Characterization of an Unknown Region Linked to the Glycoside Hydrolase Family 17 β-1,3-Glucanase of Vibrio vulnificus Reveals a Novel Glucan-Binding Domain
Abstract
1. Introduction
2. Results
2.1. Bioinformatic Analysis of VvGH17
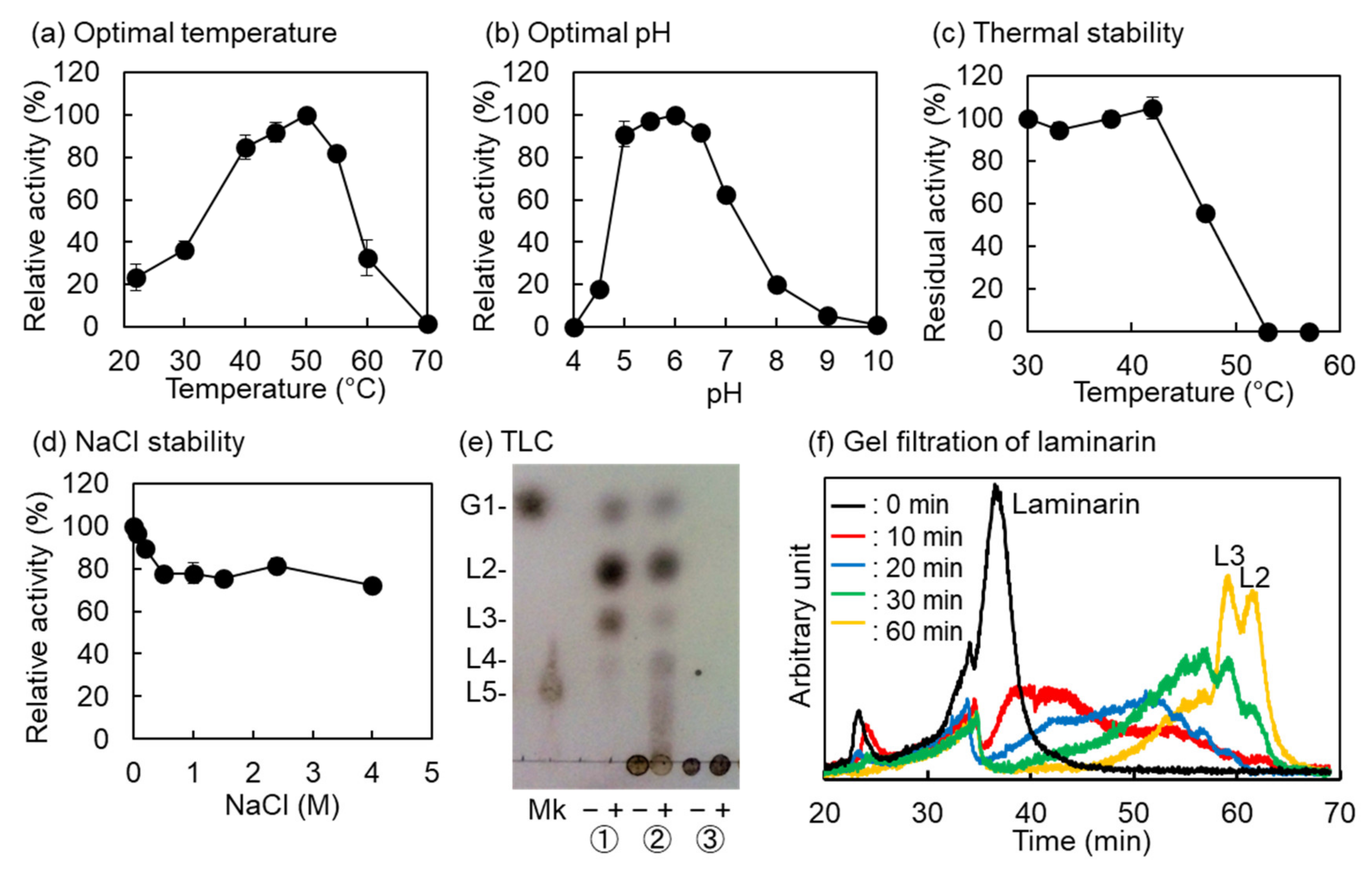
2.2. Biochemical Properties of VvGH17
2.3. C-terminal-Truncated Mutant
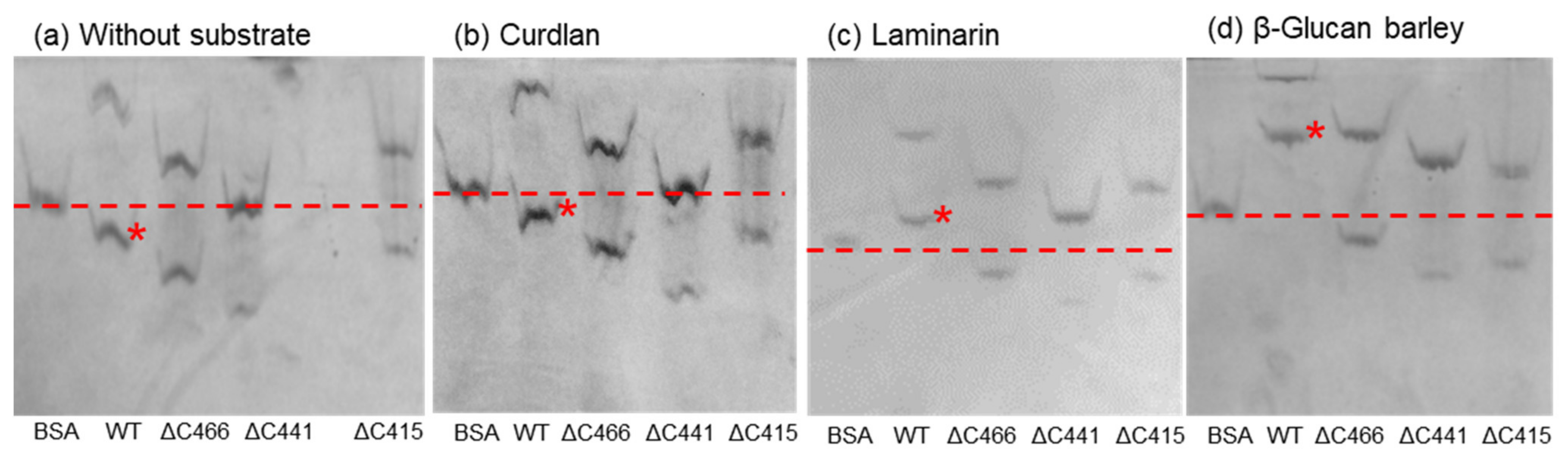
2.4. Affinity Gel Analysis of C-Terminal-Truncated Mutants of VvGH17
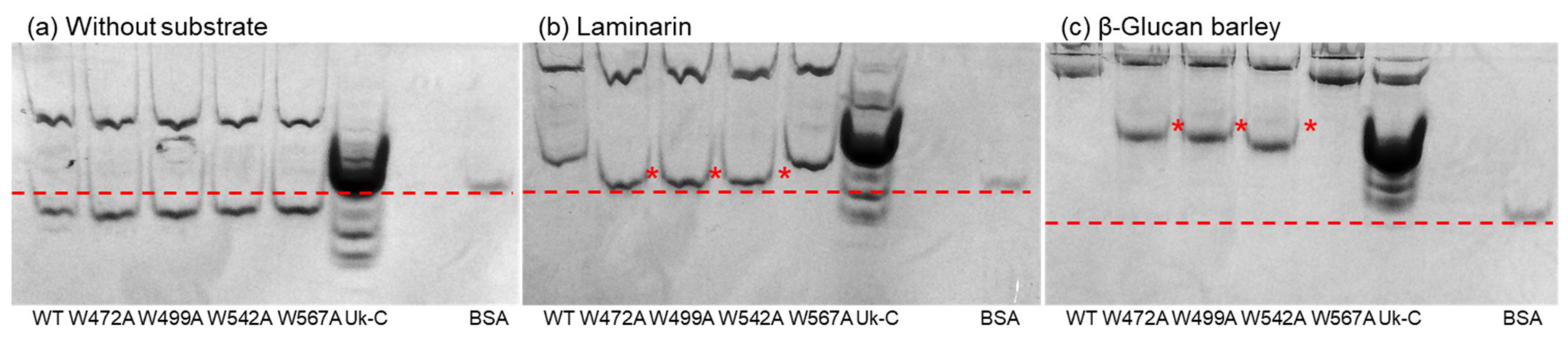
2.5. Affinity Gel Analysis of Uk-C and Point Mutants of VvGH17
2.6. Prediction of Uk-C Structure and Function
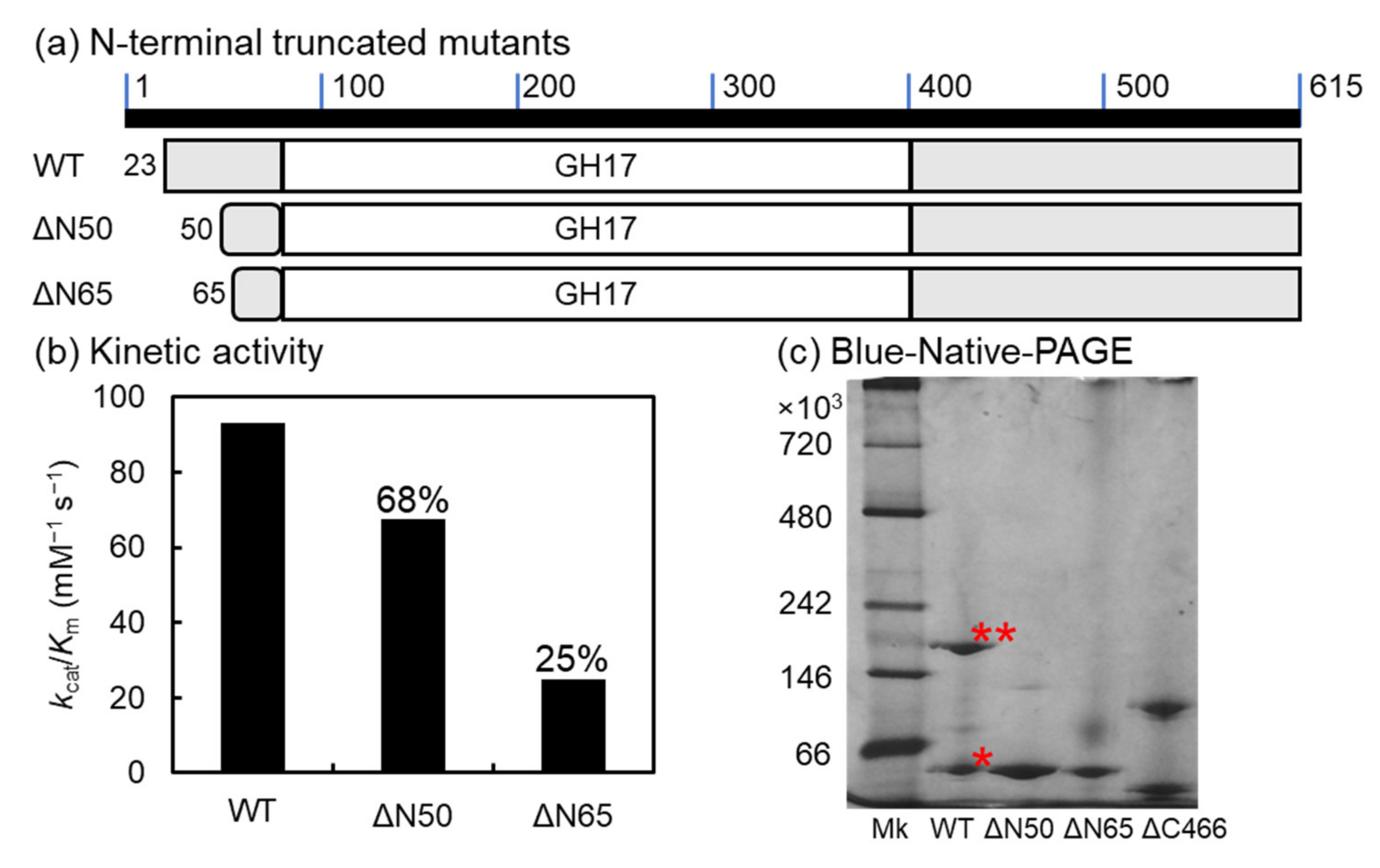
2.7. N-Terminal-Truncated Mutants of VvGH17
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bioinformatic Analysis of VvGH17
4.3. Construction, Expression, and Purification of VvGH17
4.4. Construction of VvGH17 Mutants
4.5. VvGH17 Standard Activity Assay
4.6. Analysis of Hydrolysis Products by TLC and Gel Filtration Chromatography
4.7. CD Spectroscopy
4.8. Polyacrylamide Gel Electrophoresis (PAGE) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef] [PubMed]
- Stefan, B.; Jan, T.; Sarah, C.; Karen, W.; Hvitfeldt, I.M.; Tilmann, H.; Kai-Uwe, H.; Jan-Hendrik, H. Laminarin is a major molecule in the marine carbon cycle. Proc. Natl. Acad. Sci. USA 2020, 117, 6599–6607. [Google Scholar] [CrossRef]
- Lim, H.G.; Kwak, D.H.; Park, S.; Woo, S.; Yang, J.-S.; Kang, C.W.; Kim, B.; Noh, M.H.; Seo, S.W.; Jung, G.Y. Vibrio sp. dhg as a platform for the biorefinery of brown macroalgae. Nat. Commun. 2019, 10, 2486. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Viborg, A.H.; Terrapon, N.; Lombard, V.; Michel, G.; Czjzek, M.; Henrissat, B.; Brumer, H. A subfamily roadmap of the evolutionarily diverse glycoside hydrolase family 16 (GH16). J. Biol. Chem. 2019, 294, 15973–15986. [Google Scholar] [CrossRef]
- Jian, Y.; Yuqun, X.; Takuya, M.; Lijuan, L.; Masaru, T.; Ning-Yi, Z. Molecular Basis for Substrate Recognition and Catalysis by a Marine Bacterial Laminarinase. Appl. Environ. Microbiol. 2022, 86, e01796-20. [Google Scholar] [CrossRef]
- Burkhardt, C.; Schäfers, C.; Claren, J.; Schirrmacher, G.; Antranikian, G. Comparative Analysis and Biochemical Characterization of Two Endo-β-1,3-Glucanases from the Thermophilic Bacterium Fervidobacterium sp. Catalysts 2019, 9, 830. [Google Scholar] [CrossRef]
- Liberato, M.V.; Teixeira Prates, E.; Gonçalves, T.A.; Bernardes, A.; Vilela, N.; Fattori, J.; Ematsu, G.C.; Chinaglia, M.; Machi Gomes, E.R.; Migliorini Figueira, A.C.; et al. Insights into the dual cleavage activity of the GH16 laminarinase enzyme class on β-1,3 and β-1,4 glycosidic bonds. J. Biol. Chem. 2021, 296, 100385. [Google Scholar] [CrossRef]
- Mitsuya, D.; Sugiyama, T.; Zhang, S.; Takeuchi, Y.; Okai, M.; Urano, N.; Ishida, M. Enzymatic properties and the gene structure of a cold-adapted laminarinase from Pseudoalteromonas species LA. J. Biosci. Bioeng. 2018, 126, 169–175. [Google Scholar] [CrossRef]
- Oda, M.; Inaba, S.; Kamiya, N.; Bekker, G.-J.; Mikami, B. Structural and thermodynamic characterization of endo-1,3-β-glucanase: Insights into the substrate recognition mechanism. Biochim. Biophys. Acta-Proteins Proteom. 2018, 1866, 415–425. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Lyu, Q. Biochemical Characterization of a Novel Endo-1,3-β-Glucanase from the Scallop Chlamys farreri. Mar. Drugs 2020, 18, 466. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Thakur, R.; Kumar, G. Human gut Bacteroides uniformis utilizes mixed linked β-glucans via an alternative strategy. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100282. [Google Scholar] [CrossRef]
- Unfried, F.; Becker, S.; Robb, C.S.; Hehemann, J.-H.; Markert, S.; Heiden, S.E.; Hinzke, T.; Becher, D.; Reintjes, G.; Krüger, K.; et al. Adaptive mechanisms that provide competitive advantages to marine bacteroidetes during microalgal blooms. ISME J. 2018, 12, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Xie, Z.-X.; Li, Y.-Y.; Chen, X.-H.; Sun, G.; Lin, L.; Giovannoni, S.J.; Wang, D.-Z. Polysaccharide utilization by a marine heterotrophic bacterium from the SAR92 clade. FEMS Microbiol. Ecol. 2021, 97, fiab120. [Google Scholar] [CrossRef]
- Déjean, G.; Tamura, K.; Cabrera, A.; Jain, N.; Pudio, N.A.; Pereira, G.; Viborg, A.H.; van Petegem, F.; Martens, E.C.; Brumer, H. Synergy between Cell Surface Glycosidases and Glycan-Binding Proteins Dictates the Utilization of Specific Beta(1,3)-Glucans by Human Gut Bacteroides. MBio 2022, 11, e00095-20. [Google Scholar] [CrossRef]
- Armstrong, Z.; Liu, F.; Kheirandish, S.; Chen, H.M.; Mewis, K.; Duo, T.; Morgan-Lang, C.; Hallam, S.J.; Withers, S.G. High-Throughput Recovery and Characterization of Metagenome-Derived Glycoside Hydrolase-Containing Clones as a Resource for Biocatalyst Development. mSystems 2022, 4, e00082-19. [Google Scholar] [CrossRef]
- Kappelmann, L.; Krüger, K.; Hehemann, J.-H.; Harder, J.; Markert, S.; Unfried, F.; Becher, D.; Shapiro, N.; Schweder, T.; Amann, R.I.; et al. Polysaccharide utilization loci of North Sea Flavobacteriia as basis for using SusC/D-protein expression for predicting major phytoplankton glycans. ISME J. 2019, 13, 76–91. [Google Scholar] [CrossRef]
- Bagnaresi, P.; Biselli, C.; Orrù, L.; Urso, S.; Crispino, L.; Abbruscato, P.; Piffanelli, P.; Lupotto, E.; Cattivelli, L.; Valè, G. Comparative Transcriptome Profiling of the Early Response to Magnaporthe oryzae in Durable Resistant vs Susceptible Rice (Oryza sativa L.) Genotypes. PLoS ONE 2012, 7, e51609. [Google Scholar] [CrossRef]
- Wu, J.; Lee, D.Y.; Wang, Y.; Kim, S.T.; Baek, S.-B.; Kim, S.G.; Kang, K.Y. Protein profiles secreted from phylloplane of rice leaves free from cytosolic proteins: Application to study rice-Magnaporthe Oryzae interactions. Physiol. Mol. Plant Pathol. 2014, 88, 28–35. [Google Scholar] [CrossRef]
- Marqués-Gálvez, J.E.; Miyauchi, S.; Paolocci, F.; Navarro-Ródenas, A.; Arenas, F.; Pérez-Gilabert, M.; Morin, E.; Auer, L.; Barry, K.W.; Kuo, A.; et al. Desert truffle genomes reveal their reproductive modes and new insights into plant–fungal interaction and ectendomycorrhizal lifestyle. New Phytol. 2021, 229, 2917–2932. [Google Scholar] [CrossRef] [PubMed]
- Sadovskaya, I.; Vinogradov, E.; Li, J.; Hachani, A.; Kowalska, K.; Filloux, A. High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: The ndvB gene is involved in the production of highly glycerol-phosphorylated β-(1→3)-glucans, which bind aminoglycosides. Glycobiology 2010, 20, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Hreggvidsson, G.O.; Dobruchowska, J.M.; Fridjonsson, O.H.; Jonsson, J.O.; Gerwig, G.J.; Aevarsson, A.; Kristjansson, J.K.; Curti, D.; Redgwell, R.R.; Hansen, C.-E.; et al. Exploring novel non-Leloir β-glucosyltransferases from proteobacteria for modifying linear (β1→3)-linked gluco-oligosaccharide chains. Glycobiology 2011, 21, 304–328. [Google Scholar] [CrossRef]
- Qin, Z.; Yan, Q.; Yang, S.; Jiang, Z. Modulating the function of a β-1,3-glucanosyltransferase to that of an endo-β-1,3-glucanase by structure-based protein engineering. Appl. Microbiol. Biotechnol. 2016, 100, 1765–1776. [Google Scholar] [CrossRef]
- Badur, A.H.; Ammar, E.M.; Yalamanchili, G.; Hehemann, J.-H.; Rao, C.V. Characterization of the GH16 and GH17 laminarinases from Vibrio breoganii 1C10. Appl. Microbiol. Biotechnol. 2020, 104, 161–171. [Google Scholar] [CrossRef]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, L.; Stokke, R.; Jensen, M.S.; Westereng, B.; Jameson, J.-K.; Steen, I.H.; Eijsink, V.G.H.; Stabb, E.V. Discovery of a Thermostable GH10 Xylanase with Broad Substrate Specificity from the Arctic Mid-Ocean Ridge Vent System. Appl. Environ. Microbiol. 2019, 85, e02970-18. [Google Scholar] [CrossRef]
- Leth, M.L.; Ejby, M.; Workman, C.; Ewald, D.A.; Pedersen, S.S.; Sternberg, C.; Bahl, M.I.; Licht, T.R.; Aachmann, F.L.; Westereng, B.; et al. Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat. Microbiol. 2018, 3, 570–580. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Wang, Z.; Robertson, K.L.; Liu, C.; Liu, J.L.; Johnson, B.J.; Leary, D.H.; Compton, J.R.; Vuddhakul, V.; Legler, P.M.; Vora, G.J. A novel Vibrio beta-glucosidase (LamN) that hydrolyzes the algal storage polysaccharide laminarin. FEMS Microbiol. Ecol. 2015, 91, fiv087. [Google Scholar] [CrossRef][Green Version]
- Barral, P.; Suárez, C.; Batanero, E.; Alfonso, C.; Alché, J.d.D.; Rodríguez-García, M.I.; Villalba, M.; Rivas, G.; Rodríguez, R. An olive pollen protein with allergenic activity, Ole e 10, defines a novel family of carbohydrate-binding modules and is potentially implicated in pollen germination. Biochem. J. 2005, 390, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, Y.-J.; Chun, J.; Aeok, Y.-J.; Lee, J.K.; Kim, K.-S.; Lee, K.-H.; Park, S.-J.; Choi, S.H. Complete Genome Sequence of Vibrio vulnificus MO6-24/O. J. Bacteriol. 2011, 193, 2062–2063. [Google Scholar] [CrossRef] [PubMed]
- Venditto, I.; Luis, A.S.; Rydahl, M.; Schückel, J.; Fernandes, V.O.; Vidal-Melgosa, S.; Bule, P.; Goyal, A.; Pires, V.M.R.; Dourado, C.G.; et al. Complexity of the Ruminococcus flavefaciens cellulosome reflects an expansion in glycan recognition. Proc. Natl. Acad. Sci. USA 2016, 113, 7136–7141. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Nurizzo, D.; Notenboom, V.; Ducros, V.; Rose, D.R.; Kilburn, D.G.; Davies, G.J. Differential Oligosaccharide Recognition by Evolutionarily-related β-1,4 and β-1,3 Glucan-binding Modules. J. Mol. Biol. 2002, 319, 1143–1156. [Google Scholar] [CrossRef]
- Van Bueren, A.L.; Morland, C.; Gilbert, H.J.; Boraston, A.B. Family 6 Carbohydrate Binding Modules Recognize the Non-reducing End of β-1,3-Linked Glucans by Presenting a Unique Ligand Binding Surface. J. Biol. Chem. 2005, 280, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Charnock, S.J.; Bolam, D.N.; Turkenburg, J.P.; Gilbert, H.J.; Ferreira, L.M.A.; Davies, G.J.; Fontes, C.M.G.A. The X6 “Thermostabilizing” Domains of Xylanases Are Carbohydrate-Binding Modules: Structure and Biochemistry of the Clostridium thermocellum X6b Domain. Biochemistry 2000, 39, 5013–5021. [Google Scholar] [CrossRef]
- Luís, A.S.; Venditto, I.; Temple, M.J.; Rogowski, A.; Baslé, A.; Xue, J.; Knox, J.P.; Prates, J.A.M.; Ferreira, L.M.A.; Fontes, C.M.G.A.; et al. Understanding How Noncatalytic Carbohydrate Binding Modules Can Display Specificity for Xyloglucan. J. Biol. Chem. 2013, 288, 4799–4809. [Google Scholar] [CrossRef]
- Duan, C.-J.; Feng, Y.-L.; Cao, Q.-L.; Huang, M.-Y.; Feng, J.-X. Identification of a novel family of carbohydrate-binding modules with broad ligand specificity. Sci. Rep. 2016, 6, 19392. [Google Scholar] [CrossRef]
- Kumagai, Y.; Okuyama, M.; Kimura, A. Heat treatment of curdlan enhances the enzymatic production of biologically active β-(1,3)-glucan oligosaccharides. Carbohydr. Polym. 2016, 146, 396–401. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H. Predicting Secretory Proteins with SignalP. In Protein Function Prediction, 1st ed.; Kihara, D., Ed.; Methods in Molecular Biology; Springer: Cham, Switzerland, 2017; Volume 1611, pp. 59–73. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Jones, D.T. The PSIPRED Protein Analysis Workbench: 20 years on. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Usuki, H.; Yamamoto, Y.; Yamasato, A.; Arima, J.; Mukaihara, T.; Hatanaka, T. Characterization of calcium ion sensitive region for β-Mannanase from Streptomyces thermolilacinus. Biochim. Biophys. Acta-Proteins Proteom. 2011, 1814, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Waffenschmidt, S.; Jaenicke, L. Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal. Biochem. 1987, 165, 337–340. [Google Scholar] [CrossRef]
- Zhang, M.; Chekan, J.R.; Dodd, D.; Hong, P.-Y.; Radlinski, L.; Revindran, V.; Nair, S.K.; Mackie, R.I.; Cann, I. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, E3708–E3717. [Google Scholar] [CrossRef]




| kcat/Km (mM−1 s−1) | kcat (s−1) | Km (mM−1) | |
|---|---|---|---|
| VvGH17 | 93.0 | 148 | 1.60 |
| ΔC466 | 35.3 | 81.7 | 2.49 |
| ΔC441 | 32.7 | 87.6 | 2.87 |
| ΔC415 | 0.2 | 1.4 | 9.39 |
| ΔN50 | 67.5 | 143.3 | 2.12 |
| ΔN65 | 24.7 | 86.8 | 3.52 |
| Primer Name | Primer Sequence (5′-3′) | Purpose |
|---|---|---|
| ΔC466-S | TGATGGCAAGCTTGCGGCCGCACTC | Truncation of 149 AAs from C-terminus |
| ΔC466-AS | GCAAGCTTGCCATCAAACGCGCCGGC | |
| ΔC441-S | GACCGGCAAGCTTGCGGCCGCACTC | Truncation of 174 AAs from C-terminus |
| ΔC441-AS | GCAAGCTTGCCGGTCAGTAATGCACT | |
| ΔC415-S | TGCTCCGTGAAAGCTTGCGGCCGCACTC | Truncation of 200 AAs from C-terminus |
| ΔC415-AS | GCAAGCTTTCACGGAGCAAGAACGGAAT | |
| ΔN50-S | CCATATGGGCAACTATCCGACAGCT | Truncation of 50 AAsf rom N-terminus |
| ΔN50-AS | TAGTTGCCCATATGGCTGCCGCGCGG | |
| ΔN65-S | CCATATGGGCAACGCGAATTATCCG | Truncation of 65 AAs from N-terminus |
| ΔN65-AS | GCGTTGCCCATATGGCTGCCGCGCGG | |
| UK-C-S | CCATATGGCCGGCGCGTTTGATGGC | Truncation of 410 AAs from N-terminus |
| UK-C-AS | GCGCCGGCCATATGGCTGCCGCGCGG | |
| W472A-S | ATCGCAgcgGAAGGTACCGCCTATCTG | Mutation of Trp472 to Ala472 |
| W472A-AS | ACCTTCcgcTGCGATCGCTTCCCCGCC | |
| W499A-S | TGGGGTgcgGGAGCGGGCGTCGTGCTC | Mutation of Trp499 to Ala499 |
| W499A-AS | CGCTCCcgcACCCCAGTCTTTTGCAGT | |
| W542A-S | GGCCTGgcgGGCAACAACGACCGTCCG | Mutation of Trp542 to Ala542 |
| W542A-AS | GTTGCCcgcCAGGCCGGTCTGAAATCC | |
| W567A-S | ACCGAAgcgACAGCCTACACGATTCCG | Mutation of Trp567 to Ala567 |
| W567A-AS | GGCTGTcgcTTCGGTTGAAATGGCACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai, Y.; Kishimura, H.; Lang, W.; Tagami, T.; Okuyama, M.; Kimura, A. Characterization of an Unknown Region Linked to the Glycoside Hydrolase Family 17 β-1,3-Glucanase of Vibrio vulnificus Reveals a Novel Glucan-Binding Domain. Mar. Drugs 2022, 20, 250. https://doi.org/10.3390/md20040250
Kumagai Y, Kishimura H, Lang W, Tagami T, Okuyama M, Kimura A. Characterization of an Unknown Region Linked to the Glycoside Hydrolase Family 17 β-1,3-Glucanase of Vibrio vulnificus Reveals a Novel Glucan-Binding Domain. Marine Drugs. 2022; 20(4):250. https://doi.org/10.3390/md20040250
Chicago/Turabian StyleKumagai, Yuya, Hideki Kishimura, Weeranuch Lang, Takayoshi Tagami, Masayuki Okuyama, and Atsuo Kimura. 2022. "Characterization of an Unknown Region Linked to the Glycoside Hydrolase Family 17 β-1,3-Glucanase of Vibrio vulnificus Reveals a Novel Glucan-Binding Domain" Marine Drugs 20, no. 4: 250. https://doi.org/10.3390/md20040250
APA StyleKumagai, Y., Kishimura, H., Lang, W., Tagami, T., Okuyama, M., & Kimura, A. (2022). Characterization of an Unknown Region Linked to the Glycoside Hydrolase Family 17 β-1,3-Glucanase of Vibrio vulnificus Reveals a Novel Glucan-Binding Domain. Marine Drugs, 20(4), 250. https://doi.org/10.3390/md20040250








