Marine Microbial-Derived Resource Exploration: Uncovering the Hidden Potential of Marine Carotenoids
Abstract
:1. Introduction
2. Carotenoids
2.1. Characteristics and Biological Function of Carotenoids
2.2. Carotenoid Synthesis Gene Cluster in Marine Microorganisms
3. Marine-Derived Carotenoid Screening
3.1. In Vitro Carotenoid Screening
3.1.1. Culture-Dependent Approaches
3.1.2. Culture-Independent Approaches
3.2. In Silico Carotenoid Screening
4. Industrial Production of Carotenoid
4.1. Economic Potential of Marine Carotenoids
4.2. Carotenoids Function and Uses in Health and Pharmaceutical Industry
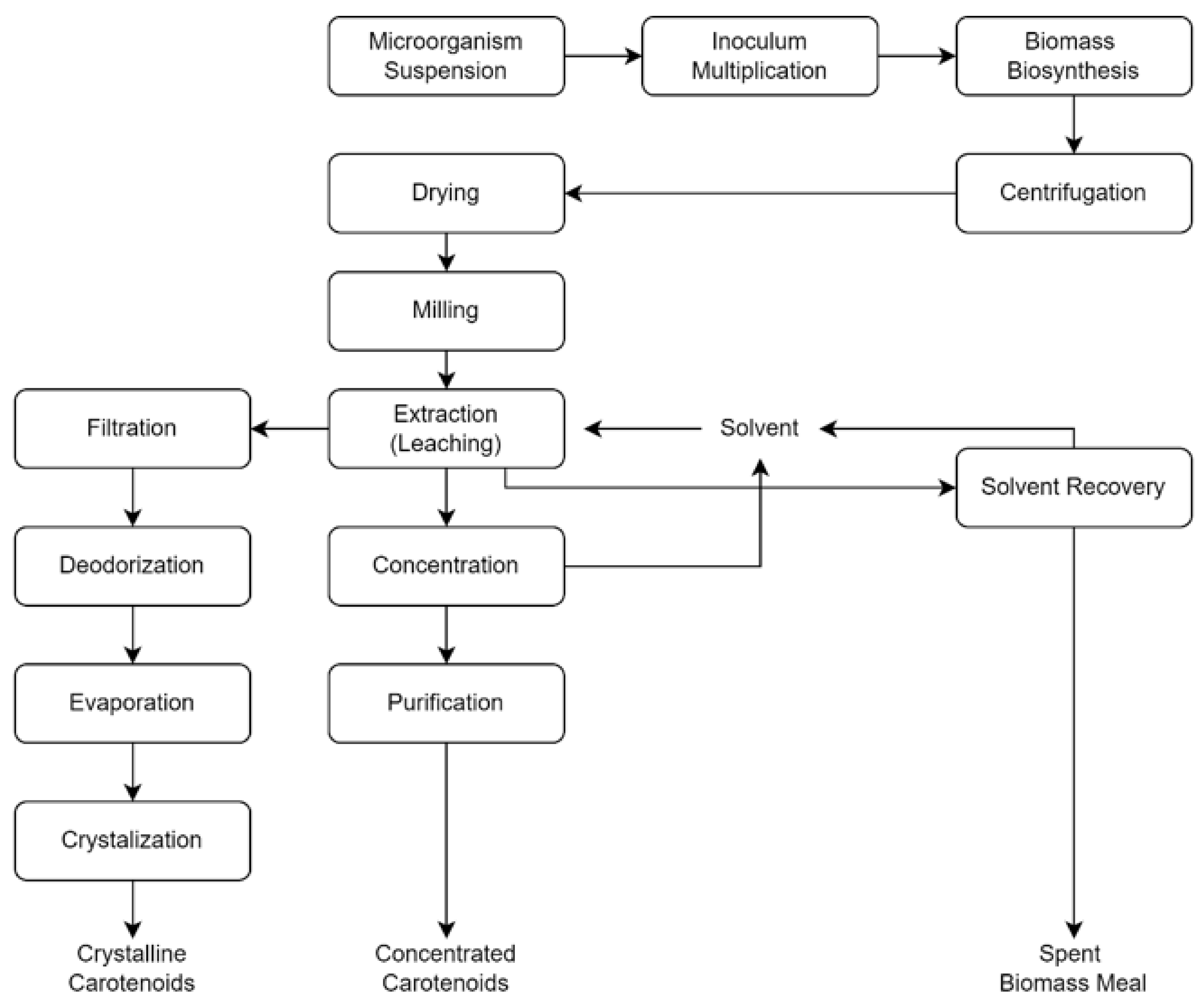
4.3. Production System and Mechanism in Industrial Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bérdy, J. Thoughts and Facts about Antibiotics: Where We Are Now and Where We Are Heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.-E.; Kellermann, M.Y.; Schupp, P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. In YOUMARES 9—The Oceans: Our Research, Our Future: Proceedings of the 2018 Conference for Young Marine Researcher in Oldenburg, Germany; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 159–180. ISBN 978-3-030-20389-4. [Google Scholar]
- López-García, P.; López-López, A.; Moreira, D.; Rodríguez-Valera, F. Diversity of Free-Living Prokaryotes from a Deep-Sea Site at the Antarctic Polar Front. FEMS Microbiol. Ecol. 2001, 36, 193–202. [Google Scholar] [CrossRef]
- Bano, N.; Hollibaugh, J.T. Phylogenetic Composition of Bacterioplankton Assemblages from the Arctic Ocean. Appl. Environ. Microbiol. 2002, 68, 505–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.M.; Rappé, M.S.; Connon, S.A.; Vergin, K.L.; Siebold, W.A.; Carlson, C.A.; Giovannoni, S.J. SAR11 Clade Dominates Ocean Surface Bacterioplankton Communities. Nature 2002, 420, 806–810. [Google Scholar] [CrossRef]
- Pérez-Matos, A.E.; Rosado, W.; Govind, N.S. Bacterial Diversity Associated with the Caribbean Tunicate Ecteinascidia Turbinata. Antonie Van Leeuwenhoek 2007, 92, 155–164. [Google Scholar] [CrossRef]
- Radjasa, O.K.; Martens, T.; Grossart, H.-P.; Brinkhoff, T.; Sabdono, A.; Simon, M. Antagonistic Activity of a Marine Bacterium Pseudoalteromonas Luteoviolacea TAB4. 2 Associated with Coral Acropora sp. J. Biol. Sci. 2007, 7, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Ul Hassan, S.S.; Shaikh, A.L. Marine Actinobacteria as a Drug Treasure House. Biomed. Pharmacother. 2017, 87, 46–57. [Google Scholar] [CrossRef]
- Skropeta, D.; Wei, L. Recent Advances in Deep-Sea Natural Products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. 1.12—Secondary Metabolites. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Butler, M., Eds.; Academic Press: Burlington, NJ, USA, 2011; pp. 155–167. ISBN 978-0-08-088504-9. [Google Scholar]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Chu, L.; Huang, J.; Muhammad, M.; Deng, Z.; Gao, J. Genome Mining as a Biotechnological Tool for the Discovery of Novel Marine Natural Products. Crit. Rev. Biotechnol. 2020, 40, 571–589. [Google Scholar] [CrossRef]
- Niu, F.-X.; Lu, Q.; Bu, Y.-F.; Liu, J.-Z. Metabolic Engineering for the Microbial Production of Isoprenoids: Carotenoids and Isoprenoid-Based Biofuels. Synth. Syst. Biotechnol. 2017, 2, 167–175. [Google Scholar] [CrossRef]
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of Marine Invertebrate-Derived Biosynthetic Products: Their Biomedical Potential and Possible Production by Microbial Associants. Bioorganic Med. Chem. 2011, 19, 6658–6674. [Google Scholar] [CrossRef] [Green Version]
- Kusmita, L.; Mutiara, E.V.; Nuryadi, H.; Pratama, P.A.; Wiguna, A.S.; Radjasa, O.K. Characterization of Carotenoid Pigments from Bacterial Symbionts of Soft-Coral Sarcophyton Sp. from North Java Sea. Int. Aquat. Res. 2017, 9, 61–69. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Vegetables; Springer Science + Business Media: New York, NY, USA, 1991. [Google Scholar]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of Methods for Analysis of Carotenoids. TrAC Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Wagner, K.-H.; Elmadfa, I. Biological Relevance of Terpenoids. Ann. Nutr. Metab. 2003, 47, 95–106. [Google Scholar] [CrossRef]
- Feltl, L.; Pacakova, V.; Stulik, K.; Volka, K. Reliability of Carotenoid Analyses: A Review. Curr. Anal. Chem. 2005, 1, 93–102. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Vegetables; Springer: Boston, MA, USA, 2012; ISBN 978-1-4613-5842-8. [Google Scholar]
- Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, Medicinal Properties and Their Application in Food and Nutraceutical Industry. J. Med. Plants Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1118733304. [Google Scholar]
- Chasse, G.A.; Mak, M.L.; Deretey, E.; Farkas, I.; Torday, L.L.; Papp, J.G.; Sarma, D.S.; Agarwal, A.; Chakravarthi, S.; Agarwal, S.; et al. An Ab Initio Computational Study on Selected Lycopene Isomers. J. Mol. Struct. THEOCHEM 2001, 571, 27–37. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of Carotenoids in Carrot: An Underground Story Comes to Light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef]
- Yang, X.; Lei, H.; Gao, P.; Thomas, D.G.; Mobley, D.L.; Baker, N.A. Atomic Radius and Charge Parameter Uncertainty in Biomolecular Solvation Energy Calculations. J. Chem. Theory Comput. 2018, 14, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Horton, P. Dynamic Behavior of Photosystem II Light Harvesting. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 178–183. [Google Scholar]
- Bouvier, F.; D’Harlingue, A.; Backhaus, R.A.; Kumagai, M.H.; Camara, B. Identification of Neoxanthin Synthase as a Carotenoid Cyclase Paralog. Eur. J. Biochem. 2000, 267, 6346–6352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, Occurrence, and Potential Health Benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [Green Version]
- Wilding, E.I.; Brown, J.R.; Bryant, A.P.; Chalker, A.F.; Holmes, D.J.; Ingraham, K.A.; Iordanescu, S.; So, C.Y.; Rosenberg, M.; Gwynn, M.N. Identification, Evolution, and Essentiality of the Mevalonate Pathway for Isopentenyl Diphosphate Biosynthesis in Gram-Positive Cocci. J. Bacteriol. 2000, 182, 4319–4327. [Google Scholar] [CrossRef] [Green Version]
- Rohmer, M.; Seemann, M.; Horbach, S.; Bringer-Meyer, S.; Sahm, H. Glyceraldehyde 3-Phosphate and Pyruvate as Precursors of Isoprenic Units in an Alternative Non-Mevalonate Pathway for Terpenoid Biosynthesis. J. Am. Chem. Soc. 1996, 118, 2564–2566. [Google Scholar] [CrossRef]
- Giraud, E.; Hannibal, L.; Fardoux, J.; Jaubert, M.; Jourand, P.; Dreyfus, B.; Sturgis, J.N.; Verméglio, A. Two Distinct crt Gene Clusters for Two Different Functional Classes of Carotenoid in Bradyrhizobium. J. Biol. Chem. 2004, 279, 15076–15083. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, C.; Zhang, X.; Tan, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys Nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of Stress on Astaxanthin Production in Haematococcus Pluvialis Grown under Different Culture Conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Du, X.; Li, P.; Liang, B.; Cheng, X.; Du, L.; Huang, D.; Wang, L.; Wang, S. Complete Genome Sequence and Transcriptomics Analyses Reveal Pigment Biosynthesis and Regulatory Mechanisms in an Industrial Strain, Monascus purpureus YY-1. Sci. Rep. 2015, 5, 8331. [Google Scholar] [CrossRef]
- Zakar, T.; Laczko-Dobos, H.; Toth, T.N.; Gombos, Z. Carotenoids Assist in Cyanobacterial Photosystem II Assembly and Function. Front. Plant Sci. 2016, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Structures, Nomenclature and General Chemistry of Carotenoids and Their Esters. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Hannibal, L.; Lorquin, J.; D’Ortoli, N.A.; Garcia, N.; Chaintreuil, C.; Masson-Boivin, C.; Dreyfus, B.; Giraud, E. Isolation and Characterization of Canthaxanthin Biosynthesis Genes from the Photosynthetic Bacterium Bradyrhizobium sp. Strain ORS278. J. Bacteriol. 2000, 182, 3850–3853. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid Profile, Antioxidant Capacity, and Chromoplasts of Pink Guava (Psidium guajava L. Cv. ‘Criolla’) during Fruit Ripening. J. Agric. Food Chem. 2017, 65, 3737–3747. [Google Scholar] [CrossRef]
- Bode, S.; Quentmeier, C.C.; Liao, P.-N.; Hafi, N.; Barros, T.; Wilk, L.; Bittner, F.; Walla, P.J. On the Regulation of Photosynthesis by Excitonic Interactions between Carotenoids and Chlorophylls. Proc. Natl. Acad. Sci. USA 2009, 106, 12311–12316. [Google Scholar] [CrossRef] [Green Version]
- Aro, E.-M.; Suorsa, M.; Rokka, A.; Allahverdiyeva, Y.; Paakkarinen, V.; Saleem, A.; Battchikova, N.; Rintamäki, E. Dynamics of Photosystem II: A Proteomic Approach to Thylakoid Protein Complexes. J. Exp. Bot. 2005, 56, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis-Mansur, M.C.P.P.; Cardoso-Rurr, J.S.; Silva, J.V.M.A.; de Souza, G.R.; da Silva Cardoso, V.; Mansoldo, F.R.P.; Pinheiro, Y.; Schultz, J.; Lopez Balottin, L.B.; da Silva, A.J.R.; et al. Carotenoids from UV-Resistant Antarctic Microbacterium sp. LEMMJ01. Sci. Rep. 2019, 9, 9554. [Google Scholar] [CrossRef] [PubMed]
- Sedkova, N.; Tao, L.; Rouvière, P.E.; Cheng, Q. Diversity of Carotenoid Synthesis Gene Clusters from Environmental Enterobacteriaceae Strains. Appl. Environ. Microbiol. 2005, 71, 8141–8146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Selão, T.T.; Selstam, E.; Norling, B. Subcellular Localization of Carotenoid Biosynthesis in Synechocystis sp. PCC 6803. PLoS ONE 2015, 10, e0130904. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Adachi, K.; Kasai, H.; Shizuri, Y.; Shindo, K.; Sawabe, A.; Komemushi, S.; Miki, W.; Misawa, N. Elucidation of a Carotenoid Biosynthesis Gene Cluster Encoding a Novel Enzyme, 2, 2′-β-Hydroxylase, from Brevundimonas sp. Strain SD212 and Combinatorial Biosynthesis of New or Rare Xanthophylls. Appl. Environ. Microbiol. 2005, 71, 4286–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misawa, N.; Satomi, Y.; Kondo, K.; Yokoyama, A.; Kajiwara, S.; Saito, T.; Ohtani, T.; Miki, W. Structure and Functional Analysis of a Marine Bacterial Carotenoid Biosynthesis Gene Cluster and Astaxanthin Biosynthetic Pathway Proposed at the Gene Level. J. Bacteriol. 1995, 177, 6575–6584. [Google Scholar] [CrossRef] [Green Version]
- Pasamontes, L.; Hug, D.; Tessier, M.; Hohmann, H.-P.; Schierle, J.; van Loon, A.P.G.M. Isolation and Characterization of the Carotenoid Biosynthesis Genes of Flavobacterium sp. Strain R1534. Gene 1997, 185, 35–41. [Google Scholar] [CrossRef]
- Krubasik, P.; Sandmann, G. A Carotenogenic Gene Cluster from Brevibacterium Linens with Novel Lycopene Cyclase Genes Involved in the Synthesis of Aromatic Carotenoids. Mol. Gen. Genet. MGG 2000, 263, 423–432. [Google Scholar] [CrossRef]
- Tao, L.; Yao, H.; Kasai, H.; Misawa, N.; Cheng, Q. A Carotenoid Synthesis Gene Cluster from Algoriphagus sp. KK10202C with a Novel Fusion-Type Lycopene β-Cyclase Gene. Mol. Genet. Genom. 2006, 276, 79–86. [Google Scholar] [CrossRef]
- Zampieri, A.; Babbucci, M.; Carraro, L.; Milan, M.; Fasolato, L.; Cardazzo, B. Combining Culture-Dependent and Culture-Independent Methods: New Methodology Insight on the Vibrio Community of Ruditapes philippinarum. Foods 2021, 10, 1271. [Google Scholar] [CrossRef]
- Kusmita, L.; Nuryadi, H.; Abi Widyananto, P.; Muchlissin, S.; Sabdono, A.; Trianto, A.; Karna Radjasa, O. Bioactivity of Carotenoid Produced by Soft Coral Symbiotic Microorganisms from Panjang and Karimunjawa Island, Centra Java, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 732–740. [Google Scholar] [CrossRef]
- Figdor, D.; Gulabivala, K. Survival against the Odds: Microbiology of Root Canals Associated with Post-Treatment Disease. Endod. Top. 2008, 18, 62–77. [Google Scholar] [CrossRef]
- Joint, I.; Mühling, M.; Querellou, J. Culturing Marine Bacteria—An Essential Prerequisite for Biodiscovery. Microb. Biotechnol. 2010, 3, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Kisand, V.; Wikner, J. Combining Culture-Dependent and-Independent Methodologies for Estimation of Richness of Estuarine Bacterioplankton Consuming Riverine Dissolved Organic Matter. Appl. Environ. Microbiol. 2003, 69, 3607–3616. [Google Scholar] [CrossRef] [Green Version]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun Metagenomics, from Sampling to Analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Hwang, S.; Kim, J.; Cho, S.; Palsson, B.; Cho, B.-K. Mini Review: Genome Mining Approaches for the Identification of Secondary Metabolite Biosynthetic Gene Clusters in Streptomyces. Comput. Struct. Biotechnol. J. 2020, 18, 1548–1556. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Merwin, N.J.; Johnston, C.W.; Magarvey, N.A. PRISM 3: Expanded Prediction of Natural Product Chemical Structures from Microbial Genomes. Nucleic Acids Res. 2017, 45, W49–W54. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving Cluster Detection and Comparison Capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into Secondary Metabolism from a Global Analysis of Prokaryotic Biosynthetic Gene Clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Hannigan, G.D.; Prihoda, D.; Palicka, A.; Soukup, J.; Klempir, O.; Rampula, L.; Durcak, J.; Wurst, M.; Kotowski, J.; Chang, D. A Deep Learning Genome-Mining Strategy for Biosynthetic Gene Cluster Prediction. Nucleic Acids Res. 2019, 47, e110. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.W.; Lee, P.C. Genome Mining Reveals Two Missing CrtP and AldH Enzymes in the C30 Carotenoid Biosynthesis Pathway in Planococcus faecalis AJ003T. Molecules 2020, 25, 5892. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, M.; Lu, W.; Chen, M.; Yan, Y.; Lin, M.; Zhang, W.; Zhou, Z. Comparative Genome Characterization of Echinicola marina sp. Nov., Isolated from Deep-Sea Sediment Provide Insight into Carotenoid Biosynthetic Gene Cluster Evolution. Sci. Rep. 2021, 11, 24188. [Google Scholar] [CrossRef] [PubMed]
- Niero, H.; da Silva, M.A.C.; de Felicio, R.; Trivella, D.B.B.; de Souza Lima, A.O. Carotenoids Produced by the Deep-Sea Bacterium Erythrobacter citreus LAMA 915: Detection and Proposal of Their Biosynthetic Pathway. Folia Microbiol. 2021, 66, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Takagi, C.; Aikawa, M.; Araki, K.; Choi, S.-K.; Itaya, M.; Shindo, K.; Misawa, N. Heterologous Production of Novel and Rare C30-Carotenoids Using Planococcus Carotenoid Biosynthesis Genes. Microb. Cell Fact. 2021, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tian, L.; Xu, J.; Xie, C.; Jiang, L.; Huang, H. Analysis and Expression of the Carotenoid Biosynthesis Genes from Deinococcus wulumuqiensis R12 in Engineered Escherichia coli. AMB Express 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, Y.; Li, X.; Zhu, F.; Cheng, Y.; Liu, Y.; Deng, Z.; Liu, T. Genome Mining of Astaxanthin Biosynthetic Genes from Sphingomonas sp. ATCC 55669 for Heterologous Overproduction in Escherichia coli. Biotechnol. J. 2016, 11, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Iftime, D.; Kulik, A.; Härtner, T.; Rohrer, S.; Niedermeyer, T.H.J.; Stegmann, E.; Weber, T.; Wohlleben, W. Identification and Activation of Novel Biosynthetic Gene Clusters by Genome Mining in the Kirromycin Producer Streptomyces collinus Tü 365. J. Ind. Microbiol. Biotechnol. 2016, 43, 277–291. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Tokovenko, B.; Brötz, E.; Rückert, C.; Kalinowski, J.; Luzhetskyy, A. Genome Rearrangements of Streptomyces Albus J1074 Lead to the Carotenoid Gene Cluster Activation. Appl. Microbiol. Biotechnol. 2014, 98, 795–806. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Markets and Markets Carotenoids Market by Type (Astaxanthin, Beta-Carotene, Lutein, Lycopene, Canthaxanthin, and Zeaxanthin), Application (Feed, Food & Beverages, Dietary Supplements, Cosmetics, and Pharmaceuticals), Source, Formulation, and Region—Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html (accessed on 1 March 2022).
- Carotenoids Market Size Is Projected to Reach USD 1168.7 Million by 2026 at CAGR 0.6%|Valuates Reports. Available online: https://www.prnewswire.com/in/news-releases/carotenoids-market-size-is-projected-to-reach-usd-1168-7-million-by-2026-at-cagr-0-6-valuates-reports-810425072.html (accessed on 1 March 2022).
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial Potential of Carotenoid Pigments from Microalgae: Current Trends and Future Prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Markets and Markets Astaxanthin Market by Source (Natural, Synthetic), Form (Dry, Liquid), Method of Production (Microalgae Cultivation, Chemical Synthesis, Fermentation), Application (Dietary Supplements, Food & Beverages, Cosmetics), and Region—Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/astaxanthin-market-162119410.html (accessed on 1 March 2022).
- Saini, R.K.; Keum, Y.-S. Microbial Platforms to Produce Commercially Vital Carotenoids at Industrial Scale: An Updated Review of Critical Issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Gammone, M.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health Benefits of Carotenoids and Potential Application in Poultry Industry: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor Activity of Astaxanthin and Its Mode of Action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef]
- Monroy-Ruiz, J.; Sevilla, M.-Á.; Carrón, R.; Montero, M.-J. Astaxanthin-Enriched-Diet Reduces Blood Pressure and Improves Cardiovascular Parameters in Spontaneously Hypertensive Rats. Pharmacol. Res. 2011, 63, 44–50. [Google Scholar] [CrossRef]
- Nishino, H.; Tsushima, M.; Matsuno, T.; Tanaka, Y.; Okuzumi, J.; Murakoshi, M.; Satomi, Y.; Takayasu, J.; Tokuda, H.; Nishino, A.; et al. Anti-Neoplastic Effect of Halocynthiaxanthin, a Metabolite of Fucoxanthin. Anti-Cancer Drugs 1992, 3, 493–498. [Google Scholar] [CrossRef]
- Maoka, T.; Nishino, A.; Yasui, H.; Yamano, Y.; Wada, A. Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates. Mar. Drugs 2016, 14, 93. [Google Scholar] [CrossRef] [Green Version]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The Body of Evidence to Support a Protective Role for Lutein and Zeaxanthin in Delaying Chronic Disease. Overview. J. Nutr. 2002, 132, 518S–524S. [Google Scholar] [CrossRef]
- Shindo, K.; Kimura, M.; Iga, M. Potent Antioxidative Activity of Cacalol, a Sesquiterpene Contained in Cacalia delphiniifolia Sleb et Zucc. Biosci. Biotechnol. Biochem. 2004, 68, 1393–1394. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zheng, J.; Luo, X.; Manabe, Y.; Hirata, T.; Sugawara, T. Absorption and Tissue Distribution of Siphonaxanthin from Green Algae. Mar. Drugs 2020, 18, 291. [Google Scholar] [CrossRef]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare Carotenoids, (3R)-Saproxanthin and (3R,2′S)-Myxol, Isolated from Novel Marine Bacteria (Flavobacteriaceae) and Their Antioxidative Activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and Production Methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, L.A.C.; Karp, S.G.; Vendruscolo, F.; Kanno, K.Y.F.; Zoz, L.I.C.; Carvalho, J.C. Biotechnological Production of Carotenoids and Their Applications in Food and Pharmaceutical Products. In Carotenoids; InTech: London, UK, 2017. [Google Scholar]
- Da Costa Cardoso, L.A.; Karen, Y.F.K.; Susan, G.K. Microbial Production of Carotenoids A Review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, P.; Bernstein, P.S. Microbial Xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.-H.; Kim, S.-W. Synthetic Biology and Metabolic Engineering for Marine Carotenoids: New Opportunities and Future Prospects. Mar. Drugs 2014, 12, 4810–4832. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Kubo, A.; Watanabe, A.; Sakuno, H.; Minobe, Y.; Sahara, T.; Murata, M.; Araki, M.; Harada, H.; Terada, Y.; et al. Pathway Engineering for High-Yield Production of Lutein in Escherichia coli. Synth. Biol. 2021, 6, ysab012. [Google Scholar] [CrossRef]
- Dou, W.; Zhu, Q.; Zhang, M.; Jia, Z.; Guan, W. Screening and Evaluation of the Strong Endogenous Promoters in Pichia Pastoris. Microb. Cell Fact. 2021, 20, 156. [Google Scholar] [CrossRef]
- Nishizaki, T.; Tsuge, K.; Itaya, M.; Doi, N.; Yanagawa, H. Metabolic Engineering of Carotenoid Biosynthesis in Escherichia coli by Ordered Gene Assembly in Bacillus subtilis. Appl. Environ. Microbiol. 2007, 73, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef]
- Song, Y.; He, S.; Abdallah, I.I.; Jopkiewicz, A.; Setroikromo, R.; van Merkerk, R.; Tepper, P.G.; Quax, W.J. Engineering of Multiple Modules to Improve Amorphadiene Production in Bacillus subtilis Using CRISPR-Cas9. J. Agric. Food Chem. 2021, 69, 4785–4794. [Google Scholar] [CrossRef]
- Kabir, M.; Uddin, M.; Jeandet, P.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Sayed, A.A.; Abdel-Daim, M.M.; Simal-Gandara, J. Anti-Alzheimer’s molecules derived from marine life: Understanding molecular mechanisms and therapeutic potential. Mar. Drugs 2021, 19, 251. [Google Scholar] [CrossRef]
- Bahbah, E.I.; Ghozy, S.; Attia, M.S.; Negida, A.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Abdel-Daim, M.M.; Uddin, M.; Simal-Gandara, J. Molecular mechanisms of astaxanthin as a potential neurotherapeutic agent. Mar. Drugs 2021, 19, 201. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X 2022, 13, 100217. [Google Scholar] [CrossRef]




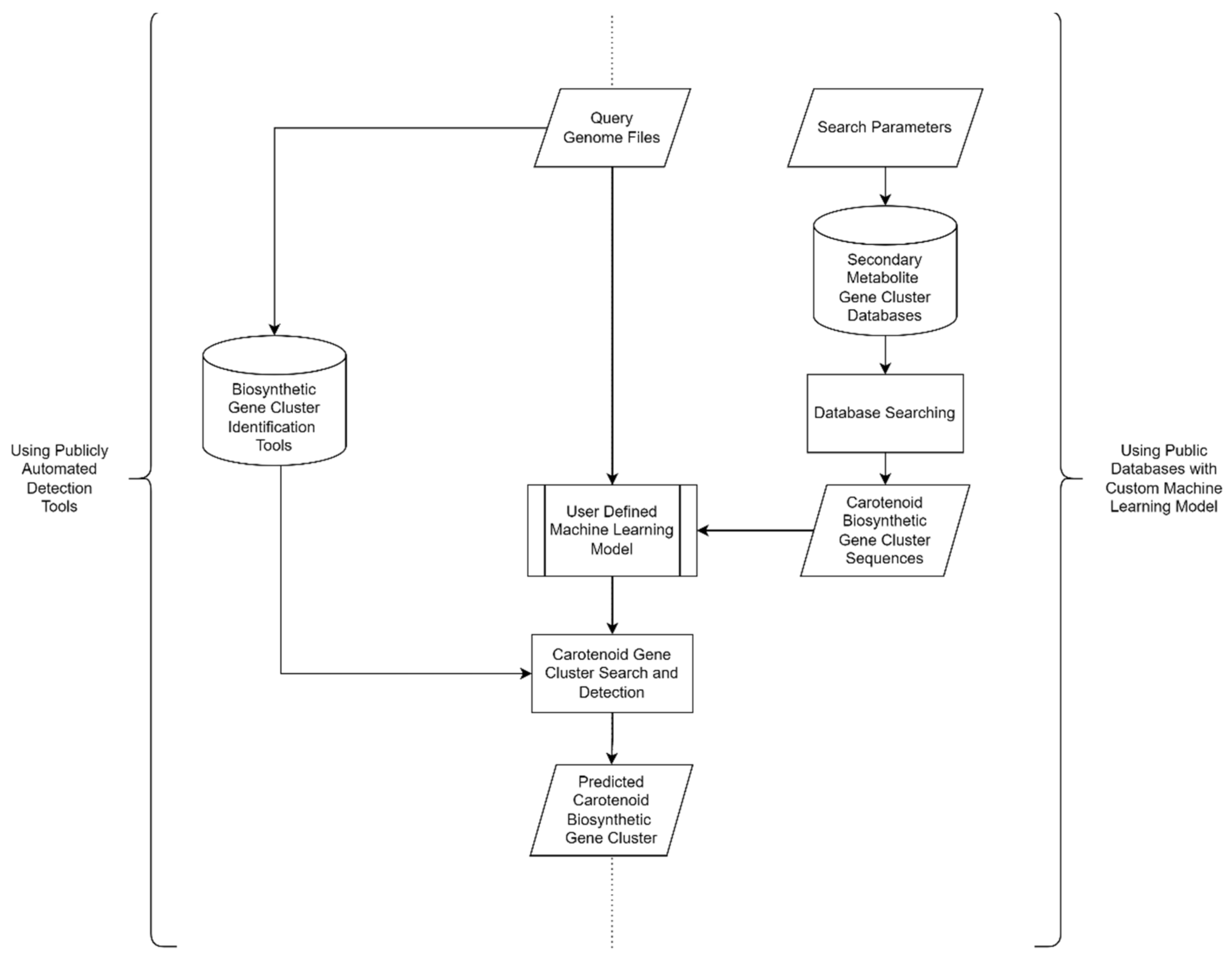
| No. | Name | Database URL |
|---|---|---|
| 1 | antiSMASH database | http://antismash-db.secondarymetabolites.org/ |
| 2 | Bactibase | http://bactibase.pfba-lab-tun.org |
| 3 | ClusterMine360 | http://www.clustermine360.ca/ |
| 4 | ClustScan Database | http://csdb.bioserv.pbf.hr/csdb/ClustScanWeb.html |
| 5 | DoBISCUIT | http://www.bio.nite.go.jp/pks/ |
| 6 | IMG-ABC | https://img.jgi.doe.gov/abc |
| 7 | MIBiG | https://mibig.secondarymetabolites.org/ |
| No. | Tool | Web URL |
|---|---|---|
| 1 | antiSMASH 6.0 | http://antismash.secondarymetabolites.org/ |
| 2 | Artemis | http://www.sanger.ac.uk/science/tools/artemis |
| 3 | ClusterFinder | http://github.com/petercim/ClusterFinder |
| 4 | ClusterMine 360 | http://clustermine360.ca/ |
| 5 | eSNaPD | http://esnapd2.rockefeller.edu/ |
| 6 | FramePlot 4.0beta | http://nocardia.nih.go.jp/fp4 |
| 7 | IMG-ABC | https://img.jgi.doe.gov/cgi-bin/abc/main.cgi |
| 8 | MultiGeneBlast | http://multigeneblast.sourceforge.net/ |
| 9 | NP.Searcher | http://dna.sherman.lsi.umich.edu/ |
| 10 | NaPDoS | http://napdos.ucsd.edu/ |
| 11 | SMURF | https://www.jcvi.org/smurf |
| 12 | HMMER | http://www.ebi.ac.uk/Tools/hmmer/search/jackhmmer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steven, R.; Humaira, Z.; Natanael, Y.; Dwivany, F.M.; Trinugroho, J.P.; Dwijayanti, A.; Kristianti, T.; Tallei, T.E.; Emran, T.B.; Jeon, H.; et al. Marine Microbial-Derived Resource Exploration: Uncovering the Hidden Potential of Marine Carotenoids. Mar. Drugs 2022, 20, 352. https://doi.org/10.3390/md20060352
Steven R, Humaira Z, Natanael Y, Dwivany FM, Trinugroho JP, Dwijayanti A, Kristianti T, Tallei TE, Emran TB, Jeon H, et al. Marine Microbial-Derived Resource Exploration: Uncovering the Hidden Potential of Marine Carotenoids. Marine Drugs. 2022; 20(6):352. https://doi.org/10.3390/md20060352
Chicago/Turabian StyleSteven, Ray, Zalfa Humaira, Yosua Natanael, Fenny M. Dwivany, Joko P. Trinugroho, Ari Dwijayanti, Tati Kristianti, Trina Ekawati Tallei, Talha Bin Emran, Heewon Jeon, and et al. 2022. "Marine Microbial-Derived Resource Exploration: Uncovering the Hidden Potential of Marine Carotenoids" Marine Drugs 20, no. 6: 352. https://doi.org/10.3390/md20060352
APA StyleSteven, R., Humaira, Z., Natanael, Y., Dwivany, F. M., Trinugroho, J. P., Dwijayanti, A., Kristianti, T., Tallei, T. E., Emran, T. B., Jeon, H., Alhumaydhi, F. A., Radjasa, O. K., & Kim, B. (2022). Marine Microbial-Derived Resource Exploration: Uncovering the Hidden Potential of Marine Carotenoids. Marine Drugs, 20(6), 352. https://doi.org/10.3390/md20060352











