One-Step Preparative Separation of Fucoxanthin from Three Edible Brown Algae by Elution-Extrusion Countercurrent Chromatography
Abstract
:1. Introduction
2. Results and Discussions
2.1. Selection of the Two-Phase Solvent System
2.2. Optimization of Elution-Extrusion Countercurrent Chromatography
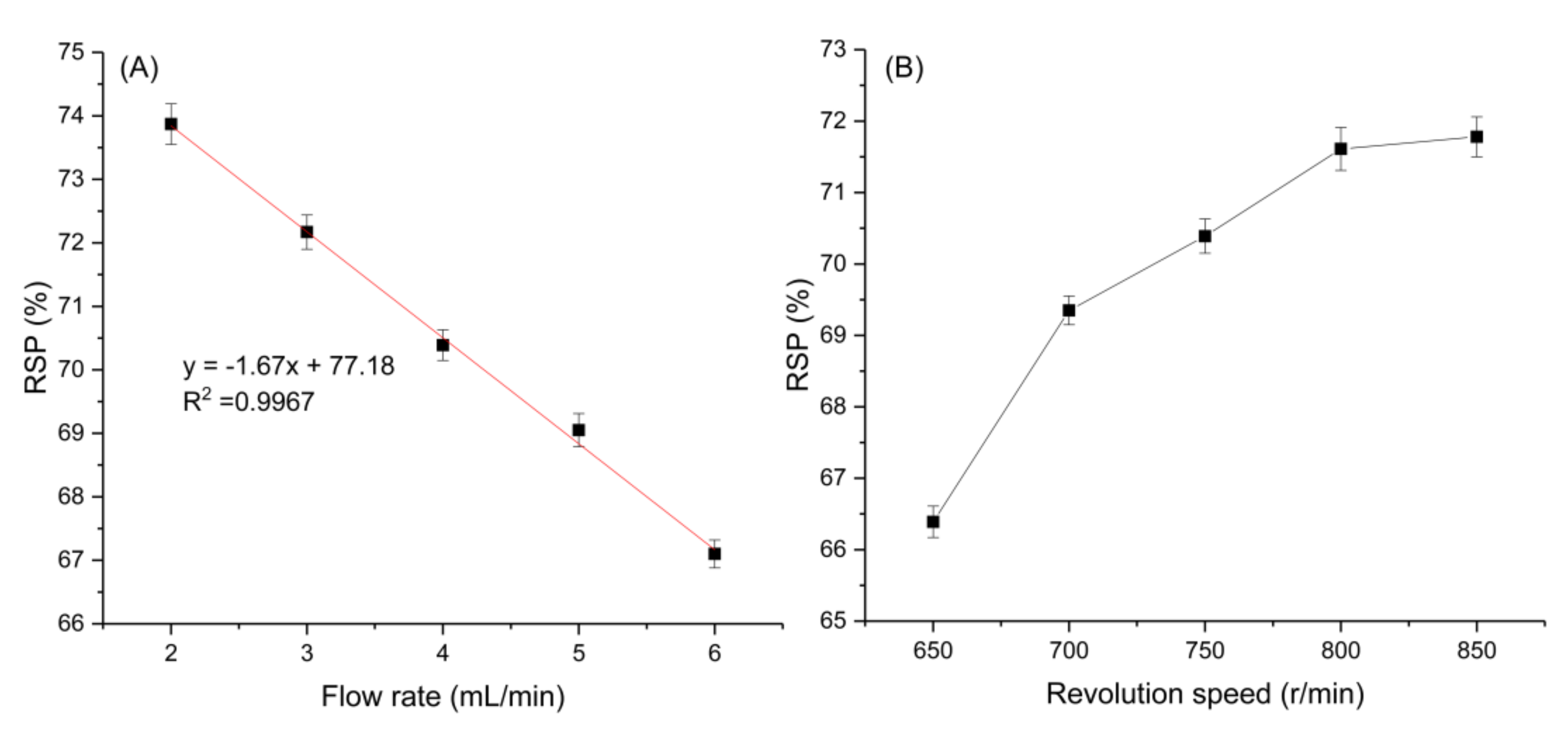
2.2.1. Effect of the Flow Rate of the Mobile Phase on the Retention of the Stationary Phase
2.2.2. Effect of Revolution Speed on the Retention of the Stationary Phase
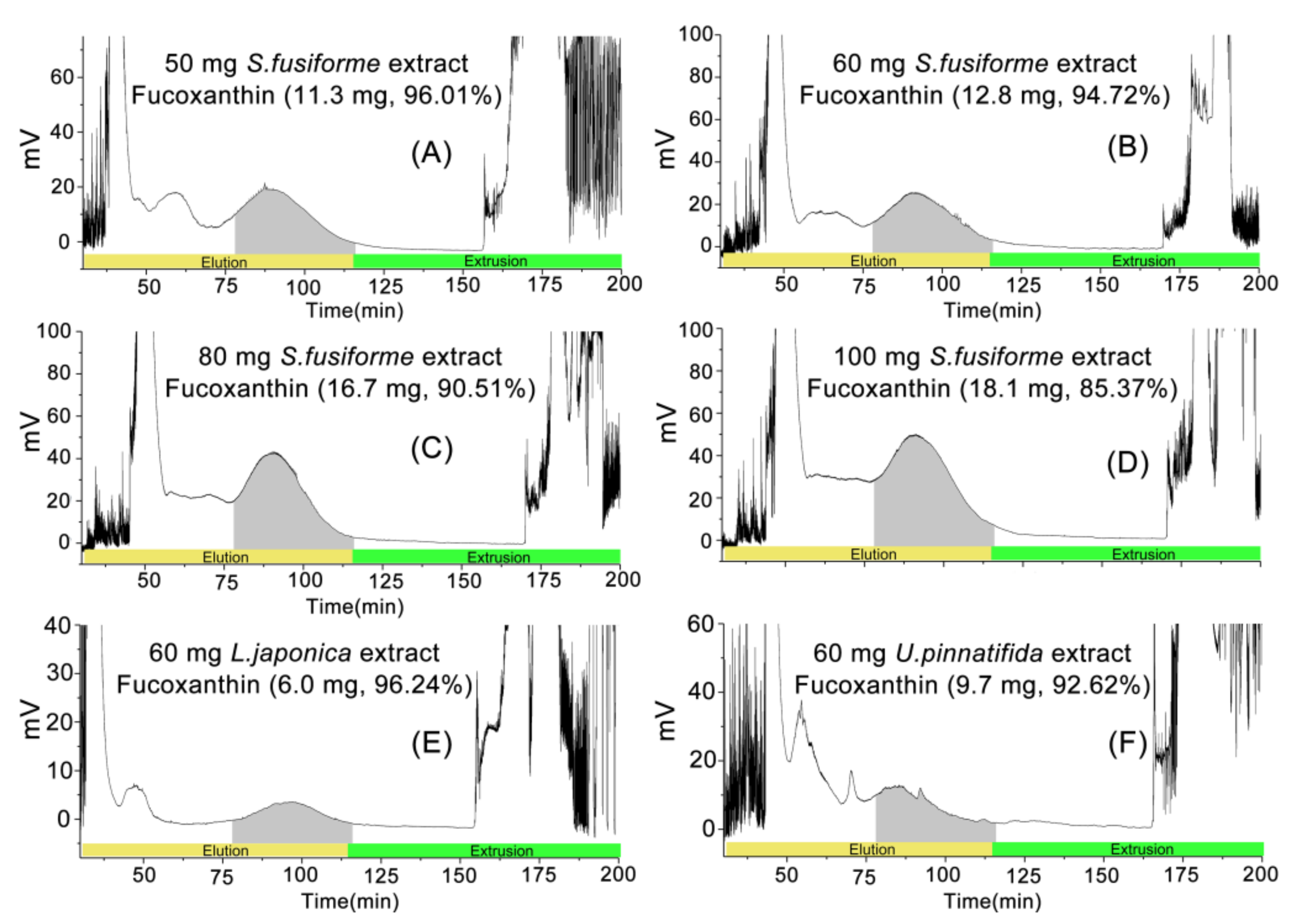
2.3. Elution-Extrusion Countercurrent Chromatography Separation of Fucoxanthin
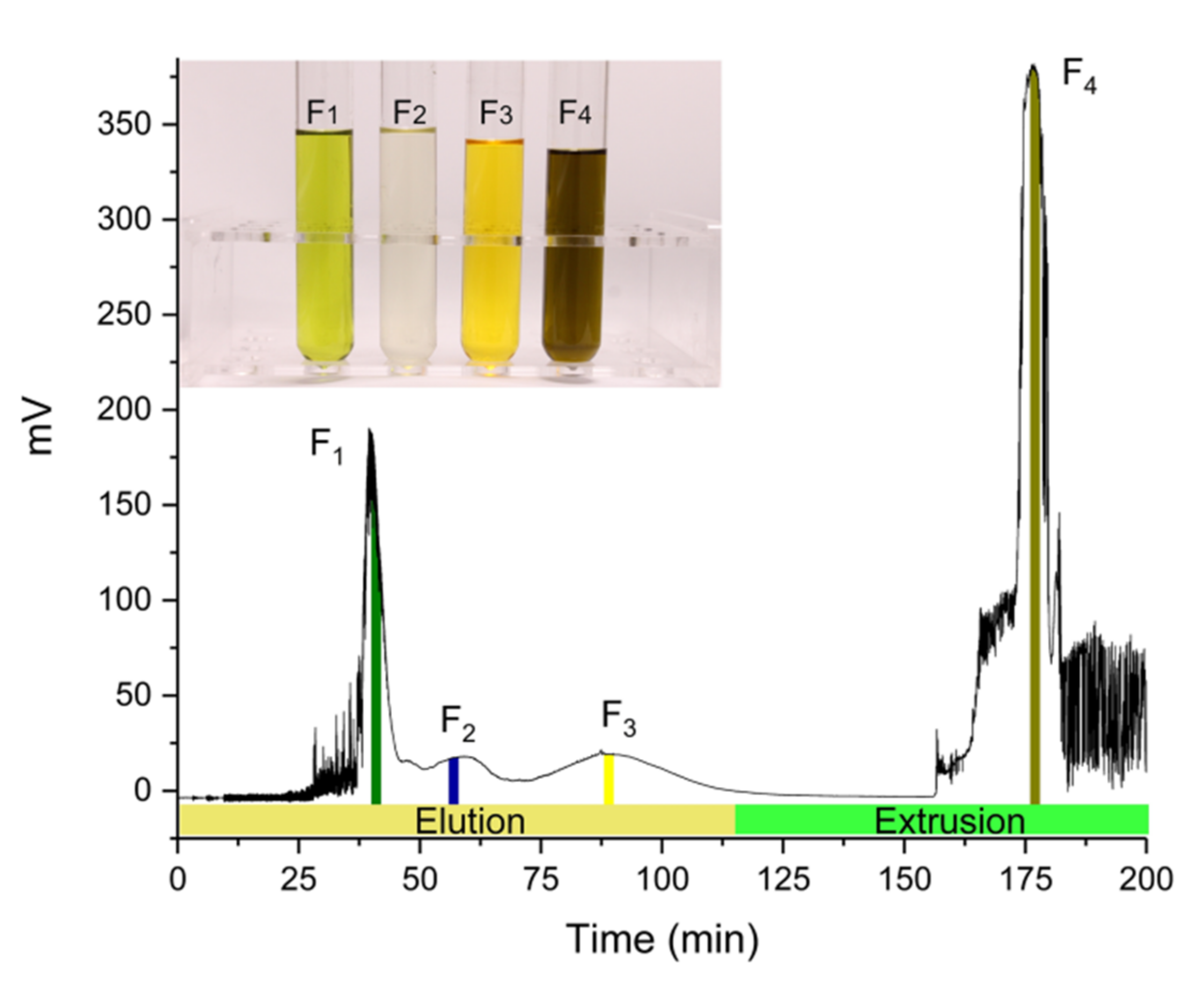
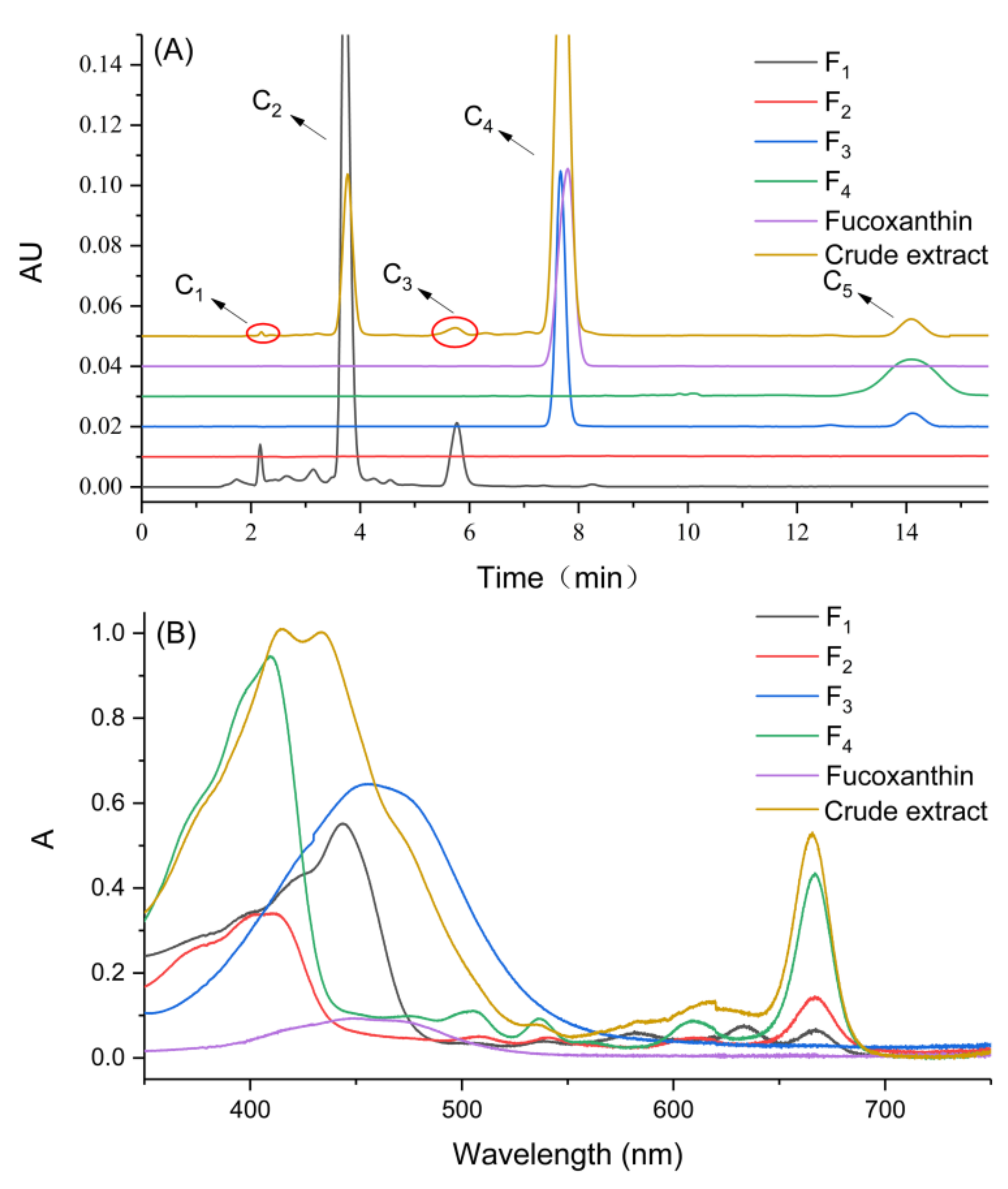
2.4. Analysis of HPLC and UV-Vis Full Scan
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Crude Extract
3.3. Quantitation of Fucoxanthin by High-Performance Liquid Chromatography
3.4. Selection of the Two-Phase Solvent System
3.5. Preparation of Elution Solvent and Sample Solution
3.6. Optimization of Elution-Extrusion Countercurrent Chromatography
3.7. Verification of the Optimal EECCC Method
3.8. Detection of Elution-Extrusion Countercurrent Chromatography Fractions
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Mestechkina, N.M.; Shcherbukhin, V.D. Sulfated polysaccharides and their anticoagulant activity: A review. Appl. Biochem. Microbiol. 2010, 46, 267–273. [Google Scholar] [CrossRef]
- Di Valentin, M.; Meneghin, E.; Orian, L.; Polimeno, A.; Buchel, C.; Salvadori, E.; Kay, C.W.; Carbonera, D. Triplet-triplet energy transfer in fucoxanthin-chlorophyll protein from diatom Cyclotella meneghiniana: Insights into the structure of the complex. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, X.; Huang, C.; Zhang, X.; Lyu, Y. Lutein content in petals and leaves of marigold and analysis of lutein synthesis gene expression. Acta Physiol. Plant. 2019, 41, 128–138. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.Y.; Narasimhan, A.L.; Kim, S.; Oh, Y.K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124. [Google Scholar] [CrossRef]
- Migkos, M.P.; Kaltsonoudis, E.; Pelechas, E.; Drossou, V.; Karagianni, P.G.; Kavvadias, A.; Voulgari, P.V.; Drosos, A.A. Use of conventional synthetic and biologic disease-modifying anti-rheumatic drugs in patients with rheumatic diseases contracting COVID-19: A single-center experience. Rheumatol. Int. 2021, 41, 903–909. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Shu, G.; Li, J. Antihypertensive potential of plant foods: Research progress and prospect of plant-derived angiotensin-converting enzyme inhibition compounds. J. Agric. Food Chem. 2021, 69, 5297–5305. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and its modified formulations on inflammatory bowel disease (IBD): The story so far and future outlook. Pharmaceutics 2021, 13, 484–521. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive metabolites from marine algae as potent pharmacophores against oxidative stress-associated human diseases: A comprehensive review. Molecules 2021, 26, 37–61. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Tsai, C.-H.; Chen, H.-Y.; Wang, K.-L.; Chang, H.-Y.; Huang, Y.-J.; Hong, Y.-H.; Ali, M.; Shieh, T.-M.; Huang, T.-C.; et al. Protective effects of fucoxanthin on hydrogen peroxide-induced calcification of heart valve interstitial cells. Mar. Drugs 2021, 19, 307–319. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Lee, A.-S.; Lin, C.-H.; Tseng, K.-W. Cytoprotective potential of fucoxanthin in oxidative stress-induced age-related macular degeneration and retinal pigment epithelial cell senescence in vivo and in vitro. Mar. Drugs 2021, 19, 114–128. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lim, Y.-P.; Hu, M.-L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-hepatocellular carcinoma (HepG2) activities of monoterpene hydroxy lactones isolated from the marine microalga Tisochrysis lutea. Mar. Drugs 2020, 18, 567–576. [Google Scholar] [CrossRef]
- Kanno, K.Y.F.; Karp, S.G.; Rodrigues, C.; de Andrade Tanobe, V.O.; Soccol, C.R.; da Costa Cardoso, L.A. Influence of organic solvents in the extraction and purification of torularhodin from Sporobolomyces ruberrimus. Biotechnol. Lett. 2021, 43, 89–98. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Prabhu, A.; Abdul, K.S.; Rekha, P.-D. Isolation and purification of lutein from Indian spinach Basella alba. Res. J. Pharm. Technol. 2015, 8, 1379–1382. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Bharathiaraja, S.; Mahendran, S. Extraction, partially purification and study on antioxidant property of fucoxanthin from Sargassum cinereum J. Agardh. J. Chem. Pharm. Res. 2016, 8, 610–616. [Google Scholar]
- Zhang, S.; Li, L.; Cui, Y.; Luo, L.; Li, Y.; Zhou, P.; Sun, B. Preparative high-speed counter-current chromatography separation of grape seed proanthocyanidins according to degree of polymerization. Food Chem. 2017, 219, 399–407. [Google Scholar] [CrossRef]
- Du, X.; Dong, C.; Wang, K.; Jiang, Z.; Chen, Y.; Yang, Y.; Chen, F.; Ni, H. Separation and purification of astaxanthin from Phaffia rhodozyma by preparative high-speed counter-current chromatography. J. Chromatogr. B 2016, 1029–1030, 191–197. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, T.Y.; Xu, G.Q.; Ito, Y. Application of analytical and preparative high-speed counter-current chromatography for separation of lycopene from crude extract of tomato paste. J. Chromatogr. A 2001, 929, 169–173. [Google Scholar] [CrossRef]
- Zou, H.; Ma, Y.; Xu, Z.; Liao, X.; Chen, A.; Yang, S. Isolation of strawberry anthocyanins using high-speed counter-current chromatography and the copigmentation with catechin or epicatechin by high pressure processing. Food Chem. 2018, 247, 81–88. [Google Scholar] [CrossRef]
- Englert, M.; Hammann, S.; Vetter, W. Isolation of β-carotene, α-carotene and lutein from carrots by countercurrent chromatography with the solvent system modifier benzotrifluoride. J. Chromatogr. A 2015, 1388, 119–125. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Berthod, A.; Friesen, J.B.; Inui, T.; Pauli, G.F. Elution-extrusion countercurrent chromatography: Theory and concepts in metabolic analysis. Anal. Chem. 2007, 79, 3371–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zeng, H.; Jiang, S.; Zhang, L.; Yang, F.; Chen, X.; Yang, H. Separation of polyphenols from leaves of Malus hupehensis (Pamp.) Rehder by off-line two-dimensional high speed counter-current chromatography combined with recycling elution mode. Food Chem. 2015, 186, 139–145. [Google Scholar] [CrossRef]
- Shi, S.; Ma, Y.; Zhang, Y.; Liu, L.; Liu, Q.; Peng, M.; Xiong, X. Systematic separation and purification of 18 antioxidants from Pueraria lobata flower using HSCCC target-guided by DPPH–HPLC experiment. Sep. Purif. Technol. 2012, 89, 225–233. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Suo, H.; Zhang, S.; Sun, B. Separation of a family of antioxidants flavan-3-ol thio-conjugates from procyanidins by high-speed counter-current chromatography. Eur. Food Res. Technol. 2020, 246, 1017–1029. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Cui, Y.; Li, Y.; Luo, L.; Zhou, P.; Sun, B. Preparative separation of cacao bean procyanidins by high-speed counter-current chromatography. J. Chromatogr. B 2016, 1036–1037, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nagao, R.; Tomo, T.; Noguchi, E.; Suzuki, T.; Okumura, A.; Narikawa, R.; Enami, I.; Ikeuchi, M. Proteases are associated with a minor fucoxanthin chlorophyll a/c-binding protein from the diatom, Chaetoceros gracilis. Biochim. Biophys. Acta 2012, 1817, 2110–2117. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Haq, A.; Murkovic, M. Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (chicory) leaves. Eur. Food Res. Technol. 2018, 245, 365–374. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Chemat, F.; Abert-Vian, M. Ultrasonic food processing. In Alternatives to Conventional Food Processing; Proctor, A., Ed.; The Royal Society of Chemistry: London, UK, 2011; Chapter 10; pp. 387–414. ISBN 978-1-84973-037-2. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Ultrasound-assisted extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; Chapter 3; pp. 89–112. ISBN 978-1-84973-606-0. [Google Scholar]
- Shannon, E.; Abu-Ghannam, N. Enzymatic extraction of fucoxanthin from brown seaweeds. Int. J. Food Sci. Technol. 2018, 53, 2195–2204. [Google Scholar] [CrossRef] [Green Version]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Wu, X.; Gao, X.; Liu, X.; Zhang, S.; Yang, H.; Zhu, X.; Song, H.; Li, F.; Chen, Q. Quality control of Psoralea corylifolia L. based on high-speed countercurrent chromatographic fingerprinting. Molecules 2020, 25, 279–293. [Google Scholar] [CrossRef] [Green Version]
| Solvent System | Volume Ratios | K |
|---|---|---|
| n-hexane-ethanol-water | 20:11:9 | 0.23 ± 0.09 |
| 20:10:10 | 0.65 ± 0.08 | |
| 20:9:11 | 1.74 ± 0.11 | |
| 20:8:12 | 2.28 ± 0.12 | |
| 20:7:13 | 3.61 ± 0.14 |
| Samples | Loading Capacity (mg/10 mL) | Yield (mg) | Purity (%) |
|---|---|---|---|
| S. fusiforme | 50 | 11.3 b,c,* | 96.01 ± 1.80 A ** |
| 60 | 12.8 b | 94.72 ± 1.47 A | |
| 80 | 16.7 a | 90.51 ± 1.53 C | |
| 100 | 18.1 a | 85.37 ± 1.63 D | |
| L. japonica | 60 | 6.0 d | 96.24 ± 1.58 A |
| U. pinnatifida | 60 | 9.7 c | 92.62 ± 1.77 B |
| Repeatability (RSD * %, N = 3) | Precision (RSD%, N = 6) | |
|---|---|---|
| Yield (mg) | 5.2 | 5.9 |
| Purity (%) | 1.6 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Jin, Y.; Hu, D.; Ye, J.; Lu, Y.; Dai, Z. One-Step Preparative Separation of Fucoxanthin from Three Edible Brown Algae by Elution-Extrusion Countercurrent Chromatography. Mar. Drugs 2022, 20, 257. https://doi.org/10.3390/md20040257
Chen D, Jin Y, Hu D, Ye J, Lu Y, Dai Z. One-Step Preparative Separation of Fucoxanthin from Three Edible Brown Algae by Elution-Extrusion Countercurrent Chromatography. Marine Drugs. 2022; 20(4):257. https://doi.org/10.3390/md20040257
Chicago/Turabian StyleChen, Danting, Yating Jin, Di Hu, Jing Ye, Yanbin Lu, and Zhiyuan Dai. 2022. "One-Step Preparative Separation of Fucoxanthin from Three Edible Brown Algae by Elution-Extrusion Countercurrent Chromatography" Marine Drugs 20, no. 4: 257. https://doi.org/10.3390/md20040257
APA StyleChen, D., Jin, Y., Hu, D., Ye, J., Lu, Y., & Dai, Z. (2022). One-Step Preparative Separation of Fucoxanthin from Three Edible Brown Algae by Elution-Extrusion Countercurrent Chromatography. Marine Drugs, 20(4), 257. https://doi.org/10.3390/md20040257







