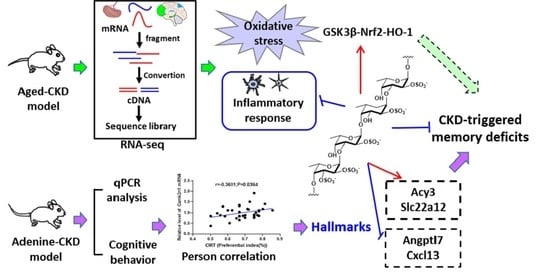
The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction
Abstract
1. Introduction
2. Results
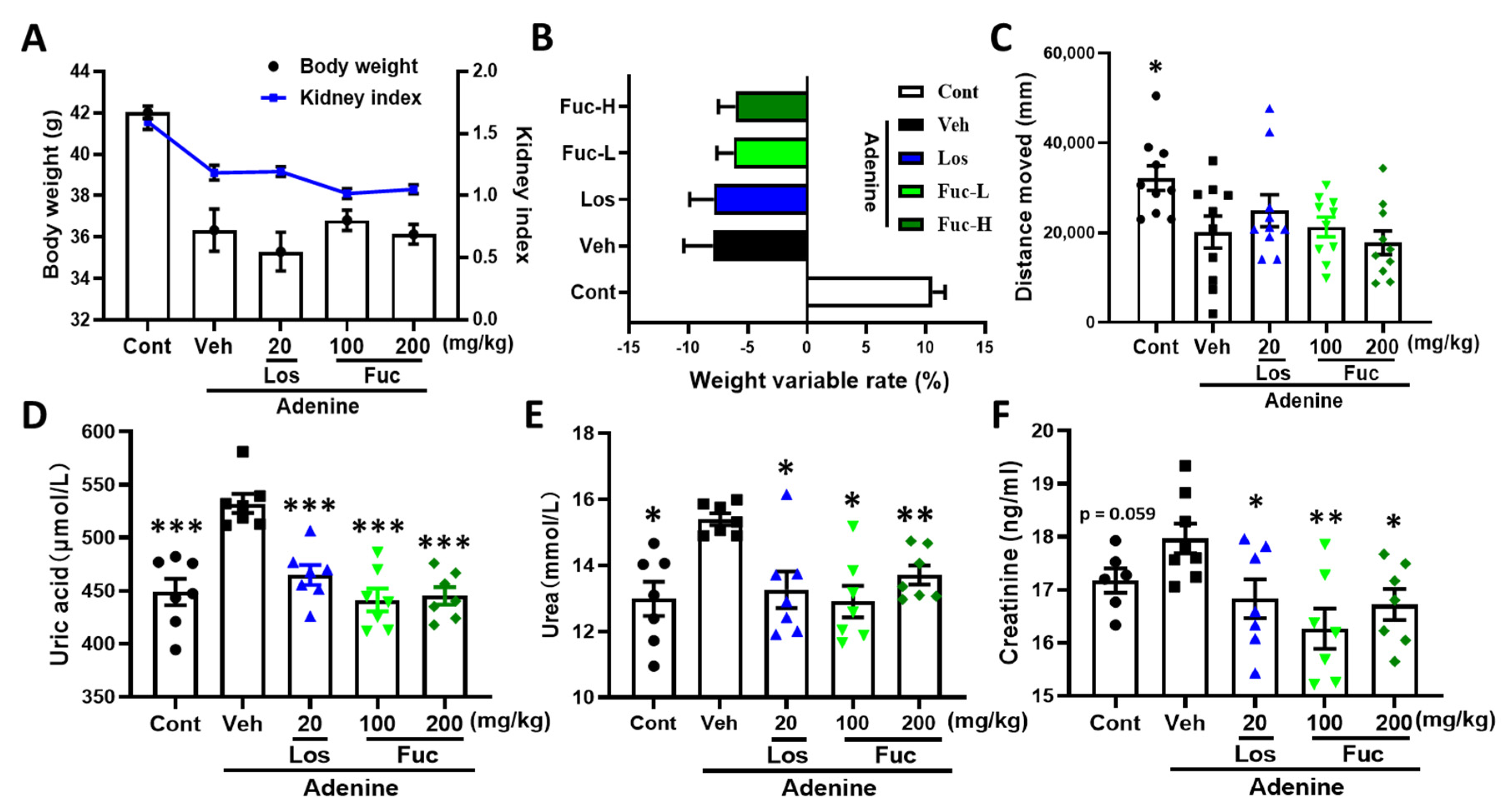
2.1. Fucoidan Ameliorated Renal Function in Adenine-Induced CKD Mice
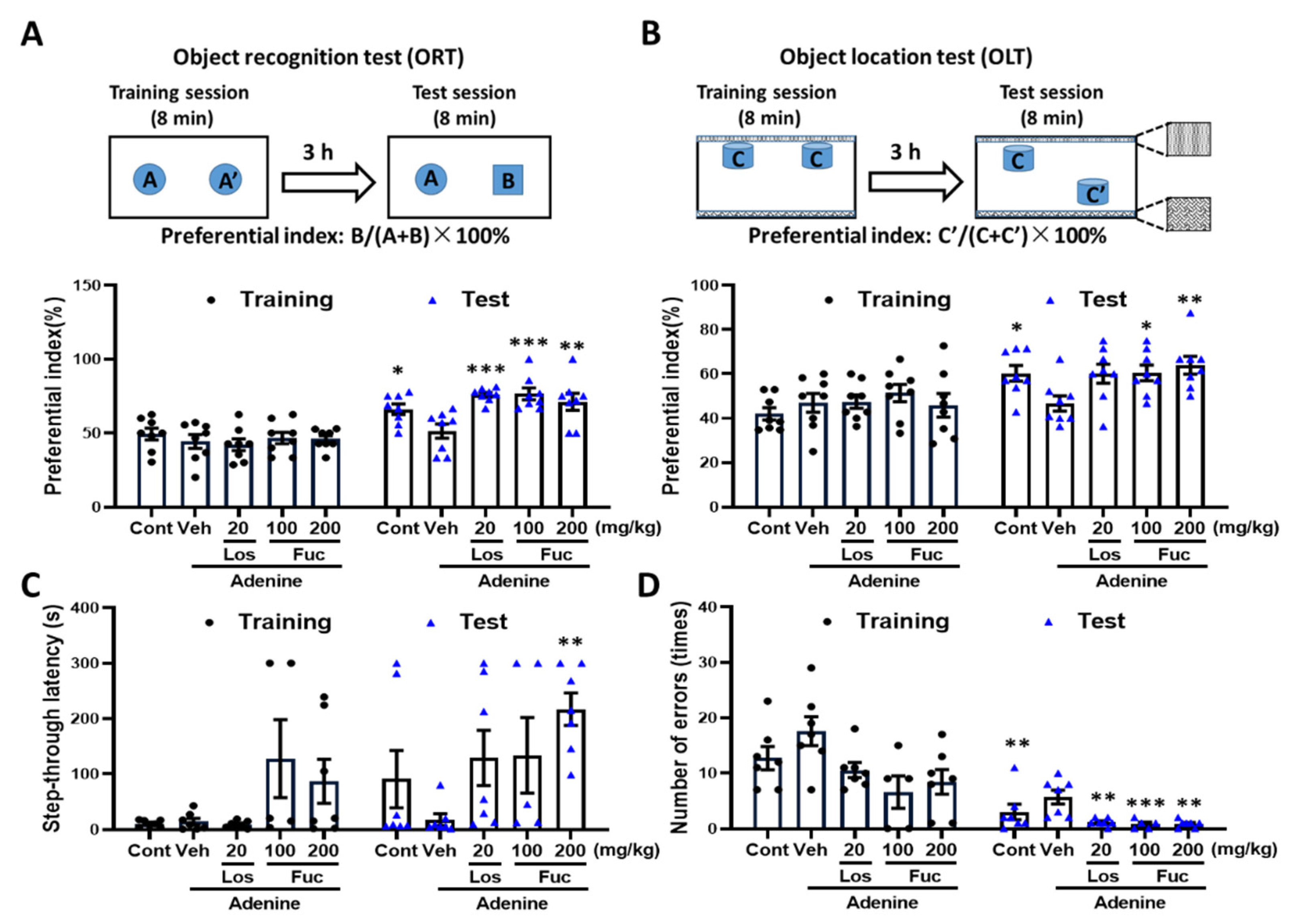
2.2. Fucoidan Ameliorated Cognitive Deficits in Adenine-Induced CKD Mice
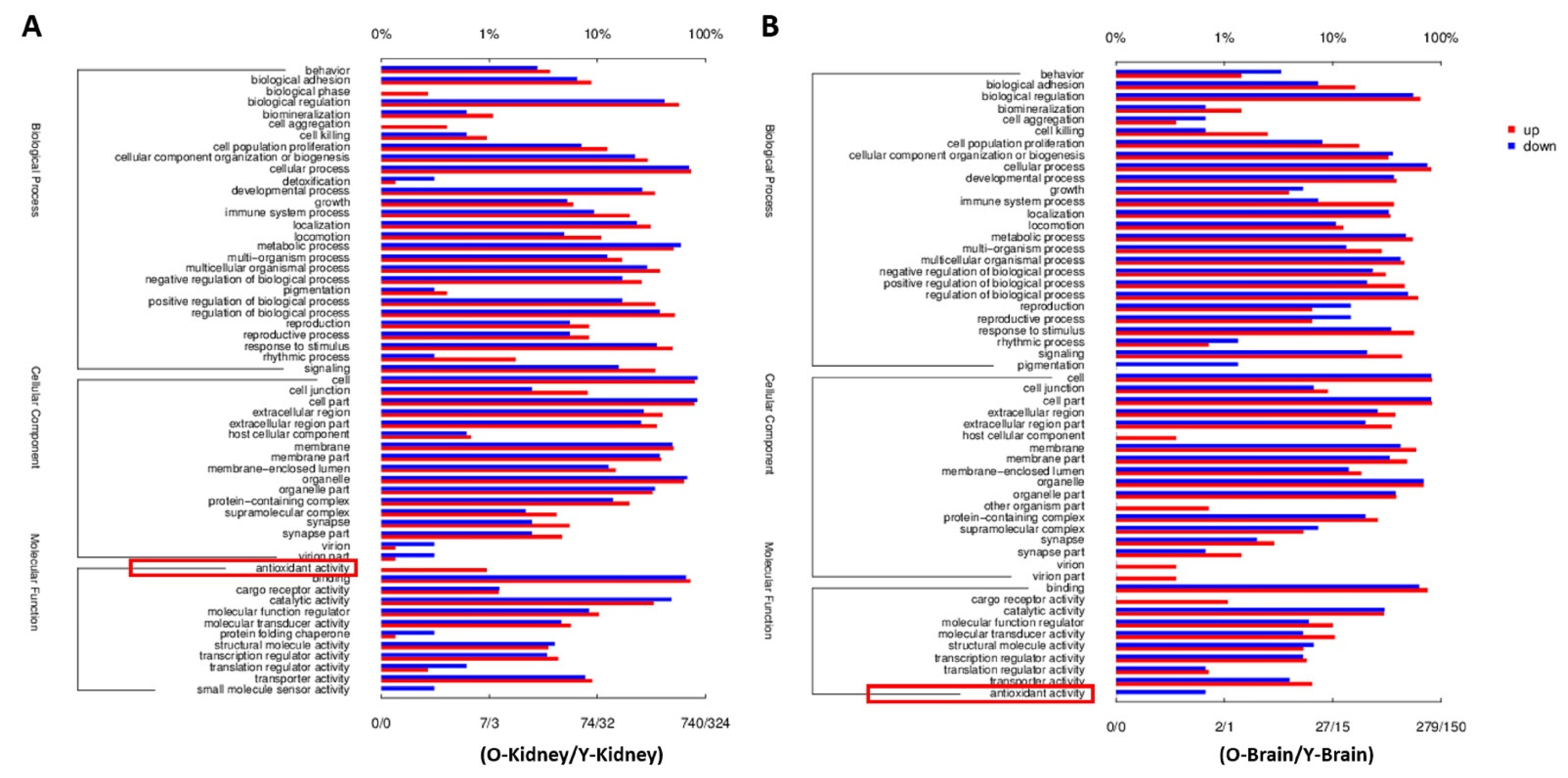
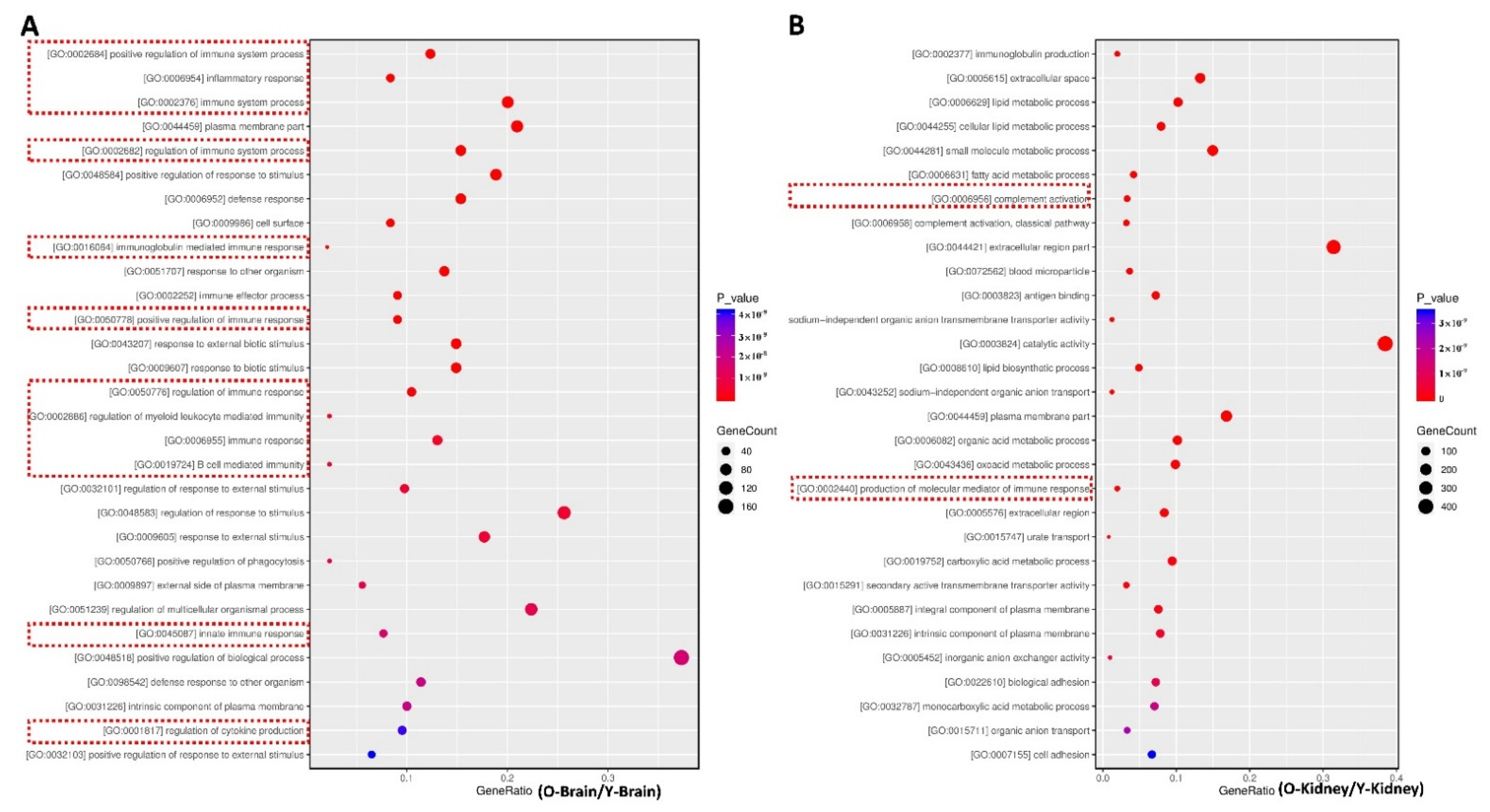
2.3. Fucoidan Regulated Brain and Kidney Genes in Aging Renal Failure Mice
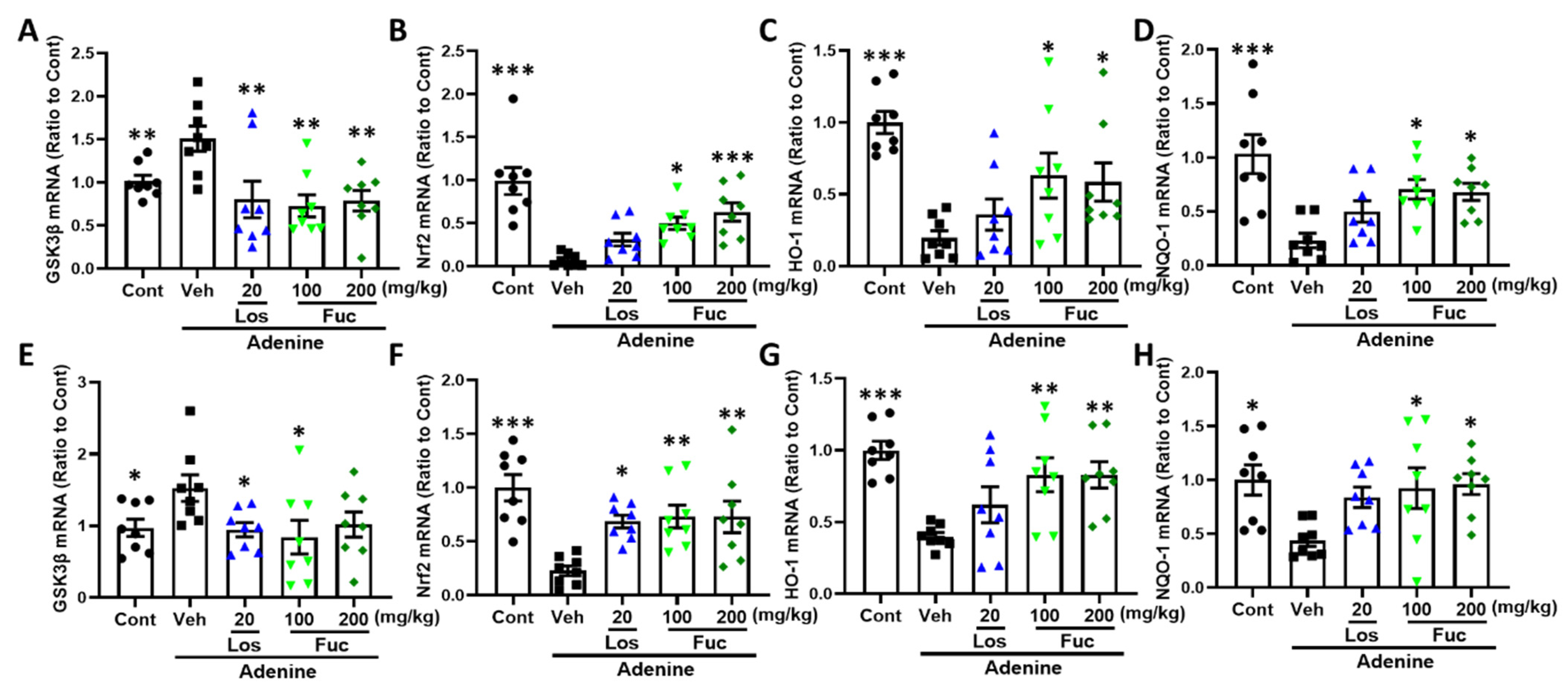
2.4. Fucoidan Ameliorated Oxidative Stress Via Nrf2-HO-1 Signaling Pathway in Adenine-Induced CKD Mice
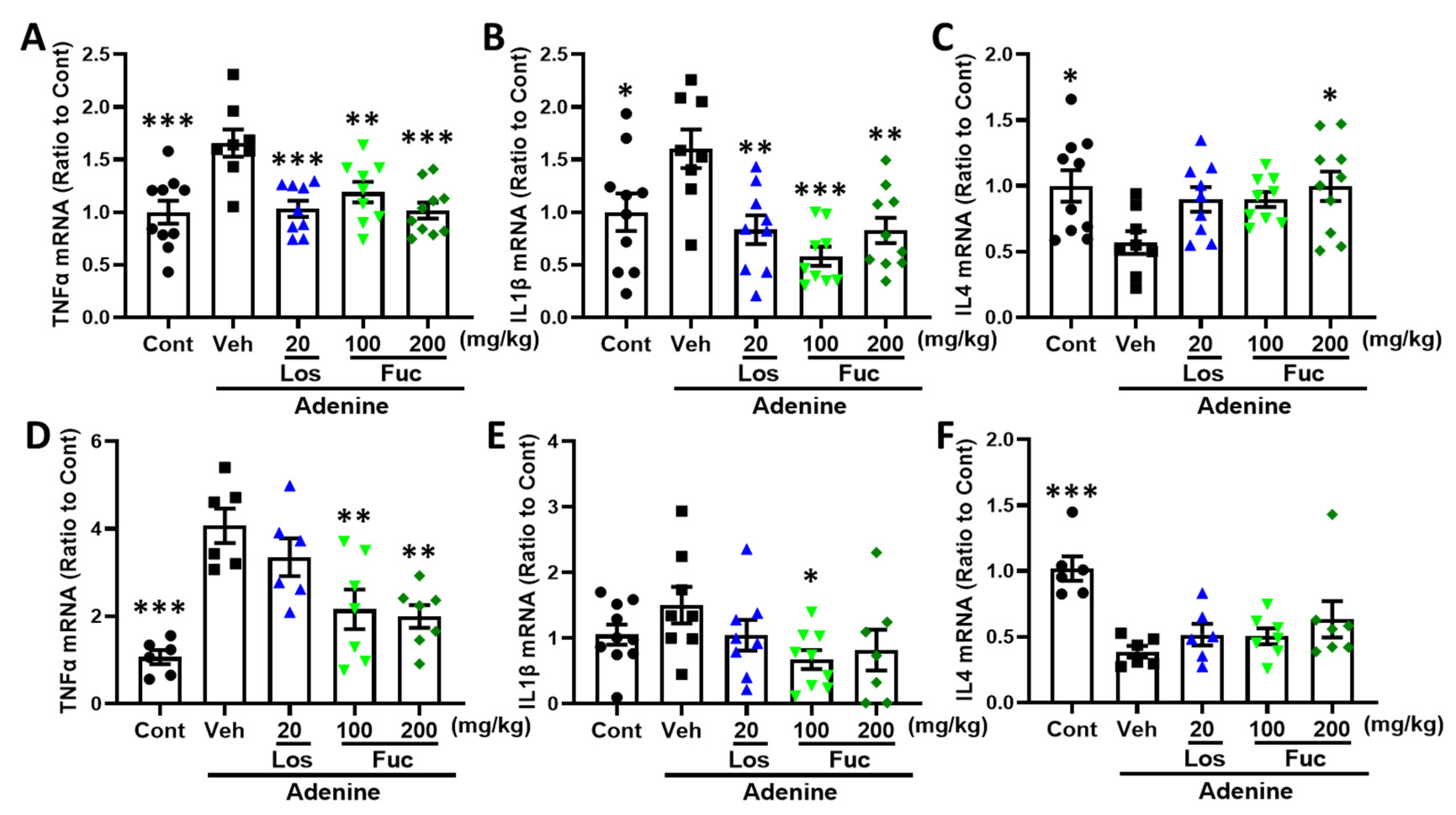
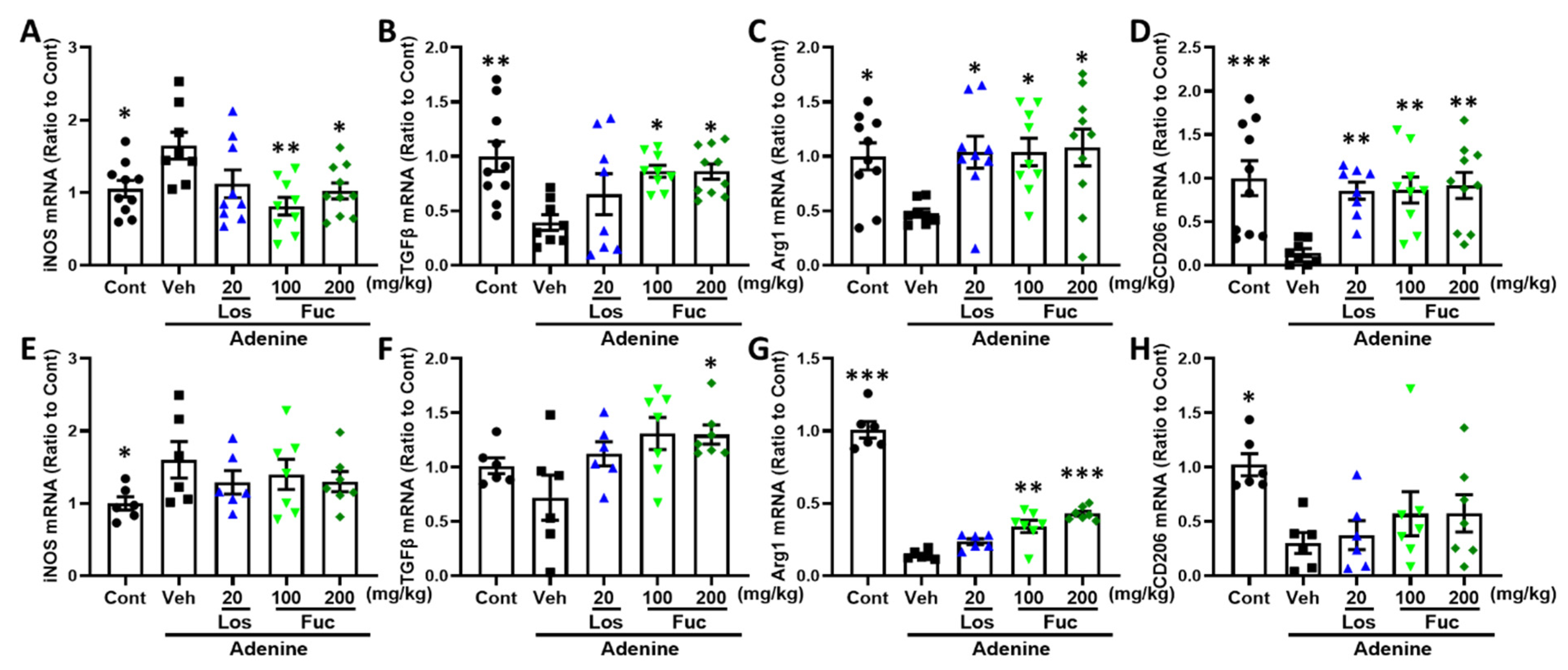
2.5. Fucoidan Inhibited Inflammatory Response in Adenine-Induced CKD Mice
2.6. Fucoidan Attenuated Cognitive-Behavior-Related Hallmarks in Adenine-Induced CKD Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Procedure
4.3. Behavioral Test
4.4. Measurement of Urea, Uric Acid, and Creatinine
4.5. RNA-Seq Analysis
4.6. ELISA Analysis of MDA, SOD, and GSH-Px
4.7. RNA Isolation and RT-qPCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2012, 80, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef]
- McQuillan, R.; Jassal, S.V. Neuropsychiatric complications of chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Lin, C.C.; Tsai, C.F.; Yang, W.C.; Wang, S.J.; Lin, F.H.; Fuh, J.L. Cognitive impairment and hippocampal atrophy in chronic kidney disease. Acta Neurol. Scand. 2017, 136, 477–485. [Google Scholar] [CrossRef]
- Hooper, S.R.; Gerson, A.C.; Butler, R.W.; Gipson, D.S.; Mendley, S.R.; Lande, M.B.; Shinnar, S.; Wentz, A.; Matheson, M.; Cox, C. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1824–1830. [Google Scholar] [CrossRef]
- Elias, M.F.; Elias, P.K.; Seliger, S.L.; Narsipur, S.S.; Dore, G.A.; Robbins, M.A. Chronic kidney disease, creatinine and cognitive functioning. Nephrol. Dial. Transplant. 2009, 24, 2446–2452. [Google Scholar] [CrossRef]
- Seliger, S.L.; Weiner, D.E. Cognitive impairment in dialysis patients: Focus on the blood vessels? Am. J. Kidney Dis. 2013, 61, 187–190. [Google Scholar] [CrossRef][Green Version]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods. 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Souza, B.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, Z.; Zhang, Y.; Song, C.; Hu, X. Protective effect of fucoidan on Aβ25-35-induced neuronal injury and neurite atrophy. J. Guangdong Ocean. U. 2021, 41, 77–83. [Google Scholar]
- Zhang, D.; Liu, H.; Luo, P.; Li, Y. Production inhibition and excretion promotion of urate by fucoidan from Laminaria japonica in adenine-induced hyperuricemic mice. Mar. Drugs 2018, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Chong, F.L.; Yuan, C.C.; Liu, Y.L.; Yang, H.M.; Wang, W.W.; Fang, Q.J.; Wu, W.; Wang, M.Z.; Tu, Y.; et al. Fucoidan ameliorates renal injury-related calcium-phosphorus metabolic disorder and bone abnormality in the CKD-MBD model rats by targeting FGF23-klotho signaling axis. Front. Pharmacol. 2021, 11, 586725. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, W.; Zhang, Q.; Jia, Y.; Sun, Y.; Weng, L.; Luo, D.; Zhou, H.; Yang, B. Low molecular weight fucoidan ameliorates diabetic nephropathy via inhibiting epithelial-mesenchymal transition and fibrotic processes. Am. J. Transl. Res. 2015, 7, 1553–1563. [Google Scholar] [PubMed]
- Wang, J.; Liu, H.; Li, N.; Zhang, Q.; Zhang, H. The protective effect of fucoidan in rats with streptozotocin-induced diabetic nephropathy. Mar. Drugs 2014, 12, 3292–3306. [Google Scholar] [CrossRef]
- Jia, T.; Olauson, H.; Lindberg, K.; Amin, R.; Edvardsson, K.; Lindholm, B.; Andersson, G.; Wernerson, A.; Sabbagh, Y.; Schiavi, S.; et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013, 14, 116. [Google Scholar] [CrossRef]
- Han, B.; Gong, M.; Li, Z.; Qiu, Y.; Zou, Z. NMR-based metabonomic study reveals intervention effects of polydatin on potassium oxonate-induced hyperuricemia in rats. Oxid. Med. Cell Longev. 2020, 2020, 6943860. [Google Scholar] [CrossRef]
- Huang, Z.; He, L.; Huang, D.; Lei, S.; Gao, J. Icariin protects rats against 5/6 nephrectomy-induced chronic kidney failure by increasing the number of renal stem cells. BMC Complement. Altern. Med. 2015, 15, 378. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, C.; Li, X.H.; Ma, Z.H.; Ge, Y.W.; Qian, Z.J.; Song, C. Heterophyllin B, a cyclopeptide from Pseudostellaria heterophylla, enhances cognitive function via neurite outgrowth and synaptic plasticity. Phytother. Res. 2021, 35, 5318–5329. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Kim, E.H.; Hannan, M.A.; Ha, H. Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting Nrf2-HO-1 signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Kiernan, M.C.; Murray, A.; Rosner, M.H.; Ronco, C. Kidney-brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 2015, 11, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Okamoto, S.I.; Cui, J.; Watanabe, Y.; Furuta, K.; Suzuki, M.; Tohyama, K.; Lipton, S.A. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc. Natl. Acad. Sci. USA 2006, 103, 768–773. [Google Scholar] [CrossRef]
- Duan, Q.; Sun, W.; Yuan, H.; Mu, X. MicroRNA-135b-5p prevents oxygen-glucose deprivation and reoxygenation-induced neuronal injury through regulation of the GSK-3β/Nrf2/ARE signaling pathway. Arch. Med. Sci. 2018, 14, 735–744. [Google Scholar] [CrossRef]
- Yang, Z.; Kuboyama, T.; Tohda, C. Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytother. Res. 2019, 33, 1114–1121. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kuo, Y.T.; Li, C.Y.; Sung, J.M.; Chang, C.C.; Wang, J.D.; Sun, C.Y.; Wu, J.L.; Chang, Y.T. Risk of dementia in patients with end-stage renal disease under maintenance dialysis-a nationwide population-based study with consideration of competing risk of mortality. Alzheimers Res. Ther. 2019, 11, 31. [Google Scholar] [CrossRef]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Ramu, S.; Murali, A.; Narasimhaiah, G.; Jayaraman, A. Toxicological evaluation of Sargassum Wightii greville derived fucoidan in wistar rats: Haematological, biochemical and histopathological evidences. Toxicol. Rep. 2020, 7, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.; Li, K.L.; Lin, Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Gideon, T.P.; Rengasamy, R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J. Med. Food 2008, 11, 638–642. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Drews, H.J.; Klein, R.; Lourhmati, A.; Buadze, M.; Schaeffeler, E.; Lang, T.; Seferyan, T.; Hanson, L.R.; Frey Ii, W.H.; de Vries, T.C.G.M.; et al. Losartan improves memory, neurogenesis and cell motility in transgenic Alzheimer′s mice. Pharmaceuticals 2021, 14, 166. [Google Scholar] [CrossRef]
- Fogari, R.; Mugellini, A.; Zoppi, A.; Derosa, G.; Pasotti, C.; Fogari, E.; Preti, P. Influence of losartan and atenolol on memory function in very elderly hypertensive patients. J. Hum. Hypertens. 2003, 17, 781–785. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Xia, Y.; Yu, G.; Zeng, K.; Luo, H.; Hu, J.; Gong, C.X.; Wang, J.Z.; Zhou, X.W.; et al. Losartan-induced hypotension leads to tau hyperphosphorylation and memory deficit. J. Alzheimers Dis. 2014, 40, 419–427. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.B.; Jin, W.H.; Niu, X.Z.; Zhang, H. Effects and mechanism of low molecular weight fucoidan in mitigating the peroxidative and renal damage induced by adenine. Carbohydr. Polym. 2011, 84, 417–423. [Google Scholar] [CrossRef]
- Dong, H.; Xue, T.; Liu, Y.; He, S.; Yi, Y.; Zhang, B.; Xin, J.; Wang, Z.; Li, X. Low molecular weight fucoidan inhibits pulmonary fibrosis in vivo and in vitro via antioxidant activity. Oxid. Med. Cell Longev. 2022, 2022, 7038834. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Chung, H.S. Fucoidan reduces oxidative stress by regulating the gene expression of HO-1 and SOD-1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol. Med. Rep. 2016, 14, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of Fucoidan from the brown seaweed Fucus vesiculosus L. of the barents sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, C.H.; Li, B.F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2008, 20, 431–436. [Google Scholar] [CrossRef]
- Qi, H.M.; Zhao, T.T.; Zhang, Q.B.; Li, Z.E.; Zhao, Z.Q.; Xing, R.E. Antioxidant activity of different molecular weight sulfated polysaccharides from ulva pertusa kjellm (chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Jin, W.H.; Zhang, H.; Zhang, Q.B. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, S.H.; Huang, C.Y.; Dong, C.D.; Huang, C.Y.; Chang, C.C.; Chang, J.S. Effect of molecular mass and sulfate content of fucoidan from Sargassum siliquosum on antioxidant, anti-lipogenesis, and anti-inflammatory activity. J. Biosci. Bioeng. 2021, 132, 359–364. [Google Scholar] [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-inflammatory activity of fucoidan extracts in vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef]
- Vigil, F.A.; Mizuno, K.; Lucchesi, W.; Valls-Comamala, V.; Giese, K.P. Prevention of long-term memory loss after retrieval by an endogenous CaMKII inhibitor. Sci. Rep. 2017, 7, 4040. [Google Scholar] [CrossRef]
- Yu, Y.L.; Lv, J.Z.; Ma, D.; Han, Y.; Wang, Z.T. Microglial ApoD induced NLRC4 inflammasome activation promotes Alzheimer′s disease progression. Res. Sq. 2021. Preprint. [Google Scholar]
- Wang, E.; Zhu, H.; Wang, X.; Gower, A.C.; Wallack, M.; Blusztajn, J.K.; Kowall, N.; Qiu, W.Q. Amylin treatment reduces neuroinflammation and ameliorates abnormal patterns of gene expression in the cerebral cortex of an Alzheimer′s disease mouse model. J. Alzheimers Dis. 2017, 56, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, A.; Kamada, M.; Kawamura, Y.; Terao, C.; Shimoda, F.; Tomita, N.; Arai, H.; Furukawa, K. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer′s disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J. Neurochem. 2016, 136, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Halbgebauer, S.; Anderl-Straub, S.; Steinacker, P.; Huss, A.M.; Neugebauer, H.; von Arnim, C.A.F.; Diehl-Schmid, J.; Grimmer, T.; Kornhuber, J. Glial fibrillary acidic protein in serum is increased in Alzheimer′s disease and correlates with cognitive impairment. J. Alzheimers Dis. 2019, 67, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, A.M.; Alberghina, L.; Papa, M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci. Lett. 2014, 565, 59–64. [Google Scholar] [CrossRef]
- Licastro, F.; Chiappelli, M.; Grimaldi, L.M.; Morgan, K.; Kalsheker, N.; Calabrese, E.; Ritchie, A.; Porcellini, E.; Salani, G.; Franceschi, M.; et al. A new promoter polymorphism in the alpha-1-antichymotrypsin gene is a disease modifier of Alzheimer′s disease. Neurobiol. Aging 2005, 26, 449–453. [Google Scholar] [CrossRef]
- Rentsendorj, A.; Sheyn, J.; Fuchs, D.T.; Daley, D.; Salumbides, B.C.; Schubloom, H.E.; Hart, N.J.; Li, S.; Hayden, E.Y.; Teplow, D.B.; et al. A novel role for osteopontin in macrophage-mediated amyloid-β clearance in Alzheimer′s models. Brain Behav. Immun. 2018, 67, 163–180. [Google Scholar] [CrossRef]
- Lisignoli, G.; Toneguzzi, S.; Piacentini, A.; Cristino, S.; Grassi, F.; Cavallo, C.; Facchini, A. CXCL12 (SDF-1) and CXCL13 (BCA-1) chemokines significantly induce proliferation and collagen type I expression in osteoblasts from osteoarthritis patients. J. Cell. Physiol. 2006, 206, 78–85. [Google Scholar] [CrossRef]
- Qian, T.; Wang, K.; Cui, J.S.; He, Y.D.; Yang, Z.Q. Angiopoietin-like protein 7 promotes an inflammatory phenotype in RAW264.7 macrophages through the P38 MAPK signaling pathway. Inflammation 2016, 39, 974–985. [Google Scholar] [CrossRef]
- Hosoyamada, M.; Ichida, K.; Enomoto, A.; Hosoya, T.; Endou, H. Function and localization of urate transporter 1 in mouse kidney. J. Am. Soc. Nephrol. 2004, 15, 261–268. [Google Scholar] [CrossRef]
- Pushkin, A.; Carpenito, G.; Abuladze, N.; Newman, D.; Tsuprun, V.; Ryazantsev, S.; Motemoturu, S.; Sassani, P.; Solovieva, N.; Dukkipati, R.; et al. Structural characterization, tissue distribution, and functional expression of murine aminoacylase III. Am. J. Physiol. Cell Physiol. 2004, 286, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Karaca, T.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Lung oxidative stress, DNA damage, apoptosis, and fibrosis in adenine-induced chronic kidney disease in mice. Front. Physiol. 2017, 8, 896. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid β production/Tau hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Yang, Z.; Feng, X.; Deng, J.; He, C.; Li, R.; Zhao, Y.; Ge, Y.; Zhang, Y.; Song, C.; et al. The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction. Mar. Drugs 2022, 20, 258. https://doi.org/10.3390/md20040258
Ma Z, Yang Z, Feng X, Deng J, He C, Li R, Zhao Y, Ge Y, Zhang Y, Song C, et al. The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction. Marine Drugs. 2022; 20(4):258. https://doi.org/10.3390/md20040258
Chicago/Turabian StyleMa, Zhihui, Zhiyou Yang, Xinyue Feng, Jiahang Deng, Chuantong He, Rui Li, Yuntao Zhao, Yuewei Ge, Yongping Zhang, Cai Song, and et al. 2022. "The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction" Marine Drugs 20, no. 4: 258. https://doi.org/10.3390/md20040258
APA StyleMa, Z., Yang, Z., Feng, X., Deng, J., He, C., Li, R., Zhao, Y., Ge, Y., Zhang, Y., Song, C., & Zhong, S. (2022). The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction. Marine Drugs, 20(4), 258. https://doi.org/10.3390/md20040258