Preclinical Development of Seriniquinones as Selective Dermcidin Modulators for the Treatment of Melanoma
Abstract
1. The Discovery of Seriniquinone and its Early Bioactivity

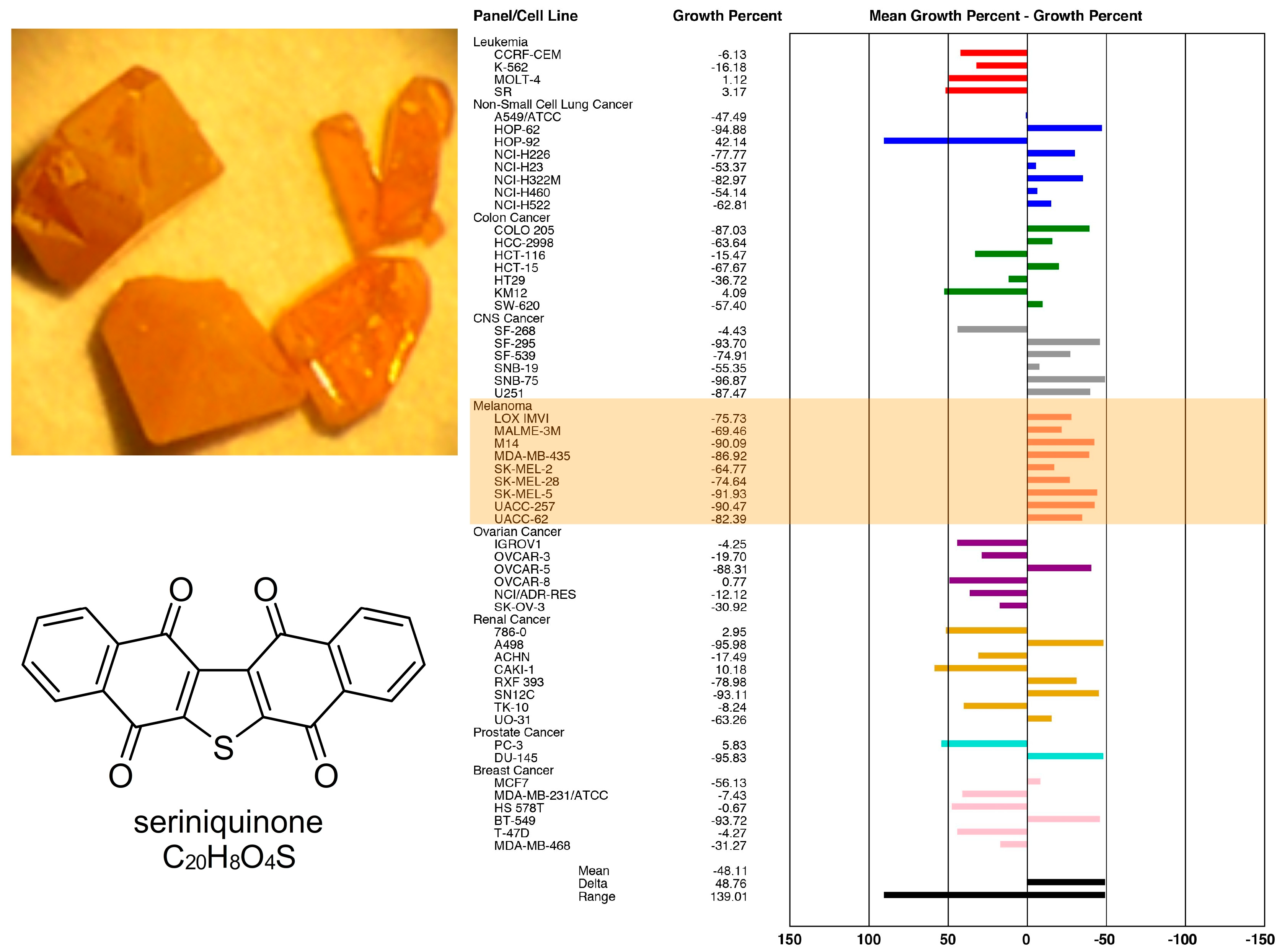
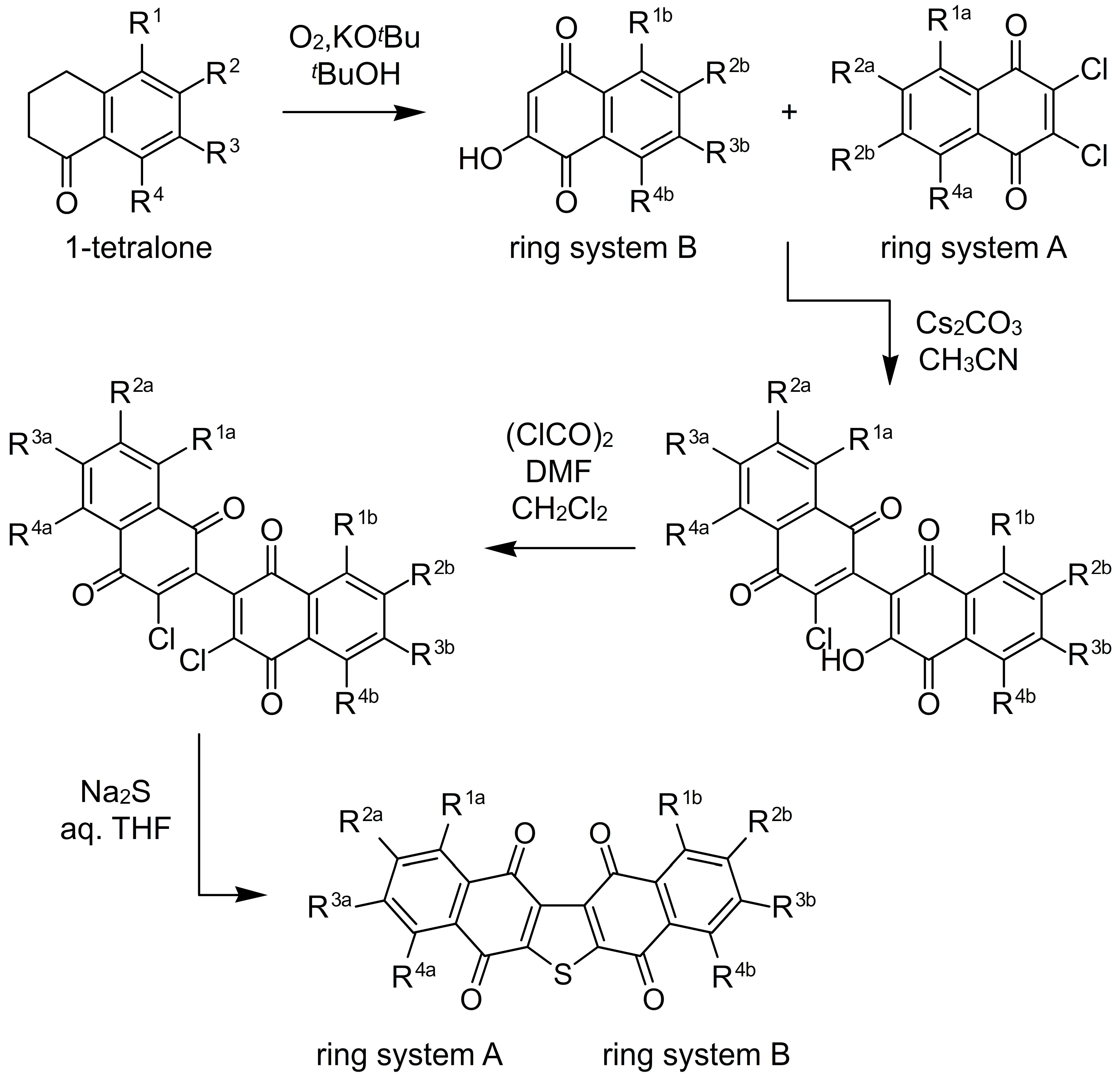
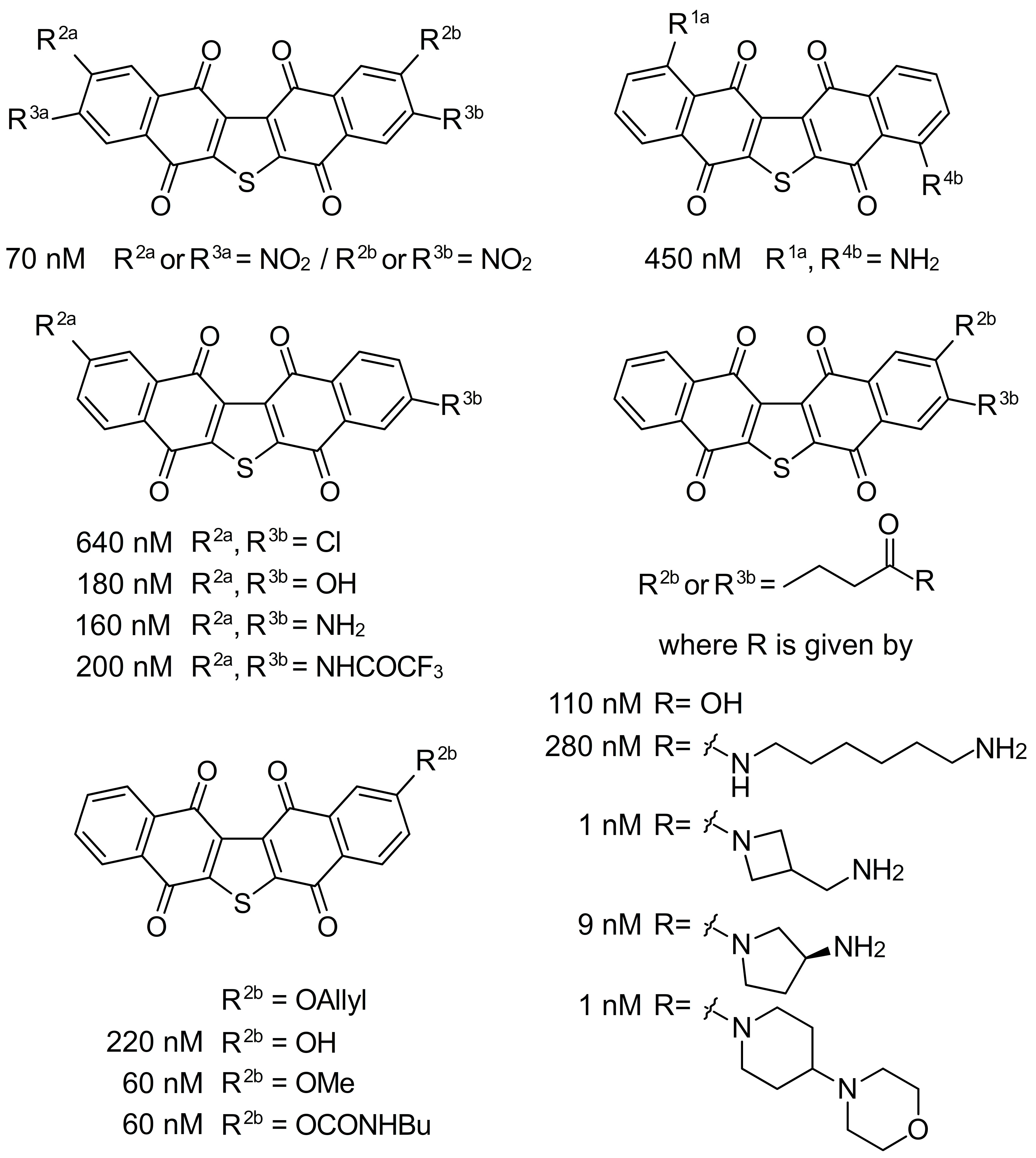
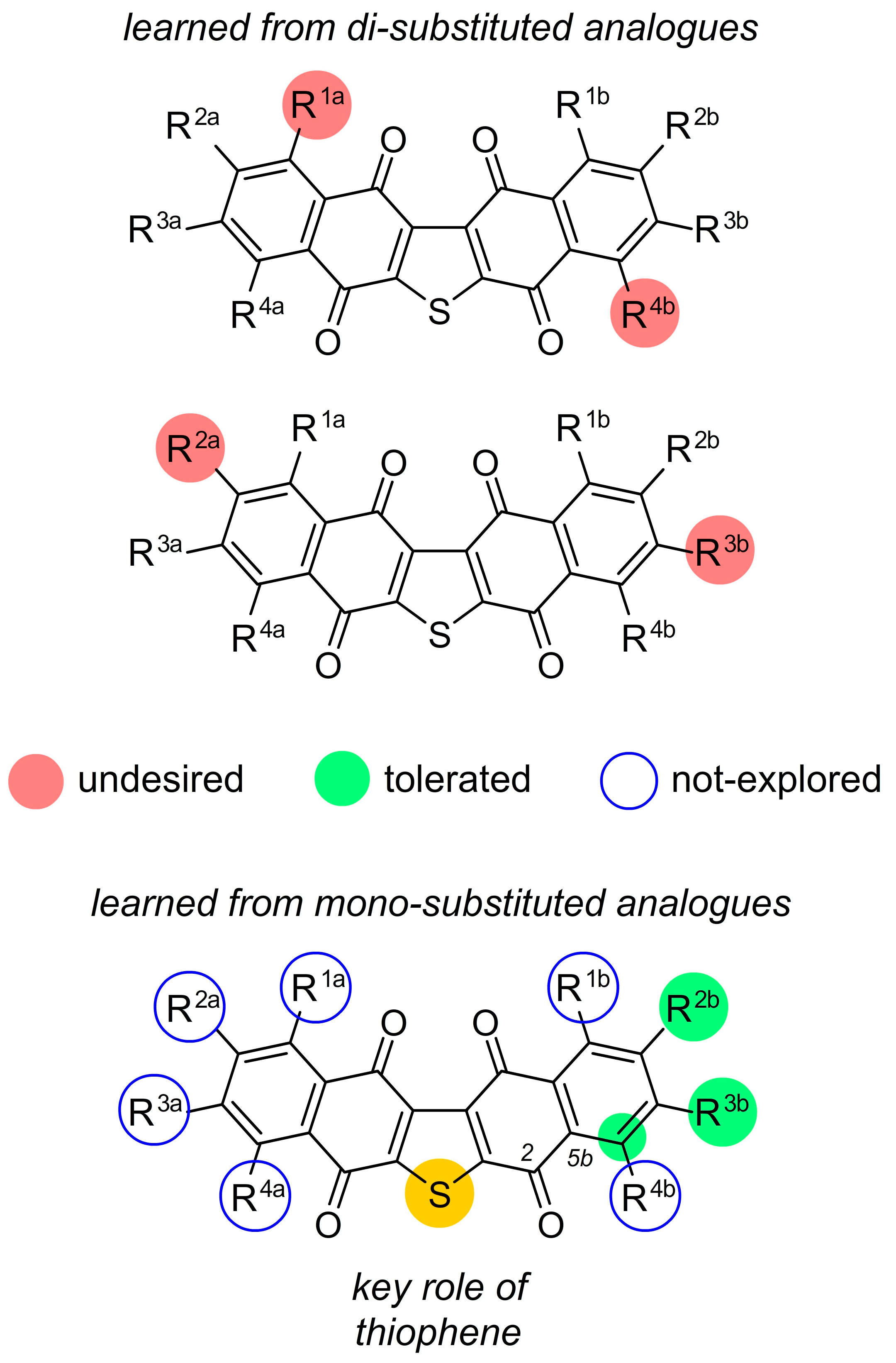
2. Facile Synthetic Access Enables Detailed Structure–Activity Relationship (SAR) Studies
3. A Unique Mode of Action: Dermcidin
4. Complex Pharmacology
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammons, J.C.; Trzoss, L.; Jimenez, P.C.; Hirata, A.S.; Costa-Lotufo, L.V.; La Clair, J.J.; Fenical, W. Advance of Seriniquinone Analogues as Melanoma Agents. ACS Med. Chem. Lett. 2019, 10, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Trzoss, L.; Fukuda, T.; Costa-Lotufo, L.V.; Jimenez, P.; La Clair, J.J.; Fenical, W. Seriniquinone, a selective anticancer agent, induces cell death by autophagocytosis, targeting the cancer-protective protein dermcidin. Proc. Natl. Acad. Sci. USA 2014, 111, 14687–14692. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Tanaka, T.; Nagai, K.; Furuichi, Y.; Terahara, T.; Anda, M.; Tsukamasa, Y.; Fukuda, T. New dihydronaphthothiophene derivatives by the biological transformation of seriniquinone using marine-derived actinomycete Streptomyces albogriseolus OM27-12. J. Antibiot. 2022, 65, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.D.; Burkart, M.D.; Leonard, M.S.; Portonovo, P.; Liang, B.; Ding, X.; Joullié, M.M.; Gulledge, B.M.; Aggen, J.B.; Chamberlin, A.R.; et al. A Central Strategy for Converting Natural Products into Fluorescent Probes. ChemBioChem 2006, 7, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.C.; MacMillan, J.B.; Gaudêncio, S.P.; Fenical, W.; La Clair, J.J. Ammosamides A and B Target Myosin. Angew. Chemie Int. Ed. 2009, 48, 728–732. [Google Scholar] [CrossRef]
- IARC. The Global Cancer Observatory (Globocan) 2020 Database. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/16-Melanoma-of-skin-fact-sheet.pdf (accessed on 13 December 2021).
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Lee, C.; Collichio, F.; Ollila, D.; Moschos, S. Historical review of melanoma treatment and outcomes. Clin. Dermatol. 2013, 31, 141–147. [Google Scholar] [CrossRef]
- Wagner, D.E.; Ramirez, G.; Weiss, A.J.; Hill, G., Jr. Combination Phase 1–II Study of Imidazole Carboxamide (NCS 45388). Oncology 1972, 26, 310–316. [Google Scholar] [CrossRef]
- Hill, G.J.; Ruess, R.; Berris, R.; Philpott, G.W.; Parkin, P. Chemotherapy of Malignant Melanoma with Dimethyl Triazeno lmidazole Carboxamide (DITC) and Nitrosourea Derivatives (BCN U, CCNU). Ann. Surg. 1974, 180, 167–174. [Google Scholar] [CrossRef]
- Schadendorf, D.; Vaubel, J.; Livingstone, E.; Zimmer, L. Advances and perspectives in immunotherapy of melanoma. Ann. Oncol. 2012, 23, x104–x108. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef]
- Curl, P.; Vujic, I.; van ‘t Veer, L.J.; Ortiz-Urda, S.; Kahn, J.G. Cost-Effectiveness of Treatment Strategies for BRAF-Mutated Metastatic Melanoma. PLoS ONE 2014, 9, e107255. [Google Scholar] [CrossRef]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology (Williston Park) 2009, 23, 488–496. [Google Scholar]
- Oh, A.; Tran, D.M.; McDowell, L.C.; Keyvani, D.; Barcelon, J.A.; Merino, O.; Wilson, L. Cost-Effectiveness of Nivolumab-Ipilimumab Combination Therapy Compared with Monotherapy for First-Line Treatment of Metastatic Melanoma in the United States. J. Manag. Care Spec. Pharm. 2017, 23, 653–664. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- da Rocha Dias, S.; Salmonson, T.; van Zwieten-Boot, B.; Jonsson, B.; Marchetti, S.; Schellens, J.H.M.; Giuliani, R.; Pignatti, F. The European Medicines Agency review of vemurafenib (Zelboraf®) for the treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma: Summary of the scientific assessment of the Committee for Medicinal Products for Huma. Eur. J. Cancer 2013, 49, 1654–1661. [Google Scholar] [CrossRef]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Lugowska, I.; Kosela-Paterczyk, H.; Kozak, K.; Rutkowski, P. Trametinib: A MEK inhibitor for management of metastatic melanoma. Onco. Targets. Ther. 2015, 8, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Shih, V.; Ten Ham, R.M.; Bui, C.T.; Tran, D.N.; Ting, J.; Wilson, L. Targeted Therapies Compared to Dacarbazine for Treatment of BRAF(V600E) Metastatic Melanoma: A Cost-Effectiveness Analysis. J. Skin Cancer 2015, 2015, 505302. [Google Scholar] [CrossRef] [PubMed]
- Fairlie, W.D.; Tran, S.; Lee, E.F. Crosstalk between apoptosis and autophagy signaling pathways. Int. Rev. Cell Mol. Biol. 2020, 352, 115–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Yang, Y.; Wan, X.; Alvarez, A.A.; Sastry, N.; Feng, H.; Hu, B.; Cheng, S.-Y. Autophagy and Hallmarks of Cancer. Crit. Rev. Oncog. 2018, 23, 247–267. [Google Scholar] [CrossRef]
- Rahmati, M.; Ebrahim, S.; Hashemi, S.; Motamedi, M.; Moosavi, M.A. New insights on the role of autophagy in the pathogenesis and treatment of melanoma. Mol. Biol. Rep. 2020, 47, 9021–9032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, P.; Zou, L.; Zhang, J.; Chen, J.; Yang, C.; He, G.; Liu, B.; Liu, J.; Chiang, C.M.; et al. Discovery of Novel Dual-Target Inhibitor of Bromodomain-Containing Protein 4/Casein Kinase 2 Inducing Apoptosis and Autophagy-Associated Cell Death for Triple-Negative Breast Cancer Therapy. J. Med. Chem. 2021, 64, 18025–18053. [Google Scholar] [CrossRef]
- Biggers, J.W.; Nguyen, T.; Di, X.; Gupton, J.T.; Henderson, S.C.; Emery, S.M.; Alotaibi, M.; White, K.L.; Brown, R.; Almenara, J.; et al. Autophagy, cell death and sustained senescence arrest in B16/F10 melanoma cells and HCT-116 colon carcinoma cells in response to the novel microtubule poison, JG-03-14. Cancer Chemother. Pharmacol. 2013, 71, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Wang, D.; Li, X.; Wang, W.; Lou, H.; Yuan, H. Malformin A1 promotes cell death through induction of apoptosis, necrosis and autophagy in prostate cancer cells. Cancer Chemother. Pharmacol. 2016, 77, 63–75. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Hirata, A.S.; Rezende-Teixeira, P.; Machado-Neto, J.A.; Jimenez, P.C.; Clair, J.J.L.; Fenical, W.; Costa-Lotufo, L.V. Seriniquinones as Therapeutic Leads for Treatment of BRAF and NRAS Mutant Melanomas. Molecules 2021, 26, 7362. [Google Scholar] [CrossRef]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic significance of BRAF and NRAS mutations in melanoma: A German study from routine care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef] [PubMed]
- Ryabaya, O.O.; Inshakov, A.N.; Egorova, A.V.; Emelyanova, M.A.; Nasedkina, T.V.; Zasedatelev, A.S.; Khochenkov, D.A.; Stepanova, E.V. Autophagy inhibitors chloroquine and LY294002 enhance temozolomide cytotoxicity on cutaneous melanoma cell lines in vitro. Anticancer. Drugs 2017, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Piao, S.-F.; Dey, S.; Mcafee, Q.; Karakousis, G.; Villanueva, J.; Hart, L.S.; Levi, S.; Hu, J.; Zhang, G.; et al. Targeting ER stress–induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Invest. 2014, 124, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, R.; Leone, R.; Chang, Y.C.; Fecher, L.A.; Schuchter, L.M.; Kramer, A.; Tan, K.-S.; Heitjan, D.F.; Rodgers, G.; Gallagher, M.; et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy 2014, 10, 1369–1379. [Google Scholar] [CrossRef]
- Strohecker, A.M.; White, E. Targeting Mitochondrial Metabolism by Inhibiting Autophagy in BRAF-Driven Cancers. Cancer Discov. 2014, 4, 766–772. [Google Scholar] [CrossRef]
- Xie, X.; Koh, J.Y.; Price, S.; White, E.; Mehnert, J.M. Atg7 Overcomes Senescence and Promotes Growth of BrafV600E-Driven Melanoma. Cancer Discov. 2015, 5, 410–423. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, Y.; Cao, L.; Yang, L.; Yang, M.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell. Oncol. 2015, 2, e1054549. [Google Scholar] [CrossRef]
- Zheng, Z.-Y.; Li, J.; Li, F.; Zhu, Y.; Cui, K.; Wong, S.T.; Chang, E.C.; Liao, Y.-H. Induction of N-Ras degradation by flunarizine-mediated autophagy. Sci. Rep. 2018, 8, 16932. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Lown, J.W. The mechanism of action of quinone antibiotics. Mol. Cell. Biochem. 1983, 55, 17–40. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Moreira da Silva, R.; Carrão, D.B.; Habenschus, M.D.; Jimenez, P.C.; Lopes, N.P.; Fenical, W.; Costa-Lotufo, L.V.; de Oliveira, A.R.M. Prediction of seriniquinone-drug interactions by in vitro inhibition of human cytochrome P450 enzymes. Toxicol. In Vitro 2020, 65, 104820. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Apolinário, A.C.; Hirata, A.S.; Anjos Miguel, R.D.; Costa-Lotufo, L.V.; Pessoa, A.; La Clair, J.J.; Fenical, W.; Lopes, L.B. Exploring the benefits of nanotechnology for cancer drugs in different stages of the drug development pipeline. Nanomedicine 2020, 15, 2539–2542. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Hodge, L.; Speicher, D.; Reim, D.; Tyler-Polsz, C.; Levitt, P.; Eagleson, K.; Kennedy, S.; Wang, Y. Identification of a Survival-Promoting Peptide in Medium Conditioned by Oxidatively Stressed Cell Lines of Nervous System Origin. J. Neurosci. 1998, 18, 7047–7060. [Google Scholar] [CrossRef]
- Cariuk, P.; Lorite, M.; Todorov, P.; Field, W.; Wigmore, S.; Tisdale, M. Induction of cachexia in mice by a product isolated from the urine of cachectic cancer patients. Br. J. Cancer 1997, 76, 606–613. [Google Scholar] [CrossRef]
- Lorite, M.; Thompson, M.; Drake, J.; Carling, G.; Tisdale, M. Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br. J. Cancer 1998, 78, 850–856. [Google Scholar] [CrossRef]
- Porter, D.; Weremowicz, S.; Chin, K.; Seth, P.; Keshaviah, A.; Lahti-Domenici, J.; Bae, Y.K.; Monitto, C.L.; Merlos-Suarez, A.; Chan, J.; et al. A neural survival factor is a candidate oncogene in breast cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 10931–10936. [Google Scholar] [CrossRef]
- Brauer, H.A.; D’Arcy, M.; Libby, T.E.; Thompson, H.J.; Yasui, Y.Y.; Hamajima, N.; Li, C.I.; Troester, M.A.; Lampe, P.D. Dermcidin expression is associated with disease progression and survival among breast cancer patients. Breast Cancer Res. Treat. 2014, 144, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Deans, D.A.C.; Wigmore, S.J.; Gilmour, H.; Tisdale, M.J.; Fearon, K.C.H.; Ross, J.A. Expression of the proteolysis-inducing factor core peptide mRNA is upregulated in both tumour and adjacent normal tissue in gastro-oesophageal malignancy. Br. J. Cancer 2006, 94, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, W.; Kuai, X.; Ji, Y.; Zhu, Z.; Mao, Z.; Wang, Z. Dermcidin as a novel binding protein of lncRNA STCAT3 and its effect on prognosis in gastric cancer. Oncol. Rep. 2018, 40, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-L.; Qiu, F.-H.; Dayarathna, T.K.; Wu, J.; Kuang, M.; Li, S.S.C.; Peng, B.-G.; Nie, J. Identification of Dermcidin as a novel binding protein of Nck1 and characterization of its role in promoting cell migration. Biochim. Biophys. Acta 2011, 1812, 703–710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, F.; Qiu, F.; Liu, L.; Liu, J.; Xu, J.; Huang, X. The Role of Dermcidin in the Diagnosis and Staging of Hepatocellular Carcinoma. Genet. Test. Mol. Biomark. 2018, 22, 218–223. [Google Scholar] [CrossRef]
- López-Sánchez, L.M.; Jurado-Gámez, B.; Feu-Collado, N.; Valverde, A.; Cañas, A.; Fernández-Rueda, J.L.; Aranda, E.; Rodríguez-Ariza, A. Exhaled breath condensate biomarkers for the early diagnosis of lung cancer using proteomics. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L664–L676. [Google Scholar] [CrossRef]
- Núñez-Naveira, L.; Mariñas-Pardo, L.A.; Montero-Martínez, C. Mass Spectrometry Analysis of the Exhaled Breath Condensate and Proposal of Dermcidin and S100A9 as Possible Markers for Lung Cancer Prognosis. Lung 2019, 197, 523–531. [Google Scholar] [CrossRef]
- Ortega-Martínez, I.; Gardeazabal, J.; Erramuzpe, A.; Sanchez-Diez, A.; Cortés, J.; García-Vázquez, M.D.; Pérez-Yarza, G.; Izu, R.; Luís Díaz-Ramón, J.; de la Fuente, I.M.; et al. Vitronectin and dermcidin serum levels predict the metastatic progression of AJCC I–II early-stage melanoma. Int. J. Cancer 2016, 139, 1598–1607. [Google Scholar] [CrossRef]
- Mancuso, F.; Lage, S.; Rasero, J.; Díaz-Ramón, J.L.; Apraiz, A.; Pérez-Yarza, G.; Ezkurra, P.A.; Penas, C.; Sánchez-Diez, A.; García-Vazquez, M.D.; et al. Serum markers improve current prediction of metastasis development in early-stage melanoma patients: A machine learning-based study. Mol. Oncol. 2020, 14, 1705–1718. [Google Scholar] [CrossRef]
- Stewart, G.D.; Skipworth, R.J.E.; Pennington, C.J.; Lowrie, A.G.; Deans, D.A.C.; Edwards, D.R.; Habib, F.K.; Riddick, A.C.P.; Fearon, K.C.H.; Ross, J.A. Variation in dermcidin expression in a range of primary human tumours and in hypoxic/oxidatively stressed human cell lines. Br. J. Cancer 2008, 99, 126–132. [Google Scholar] [CrossRef]
- Stewart, G.D.; Lowrie, A.G.; Riddick, A.C.P.; Fearon, K.C.H.; Habib, F.K.; Ross, J.A. Dermcidin expression confers a survival advantage in prostate cancer cells subjected to oxidative stress or hypoxia. Prostate 2007, 67, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Bancovik, J.; Moreira, D.F.; Carrasco, D.; Yao, J.; Porter, D.; Moura, R.; Camargo, A.; Fontes-Oliveira, C.C.; Malpartida, M.G.; Carambula, S.; et al. Dermcidin exerts its oncogenic effects in breast cancer via modulation of ERBB signaling. BMC Cancer 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, A.G.; Wigmore, S.J.; Wright, D.J.; Waddell, I.D.; Ross, J.A. Dermcidin expression in hepatic cells improves survival without N-glycosylation, but requires asparagine residues. Br. J. Cancer 2006, 94, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Lager, T.W.; Conner, C.; Keating, C.R.; Warshaw, J.N.; Panopoulos, A.D. Cell surface GRP78 and Dermcidin cooperate to regulate breast cancer cell migration through Wnt signaling. Oncogene 2021, 40, 4050–4059. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirata, A.S.; La Clair, J.J.; Jimenez, P.C.; Costa-Lotufo, L.V.; Fenical, W. Preclinical Development of Seriniquinones as Selective Dermcidin Modulators for the Treatment of Melanoma. Mar. Drugs 2022, 20, 301. https://doi.org/10.3390/md20050301
Hirata AS, La Clair JJ, Jimenez PC, Costa-Lotufo LV, Fenical W. Preclinical Development of Seriniquinones as Selective Dermcidin Modulators for the Treatment of Melanoma. Marine Drugs. 2022; 20(5):301. https://doi.org/10.3390/md20050301
Chicago/Turabian StyleHirata, Amanda S., James J. La Clair, Paula C. Jimenez, Leticia Veras Costa-Lotufo, and William Fenical. 2022. "Preclinical Development of Seriniquinones as Selective Dermcidin Modulators for the Treatment of Melanoma" Marine Drugs 20, no. 5: 301. https://doi.org/10.3390/md20050301
APA StyleHirata, A. S., La Clair, J. J., Jimenez, P. C., Costa-Lotufo, L. V., & Fenical, W. (2022). Preclinical Development of Seriniquinones as Selective Dermcidin Modulators for the Treatment of Melanoma. Marine Drugs, 20(5), 301. https://doi.org/10.3390/md20050301






