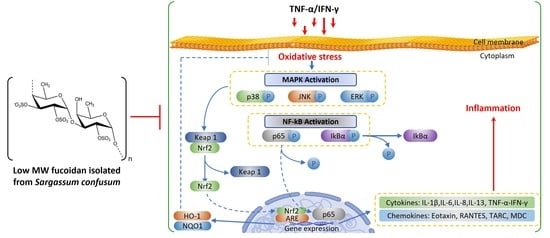
Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. SCF Effectively Increases Cell Viability by Suppressing the Production of Intracellular ROS in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
2.2. SCF Downregulates the Expression of Inflammatory Cytokines and Chemokines in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
2.3. SCF Suppresses the Activation of MAPK and NF-κB Signaling Pathways in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
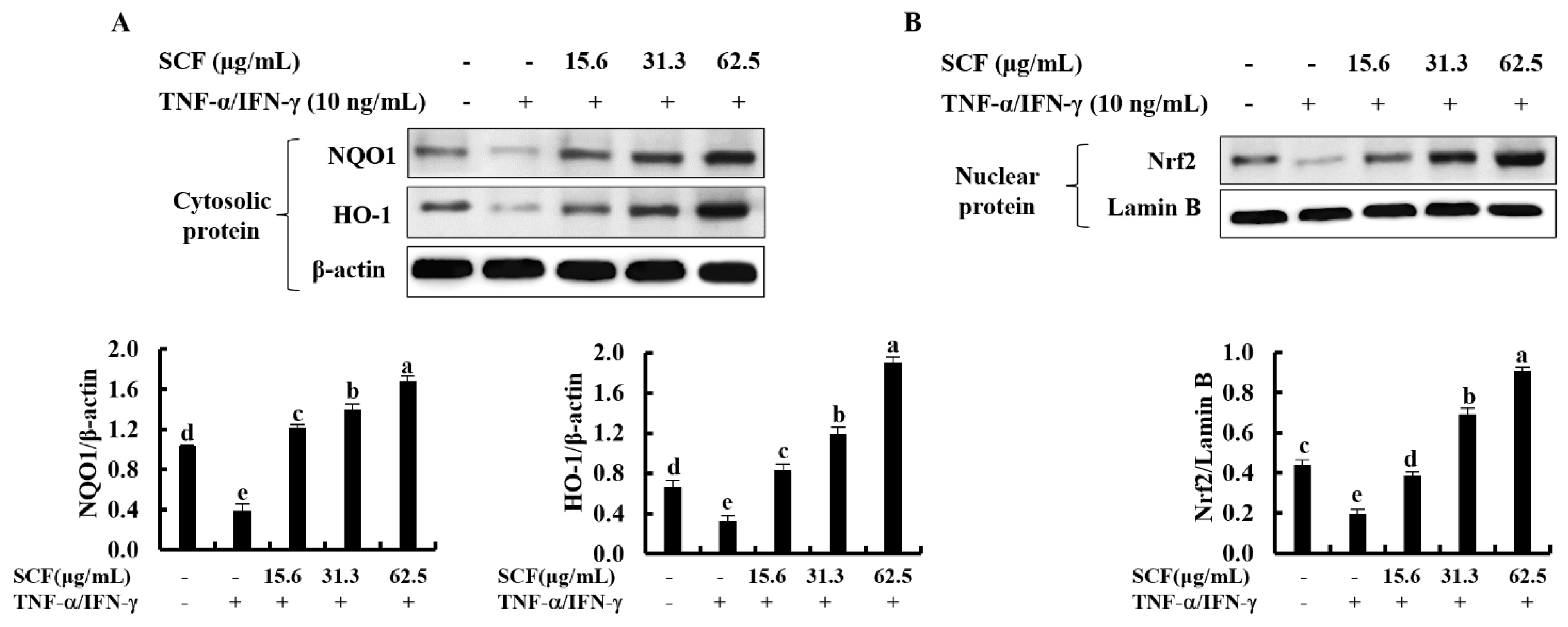
2.4. SCF Activates the Nrf2/HO-1 Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
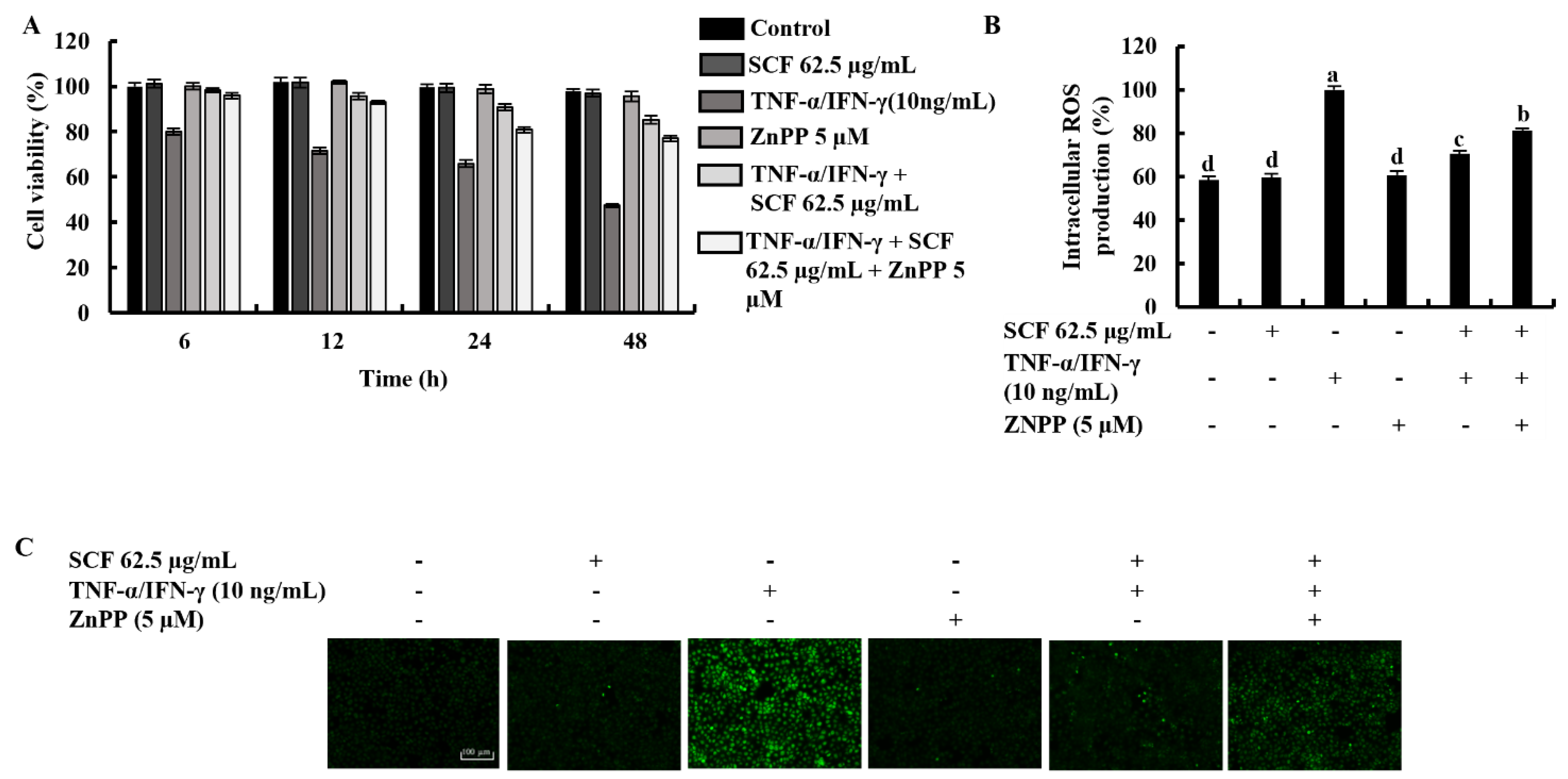
2.5. Effect of the ZnPP in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes with the Treatment of SCF
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and SCF Treatment
4.3. Cell Viability Assay
4.4. Analysis of Intracellular ROS Production
4.5. Western Blot Analysis
4.6. RNA Extraction and RT-PCR Analysis
4.7. ELISA Analysis
4.8. Immunofluorescence Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, S.-H.; Kim, H.-S.; Lee, W.; Han, E.J.; Kim, S.-Y.; Fernando, I.S.; Ahn, G.; Kim, K.-N. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, M.; Loewa, A.; Nováčková, A.; Kováčik, A.; Kaessmeyer, S.; Erdmann, G.; Vávrová, K.; Hedtrich, S. Impact of intercellular crosstalk between epidermal keratinocytes and dermal fibroblasts on skin homeostasis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118722. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Werfel, T. Interaction of keratinocytes with infiltrating lymphocytes in allergic eczematous skin diseases. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Dai, Y.-W.; Peng, H.-L.; Kang, C.-W.; Kuo, C.-Y.; Liou, C.-J. Phloretin ameliorates chemokines and ICAM-1 expression via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human keratinocytes. Int. Immunopharmacol. 2015, 27, 32–37. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Jung, K.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Lee, W.; Jee, Y.; Jeon, Y.-J.; Lee, J.; Kim, T. Sargassum horneri ethanol extract ameliorates TNF-α/IFN-γ-induced inflammation in human keratinocytes and TPA-induced ear edema in mice. Food Biosci. 2021, 39, 100831. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar]
- Kwon, D.H.; Cha, H.-J.; Choi, E.O.; Leem, S.-H.; Kim, G.-Y.; Moon, S.-K.; Chang, Y.-C.; Yun, S.-J.; Hwang, H.J.; Kim, B.W. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef]
- Luo, J.-F.; Shen, X.-Y.; Lio, C.K.; Dai, Y.; Cheng, C.-S.; Liu, J.-X.; Yao, Y.-D.; Yu, Y.; Xie, Y.; Luo, P. Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.-H.; Jeon, Y.-J.; Kim, S.-Y. (–)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-γ/TNF-α-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.-H.; Kang, I.-J.; Won, M.-H.; Yi, J.-S. Suppression of thymus-and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef]
- Sebastiani, S.; Albanesi, C.; De Pità, O.; Puddu, P.; Cavani, A.; Girolomoni, G. The role of chemokines in allergic contact dermatitis. Arch. Dermatol. Res. 2002, 293, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Komine, M.; Kakinuma, T.; Kagami, S.; Hanakawa, Y.; Hashimoto, K.; Tamaki, K. Mechanism of thymus-and activation-regulated chemokine (TARC)/CCL17 production and its modulation by roxithromycin. J. Investig. Dermatol. 2005, 125, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, M.-S.; Jeong, G.-S.; Yoon, J. Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J. Ethnopharmacol. 2015, 171, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-r.; Lee, T.H.; Bang, M.-H.; Kim, H.; Son, Y.; Chung, D.K.; Kim, J. Suppression of thymus-and activation-regulated chemokine (TARC/CCL17) production by 3-O-β-D-glucopyanosylspinasterol via blocking NF-κB and STAT1 signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes. Biochem. Biophys. Res. Commun. 2012, 427, 236–241. [Google Scholar] [CrossRef]
- Yang, J.-H.; Hwang, Y.-H.; Gu, M.-J.; Cho, W.-K.; Ma, J.Y. Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine 2015, 22, 1262–1268. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, S.-H.; Huang, C.-Y.; Dong, C.-D.; Huang, C.-Y.; Chang, C.-C.; Chang, J.-S. Effect of molecular mass and sulfate content of fucoidan from Sargassum siliquosum on antioxidant, anti-lipogenesis, and anti-inflammatory activity. J. Biosci. Bioeng. 2021, 132, 359–364. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Chen, M.; Jia, R.; Liu, B.; Zhao, C. The antidiabetic activity of brown seaweed Sargassum confusum polysaccharide hydrolysates in insulin resistance HepG2 cells in vitro. Res. J. Biotechnol. 2017, 12, 1–9. [Google Scholar]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R. Human keratinocyte UVB-protective effects of a low molecular weight fucoidan from Sargassum horneri purified by step gradient ethanol precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V. Anti-inflammatory activity of fucoidan extracts in vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.-J.; Ahn, G. Fucoidan Fractionated from Sargassum coreanum via Step-Gradient Ethanol Precipitation Indicate Promising UVB-Protective Effects in Human Keratinocytes. Antioxidants 2021, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.-Y.; Kim, Y.-S.; Lee, H.-G.; Lee, J.-S.; Jeon, Y.-J. Anti-photoaging and anti-melanogenesis effects of fucoidan isolated from Hizikia fusiforme and its underlying mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.-J.; Lee, K.; Cheong, S.H.; Ahn, G. Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. Int. J. Biol. Macromol. 2021, 168, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Fernando, I.S.; Wang, L.; Abetunga, D.; Kim, W.-S.; Lee, D.-S.; Jeon, Y.-J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.-S.; Vaas, A.; De Silva, H.; Nanayakkara, C.; Abeytunga, D.; Lee, W.; Ahn, G.; Lee, D.-S. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 2019, 233, 116714. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Raingeaud, J.; Pierre, J. Interleukin-4 downregulates TNFα-induced IL-8 production in keratinocytes. FEBS Lett. 2005, 579, 3953–3959. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Di, H.; Zou, Y.; Zhang, H.; Zhao, J. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Chung, H.S. Anti-inflammatory activity of fucoidan with blocking NF-κB and STAT1 in human keratinocytes cells. Nat. Prod. Sci. 2015, 21, 205–209. [Google Scholar]
- Lim, H.-S.; Yeji, K.; Seo, C.-S.; Yoo, S.-R.; Jin, S.-E.; Shin, H.-K.; Jeong, S.-J. Chungsimyeonja-eum inhibits inflammatory responses in RAW 264.7 macrophages and HaCaT keratinocytes. BMC Complement. Altern. Med. 2015, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Ribet, V.; Alvarez-Georges, S.; Sibaud, V.; Guerrero, D.; Schmitt, A.M.; Redoules, D. Modulation of Interleukin-8 and staphylococcal flora by Avène hydrotherapy in patients suffering from chronic inflammatory dermatoses. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; D’Amore, M. Altered IkBα expression promotes NF-kB activation in monocytes from primary Sjögren’s syndrome patients. Pathology 2012, 44, 557–561. [Google Scholar] [CrossRef]
- Simmons, L.J.; Surles-Zeigler, M.C.; Li, Y.; Ford, G.D.; Newman, G.D.; Ford, B.D. Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway. J. Neuroinflammation 2016, 13, 237. [Google Scholar] [CrossRef]
- Ryu, M.J.; Chung, H.S. Fucoidan reduces oxidative stress by regulating the gene expression of HO-1 and SOD-1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol. Med. Rep. 2016, 14, 3255–3260. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 signaling. React. Oxyg. Species (Apex NC) 2019, 8, 312. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.-J.; Hong, S.H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; Kim, B.W.; Jeon, Y.-J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, A.M.K.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Han, E.J.; Oh, G.-W.; Jung, W.-K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. https://doi.org/10.3390/md20020117
Jayasinghe AMK, Kirindage KGIS, Fernando IPS, Han EJ, Oh G-W, Jung W-K, Ahn G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Marine Drugs. 2022; 20(2):117. https://doi.org/10.3390/md20020117
Chicago/Turabian StyleJayasinghe, Arachchige Maheshika Kumari, Kirinde Gedara Isuru Sandanuwan Kirindage, Ilekuttige Priyan Shanura Fernando, Eui Jeong Han, Gun-Woo Oh, Won-Kyo Jung, and Ginnae Ahn. 2022. "Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway" Marine Drugs 20, no. 2: 117. https://doi.org/10.3390/md20020117
APA StyleJayasinghe, A. M. K., Kirindage, K. G. I. S., Fernando, I. P. S., Han, E. J., Oh, G.-W., Jung, W.-K., & Ahn, G. (2022). Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Marine Drugs, 20(2), 117. https://doi.org/10.3390/md20020117