Therapeutic Potential and Nutraceutical Profiling of North Bornean Seaweeds: A Review
Abstract
1. Introduction
2. Therapeutic Potential of North Bornean Seaweeds
2.1. Anti-Inflammatory Activity
2.2. Antioxidant Activity
2.3. Antimicrobial Activity
2.4. Anticancer Activity
2.5. Anti-Obesity and Cardiovascular Protection
2.6. Hepatoprotection
2.7. Neuroprotection
3. Nutraceutical Profiling of North Bornean Seaweeds
4. Methodology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Ito, K.; Hori, K. Seaweed: Chemical composition and potential food use. Food Rev. Int. 1989, 5, 101–144. [Google Scholar] [CrossRef]
- Saravanan, K.R.; Ilangovan, K.; Khan, A.B. Floristic and macro faunal diversity of Pondicherry mangroves, South India. Trop. Ecol. 2008, 49, 91–94. [Google Scholar]
- Dmytryk, A.; Tuhy, Ł.; Chojnacka, K. Algae as source of pharmaceuticals. In Prospects and Challenges in Algal Biotechnology; Springer: Singapore, 2017; pp. 295–310. ISBN 9789811019500. [Google Scholar]
- Murugan, K.; Iyer, V.V. Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown, and green marine algae. In Vitro Cell. Dev. Biol.-Anim. 2013, 49, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Melanie, H.; Taarji, N.; Zhao, Y.; Khalid, N.; Neves, M.A.; Kobayashi, I.; Tuwo, A.; Nakajima, M. Formulation and characterisation of O/W emulsions stabilised with modified seaweed polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 211–221. [Google Scholar] [CrossRef]
- Hussin, H.; Khoso, A. Seaweed cultivation and coastal communities in Malaysia: An overview. Asian Fish. Sci. 2017, 30, 87–100. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Gerung, G.S.; Yasir, S.; Critchley, A.T. Cultivation of tropical red seaweeds in the BIMP-EAGA region. J. Appl. Phycol. 2014, 26, 707–718. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G. Algaebase: Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 23 September 2021).
- Lim, C.L.; Koh, R.Y.; Haw, T.Y.; Boudville, L.A. Antioxidant activity of the Sea-Bird Nest (Eucheuma Cottonii) and its radical scavenging effect on human keratinocytes. J. Med. Bioeng. 2015, 4, 461–465. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mustar, S.; Mustafa Khalid, N.; Abd Rashed, A.; Mohd Noh, M.F.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Pearson, J.P. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The seaweed diet in prevention and treatment of the Neurodegenerative diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Thanigaivel, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Seaweeds as an alternative therapeutic source for aquatic disease management. Aquaculture 2016, 464, 529–536. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Anyanji, V.U.; Mustapha, N.M.; Lim, S.L.; Mohamed, S. Seaweed (Eucheuma cottonii) reduced inflammation, mucin synthesis, eosinophil infiltration and MMP-9 expressions in asthma-induced rats compared to Loratadine. J. Funct. Foods 2015, 19, 710–722. [Google Scholar] [CrossRef]
- Buwono, N.R.; Risjani, Y.; Arsad, S. Anti-inflammatory and analgesic activity from brown algae Sargassum polycystum. J. Pharm. Sci. Res. 2018, 10, 2092–2096. [Google Scholar]
- Saito, H.; Lal, T.M. Antimycotic activity of seaweed extracts (Caulerpa lentillifera and Eucheuma cottonii) against two genera of marine oomycetes, Lagenidium spp. And Haliphthoros spp. Biocontrol Sci. 2019, 24, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Amano, H.; Arashima, K.; Nisizawa, K. Antitumor activity of marine algae. Hydrobiologia 1990, 204–205, 577–584. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J. Med. Food 2010, 13, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Gany, S.A.; Ching, S.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. World Acad. Sci. Eng. Technol. 2014, 8, 1269–1275. [Google Scholar]
- Raghavendran, H.R.B.; Sathivel, A.; Devaki, T. Hepatoprotective nature of seaweed alcoholic extract on acetaminophen induced hepatic oxidative stress. J. Health Sci. 2004, 50, 42–46. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 125–131. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef]
- Ribeiro, N.A.; Abreu, T.M.; Chaves, H.V.; Bezerra, M.M.; Monteiro, H.S.A.; Jorge, R.J.B.; Benevides, N.M.B. Sulfated polysaccharides isolated from the green seaweed Caulerpa racemosa plays antinociceptive and anti-inflammatory activities in a way dependent on HO-1 pathway activation. Inflamm. Res. 2014, 63, 569–580. [Google Scholar] [CrossRef]
- Yoon, W.J.; Ham, Y.M.; Kim, K.N.; Park, S.Y.; Lee, N.H.; Hyun, C.G.; Lee, W.J. Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage RAW 264.7 cells. J. Med. Plants Res. 2009, 3, 001–008. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Seelan Sathiya Seelan, J.; Iqbal, M. Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves. Arab. J. Chem. 2020, 13, 7170–7182. [Google Scholar] [CrossRef]
- Shah, M.D.; Iqbal, M. Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem. Toxicol. 2010, 48, 3345–3353. [Google Scholar] [CrossRef]
- Shah, M.D.; Iqbal, M. Antioxidant activity, phytochemical analysis and total polyphenolics content of essential oil, methanol extract and methanol fractions from Commelina nudiflora. Int. J. Pharm. Pharm. Sci. 2018, 10, 36. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Manikandakrishnan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of antioxidant and anticancer potential of fucoidan from Sargassum polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- NIH Understanding Emerging and Re-Rmerging Infectious Diseases—National Institute of Health (NIH) Curriculum Supplement Series—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK20370/ (accessed on 14 September 2021).
- Teo, B.S.X.; Gan, R.Y.; Abdul Aziz, S.; Sirirak, T.; Mohd Asmani, M.F.; Yusuf, E. In vitro evaluation of antioxidant and antibacterial activities of Eucheuma cottonii extract and its in vivo evaluation of the wound-healing activity in mice. J. Cosmet. Dermatol. 2021, 20, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, Y.; Halimah, E.; Shifa, N.; Fushilla, Z.; Silvana, G.; Zuhrotun, A. Activity of reed algae (Eucheuma Cottonii) against some Bacteria and Fungi. J. Pharm. Sci. Res. 2019, 11, 2362–2366. [Google Scholar]
- Nakamura, K.; Nakamura, M.; Hatai, K. Zafran Lagenidium infection in eggs and larvae of mangrove crab (Scylla serrata) produced in Indonesia. Mycoscience 1995, 36, 399–404. [Google Scholar] [CrossRef]
- Lee, Y.N.; Hatai, K.; Kurata, O. Haliphthoros sabahensis sp. nov. isolated from mud crab Scylla tranquebarica eggs and larvae in Malaysia. Fish Pathol. 2017, 52, 31–37. [Google Scholar] [CrossRef]
- Sato, Y.; Morimoto, K.; Hirayama, M.; Hori, K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem. Biophys. Res. Commun. 2011, 405, 291–296. [Google Scholar] [CrossRef]
- Safitri, A.; Srihardyastutie, A.; Roosdiana, A.; Sutrisno, S. Antibacterial activity and phytochemical analysis of edible seaweed Eucheuma spinosum against Staphyloccocus aureus. J. Pure Appl. Chem. Res. 2018, 7, 308–315. [Google Scholar] [CrossRef]
- Julyasih, K.S.; Purnawati, A. Antifungal activity of seaweed against Aspergillus flavus. In Proceedings of the Nusantara Science and Technology Proceedings, Galaxy Science, Surabaya, Indonesia, 23 November 2018; pp. 11–13. [Google Scholar] [CrossRef]
- Montanha, J.A.; Bourgougnon, N.; Boustie, J.; Amoros, M. Antiviral activity of carrageenans from marine red algae. Am. J. Pharm 2009, 28, 443–451. [Google Scholar]
- Atienza, J.O.; Evangelista, R.A. Taxonomy and Screening for Antimicrobial Activities of Selected Marine Algal Species from Talaga East, Mabini, Batangas. Available online: https://animorepository.dlsu.edu.ph/etd_bachelors/3840 (accessed on 26 October 2021).
- Klongklaew, N.; Praiboon, J.; Tamtin, M.; Srisapoome, P. Antibacterial and antiviral activities of local Thai green macroalgae crude extracts in pacific white shrimp (litopenaeus vannamei). Mar. Drugs 2020, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.V. Virus diseases of farmed shrimp in the Western Hemisphere (the Americas): A review. J. Invertebr. Pathol. 2011, 106, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. A future vision for disease control in shrimp aquaculture. J. World Aquac. Soc. 2019, 50, 249–266. [Google Scholar] [CrossRef]
- Chan, Y.S.; Ong, C.W.; Chuah, B.L.; Khoo, K.S.; Chye, F.Y.; Sit, N.W. Antimicrobial, antiviral and cytotoxic activities of selected marine organisms collected from the coastal areas of Malaysia. J. Mar. Sci. Technol. 2018, 26, 128–136. [Google Scholar] [CrossRef]
- Simon, F.; Javelle, E.; Oliver, M.; Leparc-Goffart, I.; Marimoutou, C. Chikungunya virus infection. Curr. Infect. Dis. Rep. 2011, 13, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Z.; Yilmaz-Koz, F.F.; Karabay-Yavasoglu, U.N.; Ozdemir, G.; Sukatar, A. Antimicrobial and antioxidant activity of brown algae from the Aegean Sea. J. Serb. Chem. Soc. 2009, 74, 619–628. [Google Scholar] [CrossRef]
- Thirumaran, G.; Vijayabaskar, P.; Anantharaman, P. Antibacterial and antifugal activities of brown marine macroalge (Dictyota dichotoma) from the Gulf of Mannar biosphere reserve. Environ. Ecolol. 2006, 24, 37–40. [Google Scholar]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Muckelbauer, J.K.; Kremer, M.; Minor, I.; Diana, G.; Dutko, F.J.; Groarke, J.; Pevear, D.C.; Rossmann, M.G. The structure of coxsackievirus B3 at 3.5 å resolution. Structure 1995, 3, 653–667. [Google Scholar] [CrossRef]
- Thangaraju, N.; Venkatalakshmi, R.P.; Chinnasamy, A.; Kannaiyan, P. Synthesis of silver nanoparticles and the antibacterial and anticancer activities of the crude extract of Sargassum polycystum C. Agardh. Nano Biomed. Eng. 2012, 4, 89–94. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S.; Prodpran, T. Extraction, antioxidative, and antimicrobial activities of brown seaweed extracts, Turbinaria ornata and Sargassum polycystum, grown in Thailand. Int. Aquat. Res. 2015, 7, 1–16. [Google Scholar] [CrossRef]
- Sawadogo, W.; Schumacher, M.; Teiten, M.-H.; Cerella, C.; Dicato, M.; Diederich, M. A Survey of Marine natural compounds and their dserivatives with anti-cancer activity reported in 2011. Molecules 2013, 18, 3641–3673. [Google Scholar] [CrossRef]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Induction of apoptosis in MCF-7 cells by β-1,3-xylooligosaccharides prepared from Caulerpa lentillifera. Biosci. Biotechnol. Biochem. 2012, 76, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Shao, H.; Zhang, C.; Hong, P.; Xiong, H. Separation of the polysaccharides in Caulerpa racemosa and their chemical composition and antitumor activity. J. Appl. Polym. Sci. 2008, 110, 1435–1440. [Google Scholar] [CrossRef]
- Miranda, M.A.; Chiou, C.T.; Tayo, L.L.; Hsieh, C.L.; Tsai, P.W. In vitro anticancer activities of selected Philippine seaweeds against several cancer cell lines. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 108–114. [Google Scholar]
- El-Shaibany, A.; AL-Habori, M.; Al-Maqtari, T.; Al-Mahbashi, H. The Yemeni brown algae Dictyota dichotoma exhibit high in vitro anticancer activity independent of its antioxidant capability. Biomed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arsianti, A.A.; Fadilah, F.; Fatmawaty, Y.; Wibisono, L.K.; Kusmardi, S.; Azizah, N.N.; Putrianingsih, R.; Murniasih, T.; Rasyid, A.; Pangestuti, R. Phytochemical composition and anticancer activity of seaweeds Ulva lactuca and Eucheuma cottonii against breast MCF-7 and colon HCT-116 cells. Asian J. Pharm. Clin. Res. 2016, 9, 115–119. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.X.; Li, K.H.; Yi, N.Z.; Jeong, H.L.; Chang, K.S. In vitro inhibitory effect of triterpenoidal saponins from Platycodi radix on pancreatic lipase. Arch. Pharm. Res. 2005, 28, 180–185. [Google Scholar] [CrossRef]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Larson, J.J.; Yawn, B.; Therneau, T.M.; Kim, W.R. Underestimation of liver-related mortality in the United States. Gastroenterology 2013, 145, 375–382.e2. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12. [Google Scholar] [CrossRef]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Morgan, M.Y. The prognosis and outcome of alcoholic liver disease. Alcohol Alcohol Suppl. 1994, 2, 335–343. [Google Scholar] [PubMed]
- World Health Organization. Global Burden of Disease Estimates for 2000–2012; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver Injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef]
- Hsu, P.C.; Guo, Y.L. Antioxidant nutrients and lead toxicity. Toxicology 2002, 180, 33–44. [Google Scholar] [CrossRef]
- Shah, M.D.; D’Souza, U.J.A.; Iqbal, M. The potential protective effect of Commelina nudiflora L. against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, mediated by suppression of oxidative stress and inflammation. Environ. Health Prev. Med. 2017, 22, 1–19. [Google Scholar] [CrossRef]
- Wardani, G.; Farida, N.; Andayani, R.; Kuntoro, M.; Sudjarwo, S. The potency of red seaweed (Eucheuma cottonii) extracts as hepatoprotector on lead acetate-induced hepatotoxicity in mice. Pharmacognosy Res. 2017, 9, 282–286. [Google Scholar] [CrossRef]
- Shah, M.D.; Gnanaraj, C.; Haque, A.E.; Iqbal, M. Antioxidative and chemopreventive effects of Nephrolepis biserrata against carbon tetrachloride (CCl4)-induced oxidative stress and hepatic dysfunction in rats. Pharm. Biol. 2015, 53, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Codorniz, K.D.; Marquina, R.E.M.; Nolasco, A.D.G.; Palencia, P.D.D.; Mata, S.B. Evaluation of the hepatoprotective effect of methanolic extract of Caulerpa lentillifera against acetaminophen-induced liver toxicity in juvenile zebrafish (Danio rerio). J. Ilm. Farm. 2020, 16, 31–38. [Google Scholar] [CrossRef]
- Azam, M.; Hira, K.; Qureshi, S.A.; Khatoon, N.; Ara, J.; Ehteshamul-Haque, S. Ameliorative effect of marine macro-algae against carbon tetrachloride (CCl4) induced hepatic fibrosis and associated complications in rats. Turk. J. Pharm. Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine bio compounds for neuroprotection—A review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef] [PubMed]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. The tropical carrageenophyte Kappaphycus alvarezii extract promotes axodendritic maturation of hippocampal neurons in primary culture. J. Appl. Phycol. 2018, 30, 3233–3241. [Google Scholar] [CrossRef]
- Suganthy, N.; Karutha Pandian, S.; Pandima Devi, K. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef]
- Stoppe, G.; Sandholzer, H.; Staedt, J.; Winter, S.; Kiefer, J.; Rüther, E. Prescribing practice with cognition enhancers in outpatient care: Are there differences regarding type of Dementia? -Results of a representative survey in Lower Saxony, Germany. Pharmacopsychiatry 2007, 29, 150–155. [Google Scholar] [CrossRef]
- Zarotsky, V.; Sramek, J.J.; Cutler, N.R. Galantamine hydrobromide: An agent for Alzheimer’s disease. Am. J. Health Pharm. 2003, 60, 446–452. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. The potential of Eucheuma cottonii extract as a candidate for fish anesthetic agent. Aquac. Fish. 2021. [Google Scholar] [CrossRef]
- Vimala, B.L.C.; June, I.A.B.; Fairunizal, M.N.; Syahida, A.; Ismail, I. Composition of docosahexaenoic acid, eicosapentaenoic acid and palmitoleic acid in Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera ethanolic extracts by LC-ESI-MS/MS. J. Mar. Biol. Oceanogr. 2018, 7, 1. [Google Scholar] [CrossRef]
- Yum, H.W.; Na, H.K.; Surh, Y.J. Anti-inflammatory effects of docosahexaenoic acid: Implications for its cancer chemopreventive potential. Semin. Cancer Biol. 2016, 40–41, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Gumgumjee, N.; Hajar, A.S. Antibacterial activities and GC-MS analysis of phytocomponents of Ehretia abyssinica R.Br. Int. J. Appl. Biol. Phamaceutical Technol. 2015, 6, 236–242. [Google Scholar]
- Dhevika, S.; Deivasigamani, B. Phytochemical profiling and GC-MS analysis of Caulerpa racemosa. Res. J. Life Sci. Bioinformatics, Pharm. Chem. Sci. 2018, 4, 155–165. [Google Scholar] [CrossRef]
- Tassakka, A.C.M.A.R.; Sumule, O.; Massi, M.N.; Manggau, M.; Iskandar, I.W.; Alam, J.F.; Permana, A.D.; Liao, L.M. Potential bioactive compounds as SARS-CoV-2 inhibitors from extracts of the marine red alga Halymenia durvillei (Rhodophyta)—A computational study. Arab. J. Chem. 2021, 14, 103393. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Jh, Z.; Hl, S.; Sy, C.; Zeng, L.; Wang, T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef]
- Nor Qhairul Izzreen, M.N.; Vijaya Ratnam, R. Volatile compound extraction using Solid phase micro extraction coupled with gas chromatography mass spectrometry (SPME-GCMS) in local seaweeds of Kappaphycus alvarezii, Caulerpa lentillifera and Sargassum polycystem. Int. Food Res. J. 2011, 18, 1449–1456. [Google Scholar]
- Vanitha, V.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.N.; Vidhya, E.; Praseetha, P.K. Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crops Prod. 2020, 154, 112748. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and antibacterial activity of red seaweed; Kappaphycus alvarezii against pathogenic bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar] [CrossRef]
- Solari, A.; Uitdehaag, B.M.; Giuliani, G.; Pucci, E.; Taus, C. Aminopyridines for symptomatic treatment in multiple sclerosis. Cochrane Database Syst. Rev. 2002, 4, CD001330. [Google Scholar] [CrossRef]
- Das, U.N. Gamma-linolenic acid, arachidonic acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition 1990, 6, 429–434. [Google Scholar] [PubMed]
- de Souza, C.O.; Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Rosa Neto, J.C.; Calder, P.C. Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to Oleic and Palmitic acids. Mol. Nutr. Food Res. 2018, 62, 1800322. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; June Chelyn, L.; Vimala, S.; Mohd Fairulnizal, M.N.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Shimode, S.; Miyata, K.; Araki, M.; Shindo, K. Antioxidant activities of the Antheraxanthin-related carotenoids, Antheraxanthin, 9-cis-Antheraxanthin, and Mutatoxanthins. J. Oleo Sci. 2018, 67, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, C.; Kim, M.B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.K.; Lee, J.Y. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef]
- Gansukh, E.; Nile, A.; Sivanesan, I.; Rengasamy, K.R.R.; Kim, D.H.; Keum, Y.S.; Saini, R.K. Chemopreventive effect of β-cryptoxanthin on human cervical carcinoma (HeLa) cells is modulated through oxidative stress-induced apoptosis. Antioxidants 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, Y.; Azqueta, A.; Luna, L.; Bonilla, F.; Domínguez, G.; Collins, A.R. The carotenoid β-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 2009, 30, 308–314. [Google Scholar] [CrossRef]
- Ngginak, J.; Mangibulude, J.C.; Rondonuwu, F.S. The identification of carotenoids and testing of carotenoid antioxidants from Sand lobster (Panulirus homarus) egg extract. Indones. J. Mar. Sci./Ilmu Kelautan 2017, 22, 155–160. [Google Scholar] [CrossRef][Green Version]
- Pistelli, L.; Sansone, C.; Smerilli, A.; Festa, M.; Noonan, D.M.; Albini, A.; Brunet, C. Mmp-9 and il-1β as targets for diatoxanthin and related microalgal pigments: Potential chemopreventive and photoprotective agents. Mar. Drugs 2021, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Fucoxanthin inhibits tumor cell growth by inducing G 1 cell-cycle arrest with/ without apoptosis in various tumor =cells antitumor and cancer-preventative function of Fucoxanthin: A marine carotenoid. Anticancer. Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016, 16. [Google Scholar] [CrossRef]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of Eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Shibula, K.; Velavan, S. Determination of phytocomponents in methanolic extract of Annona muricata leaf Using GC-MS technique. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1251–1255. [Google Scholar]
- Singh, V.K.; Kavita, K.; Prabhakaran, R.; Jha, B. Cis-9-octadecenoic acid from the rhizospheric bacterium Stenotrophomonas maltophilia BJ01 shows quorum quenching and anti-biofilm activities. Biofouling 2013, 29, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Chanprapai, P.; Kubo, I.; Chavasiri, W. Anti-rice pathogenic microbial activity of Persicaria sp. extracts. Sci. Technol. Asia 2018, 23, 32–41. [Google Scholar] [CrossRef]
- Xu, C.; Wu, P.; Gao, J.; Zhang, L.; Ma, T.; Ma, B.; Yang, S.; Shao, G.; Yu, Y.; Huang, X.; et al. Heptadecanoic acid inhibits cell proliferation in PC-9 non-small-cell lung cancer cells with acquired gefitinib resistance. Oncol. Rep. 2019, 41, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- To, N.B.; Nguyen, Y.T.K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic acid, an odd-chain fatty acid, suppresses the stemness of MCF-7/SC human breast cancer stem-like cells through JAK2/STAT3 signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Sivakumar, R.; Jebanesan, A.; Govindarajan, M.; Rajasekar, P. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 706–710. [Google Scholar] [CrossRef]
- Chan, K.C.; Mong, M.C.; Yin, M.C. Antioxidative and anti-inflammatory neuroprotective effects of Astaxanthin and Canthaxanthin in nerve growth factor differentiated PC12 cells. J. Food Sci. 2009, 74, H225–H231. [Google Scholar] [CrossRef]
- Halim, N.A.; Zamimi, N.N.; Kamar, S.H.S. Analysis of the fatty acid composition of Caulerpa Lentillifera using gas chromatography mass spectrometry. Int. J. Allied Health Sci. 2019, 3, 806. [Google Scholar]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic acid protects against Cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.M.; Giribabu, N.; Yelumalai, S.; Shahzad, H.; Kilari, E.K.; Salleh, N. Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: Restoration of spermatogenesis, steroidogenesis in diabetic rats. Life Sci. 2021, 278, 119605. [Google Scholar] [CrossRef]
- Moreira, D.K.T.; Santos, P.S.; Gambero, A.; Macedo, G.A. Evaluation of structured lipids with behenic acid in the prevention of obesity. Food Res. Int. 2017, 95, 52–58. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar]
- Arruda, D.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.U.; Katzin, A.M.; Uliana, S.R.B. Inhibitory activity of limonene against Leishmania parasites in vitro and in vivo. Biomed. Pharmacother. 2009, 63, 643–649. [Google Scholar] [CrossRef]
- Okechukwu, P.N. Evaluation of anti-Inflammatory, analgesic, antipyretic effect of Eicosane, Pentadecane, Octacosane, and Heneicosane. Asian J. Pharm. Clin. Res. 2020, 13, 29–35. [Google Scholar] [CrossRef]
- Guarrera, M.; Turbino, L.; Rebora, A. The anti-inflammatory activity of Azulene. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 486–487. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, X.; Zhang, T.; Ye, J.; Fang, Z.; Yang, X. Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/d- galactosamine. Eur. J. Pharmacol. 2014, 724, 112–121. [Google Scholar] [CrossRef]
- Lee, J.I.; Burckart, G.J. Nuclear factor kappa B: Important transcription factor and therapeutic target. J. Clin. Pharmacol. 1998, 38, 981–993. [Google Scholar] [CrossRef]
- Aissaoui, N.; Mahjoubi, M.; Nas, F.; Mghirbi, O.; Arab, M.; Souissi, Y.; Hoceini, A.; Masmoudi, A.S.; Mosbah, A.; Cherif, A.; et al. Antibacterial potential of 2,4-Di-tert-Butylphenol and Calixarene-based prodrugs from Thermophilic Bacillus licheniformis isolated in Algerian hot spring. Geomicrobiol. J. 2019, 36, 53–62. [Google Scholar] [CrossRef]
- Nair, R.V.R.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-inflammatory and anticancer activities of Erythrodiol-3-acetate and 2,4-Di-tert-butylphenol isolated from Humboldtia unijuga. Nat. Prod. Res. 2020, 34, 2319–2322. [Google Scholar] [CrossRef]
- Dandekar, R.; Fegade, B.; Vh, B. GC-MS analysis of phytoconstituents in alcohol extract of Epiphyllum oxypetalum leaves. J. Pharmacogn. Phytochem. Mater. 2015, 4, 149–154. [Google Scholar]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-Hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.D.F.; Blank, D.E.; Peixoto, C.R.; De Jesus Da Silveira Moreira, J.; Fernandes De Moura, N. Bioactive compounds and antioxidant activity of Bunchosia glandulifera. Int. J. Food Prop. 2016, 19, 467–473. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Jeon, Y.J. Squalene isolated from marine macroalgae Caulerpa racemosa and its potent antioxidant and anti-inflammatory activities. J. Food Biochem. 2018, 42, e12628. [Google Scholar] [CrossRef]
- Murakoshi, M.; Nishino, H.; Tokuda, H.; Iwashima, A.; Okuzumi, J.; Kitano, H.; Iwasaki, R. Inhibition by squalene of the tumor-promoting activity of 12-O-Tetradecanoylphorbol-13-acetate in mouse-skin carcinogenesis. Int. J. Cancer 1992, 52, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid and methoxyacetic acid induce apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2015, 16, 11750–11765. [Google Scholar] [CrossRef] [PubMed]
- Formuso, C.; Stracquadanio, M.; Ciotta, L. Myo-inositol vs. D-chiro inositol in PCOS treatment. Minerva Ginecol. 2015, 67, 321–325. [Google Scholar]
- Thorgeirsdóttir, T.Ó.; Kristmundsdóttir, T.; Thormar, H.; Axelsdóttir, Í.; Holbrook, W.P. Antimicrobial activity of monocaprin: A monoglyceride with potential use as a denture disinfectant. Acta Odontol. Scand. 2006, 64, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Matsabisa, M.G.; Erukainure, O.L.; Ibeji, C.U.; Islam, M.S. D-mannitol modulates glucose uptake ex vivo; suppresses intestinal glucose absorption in normal and type 2 diabetic rats. Food Biosci. 2019, 29, 30–36. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Stonik, V.A. Diterpenoid hydroperoxides from the far-eastern brown alga Dictyota dichotoma. Aust. J. Chem. 2009, 62, 1185. [Google Scholar] [CrossRef]
- Bruno, F.; Castelli, G.; Migliazzo, A.; Piazza, M.; Galante, A.; Verde, V.L.; Calderone, S.; Nucatolo, G.; Vitale, F. Cytotoxic screening and in vitro evaluation of pentadecane against leishmania infantum promastigotes and amastigotes. J. Parasitol. 2015, 101, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of Fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- Lee, J.A.; Cho, Y.R.; Hong, S.S.; Ahn, E.K. Anti-Obesity Activity of Saringosterol isolated from Sargassum muticum (Yendo) Fensholt Extract in 3T3-L1 Cells. Phyther. Res. 2017, 31, 1694–1701. [Google Scholar] [CrossRef]
- Yu, F.; Lu, S.; Yu, F.; Shi, J.; McGuire, P.M.; Wang, R. Cytotoxic activity of an octadecenoic acid extract from Euphorbia kansui (Euphorbiaceae) on human tumour cell strains. J. Pharm. Pharmacol. 2010, 60, 253–259. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata suppresses LPS-induced inflammatory response in RAW 264.7 Macrophages and Sprague Dawley rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- Nazarudin, M.F.; Alias, N.H.; Balakrishnan, S.; Wan Hasnan, W.N.I.; Noor Mazli, N.A.I.; Ahmad, M.I.; Md Yasin, I.S.; Isha, A.; Aliyu-Paiko, M. Chemical, nutrient and physicochemical properties of brown seaweed, Sargassum polycystum c. Agardh (phaeophyceae) collected from port dickson, Peninsular Malaysia. Molecules 2021, 26, 5216. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, H.; Ohigashi, H.; Kobayashi, M.; Koshimizu, K.; Takahashi, E. Inactivation of T5 phage by cis-vaccenic acid, an antivirus substance from Rhodopseudomonas capsulata, and by unsaturated fatty acids and related alcohols. FEMS Microbiol. Lett. 1991, 77, 13–18. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, F.Y.; Bennett, G.J.; Bordet, T.; Pruss, R.M. Olesoxime (cholest-4-en-3-one, oxime): Analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain 2009, 147, 202–209. [Google Scholar] [CrossRef] [PubMed]
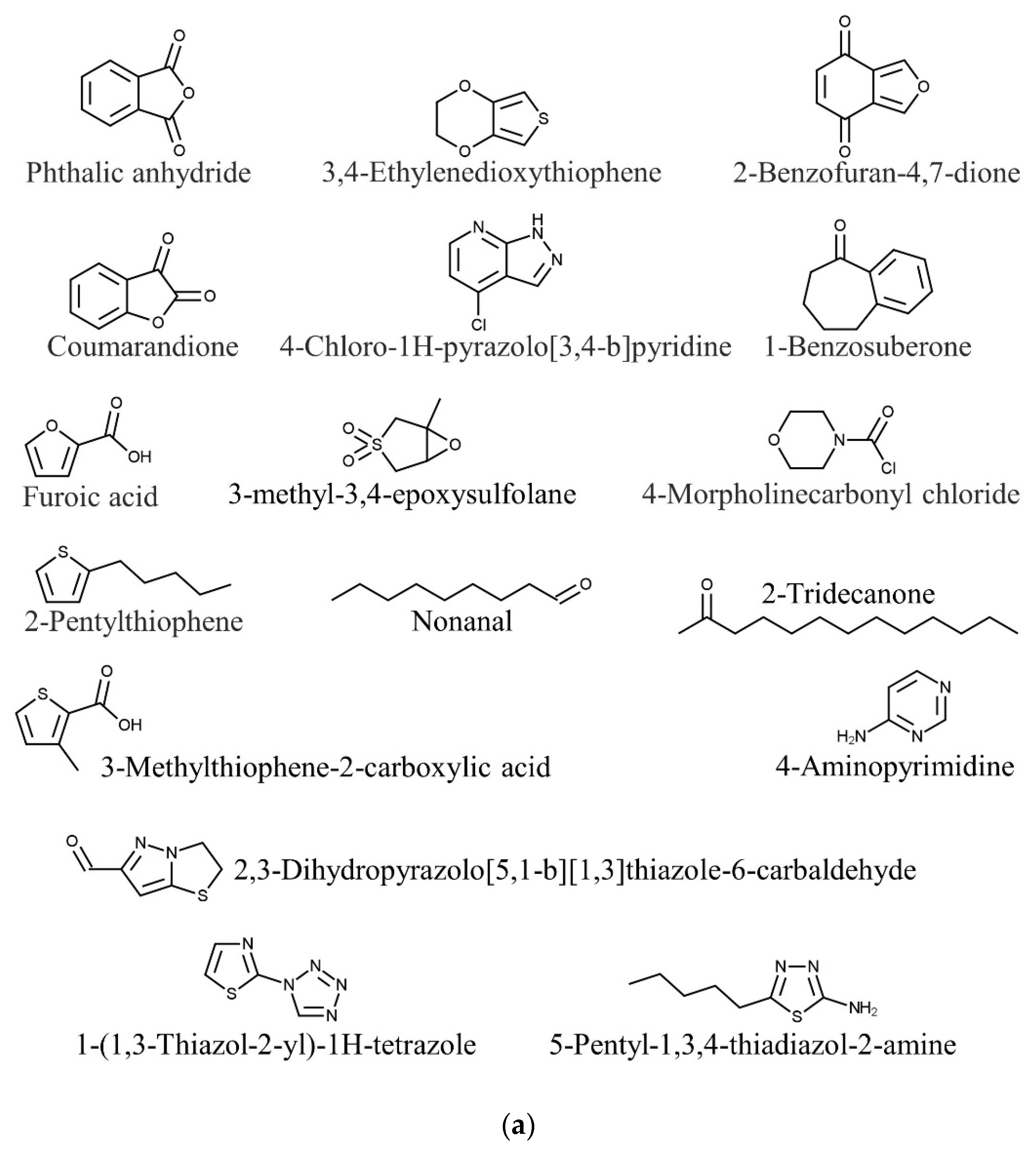
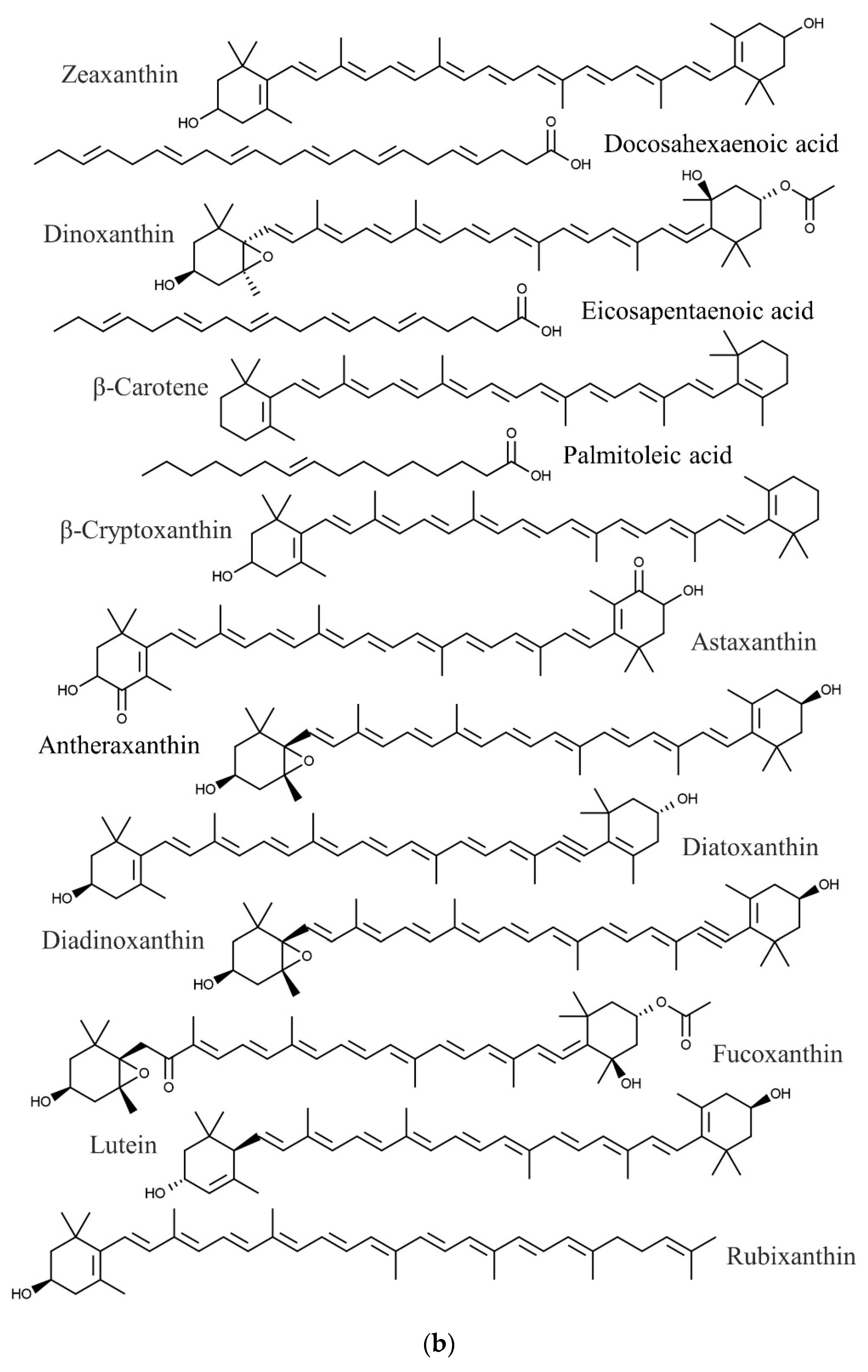
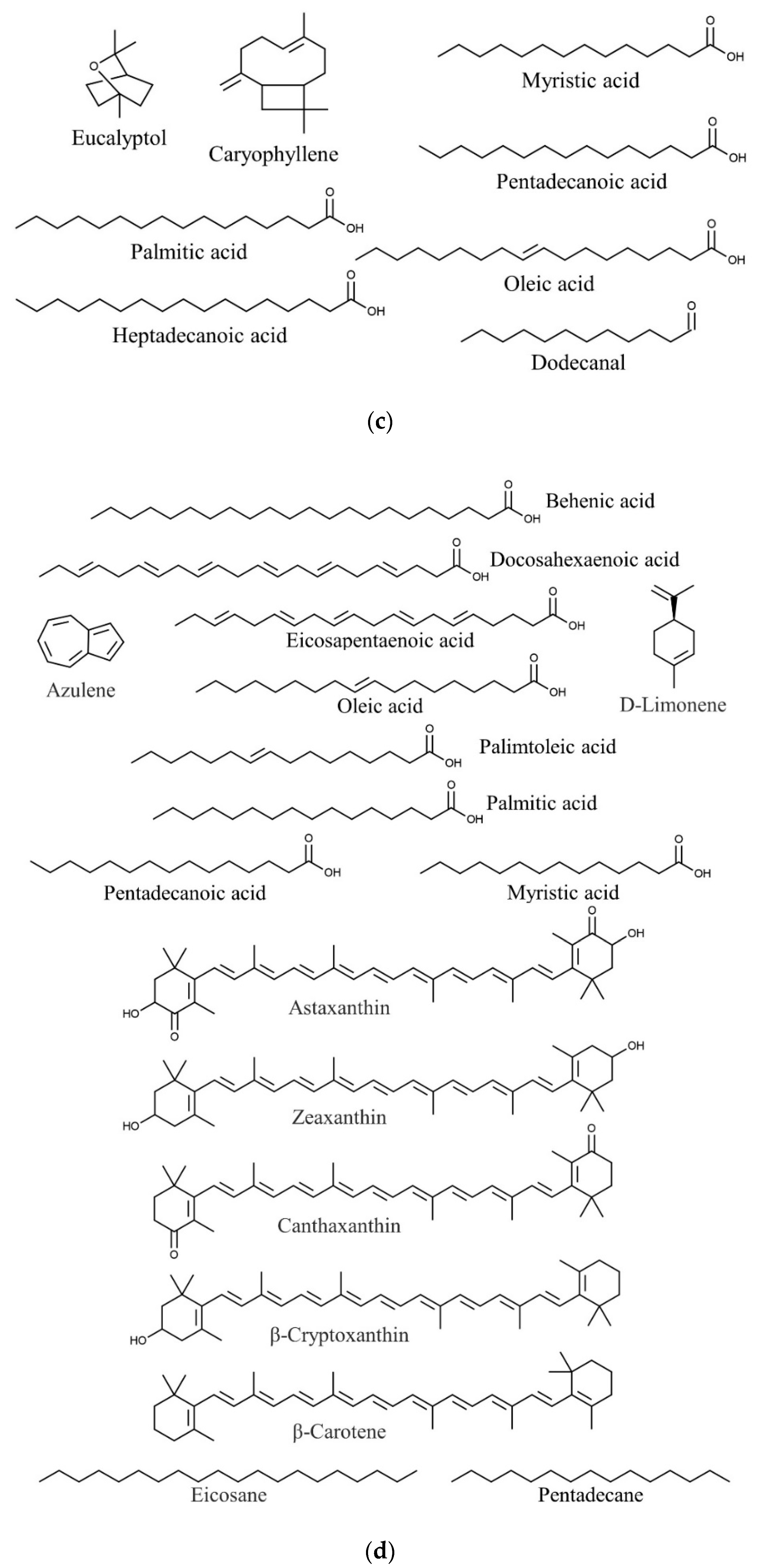

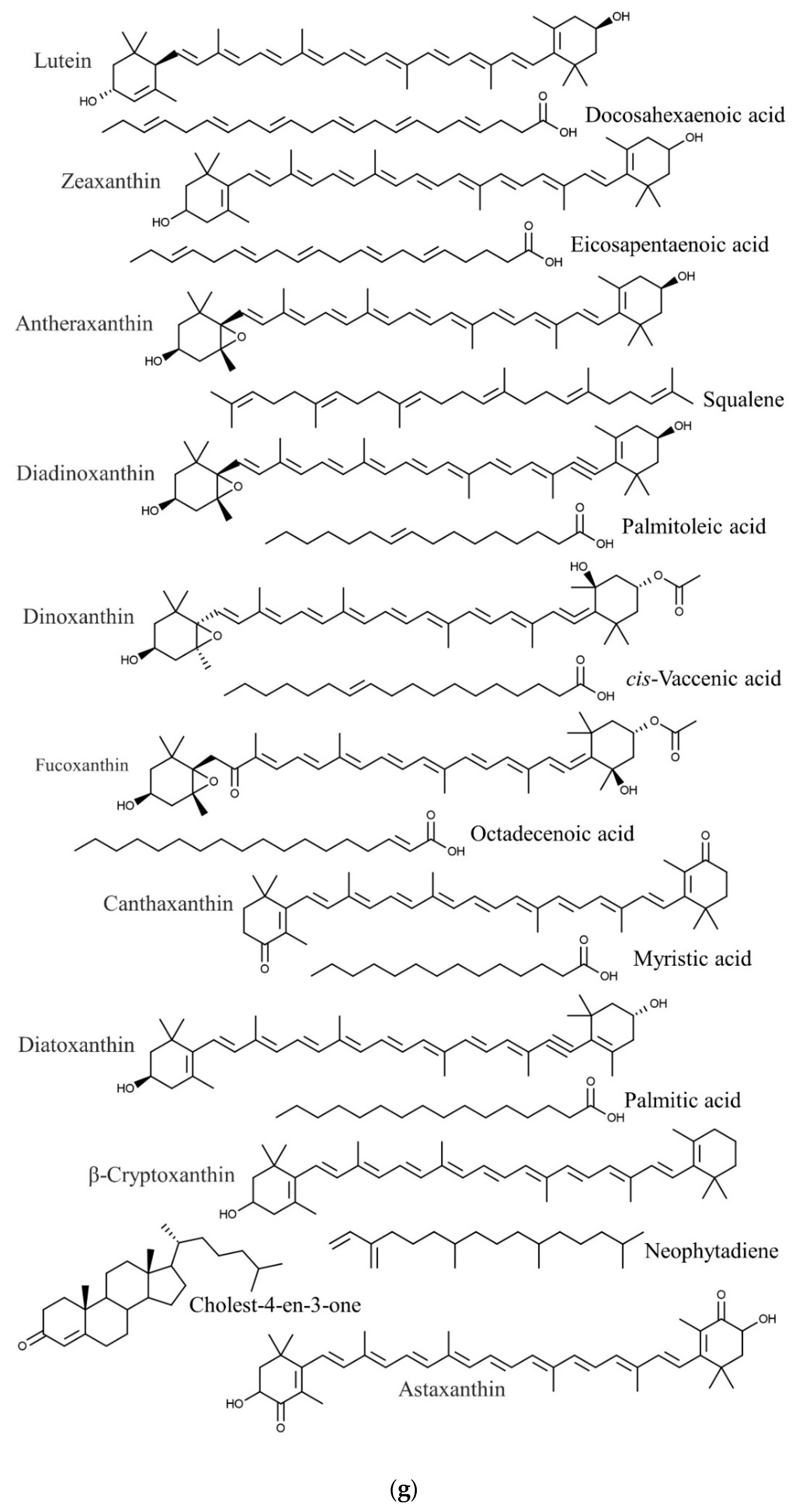
| Species | Phylum | Family | Other Names | References |
|---|---|---|---|---|
| Kappaphycus alvarezii | Rhodophyta | Solieriaceae | Eucheuma cottonii or sea bird nest | [9,10,11] |
| Eucheuma denticulatum | Rhodophyta | Solieriaceae | Eucheuma spinosum | [9,10,12] |
| Halymenia durvillaei | Rhodophyta | Halymeniaceae | [9,10] | |
| Dictyota dichotoma | Ochrophyta | Dictyotaceae | [9,10] | |
| Sargassum polycystum | Ochrophyta | Sargassaceae | [9,10] | |
| Caulerpa lentillifera | Chlorophyta | Caulerpaceae | Sea grape | [9,10] |
| Caulerpa racemosa | Chlorophyta | Caulerpaceae | Sea grape | [9,10] |
| Seaweed Species | Protective Effects | ||||||
|---|---|---|---|---|---|---|---|
| Anti Inflammatory | Anti Oxidant | Anti Microbial | Anti Cancer | Anti-Obesity and Cardiovascular Protection | Hepato Protection | Neuro- Protection | |
| Kappaphycus alvarezii | + | + | + | + | + | + | + |
| Eucheuma denticulatum | + | + | + | + | |||
| Caulerpa lentillifera | + | + | + | + | + | + | |
| Caulerpa racemosa | + | + | + | + | + | + | |
| Halymenia durvillaei | + | + | + | ||||
| Dictyota dichotoma | + | + | + | + | |||
| Sargassum polycystum | + | + | + | + | + | + | + |
| Species | Bioactive Compounds | Activity | References |
|---|---|---|---|
| Kappaphycus alvarezii | Phthalic anhydride | Antimicrobial | [85] |
| 1-Benzosuberone | Anticancer | ||
| 3,4-Ethylenedioxythiophene | Antitumor | ||
| Furoic acid | Antimicrobial | ||
| Coumarandione | Antitubercular | ||
| 2-Benzofuran-4,7-dione | Antimicrobial | ||
| 2-Pentylthiophene | Antifungal | ||
| 2,3-Dihydropyrazolo[5,1-b][1,3]thiazole-6-carbaldehyde | Anti-inflammatory, antibacterial, anticancer | ||
| 1-(1,3-Thiazol-2-yl)-1H-tetrazole | Antimicrobial | ||
| 4-Chloro-1H-pyrazolo[3,4-b]pyridine | Anticancer, antioxidant | ||
| 4-Morpholinecarbonyl chloride | Antibacterial, antidepressant | ||
| 3-Methylthiophene-2-carboxylic acid | Antibacterial | ||
| 5-Pentyl-1,3,4-thiadiazol-2-amine | Antibacterial | ||
| 3-Methyl-3,4-epoxysulfolane | Antifungal | ||
| Nonanal | Antifungal | [93,94] | |
| 2-Tridecanone | Insecticide | [94,95] | |
| 4-Aminopyrimidine | Neuroprotection | [96,97] | |
| Eucheuma denticulatum | Docosahexaenoic acid | Anticancer, cardiovascular protection, anti-inflammatory | [86,87,88] |
| Eicosapentaenoic acid | Anticancer, cardiovascular protection | [86,88,98] | |
| Palmitoleic acid | Anti-inflammatory | [86,99] | |
| Antheraxanthin | Antioxidant | [100,101] | |
| Astaxanthin | Anti-inflammatory, antioxidant | [100,102] | |
| β-Carotene | Antioxidant | [100,103] | |
| β-Cryptoxanthin | Anticancer, antioxidant | [100,104,105] | |
| Dinoxanthin | Antioxidant | [100,106] | |
| Diatoxanthin | Chemopreventive | [100,107] | |
| Diadinoxanthin | Antioxidant | [100,106] | |
| Fucoxanthin | Antitumor | [100,108] | |
| Lutein | Antioxidant | [100,106] | |
| Rubixanthin | Antioxidant | [100,109] | |
| Zeaxanthin | Antioxidant | [100,106] | |
| Halymenia durvillaei | Eucalyptol | Antimicrobial, anti-inflammatory | [91,110,111] |
| Caryophyllene | Antioxidant, anticancer, antimicrobial | [91,92] | |
| Palmitic acid | Anti-inflammatory, antioxidant, antiandrogenic 5- alpha-reductase inhibitor nematicide, pesticide, hypocholesterolemic | [17,91,112] | |
| Oleic acid | Quorum quenching and anti-biofilm potential | [91,113] | |
| Dodecanal | Antimicrobial | [91,114] | |
| Heptadecanoic acid | Anticancer | [91,115] | |
| Pentadecanoic acid | Anticancer | [91,116] | |
| Myristic acid | Larvicidal and repellent | [91,117] | |
| Caulerpa lentillifera | Docosahexaenoic acid | Anticancer, cardiovascular protection, anti-inflammatory | [86,87,88] |
| Eicosapentaenoic acid | Anticancer | [86,98] | |
| Palmitoleic acid | Anti-inflammatory | [86,99] | |
| Astaxanthin | Anti-inflammatory, antioxidant | [100,102] | |
| β-Carotene, | Antioxidant | [100,103] | |
| β-Cryptoxanthin | Anticancer, antioxidant | [100,104,105] | |
| Canthaxanthin | Antioxidant, anti-inflammatory, neuroprotection | [100,118] | |
| Zeaxanthin | Antioxidant | [100,106] | |
| Oleic acid | Antioxidant, cardiovascular protection, hepatic protection | [119,120] | |
| Pentadecanoic acid | Anticancer | [116,119] | |
| Myristic acid | Antidiabetic, anti-inflammatory | [119,121] | |
| Behenic acid | Anti-obesity | [119,122] | |
| Palmitic acid | Antitumor | [119,123] | |
| Limonene | Antiparasitic | [94,124] | |
| Heneicosane | Anti-inflammatory, analgesic, antipyretic | [94,125] | |
| Eicosane | Anti-inflammatory, analgesic, antipyretic | [94,125] | |
| Pentadecane | Anti-inflammatory, analgesic, antipyretic | [94,125] | |
| Azulene | Anti-inflammatory | [94,126] | |
| Caulerpa racemosa | Pseudoephedrine | Anti-inflammatory | [90,127] |
| Tetratetracontane | Antibacterial | [89,90] | |
| Deoxyspergualin | Nuclear factor-kappa B inhibitor | [90,128] | |
| 2,4-Di-tert-butylphenol | Antibacterial, anti-inflammatory, anticancer | [129,130] | |
| Heptacosane | Antioxidant | [131] | |
| Palmitic acid | Anti-inflammatory, antioxidant | [123,132,133] | |
| Squalene | Anti-inflammatory, antioxidant, antitumor | [134,135] | |
| Methoxyacetic acid | Anticancer | [136,137] | |
| Effective against polycystic ovary syndrome (a hormonal dysfunction among women of reproductive age) | [136,138] | ||
| Monocaprin | Antimicrobial | [136,139] | |
| D-Mannitol | Antihyperglycemic | [136,140] | |
| Dictyota dichotoma | Dictyohydroperoxide | Anticancer | [141] |
| Pentadecane | Anti-inflammatory. antiparasitic | [125,141,142] | |
| Squalene | Anti-inflammatory, antioxidant, antitumor | [134,135,141] | |
| Fucosterol | Anti-inflammatory, anticancer, hepatoprotective, antiphotoaging, anti-obesity, anti-Alzheimer’s disease, antioxidant | [141,143] | |
| Saringosterol | Anti-obesity | [141,144] | |
| Sargassum polycystum | Docosahexaenoic acid | Anticancer, cardiovascular protection, anti-inflammatory | [86,87,88] |
| Eicosapentaenoic acid | Anticancer, cardiovascular protection | [86,88,98] | |
| Palmitoleic acid | Anti-inflammatory | [86,99] | |
| Antheraxanthin | Antioxidant | [100,101] | |
| Astaxanthin | Anti-inflammatory, antioxidant | [100,102] | |
| β-Cryptoxanthin | Anticancer, antioxidant | [100,104,105] | |
| Canthaxanthin | Antioxidant, anti-inflammatory, neuroprotective | [100,118] | |
| Dinoxanthin | Antioxidant | [100,106] | |
| Diatoxanthin | Chemopreventive | [100,107] | |
| Diadinoxanthin | Antioxidant | [100,106] | |
| Fucoxanthin | Antitumor | [100,108] | |
| Lutein | Antioxidant | [100,106] | |
| Zeaxanthin | Antioxidant | [100,106] | |
| Palmitic acid | Anti-inflammatory, antioxidant, anti-androgenic 5- alpha-reductase inhibitor nematicide, pesticide, hypocholesterolemic | [17,112,132] | |
| Octadecenoic acid | Anticancer, antimicrobial | [17,112,145] | |
| Neophytadiene | Anti-inflammatory | [17,146] | |
| Myristic acid | Larvicidal and repellent | [117,147] | |
| cis-Vaccenic acid | Antiviral | [147,148] | |
| Cholest-4-en-3-one | Analgesic, neuroprotective, anti-obesity | [147,149] | |
| Squalene | Anti-inflammatory, antioxidant, antitumor | [135,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.D.; Venmathi Maran, B.A.; Shaleh, S.R.M.; Zuldin, W.H.; Gnanaraj, C.; Yong, Y.S. Therapeutic Potential and Nutraceutical Profiling of North Bornean Seaweeds: A Review. Mar. Drugs 2022, 20, 101. https://doi.org/10.3390/md20020101
Shah MD, Venmathi Maran BA, Shaleh SRM, Zuldin WH, Gnanaraj C, Yong YS. Therapeutic Potential and Nutraceutical Profiling of North Bornean Seaweeds: A Review. Marine Drugs. 2022; 20(2):101. https://doi.org/10.3390/md20020101
Chicago/Turabian StyleShah, Muhammad Dawood, Balu Alagar Venmathi Maran, Sitti Raehanah Muhamad Shaleh, Wahidatul Husna Zuldin, Charles Gnanaraj, and Yoong Soon Yong. 2022. "Therapeutic Potential and Nutraceutical Profiling of North Bornean Seaweeds: A Review" Marine Drugs 20, no. 2: 101. https://doi.org/10.3390/md20020101
APA StyleShah, M. D., Venmathi Maran, B. A., Shaleh, S. R. M., Zuldin, W. H., Gnanaraj, C., & Yong, Y. S. (2022). Therapeutic Potential and Nutraceutical Profiling of North Bornean Seaweeds: A Review. Marine Drugs, 20(2), 101. https://doi.org/10.3390/md20020101









