Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of OncoVV-AVL on HCC Cells
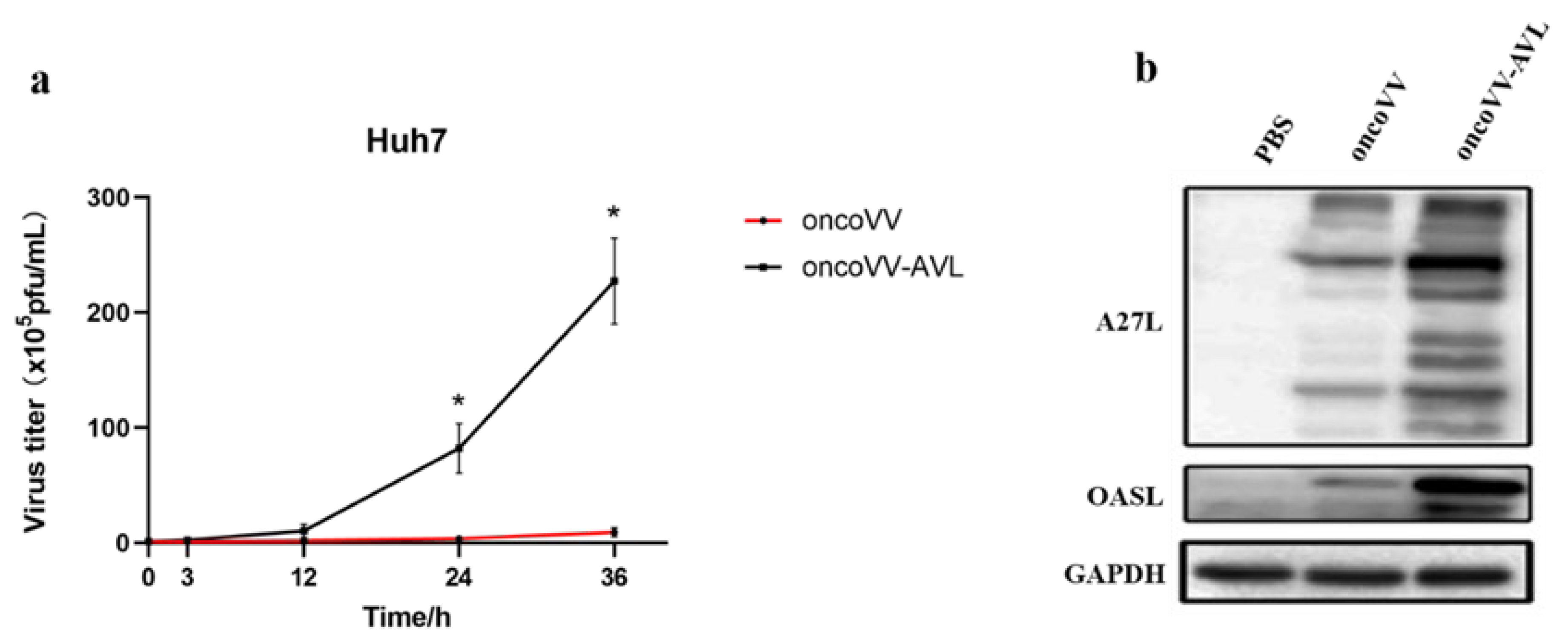
2.2. Replication Ability of OncoVV-AVL in HCC Cells
2.3. OncoVV-AVL Promotes Transcription of Type I Interferon in HCC Cells
2.4. Regulation of Antiviral Factors by OncoVV-AVL in HCC Cells
2.5. Pathways Associated with Replication Ability of OncoVV-AVL
2.6. OncoVV-AVL Suppressed HCC Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay and Flow Cytometry Determination
4.3. Detection of Viral Replication Ability
4.4. Western Blot
4.5. qRT-PCR
4.6. Dual Luciferase Reporter Gene Assay
4.7. Transcriptomics Analysis
4.8. In Vivo Tumor Formation Experiments in Animals
4.9. H&E Staining
4.10. Immunohistochemistry Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jhawar, S.R.; Thandoni, A.; Bommareddy, P.K.; Hassan, S.; Kohlhapp, F.J.; Goyal, S.; Schenkel, J.M.; Silk, A.W.; Zloza, A. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front. Oncol. 2017, 7, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buijs, P.R.A.; Verhagen, J.H.E.; van Eijck, C.H.J.; van den Hoogen, B.G. Oncolytic viruses: From bench to bedside with a focus on safety. Hum. Vacc. Immunother. 2015, 11, 1573–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.C.; McFadden, G. Editorial overview: Oncolytic viruses—replicating virus therapeutics for the treatment of cancer. Curr. Opin. Virol. 2015, 13, viii–ix. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Arulanandam, R.; Wassenaar, R.; Falls, T.; Petryk, J.; Paget, J.; Garson, K.; Cemeus, C.; Vanderhyden, B.C.; Wells, R.G.; et al. Enhancing expression of functional human sodium Iodide symporter and somatostatin receptor in recombinant oncolytic vaccinia virus for in vivo imaging of tumors. J. Nucl. Med. 2017, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Huang, B.; Deng, L.L.; Hu, Z.G. Progress in gene therapy using oncolytic vaccinia virus as vectors. J. Cancer Res. Clin. 2018, 144, 2433–2440. [Google Scholar] [CrossRef]
- Guo, Z.S.; Lu, B.F.; Guo, Z.B.; Giehl, E.; Feist, M.; Dai, E.Y.; Liu, W.L.; Storkus, W.J.; He, Y.K.; Liu, Z.Q.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Tenneti, P.; Borad, M.J.; Babiker, H.M. Exploring the role of oncolytic viruses in hepatobiliary cancers. Immunotherapy 2018, 10, 971–986. [Google Scholar] [CrossRef]
- Kloker, L.D.; Berchtold, S.; Smirnow, I.; Beil, J.; Krieg, A.; Sipos, B.; Lauer, U.M. Oncolytic vaccinia virus GLV-1h68 exhibits profound antitumoral activities in cell lines originating from neuroendocrine neoplasms. BMC Cancer 2020, 20, 628. [Google Scholar] [CrossRef]
- Beguin, J.; Gantzer, M.; Farine, I.; Foloppe, J.; Klonjkowski, B.; Maurey, C.; Quemeneur, E.; Erbs, P. Safety, biodistribution and viral shedding of oncolytic vaccinia virus TG6002 administered intravenously in healthy beagle dogs. Sci. Rep. 2021, 11, 2209. [Google Scholar] [CrossRef]
- Sharon, N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues Mantuano, N.; Natoli, M.; Zippelius, A.; Läubli, H. Tumor-associated carbohydrates and immunomodulatory lectins as targets for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001222. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.L.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biot. 2015, 99, 3755–3773. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.D.; Ly, B.M.; Hao, V.T.; Trung, D.T.; Trang, V.T.D.; Trinh, P.T.H.; Ngoc, N.T.D.; Quang, T.M. Purification, characterization and biological effect of lectin from the marine sponge stylissa flexibilis (Levi, 1961). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 216, 32–38. [Google Scholar] [CrossRef]
- Catanzaro, E.; Calcabrini, C.; Bishayee, A.; Fimognari, C. Antitumor potential of marine and freshwater lectins. Mar. Drugs 2019, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, D.N.; de Almeida, A.S.; Sousa, A.R.D.; Pereira, R.; Andrade, A.L.; Chaves, R.P.; Carneiro, R.F.; de Vasconcelos, M.A.; do Nascimento-Neto, L.G.; Pinheiro, U.; et al. Antibacterial activity of a new lectin isolated from the marine sponge chondrilla caribensis. Int. J. Biol. Macromol. 2018, 109, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Fujiwara, T.; Koide, Y.; Hasan, I.; Sugawara, S.; Rajia, S.; Kawsar, S.M.A.; Yamamoto, D.; Araki, D.; Kanaly, R.A.; et al. Internalization of a novel, huge lectin from Ibacus novemdentatus (slipper lobster) induces apoptosis of mammalian cancer cells. Glycoconj. J. 2017, 34, 85–94. [Google Scholar] [CrossRef]
- Terada, D.; Kawai, F.; Noguchi, H.; Unzai, S.; Hasan, I.; Fujii, Y.; Park, S.Y.; Ozeki, Y.; Tame, J.R. Crystal structure of MytiLec, a galactose-binding lectin from the mussel Mytilus galloprovincialis with cytotoxicity against certain cancer cell types. Sci. Rep. 2016, 6, 28344. [Google Scholar] [CrossRef]
- Chernikov, O.; Kuzmich, A.; Chikalovets, I.; Molchanova, V.; Hua, K.F. Lectin CGL from the sea mussel crenomytilus grayanus induces burkitt’s lymphoma cells death via interaction with surface glycan. Int. J. Biol. Macromol. 2017, 104, 508–514. [Google Scholar] [CrossRef]
- Li, G.C.; Cheng, J.H.; Mei, S.S.; Wu, T.; Ye, T. Tachypleus tridentatus lectin enhances oncolytic vaccinia virus replication to suppress in vivo hepatocellular carcinoma growth. Mar. Drugs 2018, 16, 200. [Google Scholar] [CrossRef] [Green Version]
- Li, G.C.; Mei, S.S.; Cheng, J.H.; Wu, T.; Luo, J.J. Haliotis discus discus sialic acid-binding lectin reduces the oncolytic vaccinia virus induced toxicity in a glioblastoma mouse model. Mar Drugs 2018, 16, 141. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.Q.; Yang, X.Y.; Duan, X.M.; Cui, L.Z.; Li, G.C. Exogenous expression of marine lectins DlFBL and SpRBL induces cancer cell apoptosis possibly through PRMT5-E2F-1 pathway. Sci. Rep. 2014, 4, 4505. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, N.N.; Liu, T.T.; Jia, X.Y.; Ye, T.; Chen, K.; Li, G.C. Oncolytic vaccinia virus expressing White-Spotted Charr lectin regulates antiviral response in tumor cells and inhibits tumor growth in vitro and in vivo. Mar. Drugs 2021, 19, 292. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xiang, Y.L.; Liu, T.T.; Wang, X.; Ren, X.Y.; Ye, T.; Li, G.C. Oncolytic vaccinia virus expressing Aphrocallistes vastus lectin as a cancer therapeutic agent. Mar. Drugs 2019, 17, 363. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Feng, H.; Xu, X.; Liu, T.; Ye, T.; Chen, K.; Li, G. Oncolytic vaccinia virus harboring Aphrocallistes vastus lectin inhibits the growth of cervical cancer cells Hela S3. Mar. Drugs 2021, 19, 532. [Google Scholar] [CrossRef]
- Vázquez, M.I.; Rivas, G.; Cregut, D.; Serrano, L.; Esteban, M. The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal alpha-helix. J. Virol. 1998, 72, 10126–10137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Shao, L.L.; Sampath, P.; Zhao, B.Y.; Patel, N.V.; Zhu, J.Z.; Behl, B.; Parise, R.A.; Beumer, J.H.; O’Sullivan, R.J.; et al. Oligoadenylate-synthetase-family protein OASL inhibits activity of the DNA sensor cGAS during DNA virus infection to limit interferon production. Immunity 2019, 50, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Fenton, S.E.; Saleiro, D.; Platanias, L.C. Type I and II interferons in the anti-tumor immune response. Cancers 2021, 13, 1037. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, J.Y. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med. J. 2011, 52, 379–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniatis, T.; Falvo, J.V.; Kim, T.H.; Kim, T.K.; Lin, C.H.; Parekh, B.S.; Wathelet, M.G. Structure and function of the interferon-beta enhanceosome. Cold Spring Harb. Symp. Quant. Biol. 1998, 63, 609–620. [Google Scholar] [CrossRef]
- Thanos, D.; Maniatis, T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 1995, 83, 1091–1100. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wan, P.; Cao, Y.; Zhang, W.; Chen, J.; Tan, L.; Wang, Y.; Sun, Z.; Zhang, Q.; Wan, Y.; et al. Hepatitis B Virus e Antigen Activates the Suppressor of Cytokine Signaling 2 to Repress Interferon Action. Sci. Rep. 2017, 7, 1729. [Google Scholar] [CrossRef] [Green Version]
- Shepardson, K.M.; Larson, K.; Johns, L.L.; Stanek, K.; Cho, H.; Wellham, J.; Henderson, H.; Rynda-Apple, A. IFNAR2 Is Required for Anti-influenza Immunity and Alters Susceptibility to Post-influenza Bacterial Superinfections. Front. Immunol. 2018, 9, 2589. [Google Scholar] [CrossRef]
- Michalska, A.; Blaszczyk, K.; Wesoly, J.; Bluyssen, H.A.R. A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses. Front. Immunol. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, K.; Grandvaux, N. STAT2 and IRF9: Beyond ISGF3. Jak-Stat 2013, 2, e27521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Xiong, T.; Yu, H.; Zhang, Q.; Zhang, K.; Li, C.; Hu, L.; Zhang, Y.; Zhang, L.; Liu, Q.; et al. Encephalomyocarditis virus 3C protease attenuates type I interferon production through disrupting the TANK-TBK1-IKKε-IRF3 complex. Biochem. J. 2017, 474, 2051–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, L.J.; Olson, M.E.; Ravindranathan, P.; Guo, H.; Kempema, A.M.; Andrews, T.E.; Chen, X.; Raj, G.V.; Harki, D.A.; Dehm, S.M. EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer. Oncotarget 2015, 6, 3811–3824. [Google Scholar] [CrossRef] [Green Version]
- Tonnus, W.; Meyer, C.; Paliege, A.; Belavgeni, A.; von Mässenhausen, A.; Bornstein, S.R.; Hugo, C.; Becker, J.U.; Linkermann, A. The pathological features of regulated necrosis. J. Pathol. 2019, 247, 697–707. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, Y.; Zhao, Q.; Sun, P.; Gao, Z.; Cui, S. Analysis of the short-term effect of photodynamic therapy on primary bronchial lung cancer. Laser. Med. Sci. 2021, 36, 753–761. [Google Scholar] [CrossRef]
- Froechlich, G.; Caiazza, C.; Gentile, C.; D’Alise, A.M.; De Lucia, M.; Langone, F.; Leoni, G.; Cotugno, G.; Scisciola, V.; Nicosia, A.; et al. Integrity of the antiviral STING-mediated DNA sensing in tumor cells is required to sustain the immunotherapeutic efficacy of Herpes Simplex oncolytic virus. Cancers 2020, 12, 3407. [Google Scholar] [CrossRef]
- Malakhova, O.A.; Yan, M.; Malakhov, M.P.; Yuan, Y.; Ritchie, K.J.; Kim, K.I.; Peterson, L.F.; Shuai, K.; Zhang, D.E. Protein ISGylation modulates the JAK-STAT signaling pathway. Gene. Dev. 2003, 17, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Nie, M.; Oravcová, M.; Jami-Alahmadi, Y.; Wohlschlegel, J.A.; Lazzerini-Denchi, E.; Boddy, M.N. FAM111A induces nuclear dysfunction in disease and viral restriction. EMBO Rep. 2021, 22, e50803. [Google Scholar] [CrossRef] [PubMed]
- Feeley, E.M.; Sims, J.S.; John, S.P.; Chin, C.R.; Pertel, T.; Chen, L.M.; Gaiha, G.D.; Ryan, B.J.; Donis, R.O.; Elledge, S.J.; et al. IFITM3 inhibits Influenza A Virus infection by preventing cytosolic entry. PLoS Pathog. 2011, 7, e1002337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, K.M.; Yang, Z.Y.; Johnson, J.R.; Geng, X.; Doitsh, G.; Krogan, N.J.; Greene, W.C. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 2014, 343, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.A.P.; Leite, F.G.G.; Andrade, L.G.; Torres, A.A.; De Sousa, L.P.; Barcelos, L.S.; Teixeira, M.M.; Ferreira, P.C.P.; Kroon, E.G.; Souto-Padron, T.; et al. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J. Virol. 2009, 83, 6883–6899. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lee, C. Extracellular signal-regulated kinase (ERK) activation is required for porcine epidemic diarrhea virus replication. Virology 2015, 484, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yu, X.; Yu, H.; Liu, B.; Zhang, Z.; Kong, C.; Li, Z. Knockdown of MAPK14 inhibits the proliferation and migration of clear cell renal cell carcinoma by downregulating the expression of CDC25B. Cancer Med. 2020, 9, 1183–1195. [Google Scholar] [CrossRef]
- Kurapati, S.; Sadaoka, T.; Rajbhandari, L.; Jagdish, B.; Shukla, P.; Ali, M.A.; Kim, Y.J.; Lee, G.; Cohen, J.I.; Venkatesan, A. Role of the JNK pathway in varicella-zoster virus lytic infection and reactivation. J. Virol. 2017, 91, e00640-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Lu, W.; Zhang, Y.; Zou, F.; Jin, Z.; Zhao, T. The Hippo pathway and viral infections. Front. Microbiol. 2017, 10, 3033. [Google Scholar] [CrossRef] [Green Version]
- Moelling, K.; Schad, K.; Bosse, M.; Zimmermann, S.; Schweneker, M. Regulation of Raf-Akt cross-talk. J. Biol. Chem. 2002, 277, 31099–31106. [Google Scholar] [CrossRef] [Green Version]
- Nussinov, R.; Tsai, C.J.; Jang, H.; Korcsmáros, T.; Csermely, P. Oncogenic KRAS signaling and YAP1/β-catenin: Similar cell cycle control in tumor initiation. Semin. Cell Dev. Biol. 2016, 58, 79–85. [Google Scholar] [CrossRef]
- Romano, D.; Nguyen, L.K.; Matallanas, D.; Halasz, M.; Doherty, C.; Kholodenko, B.N.; Kolch, W. Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat. Cell Biol. 2014, 16, 673–684. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef] [Green Version]
- Greseth, M.D.; Traktman, P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014, 10, e1004021. [Google Scholar] [CrossRef] [Green Version]
- Leung, F.W. Capsaicin as an anti-obesity drug. Prog Drug Res. 2014, 68, 171–179. [Google Scholar] [PubMed]
- Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.d.C.; Takagi, M. Effects of Capsaicin on biomimetic membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, F.J.; Villalaín, J.; Gómez-Fernández, J.C. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim. Biophys. Acta 1995, 1234, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.J.; Kuo, C.F.; Chen, W.L.; Ou, J.H.J. Enhancement of Hepatitis b virus replication by androgen and its receptor in mice. J. Virol. 2012, 86, 1904–1910. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, L.; Wang, X. Host sex steroids interact with virus infection: New insights into sex disparity in infectious diseases. Front. Microbiol. 2021, 12, 747347. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, 469–471. [Google Scholar] [CrossRef]
- Toktay, Y.; Dayanc, B.; Senturk, S. Engineering and validation of a dual luciferase reporter system for quantitative and systematic assessment of regulatory sequences in chinese hamster ovary cells. Sci. Rep. 2022, 12, 6050. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Qiu, Y.; Zhang, X.; Zhou, N.; Jia, X.; Chen, K.; Zhou, Y.; Ye, T.; Li, G. Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells. Mar. Drugs 2022, 20, 378. https://doi.org/10.3390/md20060378
Jiang R, Qiu Y, Zhang X, Zhou N, Jia X, Chen K, Zhou Y, Ye T, Li G. Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells. Marine Drugs. 2022; 20(6):378. https://doi.org/10.3390/md20060378
Chicago/Turabian StyleJiang, Riqing, Yufeng Qiu, Xiaomei Zhang, Ningning Zhou, Xiaoyuan Jia, Kan Chen, Yanrong Zhou, Ting Ye, and Gongchu Li. 2022. "Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells" Marine Drugs 20, no. 6: 378. https://doi.org/10.3390/md20060378
APA StyleJiang, R., Qiu, Y., Zhang, X., Zhou, N., Jia, X., Chen, K., Zhou, Y., Ye, T., & Li, G. (2022). Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells. Marine Drugs, 20(6), 378. https://doi.org/10.3390/md20060378





