Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield
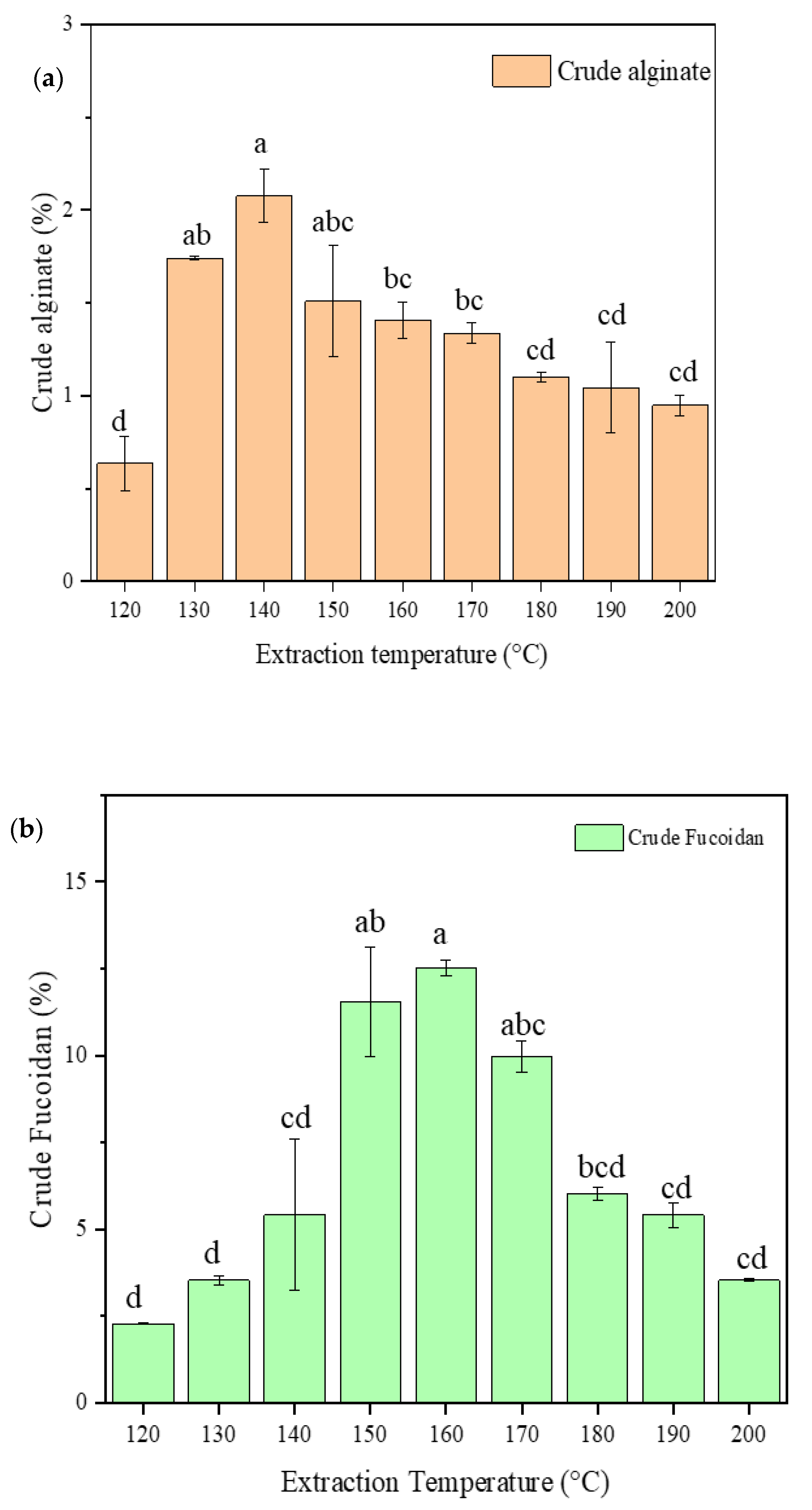
2.2. Crude Alginate and Fucoidan Content
2.3. Total Phenolic Content
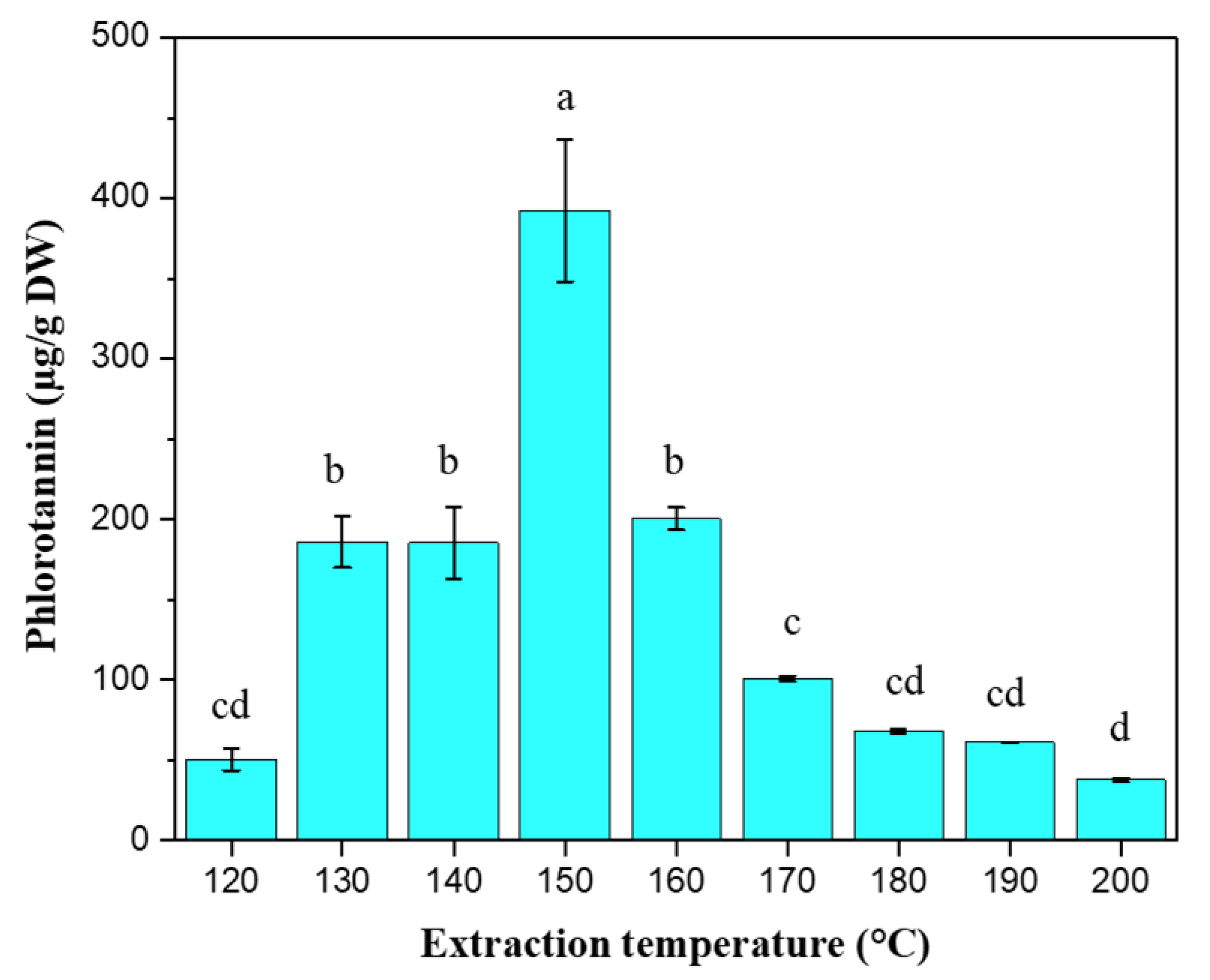
2.4. Phlorotannin Content
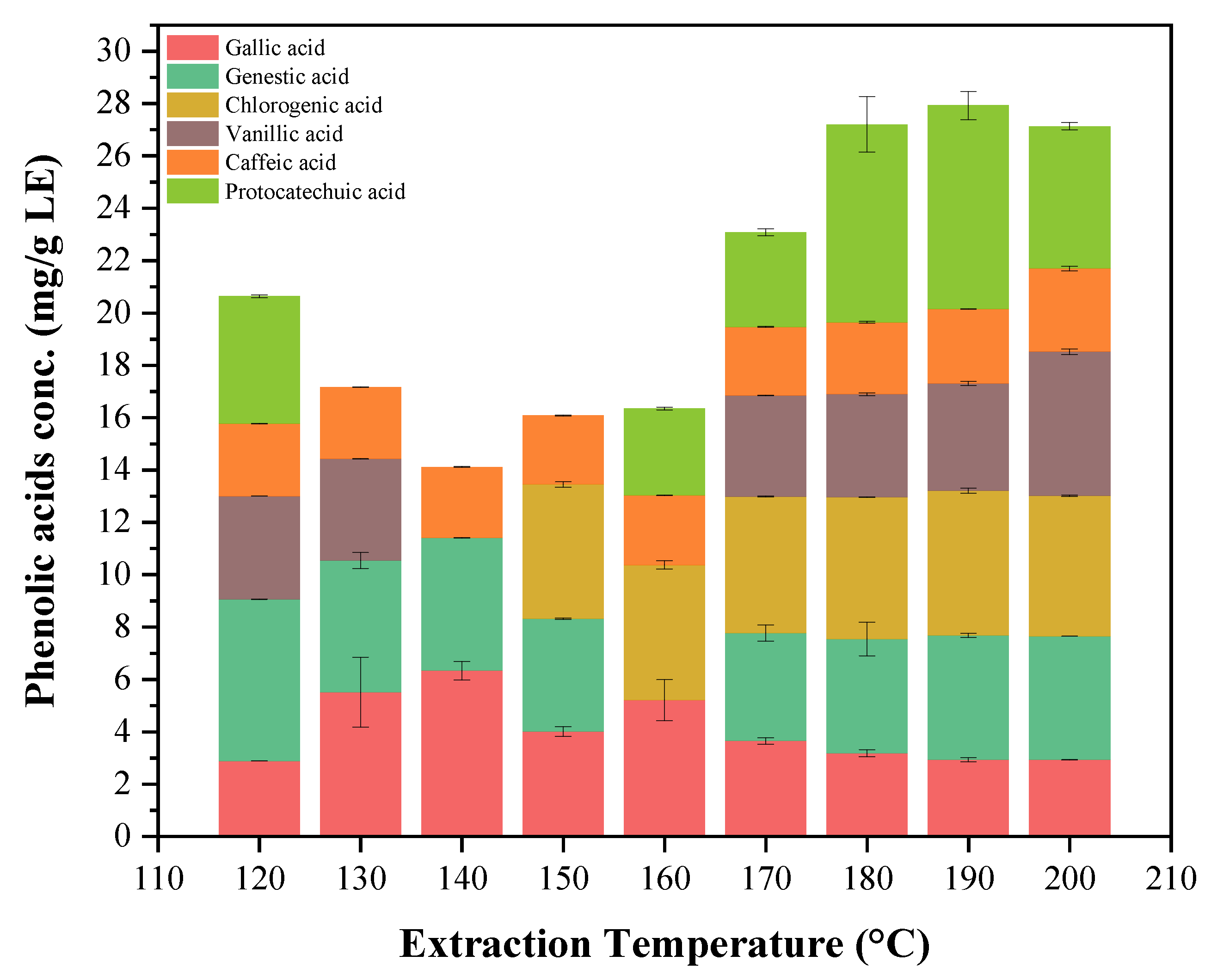
2.5. Individual Phenolic Compounds
2.6. Antioxidant Activity
2.6.1. DPPH Radicals Scavenging Activity
2.6.2. Metal-Chelating Activity
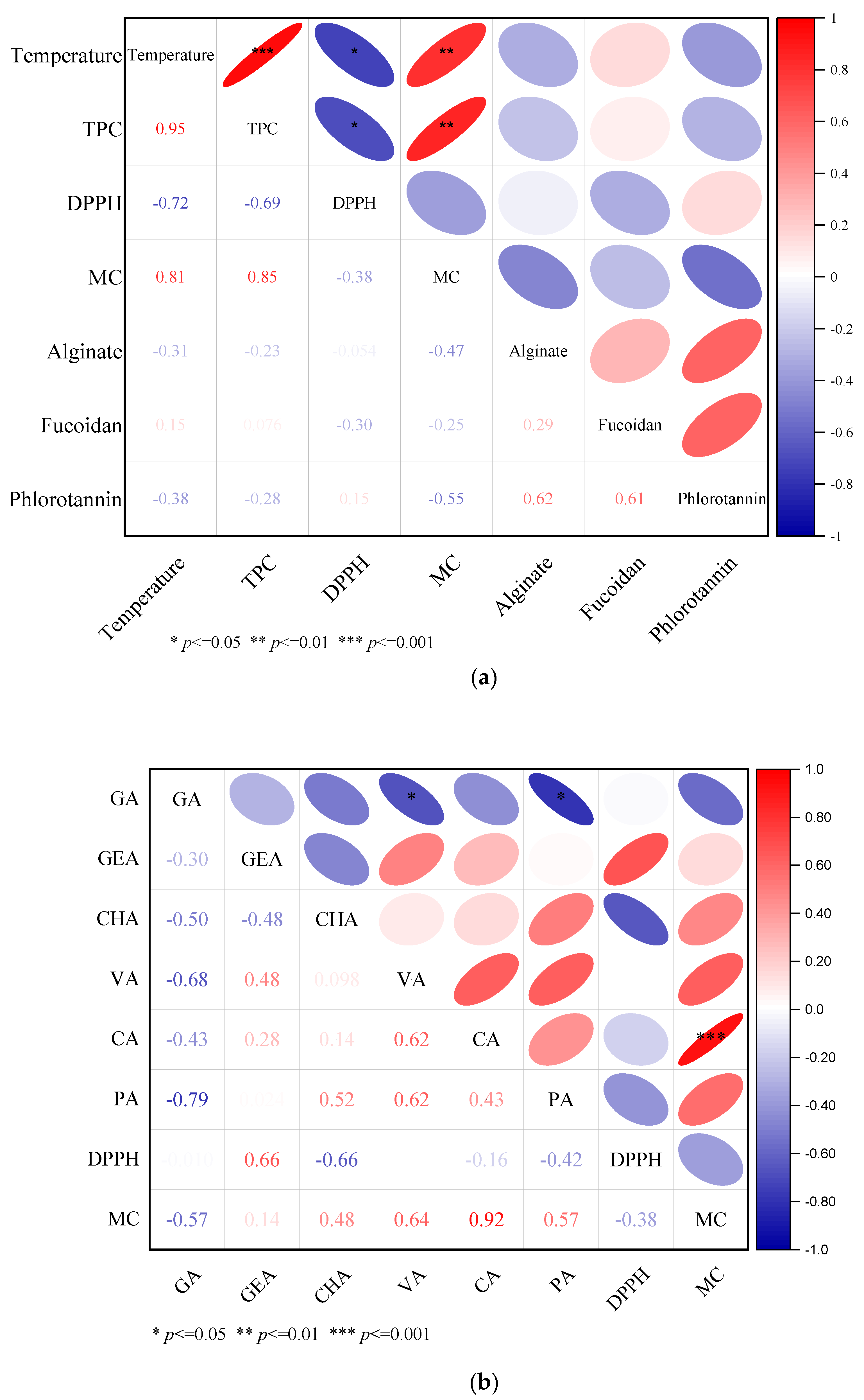
2.7. Correlation Matrix
3. Materials and Methods
3.1. Sample Preparation
3.2. Pressurized Liquid Extractions
3.3. Total Phenolic Compounds (TPC)
3.4. Phlorotannins Quantification (DMB Assay)
3.5. DPPH Radical Capacity Scavenging
3.6. Metal-Chelating Capacity
3.7. Phenolic Acid Identification
3.8. Isolation of Alginate and Fucoidan
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomic Signatures Reveal Seasonal Shifts on the Relative Abundance of High-Valued Lipids from the Brown Algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging Technologies for the Extraction of Marine Phenolics: Opportunities and Challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The Seasonal Variation of Fucoidan within Three Species of Brown Macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-Assisted Extraction of Fucoidan from Sargassum Muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure Characterization and Antioxidant Activity of Fucoidan Isolated from Undaria Pinnatifida Grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Zhang, L.; Qin, Y.; Cong, Q.; Wang, P.; Ding, K. A Fucoidan from Nemacystus Decipiens Disrupts Angiogenesis through Targeting Bone Morphogenetic Protein 4. Carbohydr. Polym. 2016, 144, 305–314. [Google Scholar] [CrossRef]
- De Yan, M.; Lin, H.Y.; Hwang, P.A. The Anti-Tumor Activity of Brown Seaweed Oligo-Fucoidan via LncRNA Expression Modulation in HepG2 Cells. Cytotechnology 2019, 71, 363–374. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-Inflammatory Effects of Fucoidan through Inhibition of NF-ΚB, MAPK and Akt Activation in Lipopolysaccharide-Induced BV2 Microglia Cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Jin, W.; Liu, B.; Li, S.; Chen, J.; Tang, H.; Jiang, D.; Zhang, Q.; Zhong, W. The Structural Features of the Sulfated Heteropolysaccharide (ST-1) from Sargassum Thunbergii and Its Neuroprotective Activities. Int. J. Biol. Macromol. 2018, 108, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of Ultrasound-Assisted Extraction Conditions for Phenolic Content and Antioxidant Activities of the Alga Hormosira Banksii Using Response Surface Methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Lajili, S.; Elkaibi, M.A.; Ben Salem, Y.; Abdelhamid, A.; Muller, C.D.; Majdoub, H.; Kraiem, J.; Bouraoui, A. Optimized Extraction, Preliminary Characterization and Evaluation of the in Vitro Anticancer Activity of Phlorotannin-Rich Fraction from the Brown Seaweed, Cystoseira sedoides. J. Aquat. Food Prod. Technol. 2019, 28, 892–909. [Google Scholar] [CrossRef]
- Sabeena, S.F.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-Assisted Extraction of Bioactive Compounds from Brown Seaweeds and Characterization. J. Appl. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Getachew, A.T.; Chun, B.-S. Characteristics of Functional Materials Recovered from Solomon Islands Red Seaweed (Kappaphycus alvarezii) Using Pressurized Hot Water Extraction. J. Appl. Phycol. 2017, 29, 1609–1621. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.-J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of Co-Solvents on Fucoxanthin and Phlorotannin Recovery from Brown Seaweed Using Supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical Water Extraction and Characterization of Bioactive Compounds from Haematococcus pluvialis Microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.-S. Characterization of Functional Materials Derived from Tropical Red Seaweed Hypnea Musciformis Produced by Subcritical Water Extraction Systems. J. Appl. Phycol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the Protein Fraction with High Antioxidant Activity from Red Seaweed Industrial Solid Residue after Agar Extraction by Subcritical Water Treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, J. Pressurized Liquids as an Alternative Green Process to Extract Antiviral Agents from the Edible Seaweed Himanthalia Elongata. J. Appl. Phycol. 2010, 23, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.-D.; Woo, H.-C.; Chun, B.-S. Subcritical Water Extraction of Fucoidan from Saccharina japonica: Optimization, Characterization and Biological Studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Bordoloi, A.; Goosen, N.J. A Greener Alternative Using Subcritical Water Extraction to Valorize the Brown Macroalgae Ecklonia Maxima for Bioactive Compounds. J. Appl. Phycol. 2020, 32, 2307–2319. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.S. Characteristics of Functional Materials Recovered from Indonesian Mangroves (Sonneratia alba and Rhizhophora mucronata) Using Subcritical Water Extraction. E3S Web Conf. 2020, 147, 03013. [Google Scholar] [CrossRef] [Green Version]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria, and Invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Springer: New York, NY, USA, 2012; pp. 55–98. [Google Scholar]
- Getachew, A.T.; Chun, B.S. Influence of Pretreatment and Modifiers on Subcritical Water Liquefaction of Spent Coffee Grounds: A Green Waste Valorization Approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus Spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Truus, K.; Vaher, M.; Taure, I. ALGAL BIOMASS FROM Fucus vesiculosus (Phaeophyta): Investigation of the mineral and alginate components. Proc. Estonian Acad. Sci. Chem. 2001, 50, 95–103. [Google Scholar]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel Procedures for the Extraction of Fucoidan from Brown Algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Chemical composition and antioxidant activity of sulphated polysaccharides extracted from fucus vesiculosus using different hydrothermal processes. Chem. Papers 2013, 68, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of Sulfated Polysaccharides by Autohydrolysis of Brown Seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.M.; Ribeiro, A.R.; Patinha, C.; Silva, A.M.S.; Cardoso, S.M.; Costa, R. Water Extraction Kinetics of Bioactive Compounds of Fucus vesiculosus. Molecules 2019, 24, 3408. [Google Scholar] [CrossRef] [Green Version]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2012, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Sánchez-Bonet, D.; García-Oms, S.; Belda-Antolí, M.; Padrón-Sanz, C.; Lloris-Carsi, J.M.; Cejalvo-Lapeña, D. RP-HPLC-DAD Determination of the Differences in the Polyphenol Content of Fucus vesiculosus Extracts with Similar Antioxidant Activity. J. Chromatogr. B 2021, 1184, 122978. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on in Vitro Bioactivities and Chemical Components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Lann, K.L.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and Antitumoural Activities of Some Phaeophyta from Brittany Coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Agregán, R.; Barba, F.J.; Gavahian, M.; Franco, D.; Khaneghah, A.M.; Carballo, J.; Ferreira, I.C.F.R.; da Silva Barretto, A.C.; Lorenzo, J.M. Fucus vesiculosus Extracts as Natural Antioxidants for Improvement of Physicochemical Properties and Shelf Life of Pork Patties Formulated with Oleogels. J. Sci. Food Agric. 2019, 99, 4561–4570. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total Phenolic Compounds, Radical Scavenging and Metal Chelation of Extracts from Icelandic Seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of Phenolic Antioxidants Extraction from Fucus vesiculosus by Pressurized Liquid Extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of Bioactive Properties in Brown Algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary Fiber and Antioxidant Capacity in Fucus vesiculosus Products. Int. J. Sci. Food Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant Activity of Fresh and Processed Edible Seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Habeebullah, S.F.K.; Alagarsamy, S.; Arnous, A.; Jacobsen, C. Enzymatic Extraction of Antioxidant Ingredients from Danish Seaweeds and Characterization of Active Principles. Algal Res. 2021, 56, 102292. [Google Scholar] [CrossRef]
- Hermund, D.B.; Heung, S.Y.; Thomsen, B.R.; Akoh, C.C.; Jacobsen, C. Improving Oxidative Stability of Skin-Care Emulsions with Antioxidant Extracts from Brown Alga Fucus vesiculosus. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 1509–1520. [Google Scholar] [CrossRef] [Green Version]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Affinity Purification and Characterisation of Chelating Peptides from Chickpea Protein Hydrolysates. Food Chem. 2011, 129, 485–490. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Selmeczi, K.; Collin, S.; Pasc, A.; Muhr, L.; Boschi-Muller, S. SPR Screening of Metal Chelating Peptides in a Hydrolysate for Their Antioxidant Properties. Food Chem. 2018, 239, 478–485. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-Derived Antioxidant Peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [Green Version]
- Walters, M.E.; Esfandi, R.; Tsopmo, A. Potential of Food Hydrolyzed Proteins and Peptides to Chelate Iron or Calcium and Enhance Their Absorption. Foods 2018, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; Di Felice, M.; Scaccini, C. Inhibition of Human Low-Density Lipoprotein Oxidation by Caffeic Acid and Other Hydroxycinnamic Acid Derivatives. Free Rad. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Almajano, M.P.; Carbó, R.; Delgado, M.E.; Gordon, M.H. Effect of PH on the Antimicrobial Activity and Oxidative Stability of Oil-in-Water Emulsions Containing Caffeic Acid. J. Food Sci. 2007, 72, C258–C263. [Google Scholar] [CrossRef]
- Yesiltas, B.; Sørensen, A.D.M.; García-Moreno, P.J.; Anankanbil, S.; Guo, Z.; Jacobsen, C. Modified Phosphatidylcholine with Different Alkyl Chain Length and Covalently Attached Caffeic Acid Affects the Physical and Oxidative Stability of Omega-3 Delivery 70% Oil-in-Water Emulsions. Food Chem. 2019, 289, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, A.D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant Properties and Efficacies of Synthesized Alkyl Caffeates, Ferulates, and Coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Undeland, I.; Larsson, K.; Storrø, I.; Rustad, T.; Jacobsen, C.; Kristinová, V.; Gallardo, J.M. Activity of Caffeic Acid in Different Fish Lipid Matrices: A Review. Food Chem. 2012, 131, 730–740. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and Characterization of Phlorotannins from Brown Algae Cystoseira Abies-Marina by Comprehensive Two-Dimensional Liquid Chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Extraction Temperature (°C) | DPPH IC50 (mg/mL) | MC IC50 (mg/mL) |
|---|---|---|
| 120 | 0.23 ± 0.08 a | 2.45 ± 0.03 cd |
| 130 | 0.19 ± 0.00 ab | 1.39 ± 0.04 d |
| 140 | 0.19 ± 0.08 ab | 1.50 ± 0.06 d |
| 150 | 0.20 ± 0.01 ab | 1.56 ± 0.11 d |
| 160 | 0.14 ± 0.02 b | 1.69 ± 0.15 d |
| 170 | 0.18 ± 0.02 ab | 4.55 ± 0,19 cd |
| 180 | 0.17 ± 0.04 b | 6.35 ± 0.40 c |
| 190 | 0.14 ± 0.02 b | 12.09 ± 1.23 b |
| 200 | 0.17 ± 0.03 ab | 23.05 ± 3.79 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getachew, A.T.; Holdt, S.L.; Meyer, A.S.; Jacobsen, C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Mar. Drugs 2022, 20, 263. https://doi.org/10.3390/md20040263
Getachew AT, Holdt SL, Meyer AS, Jacobsen C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Marine Drugs. 2022; 20(4):263. https://doi.org/10.3390/md20040263
Chicago/Turabian StyleGetachew, Adane Tilahun, Susan Løvstad Holdt, Anne Strunge Meyer, and Charlotte Jacobsen. 2022. "Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus" Marine Drugs 20, no. 4: 263. https://doi.org/10.3390/md20040263
APA StyleGetachew, A. T., Holdt, S. L., Meyer, A. S., & Jacobsen, C. (2022). Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Marine Drugs, 20(4), 263. https://doi.org/10.3390/md20040263









