Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays
Abstract
1. Introduction
2. Results and Discussion
2.1. Amounts of Proteins and Peptides
2.2. Analyses of Antiradical Activities
2.3. ACE Inhibition Activity Measurement of SHH3
2.4. Size, Polydispersity, Encapsulation Efficiency and Suspension Stability
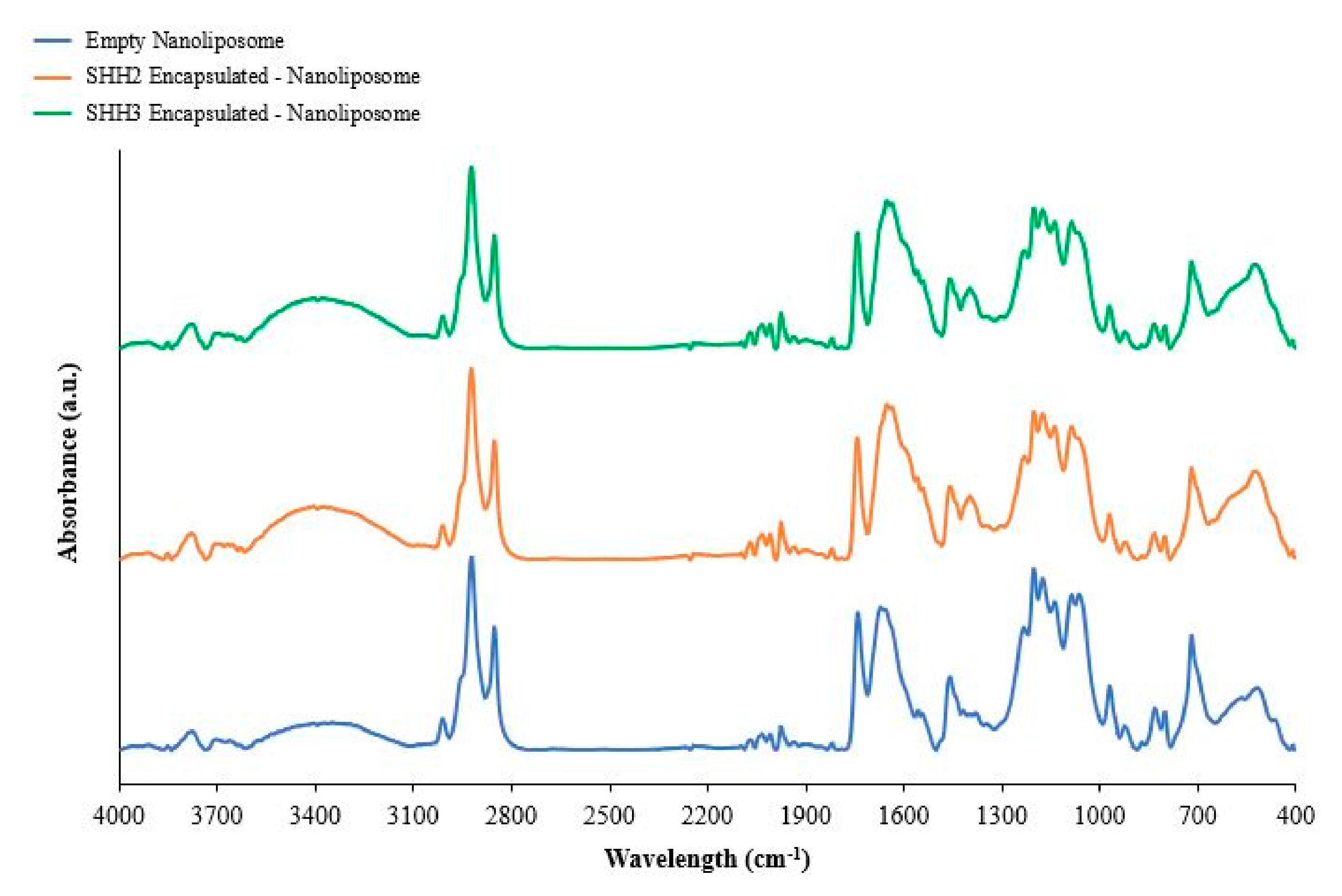
2.5. Fourier Transform Infrared of Nanoliposomes
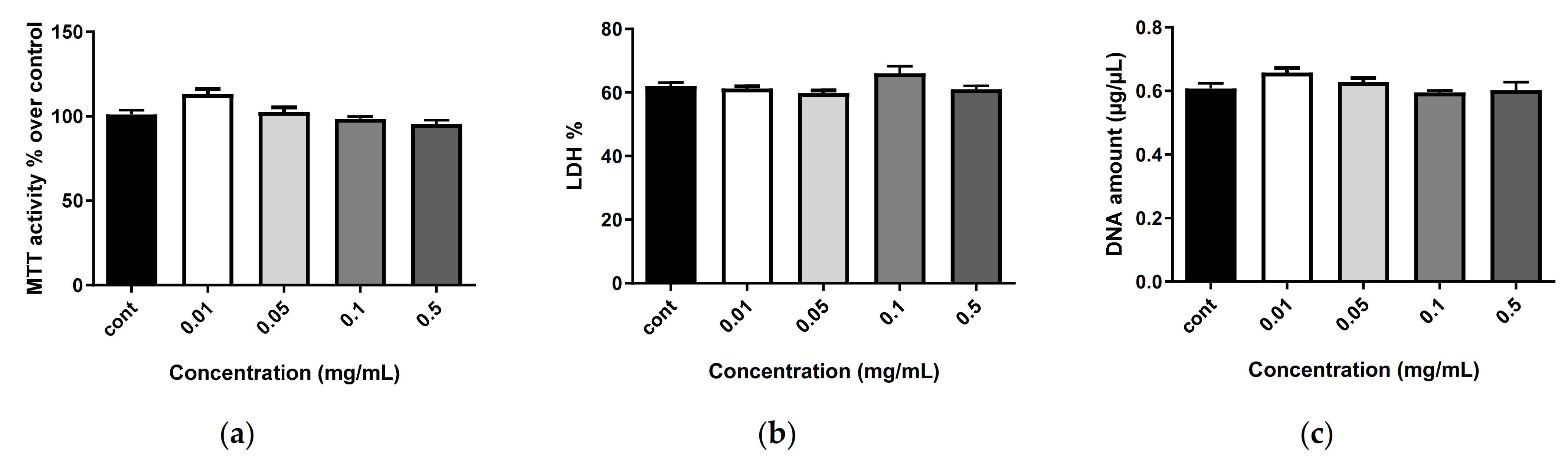
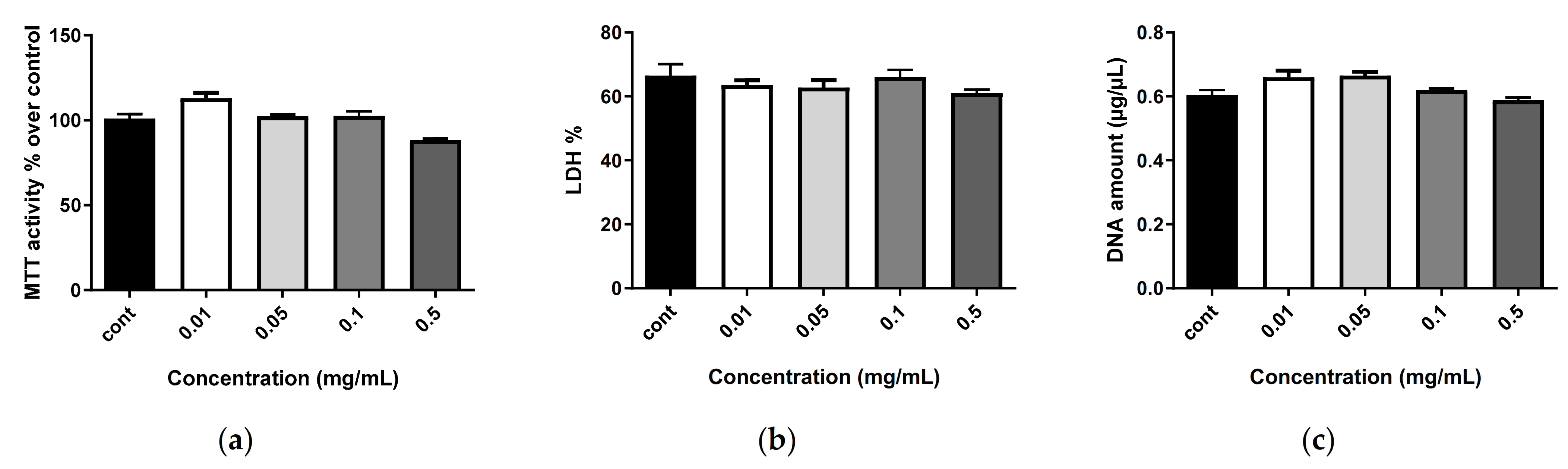
2.6. Biocompatibility of Nanostructures
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Salmon Head Hydrolysate (SHH)
3.3. Determination of Characteristics of SHHs
3.3.1. Amounts of Proteins and Peptides and Nature of Nitrogen Composition
Protein Content Using BCA Kit Method
Protein Content Using Kjeldahl Method
3.3.2. Thermogravimetric Analysis (TGA)
3.4. Bioactivity Assessment
3.4.1. ABTS•+ Scavenging Activity Assay
3.4.2. Determination of Oxygen Radical Absorbance Capacity (ORAC)
3.4.3. DPPH Radical Scavenging Activity
3.4.4. Iron Metal Chelation Activity (MCA)
3.4.5. Angiotensin-Converting Enzyme (ACE) Inhibitory Activity
3.5. Encapsulation and Characterization of SHH Nanoliposomes
3.5.1. Encapsulation of SHH Fractions
3.5.2. Characterization of SHH Fraction-Encapsulated Nanoliposomes
Encapsulation Efficiency
Dynamic Light Scattering and Zeta Potential Measurements
Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Isolation and Culture of Human MSCs
3.7. Biocompatibility Assays
3.7.1. Cytotoxicity Assay
3.7.2. Cell Proliferation
3.7.3. Cell Metabolic Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- FAO. State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Thomas, B.; Manner, A. Spécial Coproduits de La Pêche: Centralisation et Voies de Valorisation. Available online: http://www.ivamer.fr/media/etude_ivamer_nfm__098259500_1458_21082012.pdf (accessed on 19 March 2019).
- Linder, M.; Fanni, J.; Parmentier, M. Fractions Lipidiques Obtenues à Partir Des Co-Produits de La Filière Halieutique. Oléagineux Corps Gras Lipides 2006, 13, 12–15. [Google Scholar] [CrossRef][Green Version]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The Role of Omega-3 in the Prevention and Treatment of Sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Riva, A.; Petrangolini, G.; Di Paolo, E.; Gasparri, C. Effects of N-3 EPA and DHA Supplementation on Fat Free Mass and Physical Performance in Elderly. A Systematic Review and Meta-Analysis of Randomized Clinical Trial. Mech. Ageing Dev. 2021, 196, 111476. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C. Acides Gras Oméga 3 et Pathologie Cardiovasculaire: La Part Du Vrai: Omega-3 Fatty Acids and Cardiovascular Diseases: The Part of Consistency. Médecine Mal. Métaboliques 2011, 5, 269–277. [Google Scholar] [CrossRef]
- Hooper, C.; De Souto Barreto, P.; Coley, N.; Cantet, C.; Cesari, M.; Andrieu, S.; Vellas, B.; Group, M.S. Cognitive Changes with Omega-3 Polyunsaturated Fatty Acids in Non-Demented Older Adults with Low Omega-3 Index. J. Nutr. Health Aging 2017, 21, 988–993. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and Anti-Freezing Peptides from Salmon Collagen Hydrolysate Prepared by Bacterial Extracellular Protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Pliszka, M.; Vegarud, G.E. Antioxidant Properties of Salmon (Salmo salar L.) Protein Fraction Hydrolysates Revealed Following Their Ex Vivo Digestion and in Vitro Hydrolysis: Antioxidant Properties of Salmon Protein Hydrolysates. J. Sci. Food Agric. 2016, 96, 2764–2772. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Hasan, F.M.; Gill, T.A.; Aluko, R.E. Antioxidant Properties of Salmon (Salmo salar) Protein Hydrolysate and Peptide Fractions Isolated by Reverse-Phase HPLC. Food Res. Int. 2013, 52, 315–322. [Google Scholar] [CrossRef]
- Darewicz, M.; Borawska, J.; Vegarud, G.; Minkiewicz, P.; Iwaniak, A. Angiotensin I-Converting Enzyme (ACE) Inhibitory Activity and ACE Inhibitory Peptides of Salmon (Salmo salar) Protein Hydrolysates Obtained by Human and Porcine Gastrointestinal Enzymes. Int. J. Mol. Sci. 2014, 15, 14077–14101. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, S.; Xu, J.; Zeng, M.; Song, H.; Zhao, Y. Production of Cysteine-Rich Antimicrobial Peptide by Digestion of Oyster (Crassostrea gigas) with Alcalase and Bromelin. Food Control 2008, 19, 231–235. [Google Scholar] [CrossRef]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant, Immunomodulatory and Antiproliferative Effects of Gelatin Hydrolysates from Seabass (Lates calcarifer) Skins. Int. J. Food Sci. Technol. 2016, 51, 1545–1551. [Google Scholar] [CrossRef]
- Roblet, C.; Akhtar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhancement of Glucose Uptake in Muscular Cell by Peptide Fractions Separated by Electrodialysis with Filtration Membrane from Salmon Frame Protein Hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Analysis of Lipids Extracted from Salmon (Salmo salar) Heads by Commercial Proteolytic Enzymes. Eur. J. Lipid Sci. Technol. 2006, 108, 766–775. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Tsai, Y.-H.; Hong, Y.-H.; Hsieh, S.-L.; Huang, R.-H. Characterization and Antioxidant and Angiotensin I-Converting Enzyme (ACE)-Inhibitory Activities of Gelatin Hydrolysates Prepared from Extrusion-Pretreated Milkfish (Chanos chanos) Scale. Mar. Drugs 2018, 16, 346. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and Characterization of Fish Scale Collagen of Higher Thermal Stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef]
- Yvon, M.; Chabanet, C.; Pélissier, J.-P. Solubility of Peptides in Trichloroacetic Acid (TCA) Solutions Hypothesis on the Precipitation Mechanism. Int. J. Pept. Protein Res. 1989, 34, 166–176. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Ma, Q.; Zhuang, Y. Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen. Mar. Drugs 2016, 14, 186. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Bioactive Peptides from Cartilage Protein Hydrolysate of Spotless Smoothhound and Their Antioxidant Activity In Vitro. Mar. Drugs 2018, 16, 100. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Vercruysse, L.; Van Camp, J.; Smagghe, G. ACE Inhibitory Peptides Derived from Enzymatic Hydrolysates of Animal Muscle Protein: A Review. J. Agric. Food Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure–Activity Relationship of Antioxidant Dipeptides: Dominant Role of Tyr, Trp, Cys and Met Residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.-M. Determination of Reducing Power and Metal Chelating Ability of Antioxidant Peptides: Revisited Methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ben Khaled, H.; Ktari, N.; Ghorbel-Bellaaj, O.; Jridi, M.; Lassoued, I.; Nasri, M. Composition, Functional Properties and in Vitro Antioxidant Activity of Protein Hydrolysates Prepared from Sardinelle (Sardinella aurita) Muscle. J. Food Sci. Technol. 2014, 51, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Andersen, L.L.; Nielsen, H.H.; Jacobsen, C.; Jakobsen, G.; Johansson, I.; Jessen, F. Antioxidant Activity of Cod (Gadus morhua) Protein Hydrolysates: In Vitro Assays and Evaluation in 5% Fish Oil-in-Water Emulsion. Food Chem. 2014, 149, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ohba, R.; Deguchi, T.; Kishikawa, M.; Arsyad, F.; Morimura, S.; Kida, K. Physiological Functions of Enzymatic Hydrolysates of Collagen or Keratin Contained in Livestock and Fish Waste. Food Sci. Technol. Res. 2003, 9, 91–93. [Google Scholar] [CrossRef][Green Version]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- Dumont, C.; Bourgeois, S.; Fessi, H.; Jannin, V. Lipid-Based Nanosuspensions for Oral Delivery of Peptides, a Critical Review. Int. J. Pharm. 2018, 541, 117–135. [Google Scholar] [CrossRef]
- Da Rosa Zavareze, E.; Telles, A.C.; El Halal, S.L.M.; Da Rocha, M.; Colussi, R.; De Assis, L.M.; Suita de Castro, L.A.; Guerra Dias, A.R.; Prentice-Hernández, C. Production and Characterization of Encapsulated Antioxidative Protein Hydrolysates from Whitemouth Croaker (Micropogonias furnieri) Muscle and Byproduct. LWT-Food Sci. Technol. 2014, 59, 841–848. [Google Scholar] [CrossRef]
- Mosquera, M.; Giménez, B.; Da Silva, I.M.; Boelter, J.F.; Montero, P.; Gómez-Guillén, M.C.; Brandelli, A. Nanoencapsulation of an Active Peptidic Fraction from Sea Bream Scales Collagen. Food Chem. 2014, 156, 144–150. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ramezanzade, L.; Nikkhah, M. Nano-Liposomal Entrapment of Bioactive Peptidic Fraction from Fish Gelatin Hydrolysate. Int. J. Biol. Macromol. 2017, 105, 1455–1463. [Google Scholar] [CrossRef]
- Bouarab, L.; Maherani, B.; Kheirolomoom, A.; Hasan, M.; Aliakbarian, B.; Linder, M.; Arab-Tehrany, E. Influence of Lecithin–Lipid Composition on Physico-Chemical Properties of Nanoliposomes Loaded with a Hydrophobic Molecule. Colloids Surf. B Biointerfaces 2014, 115, 197–204. [Google Scholar] [CrossRef]
- Colas, J.C.; Shi, W.; Rao, V.M.; Omri, A.; Mozafari, M.R.; Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their acterial targeting. Micron 2007, 38, 841–847. [Google Scholar] [CrossRef]
- ISO 1871:2009. Available online: http://www.iso.org/cms/render/live/fr/sites/isoorg/contents/data/standard/04/13/41320.html (accessed on 19 March 2019).
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-Radical Absorbance Capacity Assay for Antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Hasan, M.; Belhaj, N.; Benachour, H.; Barberi-Heyob, M.; Kahn, C.J.F.; Jabbari, E.; Linder, M.; Arab-Tehrany, E. Liposome Encapsulation of Curcumin: Physico-Chemical Characterizations and Effects on MCF7 Cancer Cell Proliferation. Int. J. Pharm. 2014, 461, 519–528. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.C.K.; Thibodeau, J.; Bazinet, L.; Udenigwe, C.C. Liposome Encapsulation of Anionic and Cationic Whey Peptides: Influence of Peptide Net Charge on Properties of the Nanovesicles. LWT 2018, 87, 40–46. [Google Scholar] [CrossRef]
- Bianchi, A.; Velot, É.; Kempf, H.; Elkhoury, K.; Sanchez-Gonzalez, L.; Linder, M.; Kahn, C.; Arab-Tehrany, E. Nanoliposomes from Agro-Resources as Promising Delivery Systems for Chondrocytes. Int. J. Mol. Sci. 2020, 21, 3436. [Google Scholar] [CrossRef]


| Sample | SHH1 | SHH2 | SHH3 |
|---|---|---|---|
| Protein (%) | 47.34 ± 0.93 2 | 59.07 ± 1.85 1 | 60.90 ± 1.52 2 |
| Ashes (%) | 30.85 ± 0.35 | 33.32 ± 0.94 | 32.86 ± 0.29 |
| NT (%) | NP (%) | NsH (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SHH1 | SHH2 | SHH3 | SHH1 | SHH2 | SHH3 | SHH1 | SHH2 | SHH3 |
| 76.64 ± 0.372 2 | 84.32 ± 1.181 1 | 74.36 ± 0.133 3 | 16.74 ± 0.511 1 | 11.95 ± 3.312 2 | - | 59.90 ± 0.222 2 | 72.38 ± 2.751 1 | 74.36 ± 0.133 |
| ABTS | DPPH | ||||||||||||||
| IC50 (µg/mL) | TEAC (µM TE/mg) | IC50 (µg/mL) | TEAC (µM TE/mg) | ||||||||||||
| SHH1 | SHH2 | SHH3 | GSH | SHH1 | SHH2 | SHH3 | GSH | SHH1 | SHH2 | SHH3 | GSH | SHH1 | SHH2 | SHH3 | GSH |
| 39.09 ± 3.52 3 | 27.17 ± 1.17 2 | 42.32 ± 1.35 3 | 1.78 ± 0.08 1 | 178.94 ± 3.52 2 | 186.65 ± 0.65 2 | 160.78 ± 2.41 2 | 3928.35 ± 57.64 1 | 3383.02 ± 144.16 2 | 4537.03 ± 291.35 3 | 3217.80 ± 100.48 2 | 8.27 ± 0.71 1 | 2.84 ± 0.05 2 | 2.55 ± 0.07 2 | 2.80 ± 0.04 2 | 718.36 ± 18.19 1 |
| MCA | ORAC | ||||||||||||||
| IC50 (µg/mL) | TEAC (µM TE/mg) | ||||||||||||||
| SHH1 | SHH2 | SHH3 | EDTA | SHH1 | SHH2 | SHH3 | GSH | ||||||||
| 238.71 ± 3.90 2 | 265.26 ± 5.39 3 | 301.73 ± 2.28 4 | 20.49 ± 0.16 1 | 97.23 ± 3.64 2 | 288.96 ± 37.84 1 | 254.18 ± 3.11 1 | 143.10 ± 7.33 2 | ||||||||
| Sample | Average Size (nm) | Polydispersity Index | Encapsulation Efficiency (%) | Zeta Potential (mV) |
|---|---|---|---|---|
| Control empty nanoliposomes | 118.93 ± 1.15 2 | 0.28 ± 0.01 2 | - | −46.97 ± 1.10 1 |
| SHH2 nanoliposomes | 53.91 ± 2.66 4 | 0.34 ± 0.01 3 | 13.77 ± 2.50 1 | −38.57 ± 2.83 3 |
| SHH3 nanoliposomes | 143.87 ± 3.99 1 | 0.27 ± 0.02 2 | 17.27 ± 3.08 1 | −32.30 ± 0.361 4 |
| Stretching | Control Empty Nanoliposomes | SHH2 Nanoliposomes | SHH3 Nanoliposomes |
|---|---|---|---|
| (CH3)3N | 969 | 969 | 968 |
| Broad stretch PO2 | 1090 | 1090 | 1087 * |
| PO2 | 1162 | 1162 | 1160 * |
| Phosphoester bond | 1236 | 1233 * | 1233 * |
| C=O | 1648 | 1639 ** | 1646 * |
| CH2 (symmetric) | 2852 | 2852 | 2852 |
| CH3 | 2923 | 2923 | 2922 |
| -C=C- | 1654 | 1645 ** | 1652 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanachi, A.; Bianchi, A.; Kahn, C.J.F.; Velot, E.; Arab-Tehrany, E.; Cakir-Kiefer, C.; Linder, M. Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays. Mar. Drugs 2022, 20, 249. https://doi.org/10.3390/md20040249
Hanachi A, Bianchi A, Kahn CJF, Velot E, Arab-Tehrany E, Cakir-Kiefer C, Linder M. Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays. Marine Drugs. 2022; 20(4):249. https://doi.org/10.3390/md20040249
Chicago/Turabian StyleHanachi, Amine, Arnaud Bianchi, Cyril J. F. Kahn, Emilie Velot, Elmira Arab-Tehrany, Céline Cakir-Kiefer, and Michel Linder. 2022. "Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays" Marine Drugs 20, no. 4: 249. https://doi.org/10.3390/md20040249
APA StyleHanachi, A., Bianchi, A., Kahn, C. J. F., Velot, E., Arab-Tehrany, E., Cakir-Kiefer, C., & Linder, M. (2022). Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays. Marine Drugs, 20(4), 249. https://doi.org/10.3390/md20040249








