Jellyfish from Fisheries By-Catches as a Sustainable Source of High-Value Compounds with Biotechnological Applications
Abstract
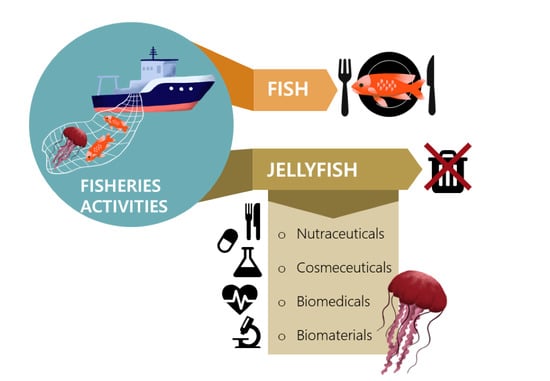
1. Introduction
2. Potential Uses of Jellyfish Collected as Fisheries By-Catches
2.1. Nutraceuticals (Including Direct Consumption as a Food)
2.2. Biomedicals
2.2.1. Green Fluorescent Proteins (GFPs)
2.2.2. Collagen
- Scaffolds. The biocompatibility between human and collagen extracted from scyphomedusae was determined about 20 years ago [95]. In this pilot study, the authors suggested the use of jellyfish collagen for scaffolds to stimulate tissue regeneration and monitored the inflammatory and immune responses to the implantation. The results encouraged the use of jelly-derived scaffolds, which found further support in following studies. The collagens extracted from Rhopilema esculentum and Nemopilema nomurai were used to design porous scaffolds for cartilage regeneration [96,97]. More recently, biphasic monolithic scaffolds made of jellyfish collagens were shown to be suitable for osteochondral engineering [98]. Jelly-derived collagen tubular scaffolds, modeled as vascular grafts, enhanced vascular endothelial cell development and its mechanical strength [99].
- Drug delivery. After marketing the collagen extracted from the scyphomedusa Rhizostoma octopus, a recent study funded by the Jellagen© company which produces it indicated that jellyfish collagen is a suitable cell matrix to culture human-induced pluripotent stem cell-derived Microglia (iMGL) that possess the morphological, surface marker expression and functional characteristics required for microglia. Jellyfish-extracted collagen showed a biological impact on human cells higher than mammalian type I collagen extracted from rat tails. Comparisons were performed by testing adhesion, cell viability and immunocytochemistry assays. These results suggest that collagen from R. octopus is a potential inert, non-reactive biomaterial suitable as a substitute for the collagen extracted from rat tail, since cells cultured on this substrate produced significant clumping and cell death [100]. Although more tests are needed to define the suitability of jellyfish collagen compared to other substrates, the fact that microglia play crucial roles within the central nervous system by ensuring synaptic plasticity, immune activity, neurogenesis and homeostasis, the potential application of jellyfish collagen may benefit the study of neural transmission and improve the treatment of diseases resulting from the degeneration of neural networks, such as Alzheimer’s disease.
2.2.3. Crude Venom
2.3. Biomaterials
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitt, K.A.; Lucas, C.H. Jellyfish Blooms; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- Cartwright, P.; Halgedahl, S.L.; Hendricks, J.R.; Jarrard, R.D.; Marques, A.C.; Collins, A.G.; Lieberman, B.S. Exceptionally Preserved Jellyfishes from the Middle Cambrian. PLoS ONE 2007, 2, e1121. [Google Scholar] [CrossRef]
- Young, G.A.; Hagadorn, J.W. The fossil record of cnidarian medusae. Palaeoworld 2010, 19, 212–221. [Google Scholar] [CrossRef]
- Jarms, G.; Morandini, A.C. World Atlas of Jellyfish: Scyphozoa except Stauromedusae; Dölling und Galitz Verlag: Hamburg, Germany, 2019. [Google Scholar]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. USA 2012, 110, 1000–1005. [Google Scholar] [CrossRef]
- Brotz, L. Jellyfish Fisheries of the World. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Hsieh, Y.-H.P.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M.; et al. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish Biol. Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Elliott, A.; Hobson, V.; Tang, K.W. Balancing fishery and conservation: A case study of the barrel jellyfish Rhizostoma octopus in South Wales. ICES J. Mar. Sci. 2017, 74, 234–241. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Hsieh, P.Y.H.; Leong, F.-M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- Edelist, D.; Angel, D.L.; Canning-Clode, J.; Gueroun, S.K.M.; Aberle, N.; Javidpour, J.; Andrade, C. Jellyfishing in Europe: Current Status, Knowledge Gaps, and Future Directions towards a Sustainable Practice. Sustainability 2021, 13, 12445. [Google Scholar] [CrossRef]
- Purcell, J.E.; Grover, J.J. Predation and food limitation as causes of mortality in larval herring at a spawning ground in British Colombia. Mar. Ecol. Prog. Ser. 1990, 59, 55–61. [Google Scholar] [CrossRef]
- D’Ambra, I.; Graham, W.M.; Carmichael, R.H.; Hernandez, F.J., Jr. Dietary overlap between jellyfish and forage fish in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2018, 587, 31–40. [Google Scholar] [CrossRef]
- Houghton, J.D.R.; Doyle, T.K.; Wilson, M.W.; Davenport, J.; Hays, G.C. Jellyfish Aggregations and Leatherback Turtle Foraging Patterns in a Temperate Coastal Environment. Ecology 2006, 87, 1967–1972. [Google Scholar] [CrossRef]
- Houghton, J.D.R.; Doyle, T.K.; Davenport, J.; Hays, G.C. The ocean sunfish Mola mola: Insights into distribution, abundance and behaviour in the Irish and Celtic Seas. J. Mar. Biol. Assoc. UK 2006, 86, 1237–1243. [Google Scholar] [CrossRef][Green Version]
- Phillips, N.; Eagling, L.; Harrod, C.; Reid, N.; Cappanera, V.; Houghton, J. Quacks snack on smacks: Mallard ducks (Anas platyrhynchos) observed feeding on hydrozoans (Velella velella). Plankton Benthos Res. 2017, 12, 143–144. [Google Scholar] [CrossRef]
- Cardona, L.; De Quevedo, I.Á.; Borrell, A.; Aguilar, A. Massive Consumption of Gelatinous Plankton by Mediterranean Apex Predators. PLoS ONE 2012, 7, e31329. [Google Scholar] [CrossRef]
- D’Ambra, I.; Graham, W.M.; Carmichael, R.H.; Hernández, F.J. Fish rely on scyphozoan hosts as a primary food source: Evidence from stable isotope analysis. Mar. Biol. 2014, 162, 247–252. [Google Scholar] [CrossRef]
- Griffin Donal, C.; Harrod, C.; Houghton Jonathan, D.R.; Capellini, I. Unravelling the macro-evolutionary ecology of fish–jellyfish associations: Life in the ‘gingerbread house’. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182325. [Google Scholar] [CrossRef]
- Lebrato, M.; Pitt, K.A.; Sweetman, A.K.; Jones, D.O.B.; Cartes, J.E.; Oschlies, A.; Condon, R.H.; Molinero, J.C.; Adler, L.; Gaillard, C.; et al. Jelly-falls historic and recent observations: A review to drive future research directions. Hydrobiologia 2012, 690, 227–245. [Google Scholar] [CrossRef]
- Sweetman, A.K.; Chelsky, A.; Pitt, K.A.; Andrade, H.; van Oevelen, D.; Renaud, P.E. Jellyfish decomposition at the seafloor rapidly alters biogeochemical cycling and carbon flow through benthic food-webs. Limnol. Oceanogr. 2016, 61, 1449–1461. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Boero, F.; Brotz, L. We should not assume that fishing jellyfish will solve our jellyfish problem. ICES J. Mar. Sci. 2015, 73, 1012–1018. [Google Scholar] [CrossRef]
- Palmieri, M.G.; Schaafsma, M.; Luisetti, T.; Barausse, A.; Harwood, A.; Sen, A.; Turner, R.K. Jellyfish Blooms and Their Impacts on Welfare Benefits: Recreation in the UK and Fisheries in Italy. In Coastal Zones Ecosystem Services: From Science to Values and Decision Making; Turner, K.R., Schaafsma, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 219–240. [Google Scholar] [CrossRef]
- Boero, F. Review of jellyfish blooms in the Mediterranean and Black Sea. In Studies and Reviews. General Fisheries Commission for the Mediterranean; FAO: Rome, Italy, 2013; Volume 92, p. 53. [Google Scholar]
- Hsieh, P.Y.H. Use of Jellyfish Collagen (Type II) in the Treatment of Rheumatoid arthritis. U.S. Patent No. 6,894,029, 17 May 2005. [Google Scholar]
- Ayed, Y.; Dellai, A.; Ben Mansour, H.; Bacha, H.; Abid, S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1775). Ann. Clin. Microbiol. Antimicrob. 2012, 11, 15. [Google Scholar] [CrossRef]
- Ayed, Y.; Sghaier, R.M.; Laouini, D.; Bacha, H. Evaluation of anti-proliferative and anti-inflammatory activities of Pelagia noctiluca venom in Lipopolysaccharide/Interferon-γ stimulated RAW264.7 macrophages. Biomed. Pharmacother. 2016, 84, 1986–1991. [Google Scholar] [CrossRef]
- Percy, J.; Fife, F. The Biochemical Composition and Energy Content of Arctic Marine Macrozooplankton. Arctic 1981, 34, 307–313. [Google Scholar] [CrossRef]
- Ikeda, T. Chemical composition and nutrition of zooplankton in the Bering Sea. In Biological Oceanography of the Northern North Pacific Ocean; Takenouti, A.Y., Ed.; Idenitsu Shoten: Tokyo, Japan, 1972; pp. 433–442. [Google Scholar]
- Clarke, A.; Holmes, L.J.; Gore, D.J. Proximate and elemental composition of gelatinous zooplankton from the Southern Ocean. J. Exp. Mar. Biol. Ecol. 1992, 155, 55–68. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Mooney, B.D.; Nichols, P.D. Lipids of gelatinous antarctic zooplankton: Cnidaria and Ctenophora. Lipids 2000, 35, 551–559. [Google Scholar] [CrossRef]
- Barba, F.F.M.D.; Bazi, C.C.; Pessatti, M.L.; Resgalla, C., Jr. Macromedusae of Southern Brazil: Temporal variation, population structure and biochemical composition. Braz. J. Oceanogr. 2016, 64, 127–136. [Google Scholar] [CrossRef]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates: Porifera and cnidaria. Prog. Lipid Res. 1979, 18, 1–30. [Google Scholar] [CrossRef]
- Hooper, S.; Ackman, R. Presence of trans -6-hexadecenoic acid in the White Jellyfish Aurelia aurita Lamarck and in a Caribbean Gorgonian. Lipids 1973, 8, 95. [Google Scholar] [CrossRef]
- Holland, D.L.; Davenport, J.; East, J. The fatty acid composition of the leatherback turtle Dermochelys coriacea and its jellyfish prey. J. Mar. Biol. Assoc. UK 1990, 70, 761–770. [Google Scholar] [CrossRef]
- Abdullah, A.; Nurjanah, N.; Taufik, H.; Dimas, U.A. Fatty acid profile of Jellyfish (Aurelia aurita) as a source raw material of aquatic result rich beneft. Int. J. Chem. Biomol. Sci. 2015, 1, 12–16. [Google Scholar]
- Schneider, G. Chemische Zusammensetzung und Biomasseparameter der Ohrenqualle Aurelia aurita. Helgol. Mar. Res. 1988, 42, 319–327. [Google Scholar] [CrossRef]
- Lucas, C.H. Biochemical composition of Aurelia aurita in relation to age and sexual maturity. J. Exp. Mar. Biol. Ecol. 1994, 183, 179–192. [Google Scholar] [CrossRef]
- Kariotoglou, D.M.; Mastronicolis, S.K. Sphingophosphonolipids, phospholipids, and fatty acids from aegean jellyfish Aurelia aurita. Lipids 2001, 36, 1255–1264. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Sato, H.; Yoshie-Stark, Y.; Ogushi, M.; Tanaka, Y. Differences in the biochemical compositions of two dietary jellyfish species and their effects on the growth and survival of Ibacus novemdentatus phyllosomas. Aquac. Nutr. 2016, 22, 25–33. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Caprioli, R.; Leone, A.; Piraino, S. Jellyfish Bioprospecting in the Mediterranean Sea: Antioxidant and Lysozyme-Like Activities from Aurelia coerulea (Cnidaria, Scyphozoa) Extracts. Mar. Drugs 2021, 19, 619. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef]
- Madondo, M. Biochemical composition (proteins, lipids and carbohydrates) of the Scyphozoan Jellyfish, Chrysaora hysoscella, from the Northern Benguela. BSc Thesis, University of Namibia, Windhoek, Namibia, 2010. [Google Scholar]
- Doyle, T.K.; Houghton, J.D.R.; McDevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-consumption. J. Exp. Mar. Biol. Ecol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Bailey, T.G.; Youngbluth, M.J.; Owen, G.P. Chemical composition and metabolic rates of gelatinous zooplankton from midwater and benthic boundary layer environments off Cape hatteras, North Carolina, USA. Mar. Ecol. Prog. Ser. 1995, 122, 121–134. [Google Scholar] [CrossRef]
- Sipos, J.C.; Ackman, R.G. Jellyfish (Cyanea capillata) Lipids: Fatty Acid Composition. J. Fish. Res. Board Can. 1968, 25, 1561–1569. [Google Scholar] [CrossRef]
- Malej, A. Rates of Metabolism of Jellyfish as Related to Body Weight, Chemical Composition and Temperature. In Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Athens, Greece, 1991; UNEP: Athens, Greece, 1991; pp. 253–259. [Google Scholar]
- Malej, A.; Faganeli, J.; Pezdič, J. Stable isotope and biochemical fractionation in the marine pelagic food chain: The jellyfish Pelagia noctiluca and net zooplankton. Mar. Biol. 1993, 116, 565–570. [Google Scholar] [CrossRef]
- Nakhel, I.C.; Mastronicolis, S.K.; Miniadis-Meimaroglou, S. Phospho- and phosphonolipids of the aegean pelagic scyphomedusa Pelagia noctiluca. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1988, 958, 300–307. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- De Rinaldis, G.; Leone, A.; De Domenico, S.; Bosch-Belmar, M.; Slizyte, R.; Milisenda, G.; Santucci, A.; Albano, C.; Piraino, S. Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea. Mar. Drugs 2021, 19, 498. [Google Scholar] [CrossRef] [PubMed]
- Morais, Z.B.; Pintão, A.M.; Costa, I.M.; Calejo, M.T.; Bandarra, N.M.; Abreu, P. Composition and in vitro antioxidant effects of jellyfish Catostylus tagi from Sado Estuary (SW Portugal). J. Aquat. Food Prod. Technol. 2009, 18, 90–107. [Google Scholar] [CrossRef]
- Carli, A.; Pane, L.; Valente, T.; Cotta, S. Lipid and Protein Content of Jellyfish from the Ligurian Sea. First Results. In Proceedings of the II Workshop on jellyfish in the Mediterranean Sea, Athens, Greece, 1991; UNEP: Athens, Greece, 1991; pp. 236–240. [Google Scholar]
- Prieto, L.; Enrique-Navarro, A.; Volsi, R.L.; Ortega, M.J. The Large Jellyfish Rhizostoma luteum as Sustainable a Resource for Antioxidant Properties, Nutraceutical Value and Biomedical Applications. Mar. Drugs 2018, 16, 396. [Google Scholar] [CrossRef]
- Gubareva, A.E.; Pustovalova, L.M.; Ryzhkov, Y.D. The features of the chemical composition of medusae in the sea of Azov. In Izvestiya Severo-Kavkazskogo Nauchnogo Tsentra Vysshei Shkoly Estestvennye Nauki; 1983; pp. 22–25. [Google Scholar]
- Huang, Y.-W. Cannonball Jellyfish (Stomolophus meleagris) as a Food Resource. J. Food Sci. 1988, 53, 341–343. [Google Scholar] [CrossRef]
- Reinhardt, S.B.; Van Vleet, E.S. Lipid composition of twenty-two species of Antarctic midwater zooplankton and fish. Mar. Biol. 1986, 91, 149–159. [Google Scholar] [CrossRef]
- Lucas, C.H. Biochemical composition of the mesopelagic coronate jellyfish Periphylla periphylla from the Gulf of Mexico. J. Mar. Biol. Assoc. UK 2008, 89, 77–81. [Google Scholar] [CrossRef]
- Milisenda, G.; Rosa, S.; Fuentes, V.L.; Boero, F.; Guglielmo, L.; Purcell, J.E.; Piraino, S. Jellyfish as Prey: Frequency of Predation and Selective Foraging of Boops boops (Vertebrata, Actinopterygii) on the Mauve Stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS ONE 2014, 9, e94600. [Google Scholar] [CrossRef]
- Kogovšek, T.; Tinta, T.; Klun, K.; Malej, A. Jellyfish biochemical composition: Importance of standardised sample processing. Mar. Ecol. Prog. Ser. 2014, 510, 275–288. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Chapter 13—Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, pp. 211–222. [Google Scholar]
- Simopoulos, A.P. Importance of the omega-6/omega-3 balance in health and disease: Evolutionary aspects of diet. World Rev. Nutr. Diet 2011, 102, 10–21. [Google Scholar] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Lecci, R.M.; Milisenda, G.; Piraino, S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food Res. Technol. 2019, 245, 1611–1627. [Google Scholar] [CrossRef]
- Aral, M.N.; Ford, J.A.; Whyte, J.N.C. Biochemical composition of fed and starved Aequorea victoria (Murbach et Shearer, 1902) (Hydromedusa). J. Exp. Mar. Biol. Ecol. 1989, 127, 289–299. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nichols, P.D.; Virtue, P. Lipids and trophodynamics of Antarctic zooplankton. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 1998, 120, 311–323. [Google Scholar] [CrossRef]
- Wang, M.; MacKenzie, A.D.; Jeffs, A.G. Lipid and fatty acid composition of likely zooplankton prey of spiny lobster (Jasus edwardsii) phyllosomas. Aquac. Nutr. 2014, 21, 385–400. [Google Scholar] [CrossRef]
- Svetashev, V.I. Fatty Acids of the Medusae Aurelia aurita (Linnaeus, 1758) and Rhopilemae sculentum (Kishinouye, 1891): The Presence of Families of Polyenoic Acids with 24 and 26 Carbon Atoms. Russ. J. Mar. Biol. 2019, 45, 113–117. [Google Scholar] [CrossRef]
- Ackman, R.G.; Hooper, S.N.; Sipos, J.C. Distribution of trans-6-hexadecenoic and other fatty acids in tissues and organs of the atlantic leatherback turtle Dermochelys coriacea coriacea L. Int. J. Biochem. 1972, 3, 171–179. [Google Scholar] [CrossRef]
- Ying, C.; Ying, W.; Jing, Z.; Na, W. Potential dietary influence on the stable isotopes and fatty acid compositions of jellyfishes in the Yellow Sea. J. Mar. Biol. Assoc. UK 2012, 92, 1325–1333. [Google Scholar] [CrossRef]
- Fukuda, Y.; Naganuma, T. Potential dietary effects on the fatty acid composition of the common jellyfish Aurelia aurita. Mar. Biol. 2001, 138, 1029–1035. [Google Scholar] [CrossRef]
- Tilves, U.; Fuentes, V.L.; Milisenda, G.; Parrish, C.C.; Vizzini, S.; Sabatés, A. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: Evidence from stable isotope signatures and fatty acid composition. Mar. Ecol. Prog. Ser. 2018, 591, 101–116. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, X.; Li, X.; Hou, L.; Wang, C. Determination of Aluminum in Edible Jellyfish Using Chrome Azurol S with Spot Test on Filter Paper. Anal. Sci. 2017, 33, 185–189. [Google Scholar] [CrossRef]
- Kandimalla, R.; Vallamkondu, J.; Corgiat, E.B.; Gill, K.D. Understanding Aspects of Aluminum Exposure in Alzheimer’s Disease Development. Brain Pathol. 2016, 26, 139–154. [Google Scholar] [CrossRef]
- Authority, E.F.S. Safety of aluminium from dietary intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 6, 754. [Google Scholar]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 37. [Google Scholar] [CrossRef]
- Bleve, G.; Ramires, F.A.; De Domenico, S.; Leone, A. An Alum-Free Jellyfish Treatment for Food Applications. Front. Nutr. 2021, 8, 559. [Google Scholar] [CrossRef]
- Macel, M.-L.; Ristoratore, F.; Locascio, A.; Spagnuolo, A.; Sordino, P.; D’Aniello, S. Sea as a color palette: The ecology and evolution of fluorescence. Zool. Lett. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Doyle, T.K.; De Haas, H.; Cotton, D.; Dorschel, B.; Cummins, V.; Houghton, J.D.R.; Davenport, J.; Hays, G.C. Widespread occurrence of the jellyfish Pelagia noctiluca in Irish coastal and shelf waters. J. Plankton Res. 2008, 30, 963–968. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, Z.; Latiff, A.A.; Gan, C.-Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Calejo, M.T.; Morais, Z.B.; Fernandes, A.I. Isolation and Biochemical Characterisation of a Novel Collagen from Catostylus tagi. J. Biomater. Sci. Polym. Ed. 2009, 20, 2073–2087. [Google Scholar] [CrossRef]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Nakao, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella. J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar] [CrossRef]
- Miura, S.; Kimura, S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. J. Biol. Chem. 1985, 260, 15352–15356. [Google Scholar] [CrossRef]
- Arai, M.N. A Functional Biology of Scyphozoa; Chapman & Hall: London, UK, 1996. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Son, D.H.; Yang, D.J.; Sun, J.S.; Kim, S.K.; Kang, N.; Kang, J.Y.; Choi, Y.-H.; Lee, J.H.; Moh, S.H.; Shin, D.M.; et al. A Novel Peptide, Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells. Mar. Drugs 2018, 16, 262. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.Y.; Lee, Y.M. Preparation of porous collagen/hyaluronic acid hybrid scaffolds for biomimetic functionalization through biochemical binding affinity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82B, 506–518. [Google Scholar] [CrossRef]
- Bernhardt, A.; Paul, B.; Gelinsky, M. Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects. Mar. Drugs 2018, 16, 91. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, S.Y.; Cho, S.K.; Chong, M.S.; Kim, K.S.; Kim, H.; Lee, S.B.; Lee, Y.M. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials 2007, 28, 1115–1122. [Google Scholar] [CrossRef]
- Mearns-Spragg, A.; Tilman, J.; Tams, D.; Barnes, A. The Biological Evaluation of Jellyfish Collagen as a New Research Tool for the Growth and Culture of iPSC Derived Microglia. Front. Mar. Sci. 2020, 7, 689. [Google Scholar] [CrossRef]
- Calejo, M.T.; Almeida, A.J.; Fernandes, A.I. Exploring a new jellyfish collagen in the production of microparticles for protein delivery. J. Microencapsul. 2012, 29, 520–531. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Mariottini, G.L. Hemolytic venoms from marine cnidarian jellyfish—An overview. J. Venom Res. 2014, 5, 22–32. [Google Scholar]
- Mariscal, R.N. Nematocysts. In Coelenterate Biology; Lenhoff, K., Muscatine, L., Marian, E., Davis, L., Eds.; University of Hawaii Press: Honolulu, HI, USA, 1974; pp. 129–178. [Google Scholar]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins: Expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, S.K.; Kim, M.; Pyo, M.J.; Kim, M.; Yang, S.; Won, C.-K.; Yoon, W.D.; Han, C.H.; Kang, C.; et al. Anticancer Effect of Nemopilema nomurai Jellyfish Venom on HepG2 Cells and a Tumor Xenograft Animal Model. Evid. -Based Complement. Altern. Med. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, I.; Lee, H.; Pyo, M.J.; Heo, Y.; Chae, J.; Yum, S.S.; Kang, C.; Kim, E. Proteomic Investigation to Identify Anticancer Targets of Nemopilema nomurai Jellyfish Venom in Human Hepatocarcinoma HepG2 Cells. Toxins 2018, 10, 194. [Google Scholar] [CrossRef]
- Li, C.; Li, P.; Feng, J.; Li, I.; Yu, H. Cytotoxicity of the venom from the nematocysts of jellyfish Cyanea nozakii Kishinouye. Toxicol. Ind. Health 2012, 28, 186–192. [Google Scholar]
- Ayed, Y.; Boussabbeh, M.; Zakhama, W.; Bouaziz, C.; Abid, S.; Bacha, H. Induction of cytotoxicity of Pelagia noctiluca venom causes reactive oxygen species generation, lipid peroxydation induction and DNA damage in human colon cancer cells. Lipids Health Dis. 2011, 10, 232. [Google Scholar] [CrossRef]
- Ayed, Y.; Bousabbeh, M.; Ben Mabrouk, H.; Morjen, M.; Marrakchi, N.; Bacha, H. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by the Mediterranean jellyfish Pelagia noctiluca venom and its fractions in cultured glioblastoma cells. Lipids Health Dis. 2012, 11, 84. [Google Scholar] [CrossRef]
- Hessinger, D.; Lenhoff, H.M. Mechanism of hemolysis induced by nematocyst venom: Roles of phospholipase A and direct lytic factor. Arch. Biochem. Biophys. 1976, 173, 603–613. [Google Scholar] [CrossRef]
- Carli, A.; Mariottini, G.L.; Pane, L. Ecological and Medical Aspects of Jellyfish Poisoning. In Epidemiological Studies Related to the Environmental Quality Criteria for Bathing Waters, Shellfish-Growing Waters and Edible Marine Organisms; Mediterranean Action Plan Technical Reports Series; No. 93; UNEP: Athens, Greece, 1995; pp. 1–21. [Google Scholar]
- Ames, C.L.; Klompen, A.M.L.; Badhiwala, K.; Muffett, K.; Reft, A.J.; Kumar, M.; Janssen, J.D.; Schultzhaus, J.; Field, L.D.; Muroski, M.E.; et al. Cassiosomes are stinging-cell structures in the mucus of the upside-down jellyfish Cassiopea xamachana. Commun. Biol. 2020, 3, 67. [Google Scholar] [CrossRef]
- Liu, W.; Mo, F.; Jiang, G.; Liang, H.; Ma, C.; Li, T.; Zhang, L.; Xiong, L.; Mariottini, G.L.; Zhang, J.; et al. Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea. Mar. Drugs 2018, 16, 341. [Google Scholar] [CrossRef]
- Hubot, N.; Giering, S.L.C.; Lucas, C.H. Similarities between the biochemical composition of jellyfish body and mucus. J. Plankton Res. 2022, 44, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Patwa, A.; Thiéry, A.; Lombard, F.; Lilley, M.K.S.; Boisset, C.; Bramard, J.-F.; Bottero, J.-Y.; Barthélémy, P. Accumulation of nanoparticles in “jellyfish” mucus: A bio-inspired route to decontamination of nano-waste. Sci. Rep. 2015, 5, 11387. [Google Scholar] [CrossRef] [PubMed]
- Lengar, Ž.; Klun, K.; Dogsa, I.; Rotter, A.; Stopar, D. Sequestration of Polystyrene Microplastics by Jellyfish Mucus. Front. Mar. Sci. 2021, 8, 854. [Google Scholar] [CrossRef]
| Species | Tissue | Proteins | Lipids | Carbohydrates | Proteins | Lipids | Carbohydrates | Reference |
|---|---|---|---|---|---|---|---|---|
| (% of DM) | (% of WM) | |||||||
| Hydromedusae | ||||||||
| Aequorea victoria | W | 6.6 | 2.2 | 0.7 | 0.06 | [29] | ||
| Aglantha digitale | W | 21.6–22.1 | 6.0–6.9 | 0.4–0.9 | [29] | |||
| W | 56.5 | 3.0 | 0.8 | [30] | ||||
| Botrynema brucei | W | 7.4 * | 1.8 * | 0.4 * | 0.3 ± 0.03 | 0.08 ± 0.03 | 0.02 ± 0.01 | [31] |
| Bougainvillia superciliaris | W | 7.7–14.9 | 6.8–10.0 | 0.7–1.0 | [29] | |||
| Calycopsis borchgrevinki | W | 3.1 | 0.1 | [32] | ||||
| W | 11.2 * | 2.2 * | 1.1 * | 0.5 ± 0.1 | 0.1 ± 0.03 | 0.05 ± 0.01 | [31] | |
| Dimophyes arctica | W | 5.8 | 0.3 | [32] | ||||
| Diphyes antarctica | W | 1.3 ± 0.3 | 0.07 ± 0.02 | [32] | ||||
| W | 13.4 * | 3.2 * | 1.2 * | 0.6 ± 0.04 | 0.1 ± 0.05 | 0.06 ± 0.01 | [31] | |
| Halitholus cirratus | W | 10.4–18.2 | 4.6–7.6 | 0.7–0.8 | [29] | |||
| Hybocodon polifer | W | 23.0–31.0 | 13.1–22.1 | 0.8 | [29] | |||
| Olindias sambaquiensis | W | 14.2 ± 0.02 | 1.6 ± 0.4 | 4.7 ± 0.2 | [33] | |||
| Rhacostoma atlanticum | W | 10.5 ± 0.01 | 1.4 ± 0.1 | 1.2 ± 0.1 | [33] | |||
| Sarsia princeps | W | 14.5–14.7 | 7.8–9.1 | 0.4–0.8 | [29] | |||
| Scyphomedusae | ||||||||
| Semaeostomeae | ||||||||
| Aurelia aurita | W | 0.5 | 0.0 | [34] | ||||
| W | 0.4 | [35] | ||||||
| W | 0.2 | [36] | ||||||
| W | 4.7 | 9.2 | 13.5 | 5.3 | 2.0 | 3.4 | [37] | |
| W | 5.9 | 1.9 | 2.9 | [38] | ||||
| G | 23.7 | 14.6 | [38] | |||||
| OA | 7.3 | 2.6 | [38] | |||||
| Aurelia aurita | B | 4.2 | 1.5 | [38] | ||||
| W | 2.1–28.6 | 1.2–3.4 | 0.4–1.1 | [39] | ||||
| G | 4.4–23.0 | 2.6–6.0 | 1.1–2.1 | [39] | ||||
| OA | 4.1–15.3 | 1.3–4.0 | 0.6–1.5 | [39] | ||||
| B | 2.3–8.3 | 0.9–2.9 | 0.3–0.9 | [39] | ||||
| W | 0.7 | 0.03-0.04 | [40] | |||||
| W | 3.5 | 0.4 | 19.9 | [41] | ||||
| Aurelia coerulea | W | 0.25 | [42] | |||||
| B | 0.11 | [42] | ||||||
| OA | 0.18 | [42] | ||||||
| Aurelia sp.1 | W | 5.7 | 4.1 ± 0.5 | [43] | ||||
| Chrysaora hysocella | W | 2.7 | [36] | |||||
| W | 4.6 ± 2.8 | 1.5 ± 2.1 | 0.8 ± 2.2 | 0.2 ± 0.1 | 0.04 ± 0.03 | 0.01 ± 0.01 | [44] | |
| G | 12.8 ± 6.8 | 4.5 ± 2.8 | 0.6 ± 0.04 | [44] | ||||
| B | 3.5 ± 2.6 | 0.6 ± 0.6 | 0.1 ± 0.08 | [44] | ||||
| Chrysaora lactea | W | 12.6 ± 0.01 | 1.8 ± 1.1 | 0.9 ± 0.1 | [33] | |||
| Chrysaora pacifica | W | 7.5 | 0.7 | 22.7 | [41] | |||
| Chrysaora quinquecirrha | W | 0.2 | [34] | |||||
| Cyanea capillata | G | 28.4 ± 3.9 | 0.6 | 0.9 | [45] | |||
| OA | 29.8 ± 3.1 | 1.2 ± 0.6 | 1.0 ± 0.1 | [45] | ||||
| B | 7.9 ± 1.5 | 0.2 ± 0.1 | 0.8 ± 0.1 | [45] | ||||
| W | 16.5 ± 3.0 | 0.5 ± 0.1 | 0.9 ± 0.02 | [45] | ||||
| G | 9.6 | 1.6 | 1.0 | [46] | ||||
| W | 0.3–0.8 | [47] | ||||||
| Cyanea lamarcki | W | 0.7 | [36] | |||||
| Pelagia noctiluca | W | 10.9–19.8 | 1.3–2.9 | 0.1–0.7 | [48,49] | |||
| W | 0.2 | [50] | ||||||
| Poralia rufescens | W | 0.2 | 0.4 | 0.1 | [46] | |||
| Stygiomedusa gigantea | W | 10.2 | 0.5 | [32] | ||||
| Rhizostomeae | ||||||||
| Acromitus maculosus | B | 21.4 ± 0.3 | 0.4 ± 0.2 | 17.7 | 0.8 ± 1.2 | [51] | ||
| (A. hardenbergi) | OA | 33.7 ± 1.1 | 1.1 ± 0.2 | 6.0 | 1.3 ± 1.0 | [51] | ||
| Cassiopea andromeda | W | 0.9 | 0.07 | [52] | ||||
| Catostylus tagi | W | 0.8 * | 0.4 | [53] | ||||
| B | 8.4 * | 1.0 * | 1.8 | 0.2 | [53] | |||
| OA | 18.0 * | 2.2 * | 4.3 | 0.5 | [53] | |||
| Cotylorhiza tuberculata | W | 2.2 | 12.3 ± 0.7 | [43] | ||||
| B | 7.6–12.0 | 0.5–0.7 | [54] | |||||
| OA | 20.0 | 6.4 | [54] | |||||
| G | 36.8 | 6.0 | [54] | |||||
| Lychnorhiza lucerna | W | 12.3 ± 0.03 | 2.7 ± 0.04 | [33] | ||||
| Rhizostoma luteum | W | 0.8–1.9 | [55] | |||||
| Rhizostoma octopus | G | 12.1 ± 9.8 | 0.6 ± 0.4 | 0.9 ± 0.03 | [45] | |||
| OA | 13.4 ± 0.4 | 0.3 ± 0.1 | 0.7 ± 0.3 | [45] | ||||
| B | 6.6 ± 2.3 | 0.3 ± 0.1 | 0.7 ± 0.01 | [45] | ||||
| W | 12.8 ± 2.3 | 0.3 | 0.8 | [45] | ||||
| Rhizostoma pulmo | W | 2.3 | [56] | |||||
| W | 6.0 | 4.0 ± 0.1 | [43] | |||||
| B | 8.7–13.7 | 0.7–1.0 | [54] | |||||
| OA | 27.0 | 0.8 | [54] | |||||
| G | 18.0 | 1.2 | [54] | |||||
| Rhopilema hispidum | B | 19.9 ± 0.7 | 0.5 ± 0.3 | 18.2 | 0.5 ± 0.2 | [51] | ||
| OA | 43.8 ± 0.2 | 1.4 ± 0.2 | 10.7 | 2.0 ± 1.6 | [51] | |||
| Rhopilema esculentum | B | 38.1 ± 1.1 | 0.6 ± 0.1 | 8.9 | 1.6 ± 0.8 | [51] | ||
| OA | 53.9 ± 2.1 | 1.8 ± 0.3 | 7.7 | 2.7 ± 0.9 | [51] | |||
| Stomolophus meleagris | B | 1.1 ± 0.2 | [57] | |||||
| B | 1.0 ± 0.1 | [57] | ||||||
| Coronatae | ||||||||
| Atolla wyvillei | W | 1.1 | [58] | |||||
| W | 16.9 * | 4.2 * | 1.7 * | 0.8 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.01 | [31] | |
| W | 0.3 | 0.01 | [32] | |||||
| Periphylla periphylla | W | 6.4 ± 1.7 | 2.1 ± 0.8 | 0.9 ± 0.2 | 0.3 ± 0.1 | 0.1 ± 0.06 | 0.05 ± 0.02 | [59] |
| Cubomedusae | ||||||||
| Chiropsalmus quadrumanus | W | 18.2 ± 0.02 | 1.3 ± 0.0 | 5.9 ± 0.3 | [33] | |||
| Tamoya haplonema | W | 27.7 ± 0.03 | 3.7 ± 0.4 | 4.2 ± 0.0 | [33] | |||
| Species | Fatty Acids | Reference | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12:0 | 14:0 | 15:0 | 16:0 | 17:0 | 18:0 | 20:0 | 16:1(n-7) | 18:1(n-9) | 18:1(n-7) | 20:1(n-9) | 20:1(n-7) | 22:1(n-11) | 22:1(n-9) | 18:2(n-6) | 18:3(n-3) | 18:4(n-3) | 20:4(n-6) | 20:4(n-3) | 20:5(n-3) | 22:4(n-6) | 22:5(n-6) | 22:5(n-3) | 22:6(n-3) | ||
| Hydromedusae | |||||||||||||||||||||||||
| Aequorea victoria | 1.7 | 0.8 | 7.7 | 0.9 | 8.9 | 1.0 | 2.6 | 5.4 | 1.1 | 2.1 | 3.1 | 6.2 | 0.4 | 0.3 | 0.4 | 9.6 | 0.4 | 9.6 | 0.3 | 4.4 | 1.5 | 18.8 | [67] | ||
| Arctapodema ampla | 0.3 | 5.5 | 1.2 | 14.9 | 0.5 | 5.1 | 0.3 | 2.8 | 5.7 | 2.0 | 1.8 | 2.0 | 2.7 | 0.9 | 0.1 | 0.2 | 0.3 | 16.4 | 0.2 | 26.4 | [68] | ||||
| Calycopsis borchgrevinki | 0.5 | 2.5 | 0.3 | 16.6 | 0.0 | 4.8 | 9.2 | 26.4 | 3.7 | 2.5 | 2.8 | 0.0 | 0.0 | 1.5 | 0.2 | 0.1 | 1.4 | 9.6 | 2.1 | 7.0 | [32] | ||||
| 0.1 | 2.7 | 0.6 | 11.6 | 0.6 | 7.8 | 0.2 | 5.2 | 14.3 | 1.9 | 4.4 | 6.9 | 0.9 | 0.3 | 0.9 | 0.1 | 0.8 | 9.9 | 2.1 | 5.6 | 16.1 | [68] | ||||
| Chelophyes appendiculata | 2.4 | 0.0 | 11.0 | 0.0 | 7.6 | 6.6 | 11.9 | 4.3 | 8.1 | 19.2 | [69] | ||||||||||||||
| Dimophyes arctica | 0.0 | 3.9 | 0.8 | 18.9 | 0.0 | 9.3 | 15.3 | 17.2 | 4.1 | 1.1 | 1.0 | 0.0 | 1.3 | 1.0 | 0.1 | 0.2 | 0.0 | 8.2 | 0.1 | 10.4 | [32] | ||||
| Diphyes antarctica | 0.0 | 7.6 | 1.1 | 18.0 | 0.0 | 8.8 | 4.0 | 8.7 | 2.0 | 1.2 | 3.6 | 0.2 | 0.1 | 2.2 | 0.3 | 2.1 | 1.4 | 16.5 | 0.2 | 16.9 | [32] | ||||
| 0.8 | 5.7 | 1.0 | 20.1 | 0.0 | 7.6 | 3.8 | 7.3 | 4.1 | 1.7 | 0.6 | 0.0 | 0.0 | 1.9 | 0.2 | 0.6 | 1.2 | 19.1 | 0.3 | 17.6 | [32] | |||||
| Scyphomedusae | |||||||||||||||||||||||||
| Atolla wyvillei | 0.6 | 6.1 | 0.6 | 20.8 | 0.0 | 2.8 | 0.0 | 5.3 | 11.8 | 7.2 | 3.2 | 0.2 | 0.0 | 2.5 | 1.0 | 5.0 | 0.0 | 16.6 | 0.3 | 2.4 | 5.6 | [32] | |||
| Aurelia aurita | 1.5 | 0.4 | 12.0 | 1.1 | 10.0 | 0.1 | 0.2 | 0.9 | 1.8 | 0.4 | 0.2 | 0.4 | 0.9 | 1.0 | 1.3 | 1.8 | 0.9 | 33.3 | 0.3 | 0.1 | 5.0 | 11.2 | [70] | ||
| 1.2 | 4.1 | 2.8 | 42.3 | 2.9 | 22.2 | 0.0 | 2.6 | 8.8 | 1.8 | 1.4 | 0.7 | [40] | |||||||||||||
| 2.2 | 13.7 | 7.4 | 1.0 | 3.3 | 9.7 | 3.3 | 6.8 | [69] | |||||||||||||||||
| 0.1 | 3.8 | 1.5 | 23.1 | 1.2 | 21.9 | 0.9 | 5.1 | 4.0 | 1.6 | 0.8 | 2.3 | 1.3 | 0.6 | 0.8 | 4.5 | 17.6 | 2.2 | 5.1 | [36] | ||||||
| 0.0 | 3.3 | 1.0 | 16.0 | 0.7 | 6.4 | 1.2 | 4.6 | 8.9 | 2.6 | 4.8 | 7.1 | 1.2 | 3.2 | 0.8 | 0.4 | 0.1 | 6.7 | 0.4 | 8.5 | 0.6 | 0.3 | 1.6 | 7.0 | [71] | |
| 3.2 | 1.0 | 11.5 | 0.5 | 13.3 | 0.3 | 3.1 | 4.4 | 1.3 | 0.9 | 0.4 | 15.2 | 19.4 | 1.7 | 0.5 | 5.8 | 12.8 | [72] | ||||||||
| 0.4 | 2.8 | 2.1 | 22.5 | 5.4 | 19.2 | 0.3 | 4.5 | 6.2 | 0.6 | 0.1 | 1.3 | 0.9 | 1.9 | 7.8 | 17.5 | 1.3 | [41] | ||||||||
| 2.9 | 1.0 | 24.3 | 1.8 | 15.7 | 0.3 | 4.7 | 2.2 | 2.3 | 0.4 | 8.2 | 1.0 | 0.7 | 0.4 | 4.8 | 12.7 | 1.9 | 5.2 | [73] | |||||||
| Aurelia sp. 1 | 0.0 | 2.4 | 33.0 | 1.4 | 32.7 | 0.0 | 0.0 | 3.0 | 1.7 | 1.3 | 5.5 | 0.0 | 14.6 | 4.4 | [43] | ||||||||||
| Cassiopea andromeda | 9.3 | 4.2 | 21.9 | 12.5 | 0.6 | 4.3 | 2.8 | 0.8 | 2.6 | 7.4 | 14.2 | 2.1 | 2.9 | 2.5 | 11.0 | [52] | |||||||||
| Chrysaora isoceles | 0.0 | 3.3 | 0.4 | 9.5 | 0.6 | 7.1 | 1.2 | 3.7 | 4.4 | 1.5 | 6.6 | 6.1 | 0.8 | 0.7 | 1.3 | 5.4 | 20.0 | 5.4 | 19.7 | [36] | |||||
| Chrysaora pacifica | 0.4 | 3.7 | 2.7 | 14.9 | 4.5 | 18.1 | 0.6 | 5.9 | 5.1 | 1.0 | 0.4 | 0.7 | 0.8 | 1.7 | 13.5 | 15.2 | 3.2 | [41] | |||||||
| Chrysaora quinquecirrha | 0.7 | 10.0 | 10.9 | 1.9 | 3.2 | 0.5 | 0.4 | 0.1 | 22.4 | 7.6 | 6.3 | 3.6 | 11.9 | [34] | |||||||||||
| Cotylorhiza tuberculata | 0.0 | 2.9 | 26.1 | 0.8 | 24.2 | 0.8 | 1.2 | 12.8 | 1.2 | 8.3 | 5.3 | 4.1 | 5.1 | 7.2 | [43] | ||||||||||
| Cyanea capillata | 1.7 | 1.0 | 12.9 | 1.9 | 6.1 | 2.0 | 4.0 | 6.2 | 10.5 | 2.1 | 0.4 | 0.5 | 0.4 | 7.8 | 0.6 | 15.0 | 1.3 | 1.1 | 3.7 | 14.4 | [47] | ||||
| Cyanea lamarcki | 0.1 | 3.5 | 1.3 | 19.0 | 1.0 | 12.0 | 1.8 | 4.7 | 5.5 | 3.1 | 3.4 | 2.5 | 0.4 | 0.4 | 0.6 | 8.7 | 13.8 | 3.2 | 12.1 | [36] | |||||
| Cyanea nozakii | 2.3 | 0.9 | 11.1 | 0.9 | 13.9 | 1.3 | 3.2 | 2.9 | 1.3 | 1.0 | 0.4 | 22.9 | 11.9 | 4.7 | 1.6 | 4.6 | 9.9 | [72] | |||||||
| Pelagia noctiluca | 0.2 | 0.2 | 69.2 | 0.9 | 15.2 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.6 | 6.2 | 0.0 | 0.0 | 0.3 | 2.3 | [18] | |||
| 3.0 | 2.4 | 33.2 | 4.9 | 18.0 | 0.6 | 2.1 | 5.7 | 4.2 | 1.6 | 2.2 | 2.7 | 0.9 | 0.5 | 0.1 | 0.8 | 0.1 | 1.1 | 0.1 | 0.4 | 0.7 | 0.7 | [74] | |||
| Periphylla periphylla | 0.0 | 3.8 | 0.5 | 15.7 | 0.5 | 7.8 | 0.2 | 2.4 | 15.5 | 3.2 | 4.4 | 5.1 | 1.7 | 3.3 | 1.0 | 0.2 | 0.8 | 0.7 | 20.9 | 3.4 | 0.5 | [68] | |||
| 0.0 | 1.0 | 0.2 | 13.5 | 0.8 | 21.5 | 0.6 | 0.0 | 6.7 | 2.4 | 4.5 | 4.7 | 2.4 | 4.5 | 0.3 | 0.3 | 1.5 | 1.0 | 19.4 | [68] | ||||||
| 0.1 | 3.1 | 0.5 | 13.6 | 0.4 | 7.0 | 0.2 | 2.1 | 14.3 | 2.5 | 4.7 | 5.2 | 1.9 | 3.2 | 0.9 | 0.1 | 0.6 | 0.6 | 17.7 | 2.6 | 12.1 | [68] | ||||
| Rhizostoma luteum | 0.3 | 2.0 | 0.4 | 11.0 | 0.7 | 15.0 | 0.1 | 3.1 | 11.3 | 1.0 | 0.1 | 4.6 | 9.8 | 23.7 | 3.6 | 0.3 | [55] | ||||||||
| Rhizostoma octopus | 0.3 | 5.1 | 2.0 | 27.3 | 2.3 | 21.7 | 0.0 | 3.8 | 6.8 | 3.5 | 0.8 | 1.6 | 1.6 | 2.8 | 2.8 | 9.7 | 1.3 | 5.3 | [36] | ||||||
| Rhizostoma pulmo | 1.3 | 3.1 | 33.2 | 0.0 | 30.6 | 0.0 | 5.1 | 1.9 | 2.5 | 8.8 | 0.0 | 8.6 | 4.9 | [43] | |||||||||||
| Rhopilema esculentum | 1.5 | 0.5 | 12.5 | 1.3 | 12.6 | 0.5 | 2.0 | 2.0 | 2.7 | 0.3 | 0.2 | 1.8 | 1.4 | 2.4 | 8.4 | 13.1 | 2.4 | 1.0 | 5.1 | 12.3 | [70] | ||||
| Stomolophus meleagris | 1.7 | 12.1 | 8.7 | 0.5 | 2.9 | 0.1 | 0.9 | 0.5 | 1.1 | 15.5 | 19.1 | 2.6 | 1.2 | 3.1 | 15.9 | [34] | |||||||||
| Stygiomedusa gigantea | 0.0 | 6.6 | 0.6 | 12.1 | 0.0 | 4.9 | 0.0 | 10.2 | 18.6 | 5.8 | 2.6 | 0.1 | 0.3 | 1.0 | 0.3 | 1.3 | 0.0 | 0.0 | 20.8 | 0.7 | 1.9 | 4.2 | [32] | ||
| Species | Tissue | Collagen Content | Reference | |||
|---|---|---|---|---|---|---|
| Pepsin | Acid | |||||
| (% DM) | (% WM) | (% DM) | (% WM) | |||
| Aurelia aurita | W | 0.01 | [84] | |||
| Chrysaora sp. | B | 9–19 | [85] | |||
| Pelagia noctiluca | W | 0.07 | [84] | |||
| Cassiopea andromeda | W | 2.2–6.0 | [52] | |||
| Catostylus tagi | B | 2.7 | [86] | |||
| Cotylorhiza tuberculata | B | 4.5 | [84] | |||
| OA | 19.4 | [84] | ||||
| B | <10 | [84] | ||||
| Rhizostoma pulmo | B | 8.3–31.5 | [84] | |||
| OA | 26–90 | [84] | ||||
| B | <10 | [84] | ||||
| Rhopilema asamushi | M | 35.2 | [87] | |||
| Rhopilema esculentum | M | 0.28 | 0.12 | [88] | ||
| Stomolophus meleagris | M | 46.4 | [89] | |||
| Nemopilema nomurai | M | 2.2 | [90] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambra, I.; Merquiol, L. Jellyfish from Fisheries By-Catches as a Sustainable Source of High-Value Compounds with Biotechnological Applications. Mar. Drugs 2022, 20, 266. https://doi.org/10.3390/md20040266
D’Ambra I, Merquiol L. Jellyfish from Fisheries By-Catches as a Sustainable Source of High-Value Compounds with Biotechnological Applications. Marine Drugs. 2022; 20(4):266. https://doi.org/10.3390/md20040266
Chicago/Turabian StyleD’Ambra, Isabella, and Louise Merquiol. 2022. "Jellyfish from Fisheries By-Catches as a Sustainable Source of High-Value Compounds with Biotechnological Applications" Marine Drugs 20, no. 4: 266. https://doi.org/10.3390/md20040266
APA StyleD’Ambra, I., & Merquiol, L. (2022). Jellyfish from Fisheries By-Catches as a Sustainable Source of High-Value Compounds with Biotechnological Applications. Marine Drugs, 20(4), 266. https://doi.org/10.3390/md20040266