Antimicrobial and Antioxidant Activities of N-2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Amino Acid Schiff Bases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis and Characterization
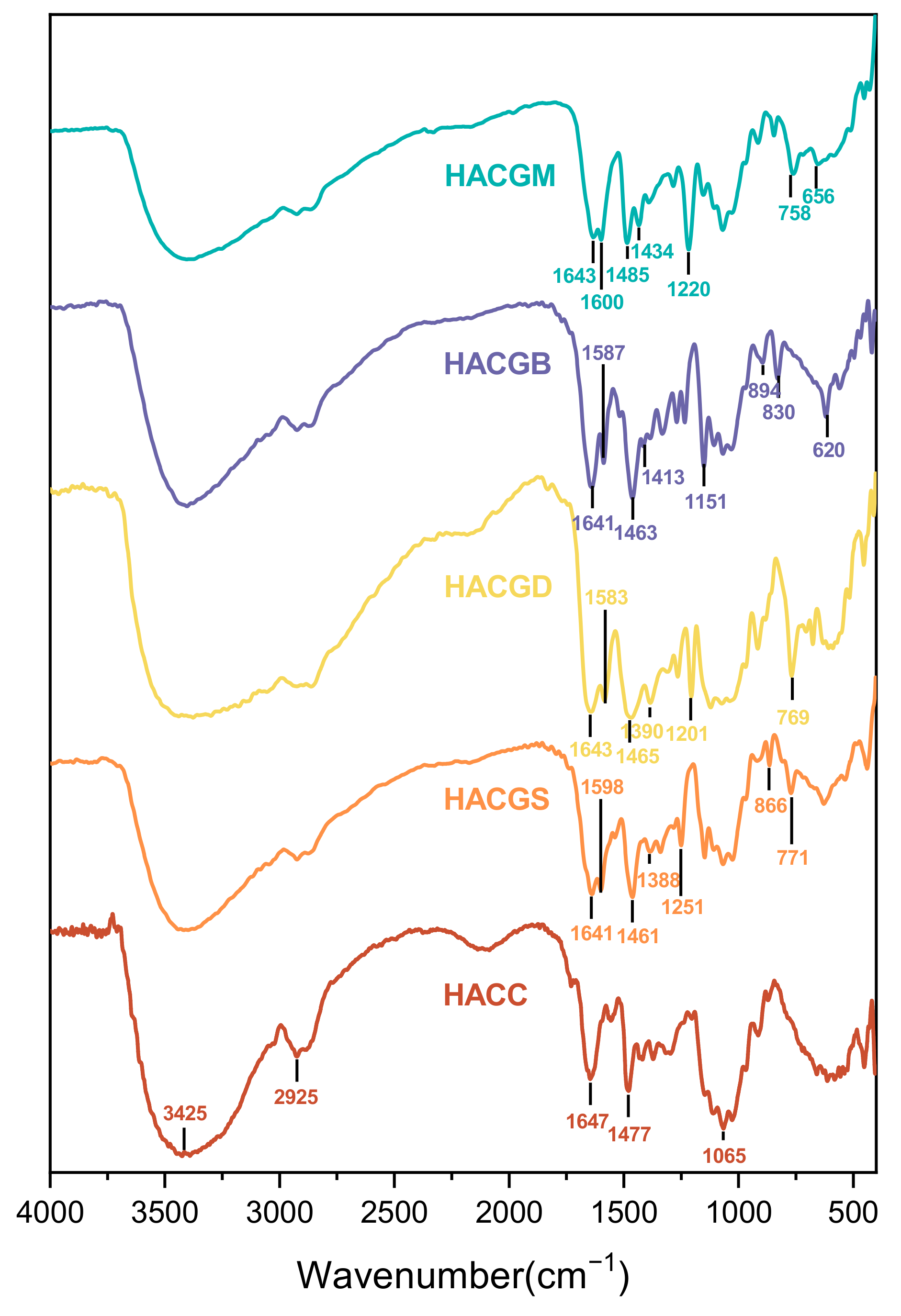
2.1.1. FT-IR Analysis
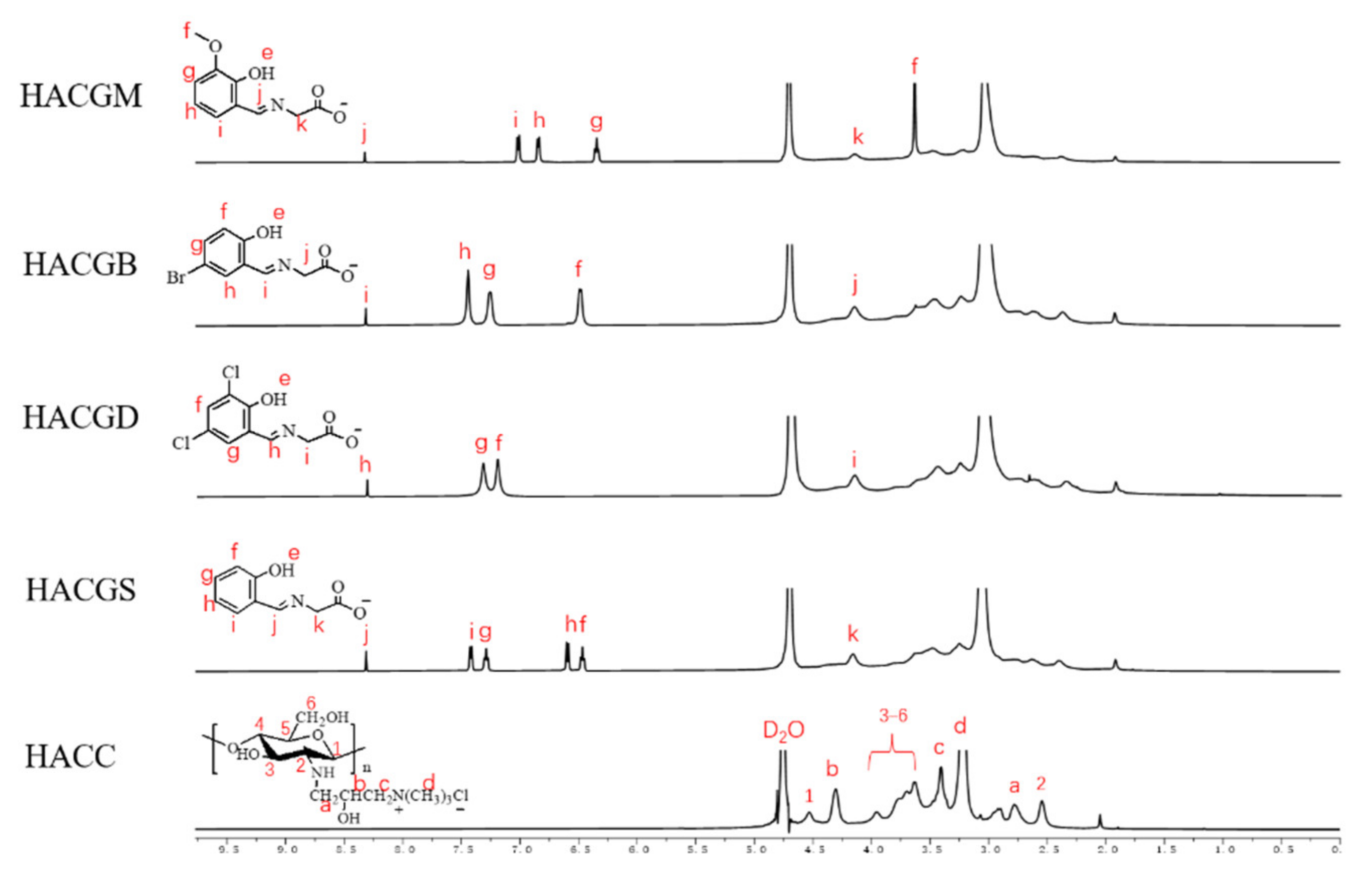
2.1.2. H NMR Analysis
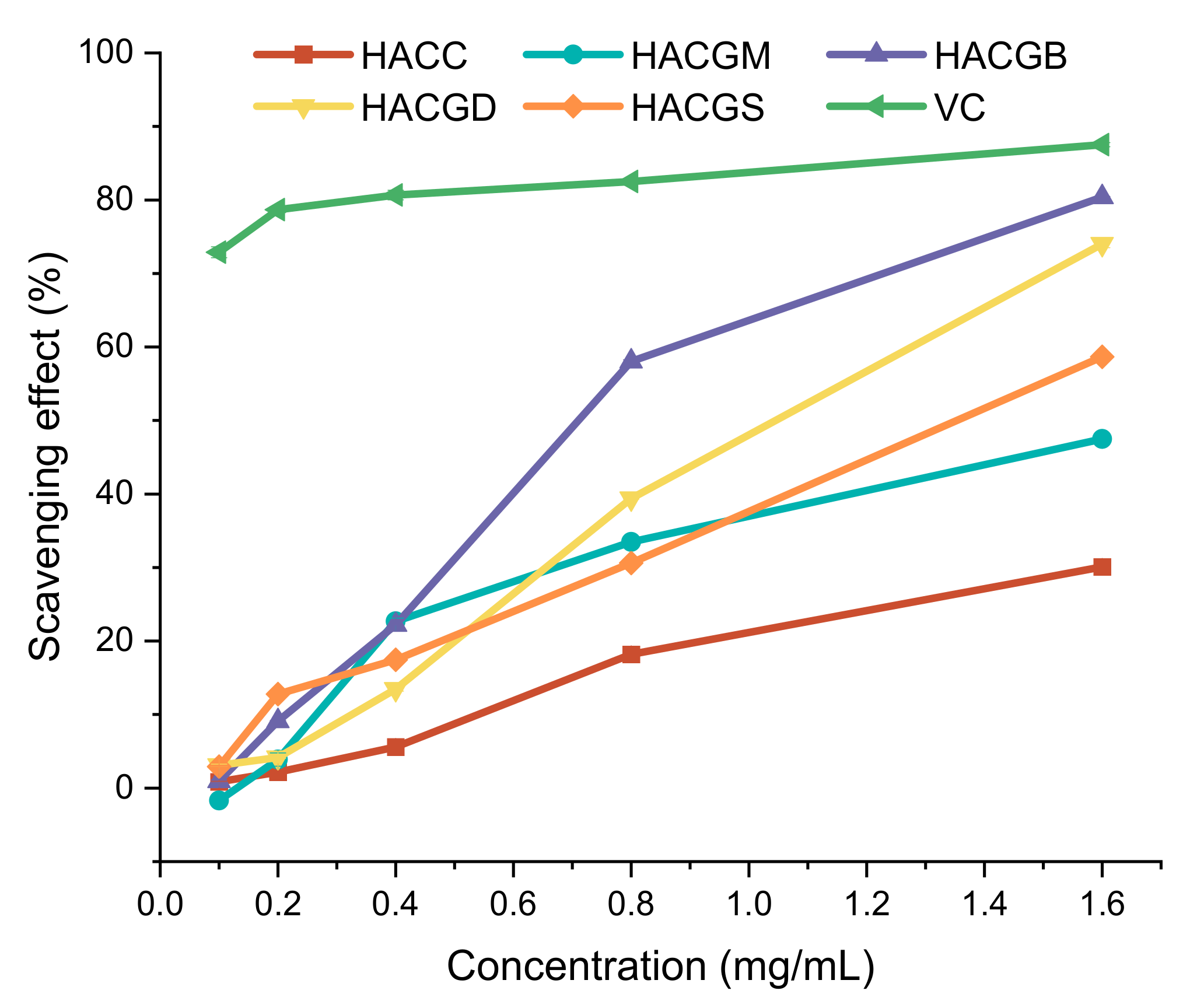
2.2. Antioxidant Capacity
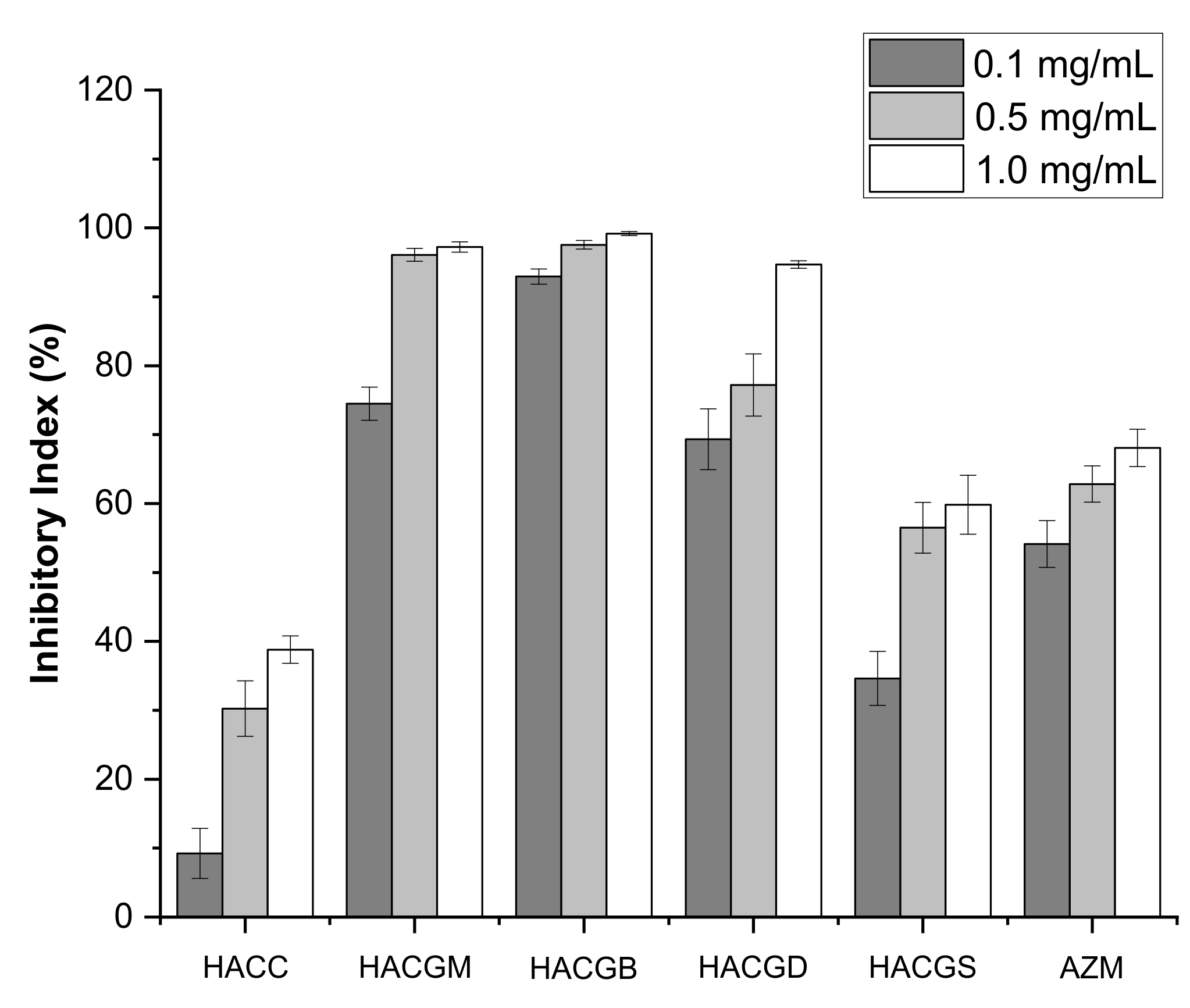
2.3. Antibacterial Activity
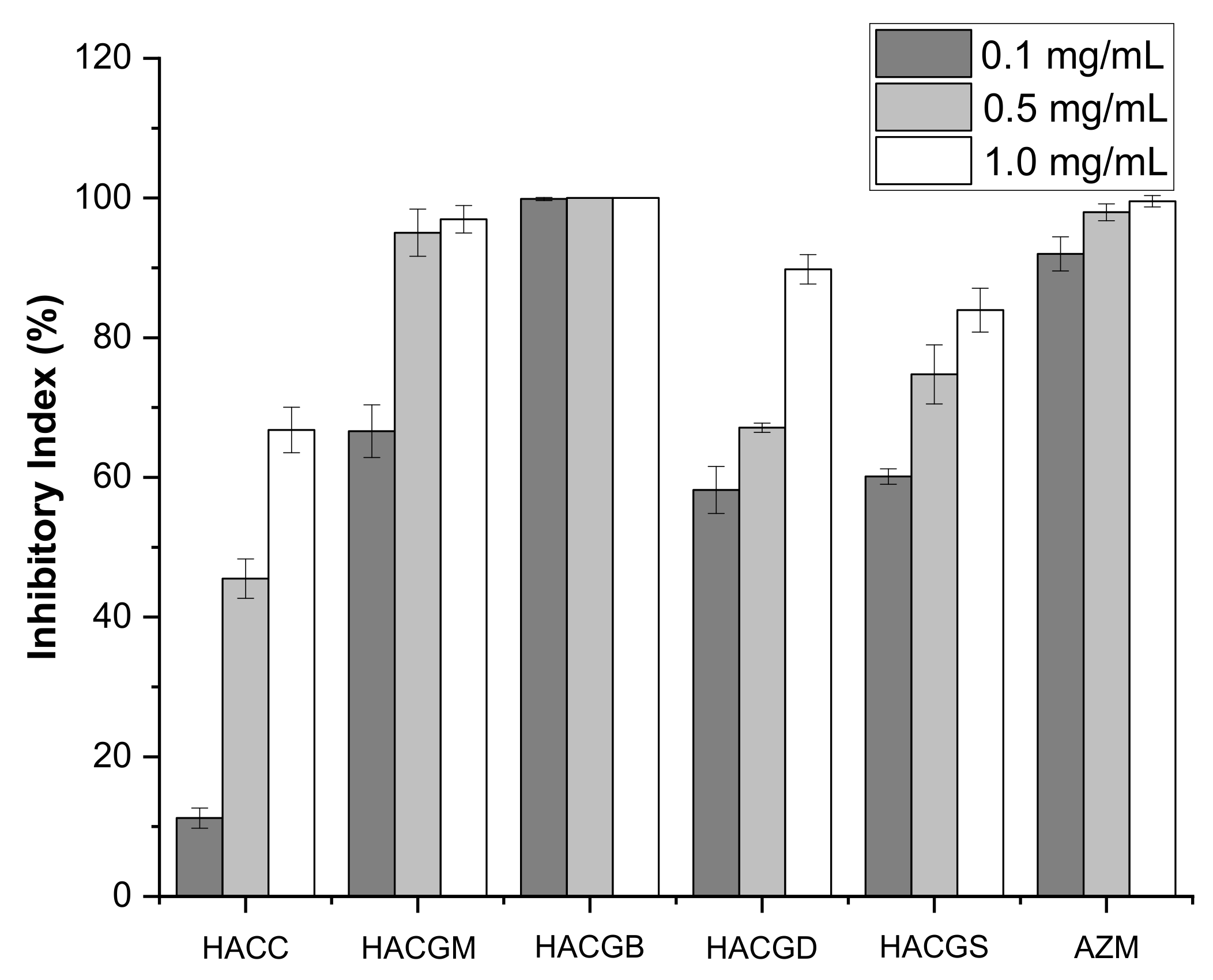
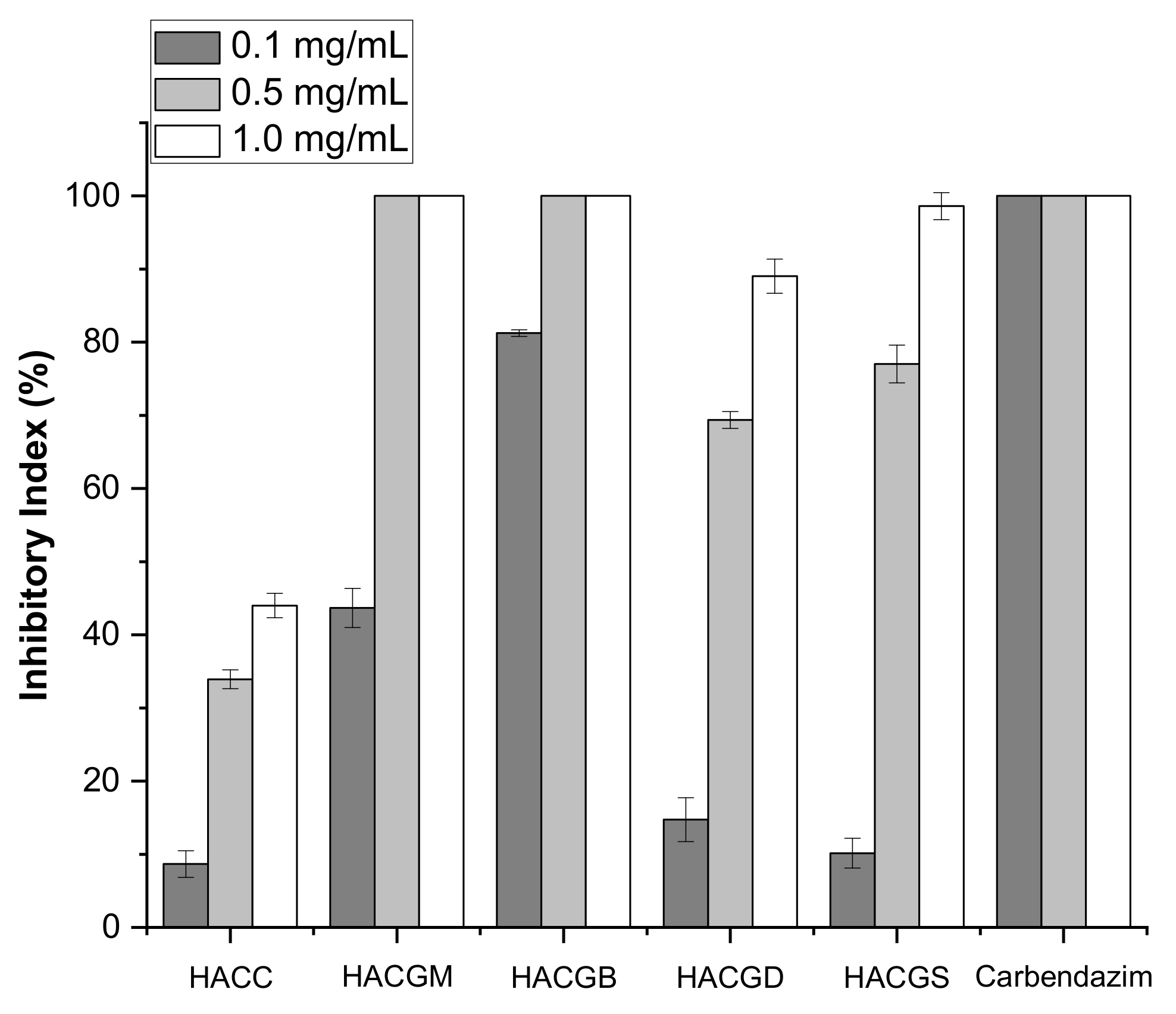
2.4. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of HACC Derivatives
3.2.1. Synthesis of HACC
3.2.2. Synthesis of Glycine Schiff Base Potassium Salt
3.2.3. Synthesis of HACC Derivatives
3.3. Analytical Methods
3.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3.2. H Nuclear Magnetic Resonance (NMR) Spectroscopy
3.4. Antioxidant Assay
3.4.1. Superoxide Radical Scavenging Ability Assay
3.4.2. DPPH Radical Scavenging Ability Assay
3.5. Antibacterial Assay
3.6. Antifungal Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, T.; Kaushik, P.; Mohan, M.K. Prevalence of E. coli O157:H7 in water sources: An overview on associated diseases, outbreaks and detection methods. Diagn. Microbiol. Infect. Dis. 2015, 82, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.H.; Azam, F. Chitin, cholera, and competence. Science 2005, 310, 1775–1777. [Google Scholar] [CrossRef]
- Leadbeater, A. Recent Developments and Challenges in Chemical Disease Control. Plant Prot. Sci. 2015, 51, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Mihdhdir, A.A. Evaluation of bateriologial and sanitary quality of drinking water stations and water tankers in Makkah Al-Mokarama. Pak. J. Biol. Sci. PJBS 2009, 12, 401–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Oh, J.W. Reviewing Chitin/Chitosan Nanofibers and Associated Nanocomposites and Their Attained Medical Milestones. Polymers 2021, 13, 2330. [Google Scholar] [CrossRef]
- Li, J.H.; Wu, Y.G.; Zhao, L.Q. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fu, C.H.; Wu, S.H.; Liu, G.H.; Guo, J.; Su, Z.Q. Determination of the Deacetylation Degree of Chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.M.; Wang, X.H.; Sun, L.P. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Tan, W.; Zhang, J.; Wei, L.; Chen, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates. Mar. Drugs 2018, 16, 315. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Dang, Q.; Liu, C.; Wang, T.; Fan, B.; Yan, J.; Xu, Y. Preparation, characterization and antibacterial activity of O-acetyl-chitosan-N-2-hydroxypropyl trimethyl ammonium chloride. Int. J. Biol. Macromol. 2015, 80, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Qu, W.; Sun, Y.; Liang, L.; Jin, Z.; Cui, S.; Zhao, K. Water-soluble N-2-Hydroxypropyl trimethyl ammonium chloride chitosan enhanced the immunogenicity of inactivated porcine parvovirus vaccine vaccination on sows against porcine parvovirus infection. Immunol. Lett. 2020, 223, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, W.; Cao, H.; Zhang, X.; Chen, G.; Wu, H.; Guo, C.; Zhang, Y.; Kang, H.; Wang, Y.; et al. Antimicrobial activity and cytotoxicity of N-2-HACC and characterization of nanoparticles with N-2-HACC and CMC as a vaccine carrier. Chem. Eng. J. 2013, 221, 331–341. [Google Scholar] [CrossRef]
- Zhao, K.; Han, J.Y.; Zhang, Y.; Wei, L.; Yu, S.; Wang, X.H.; Jin, Z.; Wang, Y.F. Enhancing Mucosal Immune Response of Newcastle Disease Virus DNA Vaccine Using N-2-Hydroxypropyl Trimethylammonium Chloride Chitosan and N,O-Carboxymethyl Chitosan Nanoparticles as Delivery Carrier. Mol. Pharm. 2018, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Favere, V.T.; Riella, H.G.; da Rosa, S. Chitosan-n-2-hydroxypropyl trimethyl ammonium chloride as adsorbent for the removal of the reactive dye from aqueous solutions. Quim. Nova 2010, 33, 1476–1481. [Google Scholar]
- Huang, R.H.; Yang, B.C.; Wang, B.; Zheng, D.S.; Zhang, Z.Q. Removal of chromium (VI) ions from aqueous solutions by N-2-hydroxypropyl trimethyl ammonium chloride chitosan-bentonite. Desalination Water Treat. 2012, 50, 329–337. [Google Scholar] [CrossRef]
- Sangeetha, Y.; Meenakshi, S.; SairamSundaram, C. Corrosion mitigation of N-(2-hydroxy-3-trimethyl ammonium)propyl chitosan chloride as inhibitor on mild steel. Int. J. Biol. Macromol. 2015, 72, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Yin, Y.H.; Pu, J.W. Preparation and synthesis of water-soluble chitosan derivative incorporated in ultrasonic-assistant wheat straw paper for antibacterial food-packaging. Nord. Pulp Pap. Res. J. 2017, 32, 606–614. [Google Scholar] [CrossRef]
- Qin, C.Q.; Xiao, Q.; Li, H.R.; Fang, M.; Liu, Y.; Chen, X.Y.; Li, Q. Calorimetric studies of the action of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride on the growth of microorganisms. Int. J. Biol. Macromol. 2004, 34, 121–126. [Google Scholar] [CrossRef]
- Peng, Z.-X.; Wang, L.; Du, L.; Guo, S.-R.; Wang, X.-Q.; Tang, T.-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Xu, J.P.; Liu, Y.; Hsu, S.H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Sun, D.J.; Yang, Y.Y.; Li, M.X.; Li, H.; Chen, L.X. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.L.; Bian, M.L.; Yang, Z.B.; Ma, X.Y.; Liu, W.K. Pt(II) and Au(III) complexes containing Schiff-base ligands: A promising source for antitumor treatment. Eur. J. Med. Chem. 2021, 211, 113098. [Google Scholar] [CrossRef]
- Lal, S.; Arora, S.; Sharma, C. Synthesis, thermal and antimicrobial studies of some Schiff bases of chitosan. J. Therm. Anal. Calorim. 2016, 124, 909–916. [Google Scholar] [CrossRef]
- Vadivel, T.; Dhamodaran, M. Synthesis, characterization and antibacterial studies of ruthenium(III) complexes derived from chitosan schiff base. Int. J. Biol. Macromol. 2016, 90, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Arif, M.; Sarfraz, M. Metal-based antibacterial and antifungal amino acid derived Schiff bases: Their synthesis, characterization and in vitro biological activity. Appl. Organomet. Chem. 2007, 21, 294–302. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Y.; Lei, F.; Dai, L. A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohydr. Polym. 2020, 230, 115671. [Google Scholar] [CrossRef] [PubMed]
- Gungor, O.; Gurkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Xing, Y.-H.; Cao, Y.-Z.; Li, Z.-P.; Wei, D.-M.; Zeng, X.-Q.; Ge, M.-F. Synthesis, structure and properties of three new oxidovanadium complexes containing a tridentate salicylaldehydeglycine. J. Mol. Struct. 2009, 938, 54–64. [Google Scholar] [CrossRef]
- Bakalorz, K.; Przypis, L.; Tomczyk, M.M.; Ksiazek, M.; Grzesik, R.; Kuznik, N. Unprecedented Water Effect as a Key Element in Salicyl-Glycine Schiff Base Synthesis. Molecules 2020, 25, 1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, Y.; Miao, Q.; Cui, J.; Tan, W.; Guo, Z. Novel 2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives: Synthesis, Characterization, Moisture Absorption and Retention Properties. Molecules 2021, 26, 4238. [Google Scholar] [CrossRef]
- Nisar, S.; Pandit, A.H.; Wang, L.-F.; Rattan, S. Strategy to design a smart photocleavable and pH sensitive chitosan based hydrogel through a novel crosslinker: A potential vehicle for controlled drug delivery. RSC Adv. 2020, 10, 14694–14704. [Google Scholar] [CrossRef] [Green Version]
- Naghipour, A.; Fakhri, A. Efficient oxidation of sulfides into sulfoxides catalyzed by a chitosan-Schiff base complex of Cu(II) supported on supramagnetic Fe3O4 nanoparticles. Environ. Chem. Lett. 2016, 14, 207–213. [Google Scholar] [CrossRef]
- Bouhdada, M.; El Amane, M.; El Hamdani, H. Synthesis, characterization, antimicrobial activity, DFT and molecular docking studies of the N-salicylidene-glycinato and their metal mixed ligand complexes with caffeine. J. Mol. Struct. 2021, 1231, 129679. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Wang, F.; Lv, L.; Liu, J.; Li, M.; Guo, A.; Jiang, J.; Shen, Y.; Guo, S. Synthesis, in vitro and in vivo evaluation of new norcantharidin-conjugated hydroxypropyltrimethyl ammonium chloride chitosan derivatives as polymer therapeutics. Int. J. Pharm. 2013, 453, 610–619. [Google Scholar] [CrossRef]
- Beyazit, N.; Cakran, H.S.; Cabir, A.; Akiscan, Y.; Demetgul, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (-)-gossypol. Carbohydr. Polym. 2020, 240, 116333. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.; Hurbain, I.; Urban, M.; Clement, A.; Housset, B. Oxidants and antioxidants-biological effects and therapeutic prospects. Ann. Chir. 1995, 49, 427–434. [Google Scholar] [PubMed]
- Tan, W.Q.; Dong, F.; Zhang, J.J.; Zhao, X.; Li, Q.; Guo, Z.Y. Physical and Antioxidant Properties of Edible Chitosan Ascorbate Films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhan, J.; Qin, Y.K.; Liu, S.; Xing, R.E.; Yu, H.H.; Chen, X.L.; Li, P.C. Synthesis and effects of the selective oxidation of chitosan in induced disease resistance against Botrytis cinerea. Carbohydr. Polym. 2021, 265, 118073. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Appaji, N.; Yagi, K. Occurrence of superoxide anion in reaction of reduced phenazine methosulfate and molecular-oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849. [Google Scholar] [CrossRef]
- Li, Q.; Mi, Y.Q.; Tan, W.Q.; Guo, Z.Y. Highly efficient free radical-scavenging property of phenolic-functionalized chitosan derivatives: Chemicalmodification and activity assessment. Int. J. Biol. Macromol. 2020, 164, 4279–4288. [Google Scholar] [CrossRef]
- Gupta, D.; Haile, A. Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr. Polym. 2007, 69, 164–171. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int. J. Biol. Macromol. 2021, 179, 292–298. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Ji, X.; Mi, Y.; Miao, Q.; Dong, F.; Tan, W.; Guo, Z. Antimicrobial and Antioxidant Activities of N-2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Amino Acid Schiff Bases. Mar. Drugs 2022, 20, 86. https://doi.org/10.3390/md20020086
Cui J, Ji X, Mi Y, Miao Q, Dong F, Tan W, Guo Z. Antimicrobial and Antioxidant Activities of N-2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Amino Acid Schiff Bases. Marine Drugs. 2022; 20(2):86. https://doi.org/10.3390/md20020086
Chicago/Turabian StyleCui, Jingmin, Xia Ji, Yingqi Mi, Qin Miao, Fang Dong, Wenqiang Tan, and Zhanyong Guo. 2022. "Antimicrobial and Antioxidant Activities of N-2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Amino Acid Schiff Bases" Marine Drugs 20, no. 2: 86. https://doi.org/10.3390/md20020086
APA StyleCui, J., Ji, X., Mi, Y., Miao, Q., Dong, F., Tan, W., & Guo, Z. (2022). Antimicrobial and Antioxidant Activities of N-2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Amino Acid Schiff Bases. Marine Drugs, 20(2), 86. https://doi.org/10.3390/md20020086






