Eugenia A. Tsvetkova, Sofya P. Nikogosova, Cao Thi Thuy Hang, Pham Duc Thinh, Dinh Thanh Trung, Tran Thi Thanh Van, Alexander S. Shashkov, and et al. 2022. "Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and Anticoagulant Activity" Marine Drugs 20, no. 6: 380. https://doi.org/10.3390/md20060380
APA StyleUstyuzhanina, N. E., Bilan, M. I., Dmitrenok, A. S., Tsvetkova, E. A., Nikogosova, S. P., Hang, C. T. T., Thinh, P. D., Trung, D. T., Van, T. T. T., Shashkov, A. S., Usov, A. I., & Nifantiev, N. E. (2022). Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and Anticoagulant Activity. Marine Drugs, 20(6), 380. https://doi.org/10.3390/md20060380







