Abstract
The search for new sources of antimicrobial compounds has become an urgent need, due to the threat that the spread of bacterial resistance represents for global health and food safety. Brown macroalgae have been proposed as a great reservoir in the search for novel antimicrobial compounds. In this study, mid-polarity extracts were performed with a selection of 20 brown macroalgae species from northern Spain. The total polyphenol, carbohydrate and protein contents were quantified by spectrophotometry. The volatile organic compounds (VOCs) of whole macroalgae were also studied as a biomarker of their metabolic state in the representative species of the tested families by gas chromatography-mass spectrometry (GC-MS). The antimicrobial potential of the extracts was assessed by a disk diffusion assay against 20 target bacteria and further determinations of the minimum inhibitory (MIC) and minimum bactericidal concentrations (MBC) were performed by a microdilution assay for the active extracts. Ericaria selaginoides, Bifurcaria bifurcata and Dictyota dichotoma showed an antimicrobial effect against six Gram-positive strains: Bacillus cereus, Bacillus subtilis, Geobacillus stearothermophilus, Listeria monocytogenes, Staphylococcus aureus and Staphylococcus haemolyticus. The phenolic content was generally higher in the extracts that showed antimicrobial activity, followed by carbohydrates and low contents of proteins. The results obtained in this study reveal the potential of brown macroalgae as a promising alternative source of antimicrobial compounds as functional ingredients for the application in industrial fields.
1. Introduction
Marine ecosystems harbor a high variety of organisms with a unique composition. Among them, macroalgae (commonly referred to as seaweeds) are important primary producers in oceanic aquatic food webs, consequently contributing to marine ecosystems [1]. In the intertidal zone, macroalgae are arranged in a vertical gradient of diverse communities subjected to either fluctuations or a gradual shift by the incidence of environmental and biological factors. To cope with these harsh and competitive marine conditions, seaweeds have developed an adaptive mechanism against biotic and abiotic stressors based on a set of physiological responses that results in the production of a wide diversity of compounds different than those observed in terrestrial environments [2].
Such chemical variety constitutes a great reservoir for the discovery of novel bioactive compounds. In fact, more than 15,000 primary and secondary metabolites from macroalgae of different chemical natures, including proteins and peptides, polysaccharides, polyphenols, polyunsaturated fatty acids or pigments, have been reported with antioxidant, antimicrobial, antifungal, anticancer or anti-inflammatory activities [3,4,5].
The search for sustainable sources and demands for compounds of a natural origin are in the spotlight, since the potential harmful side effects for health, due to the use of synthetic substances, are under discussion [6,7]. In this regard, macroalgae biomass represent a renewable, varied and versatile source that can be harvested directly in the sea in an environmentally safe way, avoiding the competitiveness for the use of land and the overexploitation of other natural resources [8]. Simultaneously, their production from aquaculture also becomes a benefit for the environment, since these aquatic organisms have the ability to assimilate carbon dioxide from anthropogenic emissions, in addition to nitrogen sequestration, contributing to mitigating climate change [9,10,11].
In addition to the use of macroalgae as a whole food, their singular composition makes them a useful resource of natural ingredients suitable for several industrial applications. In Europe, interest in seaweeds has increased exponentially over the last years and, although their exploitation is still limited, several initiatives are currently ongoing. Seaweeds used as food and food supplements and classified as novel are subject to the pre-market authorization requirements of the novel food regulation (EU) 2015/2283 [12] before they can be freely placed in the European market without the need for pre-market novel food authorization [13].
The need to intensify the search for novel food antimicrobials is increasing since food products are perishable by nature and foodborne contamination is still an important public health issue. Growing consumer demands for high-quality products, coupled with less use of synthetic food additives and minimally processed products with a longer shelf life, promote the development of alternative solutions for food safety to continue playing a key role in the food industry. Moreover, currently, there is also a social need for new antimicrobial compounds since their inadequacy and overuse have increased the spread of antimicrobial resistance, leading to the re-emergence of bacterial diseases, and causing an important clinical, economic and social impact [14,15].
The potential of marine macroalgae as a source of antimicrobials has been studied among the three main phyla. However, interest in brown macroalgae has been extensively described because of their particular composition in polyphenols such as phlorotannins; polysaccharides such as fucoidans; and pigments such as fucoxanthin, that have been widely reported to be active against pathogenic and spoilage bacteria [16], though the antimicrobial activity from crude extracts is not usually attributed to a single compound but could be related to a combination of metabolites.
In this sense, research efforts are needed to explore the richness and potential of local biodiversity in order to fulfill biomass demands and contribute to improving the overall quality and traceability of the seaweed products industry [17]. In this sense, the north of Spain coastline has been divided into four ecological zones according to their environmental differences, which makes this region a good reservoir with great macroalgal diversity [18]. Consequently, a good selection of the species is essential for further steps, and native species well-adapted to the local environment seem to be the best suited [19].
The aim of this study was to assess the antimicrobial activity of brown macroalgae mid-polarity extracts from collected macroalgal species from the northern coast of Spain, contributing with this research the knowledge of its potential as a sustainable and affordable source of novel biocompounds. This work was focused mainly on their potential against foodborne pathogens and spoilage bacteria and their use as natural preservative ingredients, although their application could be extended to other fields such as the pharmaceutical or cosmetic industries.
2. Results and Discussion
2.1. Volatile Compounds in the Biomass of Seaweeds
Though scarce research has been carried out on volatile organic compounds from macroalgae, at least 200 different volatile compounds have been reported in important commercially brown (Himanthalia elongata, Laminaria spp., Laminaria ochroleuca and Undaria pinnatifida) and red macroalgae (Porphyra umbilicalis and Palmaria palmata) [20]. The volatile organic compounds tentatively identified in whole brown macroalgae collected from the northern coast of Spain are listed in Table 1 and representative chromatograms of the evaluated families are shown in Figure 1. The VOC profile from the mid-polarity extracts was also evaluated (data not shown), although a scarce number of compounds were released under established conditions. Compound classes observed in whole algae samples are in accordance with those described in the literature, since ketones, alcohols, aldehydes, hydrocarbons, esters and halogenated compounds have been identified, with alcohols and aldehydes being the most predominant chemical structures [20]. In this study, differences in the volatile organic compound profile have been observed among taxonomical families (Figure 1), though the principal components analysis (PCA) explains only 22.62% of the variability among the samples (Figure 2). The C5 and C6 volatiles were the most common among the identified compounds, with hexanal and 1-hexanol being identified in a greater number of species, whereas compounds such as o-hydroxybiphenil (aromatic compound) have also been identified in a minor presence.

Table 1.
Volatile organic compounds identified in whole brown macroalgae samples.
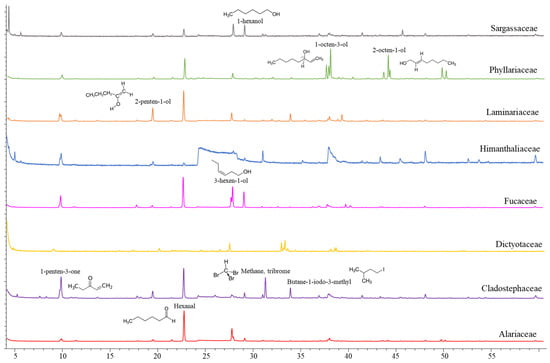
Figure 1.
Representative chromatograms of the volatile organic compound profile of whole macroalgae samples from each family.
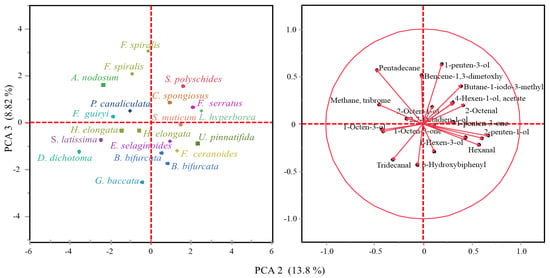
Figure 2.
Principal component analysis on volatile compounds data.
Hexanal and the pentyl leaf (C5) volatiles were detected among the evaluated species, with 1-penten-3-one being prominent in H. elongata and Cladostephus spongiosus and 3-penten-2-ona-4-methyl in the Sargassaceae family. The remaining C6 volatiles had a higher contribution to the volatile organic compound profile of the Sargassaceae species in comparison to the Fucaceae species, whereas their occurrence differs among the Laminariales species. 2-hexene-3,5,5-trimethyl was prominent in the profile of Saccharina latissima, 2-hexenal in Laminaria hyperborea and U. pinnatifida, though the major volatile compound detected in U. pinnatifida was 1-octen-3-ol. Significant emission of the C8 volatiles was also seen in Dictyota dichotoma, and both the C8 and C9 volatiles were identified in the emission profile of Sacchoriza polyschides. The saturated hydrocarbon chain length of the C15 was mostly present in the Fucaceae species, being the major volatile compound detected in Ascophyllum nodosum, Fucus guiryi and Pelvetia canaliculata and showing a remarkable content in Fucus serratus. Both the C15 and C13 saturated hydrocarbons were also observed in D. dichotoma. Volatile compounds related to organoleptic attributes, such as odor or taste, are currently of interest regarding their potential as ingredients in industrial applications. Short-chain aldehydes and their derivates are known to be important flavor compounds in several foods, and the observed differences in the volatile compound profile should affect the consumer’s acceptability of seaweed-containing food products. C6 and C9 aldehydes, such as n-hexanal and 2-nonenal detected here, have been described as contributors of flavor in Saccharina angustata (as Laminaria angustata) [21]. Thus, the six-carbon (C6), nine-carbon (C9) and pentyl leaf (C5) volatiles are responsible for the green, earth and herb notes, whereas the occurrence of the eight-carbon (C8) compounds, even at low levels, are responsible for the mushroom, musty or earthy notes of several foods.
Many of the detected volatiles are derived from the degradation of PUFAs through the lipoxygenase pathway (LOX) such as n-hexanal and a set of short-chain unsaturated aldehydes, their alcohols and esters [22,23]. Oxylipin-derived volatiles are quickly released in response to the biotic and abiotic stressors or in tissue injury due to mechanical wounding [24], but relatively low levels of these volatiles have been emitted in intact and healthy plants. Thus, the minor number of volatile organic compounds observed in this study could be attributed to the careful conditions applied in the sample collection and transport, as well as the use of cryogenic disruption of tissues and the mild experimental conditions applied in the volatile analysis from the frozen samples.
Furthermore, hexanal, together with other C6 compounds such as 2-hexenal, has been reported to show an antimicrobial effect against foodborne pathogenic microorganisms such as Escherichia coli, Salmonella enterica and Listeria monocytogenes [25], showing a great potential as a food preservative. However, these compounds have not been identified in the evaluated species of macroalgae that showed antimicrobial activity.
Halogenated compounds, brominated and iodinated species have also been identified, tribrome-methane or bromoform was observed in A. nodosum, D. dichotoma, butane-1-iodo-3-methyl in L. hyperborea, and both compounds were identified in C. spongiosus. Halogenated compounds are influenced by nutrient concentration, temperature and salinity conditions, although the release of volatile compounds is mostly affected by light variations [26]. In addition, the occurrence of halogenated volatiles has also been proposed as a biomarker of the macroalgae physiological state since they involve defense mechanisms against grazing and as a response to light exposure. Specifically, Ohsawa et al. (2001) [27] described bromoform as an agent to eliminate epiphytic organisms in the red macroalgae Corallina pilulifera. Although not related to their antimicrobial potential, it is interesting to mention that bromoform has also been identified in species of the genus Asparagopsis (A. taxiformis and A. armata), which have recently been postulated as effective feed ingredients in the dietary intervention of ruminants for methane mitigation. The feed inclusion of these species at less than 1% of the feed organic matter reduces methane emissions from sheep and cattle [28,29].
In the mid-polarity extracts, although a minor number of compounds have been released (data not shown), it is of interest to highlight that chlorinated and iodinated compounds, such as 2-propanone-1-chloro and 1-iodo-2,3-epoxypropane, have only been detected in species belonging to the Laminariaceae, L. hyperborea and S. latissima families. This finding agrees with the description of Laminariales as the major accumulators of iodine within living organisms and as a great source of iodocarbons in coastal environments [30].
2.2. Chemical Composition of Extracts
The current need for novel antimicrobial compounds has placed macroalgae in the spotlight because of their richness in bioactive compounds. Specifically, brown macroalgae are of great interest as they harbor compounds that are exclusively found within this class [31]. Among them, interest has been focused on molecules such as phlorotannins, phenolic compounds; fucoidans, sulphated polysaccharides; and fucoxanthin, carotenoid pigment, because of their numerous properties, including their antimicrobial potential [32,33,34]. Other compounds that are not exclusively from Phaeophyceae, such as proteins, peptides and fatty acids, have also been described to show antimicrobial properties [16].
Over the last decades, different extraction techniques and solvents have been used for the extraction of these compounds, with solid–liquid extraction (SLE) traditionally being performed [35]. The extraction of chemical structures depends on a wide range of factors such as the polarity of solvents, temperature, time and the extraction technique being performed. Quantification methods, as well as the availability of standards, also influence the evaluation of the obtained results since the specific identification and quantification of metabolites are not always likely to be performed due to the lack of suitable standards from the marine environment. Moreover, it is well known that numerous natural factors strongly influence macroalgae composition: biotic factors, including intrinsic (i.e., growth, reproduction state) and extrinsic factors (i.e., herbivory, epiphytism); and abiotic factors (i.e., latitude, season, irradiance, depth) [36]. This current context and the non-existence of standardized protocols originate a high variability and make it hard to establish proper comparisons with other results found in the literature. In this work, the spectrophotometric detection of the phenolic, carbohydrate and protein content of the mid-polarity extracts (Table 2) has been evaluated in selected brown macroalgae.

Table 2.
Calibration, linearity, accuracy and precision for polyphenol, carbohydrate and protein content determination by spectrophotometry.
In general, the total phenolic content was higher in most evaluated species (29.28–115.13 mg PGE/g dw), whereas S. latissima (33.43 mg GE/g dw) and some species from the order Fucales exhibited a higher carbohydrate content (15.14–19.15 mg GE/g dw). The protein content was generally minor in all samples (Table 3).

Table 3.
Phenolic, carbohydrate and protein content of mid-polarity extracts.
The values for these compounds in the present study varied between species and families as a result of the factors previously described. The most specific polyphenols of brown algae, phlorotannins, are subjected to inter-species variations [37]. The higher phenolic content has been observed in species belonging to the Fucales and within the Sargassaceae, Fucaceae and Himanthaliaceae families, whereas the contents in the Alariaceae, Laminariaceae, Cladostephaceae and Phyllariaceae families were, in general, the lowest ones, ranging between 2.52 mg PGE/g dw in S. latissima and 6.25 mg PGE/g dw in U. pinnatifida.
In the order Fucales, phlorotannins may amount to up to 20% dw [38]. In this study, the highest value was observed for Halidrys siliquosa (115.13 mg PGE/g dw), followed by Fucus vesiculosus, F. serratus and A. nodosum (42.64–54.58 mg PGE/g dw). There were also species with a low phenolic content among members of the Fucaceae family, such as Fucus ceranoides (8.44 mg PGE/g dw) and some species of F. guiryi and P. canaliculata. Whereas the intermediate values ranged between 19.28 and 34.63 mg PGE/g dw for species from the Sargassaceae and Dictyotaceae families, as well as some Fucaceae.
These results are in accordance with the values reviewed by Jacobsen et al. (2019) [39], who indicate the highest concentrations for large species of brown macroalgae, such as Fucus sp., Sargassum sp. and A. nodosum; although, in this work, Sargassum muticum had the lowest value quantified among the species of the Sargassaceae family with 13.31 mg PGE/g dw. A high variability in the phenolic content has been reported for A. nodosum. Tierney et al. (2013) [40] reported values above 100 mg PGE/g in extracts performed from a fresh sample with water and organic solvents, whereas low values can also be found, ranging between 0.11 and 0.17 mg PGE/g dw in water and acidic extracts performed by solid–liquid and ultrasound extraction methods [41]. Since there are not standardized procedures for the extraction of natural antimicrobials, the reviewed data highlight the influence of different techniques and extraction solvents on their recovery as well as their antimicrobial potential. Special attention should be paid concerning the use of high temperatures during processing. Thus, despite it helping to obtain higher yields, its application is still under discussion because of the possible thermal degradation of compounds, the hydrolysis of complex molecules in smaller structures or even polymerization reactions [42]. In methanolic extracts (60%), the reported extraction yields obtained at 60 °C were around ten times lower [43] than the recorded values at 40 °C [44], although the influence of other factors cannot be disregarded. Moreover, low yields in extracts performed with hot water have also been described [45]. In this work, extractions have been performed at room temperature to avoid a thermal effect over the molecular structures and the possible bioactivities of the extracted compounds. Thus, considering the scaling of future extraction methods at an industrial level, decreasing the energy demands would make these processes more affordable and sustainable.
Regarding the carbohydrate content, the higher values were observed in the Laminariaceae, Fucaceae and Sargassaceae families, with the highest contents being for S. latissima (33.43 mg GE/g dw), F. ceranoides (19.15 mg GE/g dw) and H. siliquosa (18.95 mg GE/g dw), followed by F. serratus and A. nodosum (15.61 and 15.14 mg GE/g dw, respectively). The results for H. siliquosa agree with the values obtained by Olsson et al. (2020) [46], that also reported the highest carbohydrate content for this species collected on the Swedish west coast. S. latissima, commonly known as sugar kelp, has a great commercial value as a source of carbohydrates used as emulsifiers and thickeners, because of their high content (30 to 50% dw), especially in alginate, which represents up to 40% dw [47]. The intermediate levels ranged between 6.05 and 13.57 mg GE/g dw, including species from all the families, and minor values (≤4.65 mg GE/g dw) were registered for S. polyschides.
In general, the results for the carbohydrate content reported in the bibliography are higher than those obtained in this work. Higher values, that amount to up to 100 times or more, have been described in ethanolic extracts prepared from commercial macroalgae such as S. latissima, U. pinnatifida, and H. elongata, collected on the Atlantic coast of Galicia. The values were also higher in other Laminariaceae species such as L. ochroleuca [48]. This fact could be mainly attributed to the extraction medium used. Due to their high polarity, the extraction of polysaccharides has been currently performed with hot water or acidic solutions, although other studies employed polar solvents such as ethanol [49]. Nevertheless, a mid-polarity extraction medium was selected in this work in order to minimize the solubilization of the major carbohydrates that occurs in brown macroalgae since their extraction could make further clean-up steps difficult. Moreover, since the extraction procedures influence the composition of the extracts [50], the lower content achieved in this work could also be a result of the selection of carbohydrates with specific chemical or functional structures.
Phaeophyceae has the lowest protein content among the three macroalgae phyla, representing 3–15% dw [51]. Proteinic structures were the minor component in these extracts. The higher values were observed among members of the Fucaceae and Himanthaliaceae families and for H. siliquosa in Sargassaceae, with amounts from 5.33 to 10.74 mg BSAE/g dw. However, a high variability is also observed for the proteinic content and there are exceptions in these families with low values, such as F. ceranoides and S. muticum (≤2.78 mg BSAE/g dw). The rest of the species from Sargassaceae also registered minor concentrations (≤2.78 mg BSAE/g dw), except for Gongolaria baccata (3.57 mg BSAE/g dw) and fertile specimens of Bifurcaria bifurcata (3.67 mg BSAE/g dw). Most of the studies found in the literature determine the total protein content in the macroalgae, which makes it difficult to establish comparisons. However, the values obtained in this study were below the percentages described above for brown macroalgae (3–15% dw), with the highest ones being in some specimens of P. canaliculata and H. elongata, with just 1% of dry weight protein content.
In addition, it is worth highlighting the differences observed regarding the life stage in B. bifurcata. No significant differences were observed in the carbohydrate and protein content between the vegetative and reproductive samples; however, the phenolic content was significantly different in the reproductive samples (p ≤ 0.01). A higher phenolic content during the reproductive stage could be related to their defense function to protect themselves, especially during the reproductive stage [52], although this effect was not observed in H. elongata since the phenolic content was higher (p ≤ 0.01) in the vegetative specimens and other factors, such as season (spring and summer), could have an influence. Similarly, the non-fertile specimens of P. canaliculata collected in Galicia in 2019 showed a lower proteinic content (6.36 ± 0.67 mg BSAE/g dw) and higher total phenolic (14.06 ± 0.31 mg PGE/g dw) content than specimens collected in Cantabria in 2017 (10.03 ± 0.67 mg BSAE/g dw and 3.17 ± 0.31 mg PGE/g dw, respectively).
Moreover, some species were collected under the different conditions of season, year or location, which affected the composition of the mid-polarity extracts. Carbohydrates and the total phenolic content were higher (p ≤ 0.01) in specimens of F. guiryi collected in 2019 (7.71 ± 0.28 mg GE/g dw and 29.33 ± 1.46 mg PGE/g dw, respectively), with respect to those collected in 2017 (≤4.65 mg GE/g dw and 2.45 ± 1.46 mg PGE/g dw, respectively). Similarly, the extractability of polyphenolic (p ≤ 0.01) and proteinic compounds (p ≤ 0.01) was higher in specimens of H. elongata collected in spring (48.31 ± 1.25 mg PGE/g dw and 10.74 ± 0.22 mg BSAE/g dw, respectively) than those collected in summer (25.47 ± 1.25 mg PGE/g dw and 8.40 ± 0.22 mg BSAE/g dw, respectively). The geographic location affected the concentrations of the total phenolic compounds (p ≤ 0.01) and carbohydrates (p ≤ 0.01) of the mid-polarity extracts of Fucus spiralis. The specimens collected in Comillas exhibited a higher content in the extractable phenolic compounds (24.89 ± 0.82 mg PGE/g dw), while the extracted carbohydrates (10.68 ± 0.27 mg GE/g dw) were higher in specimens collected in A Coruña. Differences considering these factors have been evaluated by other authors in valuable macroalgae [38,53,54], although, in this sense, more efforts are needed to determine the influence that each factor exerts over the content of certain macroalgae compounds.
2.3. Antimicrobial Activity of Macroalgae Extracts
The antimicrobial activity of the mid-polarity extracts from brown macroalgae collected in this study was observed in three species, B. bifurcata and Ericaria selaginoides from the Sargassaceae family, and D. dichotoma from the Dictyotaceae family (Table 4). Irrespective of the considered species, all the extracts exhibited the same spectrum of antimicrobial activity, which is constituted by six Gram-positive bacteria, including foodborne pathogens such as L. monocytogenes, Bacillus cereus and Staphylococcus aureus [55]; spoilage microorganisms such as Geobacillus stearothermophilus, spore-forming bacteria that remain a major challenge in the dairy industry [56]; the opportunistic pathogen Staphylococcus haemolyticus, and the spore-forming food contaminant Bacillus subtilis; thus being of relevant interest for the food industry and human health.

Table 4.
Inhibition zones obtained by disk diffusion assay (mm).
Nevertheless, despite having the same antimicrobial spectrum, the overall quantitative antimicrobial potential profile of each macroalgae extract against the six target strains was different, as determined by the MIC/MBC values obtained for E. selaginoides and B. bifurcata (Table 5). The scarce occurrence of D. dichotoma on the northern coast of Spain did not allow for the estimation of the MIC/MBC values.

Table 5.
MIC and MBC values assessed in microdilution plate assay (mg/mL).
The ability of extracts to inhibit Gram-positive strains, not observed against Gram-negative, could be associated with differences in their cell wall structure that determine their permeability, with Gram-negative bacteria being generally more resistant since their outer membrane can act as a barrier to certain compounds [57]. Reported differences in the antimicrobial effect of different macroalgae extracts denote the importance of the choice of solvent in the selectivity of the bioactive compounds. An antimicrobial effect of ethanol and dichloromethane extracts from Atlantic B. bifurcata against Gram-positive strains, such as B. subtilis and S. aureus, has also been described by El Wahidi et al. (2015) [58]. However, Alves et al. (2016) [59] reported antimicrobial activity against Gram-negative strains of dichloromethane extracts from B. bifurcata, while methanolic extracts were active against both Gram-positive and Gram-negative bacteria.
Bacillus and Geobacillus genera are ubiquitous in nature and are of concern in the food industry since they can be present in fresh and pasteurized food due to their ability to generate heat-resistant spores under harsh environmental conditions [60]. G. stearothermophilus exhibited a great sensitivity to the B. bifurcata and E. selaginoides extracts, with the lowest MIC/MBC values and inhibition halos slightly smaller than those obtained for L. monocytogenes. The diameter of the inhibition zones observed by a disk diffusion assay was 14.6–18.3 and 13.4 mm, respectively, while the MIC/MBC values ranged between 0.3–0.4 mg/mL for B. bifurcata and 0.2–0.3 mg/mL for E. selaginoides. This target strain can be found as a contaminant of dairy products and can also be isolated from dried soups and vegetables and, although it is not described as pathogenic, its occurrence in a high concentration reduces the value of the food products [61,62].
Remarkable variations in the antimicrobial activity against B. cereus were also observed on both the B. bifurcata and E. selaginoides extracts. The antimicrobial activity of B. bifurcata was higher in the extracts from the vegetative specimens, whereas the highest values observed for the fertile specimens were either for MIC (3.2 mg/mL) or MBC (6.4 mg/mL). The results also differed among the specimens of B. bifurcata and E. selaginoides collected in 2017 and 2019 and in different locations, ranging between 0.4–6.4 mg/mL and 0.9–4.5 mg/mL, respectively. B. cereus is a well-documented food-poisoning microorganism widely distributed in the environment, being responsible for most notified causes of diarrheal and emetic illnesses in developed countries [63], and is found in a wide variety of foods, such as dairy products, meat, pasteurized liquid egg, rice, ready-to-eat vegetables and spices [64].
A scarce antimicrobial effect against B. subtilis was observed for the E. selaginoides extracts, whereas the B. bifurcata extracts also showed a great variability. In general, both species showed MIC/MBC values >14.2 mg/mL, although lower MIC values were observed for B. bifurcata collected in August 2017 (0.9 mg/mL) and December 2019 (0.4 mg/mL). B. subtilis has been safely used in traditional fermented foods [65]; however, although it is not considered as pathogenic, some strains of this species may occasionally cause food poisoning [66] and it has been associated with the spoilage of UHT and canned milk products, as well as the production of toxins under mesophilic temperatures [67].
B. cereus and B. subtilis are commonly used as target strains in antimicrobial assays with macroalgae extracts; however, references about the antimicrobial effects of extracts of this nature against G. stearothermophilus have not been found in literature. Other authors have reported activity against B. subtilis when tested organic extracts performed with specimens of B. bifurcata collected in Portuguese and Moroccan regions of the Atlantic Ocean [58,59,68]. Salvador et al. (2007) [69] also reported activity against both B. cereus and B. subtilis, with solid extracts performed with E. selaginoides collected on the Atlantic coast of Spain.
Staphylococci are non-spore-forming bacteria ubiquitously distributed with a high tolerance for salt [70]. The best results for both S. haemolyticus and S. aureus were obtained from E. selaginoides specimens, collected in the same month of different years and locations. In general, lower values were registered for the B. bifurcata specimens collected in Galicia in May 2019 and, interestingly, these specimens showed similar results against Staphylococci strains despite their differences in the life stage (vegetative and fertile). S. haemolyticus is generally considered non-pathogenic since they are the predominant bacteria of fermented foods worldwide, though their isolation from skin infections in humans and animals places uncertainties around their safety. Nevertheless, S. aureus is a well-known virulent pathogen [71], able to produce a wide variety of toxins [72]. The intake of contaminated food, particularly processed meat and dairy products, is generally caused by improper handling and the subsequent storage at elevated temperatures is a major cause of food poisoning.
Unlike E. selaginoides, the B. bifurcata extracts showed scarce activity against L. monocytogenes, foodborne pathogenic bacteria found ubiquitously that can survive under refrigeration and food preservation measures. It can contaminate a wide range of foods, such as raw, chilled and ready-to-eat food (RTE) and, although the incidence of infections is low, it is especially dangerous for pregnant women and vulnerable groups, making this species responsible for many of the foodborne fatalities. Interestingly, the inhibitory capacity of the E. selaginoides extracts ranged between 2.3–3.6 mg/mL, being bactericidal at 3.6–4.5 mg/mL. No activity of these macroalgae species against L. monocytogenes has been found in the literature, but species belonging to the same genera as Listeria innocua were shown to be sensitive to the methanolic extracts of E. selaginoides [73].
Antimicrobial activity of the extracts from D. dichotoma was also observed by a disk diffusion assay against B. cereus, B. subtilis, G. stearothermophilus and L. monocytogenes, with the most sensitive being G. stearothermophilus. It also showed a bacteriostatic effect against S. aureus. Antimicrobial activity in the extracts of different polarities performed with D. dichotoma specimens collected in locations spread worldwide, such as in Spain, India, Serbia and Algeria, also reported activity against B. cereus, B. subtilis, S. aureus or L. monocytogenes [69,74,75,76].
Discrepancies between the results performed by disk diffusion and microdilution assays have been observed. In particular, the high antimicrobial activity of B. bifurcata extracts against L. monocytogenes observed by disk diffusion was not consistent with their high minimum inhibitory concentration. Moreover, the vegetative and reproductive specimens of B. bifurcata showed activity against B. cereus in the microdilution plate assay but not when they were tested by disk diffusion. Though a specific solution medium was designed to improve the solubility of the mid-polarity crude extracts and their diffusion into the aqueous agar matrix, it has to be taken into consideration that crude extracts are complex mixtures of bioactive compounds with a wide range of polarities, which do not always diffuse as easily as polar compounds. These discrepancies in the results obtained with different methods have been described by many authors who tested the antimicrobial activity of plant extracts, evidencing the effect that methodology may exert over the results, especially when crude extracts are tested [77]. Nevertheless, due to its simplicity and low cost, a disk diffusion assay is usually conducted as a preliminary screening of the antimicrobial activity, whereas a serial microdilution assay provides more accurate information about the inhibitory and bactericidal concentrations.
Furthermore, the variability in the extract composition allows for the consideration that the observed antimicrobial activity is not attributed to a single compound, but to an additive and/or synergic or antagonist action of different compounds. The molecular weight of molecules has also been proved to influence the antimicrobial activity [78]. Thus, the broad spectrum of isolated compounds achieved in the mid-polarity extracts includes pigments such as fucoxanthin [79], sugars and polyphenols, and variations in the content and the composition profile were assumed to be affected by the differences of the genetic lineages and the effect of the environmental factors. In this sense, a seasonal effect on the macroalgae development, as well as the chemical composition of seaweeds, has been reported [54], but the effect on the antimicrobial activity by environmental fluctuations is poorly understood. Thus, a similar antimicrobial effect against G. stearothermophilus was detected, irrespective of the season, year and geographical location of collection with extracts performed with the perennial algae B. bifurcata in this study. However, according to Čagalj et al. (2022) [80], the antibacterial activity of Mediterranean Cystoseira compressa was reported to be higher in summer. On the other hand, the unaffected antimicrobial activity between seasons of Dictyota indica, reported by Karkhaneh Yousefi et al. (2020) [81], is probably due to its seasonal development, mainly between spring and summer. In view of these results, no relation between the season and the antimicrobial activity could be established so far. Thus, research in this sense is still in its infancy and more efforts are needed to elucidate the compounds responsible for the antimicrobial effect observed and to determine the main factors involved in the production of these bioactive compounds. Advances in this sense are of great importance in the future in order to establish their production under controlled conditions.
3. Materials and Methods
3.1. Macroalgae Samples Collection and Processing
Twenty-seven brown algae samples (class Phaeophyceae), including twenty different species, were collected on rocky substrata in the lower intertidal and upper subtidal zones on the Galician (northwest of Spain) and Cantabrian coasts (north of Spain) in different seasons during 2017 and 2019 (Table 6). Taxonomic identification of the specimens was performed based on their morphological characteristics using keys and the specialized literature.

Table 6.
List of collected macroalgae and collection data. The taxonomic classification and currently accepted scientific names of the species, including important synonyms (=), are based on Algaebase and the rules of the International Code of Nomenclature for algae, fungi, and plants (ICN) [82].
All the individuals of each species were washed with sterile seawater to remove sand and epiphytes and wrapped in sterile cloths moistened with seawater. Then, they were kept under darkness, humid atmosphere (>84%), and cool conditions with ice packs (<15 °C) into expanded polystyrene boxes (EPS) until being transported overnight to IRTA-Monells (Girona, Spain). In order to ensure suitable delivery conditions of the seaweeds, the temperature and relative humidity inside each EPS box were monitored continuously at 10-min intervals using data loggers (Hobo Pendant UA-002-64; Hobo U23 Pro v2, Onset Computer Corporation, Bourne, MA, USA). The temperature and relative humidity during the transportation period in both summer and winter was maintained at a mean temperature of 9.0 ± 2.9 °C, with a maximum of 14.2 °C and a minimum of 5.8 °C, and a mean relative humidity of 91.6 ± 2.6%, with a maximum of 94.8% and a minimum of 84.6%.
Once the samples arrived, they were kept at −80 °C until further processing. In the laboratory, algal biomass was ground to a fine powder using a cryogenic homogenizer (SPEX SamplePrep, Metuchen, NJ, USA) and stored at −80 °C under vacuum and darkness conditions until extracts preparation.
3.2. Headspace Solid-Phase Microextraction (HS-SPME) and GC-MS Conditions
The volatile compound profile was performed using solid-phase microextraction (SPME) with a Combi PAL autosampler and separated, identified and quantified in a gas chromatograph 6850 GC-System equipped with a mass selective detector 5975C VL MSD (Agilent Technologies, Santa Clara, CA, USA). Volatile compounds were tentatively identified by comparing their mass spectra with those contained in MassHunter Quantitative Analysis B.05.01 software combined with the National Institute of Standards and Technology database (NIST 2.0 version, Gaithersburg, MD, USA).
The SPME tool was loaded with a stable-flex fiber of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS), coated with a 50/30 μm thickness (Supelco, Bellefonte, PA, USA). Before the analysis, the fiber was conditioned at 270 °C for 30 min in a SPME Fiber Conditioning Station. For headspace SPME extraction, 1 ± 0.01 g of each sample was weighed in a 10 mL amber vial (Agilent Technologies, Santa Clara, CA, USA), magnetic capped with a PTFE/silicone septum. The extraction was carried out by headspace mode at 40 °C for 40 min with magnetic stirring (250 rpm). The SPME fiber was desorbed in the injection port (splitless mode; helium pressure 34.5 kPa) at 270 °C for 5 min. The capillary column used for volatile compound separation was a DB-5MS capillary column (30 m, 250 μm i.d., 1.0 μm film thickness; J&W Scientific, Folsom, CA, USA). Helium was used as a carrier gas with a constant flow of 0.8 mL/min. The gradient temperature used for the separation was performed at 40 °C (10 min)—4 °C/min (140 °C)—10 °C/min (200 °C)—30 °C/min (250 °C, 5 min). Mass spectra of volatile compounds were acquired over the range m/z 40–450 in scan acquisition mode, with the mass detector transfer line being maintained at 280 °C, the mass source at 230 °C and the mass quad at 150 °C. Compounds were considered as correctly identified when their spectra presented a library match factor > 95%.
3.3. Extraction Procedure
Algae extracts were prepared by triplicate according to Rubiño et al. (2022) [79], using a mid-polarity extraction medium composed of a mixture of hexane–isopropanol–water (10:80:10). Three consecutive extractions of 30 mL each from 3.5 g of a frozen algae sample were performed at room temperature in an orbital homogenizer for 60 min each, and then centrifuged at 5000 rpm for 10 min and at 4 °C. Supernatants were pooled in 50 mL amber flasks.
Extracts were evaporated to dryness under vacuum conditions at room temperature (Thermo Scientific™ Savant™ SpeedVac™ SPD120 Vacuum, Thermo Fisher Scientific, Waltham, MA, USA). Dry residues were then resuspended under sterile conditions in 1 mL of a medium composed of a mixture of water–glycerol–tween 80 (80:10:10) to solubilize and facilitate the diffusion of the mid-polarity extracts through the agar.
All reagents used were of analytical grade (VWR Chemicals, Radnor, PA, USA and Merck, Darmstadt, Germany) and ultrapure water was obtained by a Milli-Q system (Millipore, Burlington, MA, USA). Because of safety concerns regarding the use of some organic solvents to produce macroalgae extracts with possible further applications, in this study, the current legislation was taken into consideration (2009/32/EC) [83] and food-grade solvents were selected.
3.4. Determination of Proximate Composition
3.4.1. Total Phenolic Content
Total phenolic content (TPC) was measured by the Folin–Ciocalteu method adapted to microtiter plate measurement [84]. An aliquot (100 μL) of the extract was placed into a 2 mL conical tub and mixed with 500 μL of the Folin–Ciocalteu reagent, diluted 1:10 (Sigma-Aldrich, San Luis, MO, USA) with water (Milli-Q system, Millipore, Burlington, MA, USA), and 400 μL of 12.5% sodium carbonate (Sigma-Aldrich, San Luis, MO, USA) solution. The mixture was allowed to stand at 45 °C for 15 min. Samples were centrifuged at 5500 rpm for 5 min to remove the excess of sodium carbonate. An amount of 250 μL was transferred into a microtiter plate and the absorbance was read at 765 nm in a Varioskan Flash spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). A calibration curve was obtained using phloroglucinol (Sigma-Aldrich, San Luis, MO, USA) as standard and the range of concentrations used was 10–80 ng/μL. Each standard and sample solution were run in duplicate and results were expressed as milligrams of phloroglucinol equivalents (PGE) per gram of seaweed dry weight (mg PGE/g dw). The limits of detection (LOD) and quantification (LOQ) were calculated according to Long & Winefordner (1983) [85], assuming k values of 3 and 10, respectively.
3.4.2. Carbohydrate Content
Total carbohydrate content was evaluated by the phenol–sulphuric acid method [86]. An aliquot (200 μL) of extract was previously dried in a 2 mL conical tube to avoid interferences with the extraction medium during the reaction and then resuspended in the same volume of water. An amount of 200 μL of phenol 5% (Sigma-Aldrich, San Luis, MO, USA) and 1 mL of sulphuric acid 96% (Panreac, Barcelona, Spain) were added and mixed with the residue. Reaction was performed for 15 min at 30 °C. An amount of 250 μL was transferred into a microtiter plate and the absorbance was read at 490 nm in a Varioskan Flash spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Each standard and sample solution were run in duplicate. A calibration curve was obtained using glucose as standard and the range of concentrations used was 25–250 ng/μL. Results were expressed as milligrams of glucose equivalents per gram of seaweed dry weight (mg GE/g dw). The detection (LOD) and quantification (LOQ) limits were determined as described in Section 3.4.1.
3.4.3. Protein Content
Total protein content was determined by Bradford method [87]. An amount of 200 μL of extract was previously dried in a 2 mL conical tube with nitrogen to avoid interferences with the extraction medium during the reaction and resuspended in the same volume of water. An amount of 1 mL of Bradford reactive diluted 1:6 with water (Bio-Rad Protein Assay; Bio Rad Laboratories, Hercules, CA, USA) was added to each sample and the mixture was allowed to stand at room temperature for 30 min. An amount of 250 μL was transferred into a microtiter plate and the absorbance was read at 595 nm in a Varioskan Flash spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Each standard and sample solution were run in duplicate. A calibration curve was obtained using bovine serum albumin (BSA; Sigma-Aldrich, San Luis, MO, USA) as standard and the range of concentrations used was 25–200 ng/μL. The results were expressed as milligrams of BSA equivalents per gram of seaweed dry weight (mg BSAE/g dw). The detection (LOD) and quantification (LOQ) limits were determined as described in Section 3.4.1.
3.5. Antimicrobial Activity Screening
3.5.1. Test Microorganisms and Culture Conditions
The antimicrobial activity of the extracts was assessed against 20 target strains, including Gram-positive and Gram-negative strains: [Gram-negative]—Escherichia coli LMG (Laboratory of Microbiology Gent) 10266, Serratia marcescens ATCC (American Type of Culture Collection) 25419, Proteus vulgaris LMG 16708 T, Pseudomonas aeruginosa CECT (Spanish Type Culture Collection) 116, Salmonella enterica serovar Enteritidis GN91 G5 (CRESA, IRTA), Salmonella enterica serovar. Thyphimurium CECT 443 H1, Citrobacter rodentium CIP (Pasteur Institute Collection) 104675, Enterobacter cloacae LMG 2783 T, Cronobacter turicensis LMG 23827 T, Cronobacter malonaticus LMG 23826, Cronobacter sakazaki LMG 5740 T and Cronobacter dublinensis LMG 23825 T; [Gram-positive]—Listeria monocytogenes CTC 1011 (Meat Technology Centre, IRTA), Bacillus cereus LMG 12335, Bacillus subtilis CECT 4002, Geobacillus stearothermophilus CECT 43, Enterococcus faecalis CECT 795, Staphylococcus aureus CECT 976 and Staphylococcus haemolyticus CECT 4900.
All the strains were cultivated from −80 °C stock cultures, cryoprotected with 20% glycerol (v/v) for 18 h in TSBYE (Triptic soy broth, VWR Chemicals, Radnor, PA, USA) and supplemented with 0.6% BactoTM yeast extract (BD Biosciences, Franklin Lakes, NJ, USA). Incubation temperature was 37 °C, except for Bacillus subtilis CECT 4002 and Bacillus cereus LMG 12335 that were incubated at 30 °C. For optimal growth of B. subtilis, shaking (300 rpm) was also required.
3.5.2. Disk Diffusion Assay
Determination of antimicrobial activity of macroalgae extracts was carried out using the overlay and disk diffusion methods. Cultures of each target strains at the early stationary phase (107–109 cfu/mL) were mixed with 20 mL of Müeller Hinton soft agar (0.7%, OXOID CM0337, Thermo Fisher Scientific, Waltham, MA, USA) and poured into sterile plates. The final concentration of bacteria in the overlay was between 104–106 cfu/mL.
Sterile paper disks (6 mm diameter, FisherbrandTM, Thermo Fisher Scientific, Waltham, MA, USA) were disposed over the agar and hydrated with 5 μL of the extract dissolution medium. Then, 20 μL of each extract were applied. The assay was performed in triplicate. One disk with extract dissolution medium was added as a negative control and 1 μL of chloramphenicol (2 mg/mL) was used as a positive control in each plate. Once prepared, plates were incubated at the optimal growth temperature for each strain for 18–24 h.
Antibacterial activity was defined by inhibition zones around the paper disk and their diameters were measured. Extracts that showed antimicrobial activity and target sensitive strains to those extracts were chosen for further determinations.
3.5.3. Determination of MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration)
MIC and MBC were evaluated for every active extract using the microdilution method in a 96-well plate according to the criteria recommended by the Clinical and Laboratory Standards Institute with modifications [88]. An amount of 150 μL of 24 h bacterial cultures grown in BHI (GranuCult™ Brain Infusion Heart Broth, Merck, Darmstadt, Germany) at the optimal growth temperature of each strain was added to the wells of the microtiter plate to obtain a final concentration of bacteria in each well of 104 cfu/mL. Row A served as the growth control for each of the assayed strains. In row B, 300 μL plus 20 μL of each extract were added and serial two-fold dilutions were performed to quantify antimicrobial activity. Assays were performed in duplicate for each strain. Plates were incubated at the optimal growth temperature for 18–24 h. The turbidity was visually observed and measured at a wavelength of 600 nm in a Varioskan Flash spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The minimum concentration that inhibited the growth of the bacteria was determined as the MIC. To assess the MBC, concentration that achieved an inactivation of 99.9% of the bacteria, plate counts for each strain were performed for all the wells with inhibited growth.
3.6. Statistical Analysis
The statistical analysis was performed using the software JMP® version 16.0.0 (SAS Institute Inc., Cary, NC, USA). All results were shown as means of replicates. Data were expressed as means ± standard deviations. One-way ANOVA was used to assess significant differences between samples, followed by the Tukey’s comparison test to carry out pairwise comparisons between means. Differences were considered significant at p ≤ 0.05. Principal component analysis (PCA) was performed to evaluate macroalgae based on VOCs composition.
4. Conclusions
The results obtained in this study highlight the antimicrobial potential of brown macroalgal species that are inhabitants of the northern coast of Spain. Remarkable growth inhibitory effects were observed in the D. dichotoma, B. bifurcata and E. selaginoides specimens against the foodborne pathogens L. monocytogenes, S. aureus and B. cereus, spore-forming bacteria G. stearothermophilus and B. subtilis, and the nosocomial pathogen S. haemolyticus. The spectrum of action provides evidence of their potential use as non-synthetic preservatives due to their ability to inhibit strains that represent a serious risk in the food industry and human health. The broad spectrum of extracted compounds achieved in the mid-polarity extracts includes pigments such as fucoxanthin, sugars and polyphenols and large variations in the extractable compounds’ profile were assumed to be due to differences in the genetic lineages and the effect of environmental factors. Higher amounts of extractable phenolic compounds (29.28–115.13 mg/g dw) were observed in most evaluated species, while the content in carbohydrates was higher in S. latissima (33.43 mg/g dw) and some species from the Fucales (15.14–19.15 mg/g dw). More efforts are required to develop a sustainable and responsible production, as well as the standardization of factors affecting the macroalgae metabolism. In addition, further purification steps will help to understand the nature of the active compounds, and to explore the potential synergistic/antagonist relationships between the chemicals. Further research is also needed to establish the most suitable methodology to test the antimicrobial activity of macroalgal extracts and to assure reproducibility and consensus among the scientific community.
Author Contributions
Conceptualization, C.P., T.A. and M.H.; methodology, S.R., T.A. and M.H.; formal analysis, S.R. and M.H.; resources, C.P.; writing—original draft preparation, S.R., C.P. and M.H.; writing—review and editing, S.R., C.P., T.A. and M.H.; supervision, C.P. and M.H.; project administration, M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness (INIA Project: RTA2015-00010-C03-01), and by the Generalitat de Catalunya (CERCA Program).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Susana Rubiño acknowledges the FPI Ph.D. grant from the Spanish Ministry of Economy and Competitiveness (BES-2017-0027).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tait, L.W.; Schiel, D.R. Ecophysiology of layered macroalgal assemblages: Importance of subcanopy species biodiversity in buffering primary production. Front. Mar. Sci. 2018, 5, 444. [Google Scholar] [CrossRef]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. Chapter eight—The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. In Seaweeds Around the World: State of Art and Perspectives; Bourgougnon, N., Ed.; Academic Press: Cambidge, MA, USA, 2020; Volume 95, pp. 247–287. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Maximiliano, J.E.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; Anadón, A.; Peteiro, C.; Rubiño, S.; et al. Brown marine algae Gongolaria baccata extract protects Caco-2 cells from oxidative stress induced by tert-butyl hydroperoxide. Food Chem. Toxicol. 2021, 156, 112460. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; De Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. J. Food Qual. 2017, 2017, 1090932. [Google Scholar] [CrossRef]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Pereira, R.; Yarish, C. Mass production of marine macroalgae. In Encyclopedia of Ecology; Five-Volume Set; Academic Press: Cambridge, MA, USA, 2008; pp. 2236–2247. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; Volume 11, pp. 27–66. [Google Scholar] [CrossRef]
- Zollmann, M.; Rubinsky, B.; Liberzon, A.; Golberg, A. Multi-Scale modeling of intensive macroalgae cultivation and marine nitrogen sequestration. Commun. Biol. 2021, 4, 848. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, 351, 72–201. [Google Scholar]
- Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe, Luxembourg, Technical Report by the Joint Research Centre (JRC), Publications Office of the European Union. 2021. Available online: https://data.europa.eu/doi/10.2760/049515 (accessed on 8 October 2022).
- Bassetti, M.; Giacobbe, D.R. A Look at the clinical, economic, and societal impact of antimicrobial resistance in 2020. Expert Opin. Pharmacother. 2020, 21, 2067–2071. [Google Scholar] [CrossRef]
- Subramaniam, G.; Girish, M. Antibiotic resistance—A cause for reemergence of infections. Indian J. Pediatr. 2020, 87, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Puente, A.; Juanes, J.A. An ecological classification of rocky shores at a regional scale: A predictive tool for management of conservation values. Mar. Ecol. 2016, 37, 311–328. [Google Scholar] [CrossRef]
- Barbier, M.; Charrier, B.; Araujo, R.; Holdt, S.L.; Jacquemin, B.; Rebours, C. Pegasus—Phycomorph European guidelines for a sustainable aquaculture of seaweeds. In COST Action FA1406; Barbier, M., Charrier, B., Eds.; European Cooperation in Science and Technology: Roscoff, France, 2019. [Google Scholar] [CrossRef]
- Garicano Vilar, E.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Volatile compounds of six species of edible seaweed: A review. Algal Res. 2020, 45, 101740. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yoshida, M.; Yotsukura, N.; Chirapart, A.; Kajiwara, T. Formation of aldehyde flavor (n-hexanal, 3Z-nonenal and 2E-nonenal) in the brown alga, Laminaria angustata. J. Appl. Phycol. 2006, 18, 409–412. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of hexanal, (E)-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Laturnus, F.; Giese, B.; Wiencke, C.; Adams, F.C. Low-molecular-weight organoiodine and organobromine compounds released by polar macroalgae—The influence of abiotic factors. Fresenius J. Anal. Chem. 2000, 368, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, N.; Ogata, Y.; Okada, N.; Itoh, N. Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: Production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide. Phytochemistry 2001, 58, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Glasson, C.R.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673. [Google Scholar] [CrossRef]
- Romanazzi, D.; Sanchez-Garcia, C.; Svenson, J.; Mata, L.; Pes, K.; Hayman, C.M.; Wheeler, T.T.; Magnusson, M. Rapid analytical method for the quantification of bromoform in the red seaweeds Asparagopsis armata and Asparagopsis taxiformis using gas chromatography–mass spectrometry. ACS Agric. Sci. Technol. 2021, 1, 436–442. [Google Scholar] [CrossRef]
- Bravo-Linares, C.M.; Mudge, S.M.; Loyola-Sepulveda, R.H. Production of volatile organic compounds (VOCs) by temperate macroalgae. The use of Solid Phase Microextraction (SPME) coupled to GC-MS as method of analysis. J. Chil. Chem. Soc. 2010, 55, 227–232. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef]
- Lann, K.L.; Ferret, C.; VanMee, E.; Spagnol, C.; Lhuillery, M.; Payri, C.; Stiger-Pouvreau, V. Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: Spatial and specific variability. Phycol. Res. 2012, 60, 37–50. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed]
- Stiger-Pouvreau, V.; Jégou, C.; Cérantola, S.; Guérard, F.; Le Lann, K. Chapter Thirteen—Phlorotannins in Sargassaceae species from Brittany (France): Interesting molecules for ecophysiological and valorisation purposes. In Sea Plants; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 379–411. [Google Scholar] [CrossRef]
- Jacobsen, J.; Sørensen, A.D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Phaeophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Belda, M.; Sanchez, D.; Bover, E.; Prieto, B.; Padrón, C.; Cejalvo, D.; Lloris, J.M. Extraction of polyphenols in Himanthalia elongata and determination by High Performance Liquid Chromatography with Diode Array Detector prior to its potential use against oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033–1034, 334–341. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Fitzgerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid—liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish West coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Saifullah; Olsen, Y.; Surilayani, D.; Handå, A. Carbohydrate of the brown seaweed, Saccharina latissima: A review. In Joint Proceedings of the 2nd and the 3rd International Conference on Food Security Innovation; Atlantis Press: Amsterdam, The Netherlands, 2021; pp. 31–34. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Sokovic, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Macroalgae as an alternative source of nutrients and compounds with bioactive potential. Proceedings 2020, 70, 46. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential Hydrothermal-Assisted Extraction followed by ultrasound and thermal technologies. Mar. Drugs. 2019, 17, 457. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Lemesheva, V.; Tarakhovskaya, E. Physiological functions of phlorotannins. Biol. Commun. 2018, 63, 70–76. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal variation of the proximate composition, mineral content, fatty acid profiles and other phytochemical constituents of selected brown macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef]
- Dodd, C.E.; Aldsworth, T.G.; Stein, R.A. (Eds.) Foodborne Diseases; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Kakagianni, M.; Koutsoumanis, K.P. Mapping the risk of evaporated milk spoilage in the Mediterranean region based on the effect of temperature conditions on Geobacillus stearothermophilus growth. Food Res. Int. 2018, 111, 104–110. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic resistance and regulation of the Gram-negative bacterial outer membrane barrier by host innate immune molecules. MBio. 2016, 7, e0154116. [Google Scholar] [CrossRef]
- El Wahidi, M.; El Amraoui, B.; El Amraoui, M.; Bamhaoud, T. Screening of antimicrobial activity of macroalgae extracts from the Moroccan Atlantic coast. Ann. Pharm. Françaises 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria bifurcata: A key macro-alga as a source of bioactive compounds and functional ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Fernández-No, I.C.; Guarddon, M.; Böhme, K.; Cepeda, A.; Calo-Mata, P.; Barros-Velázquez, J. Detection and quantification of spoilage and pathogenic Bacillus cereus, Bacillus subtilis and Bacillus licheniformis by real-time PCR. Food Microbiol. 2011, 28, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.; Seale, B.; Flint, S.; Palmer, J. Biofilms in dairy processing. In Biofilms in the Food and Beverage Industries; Woodhead Publishing Series in Food Science, Technology and Nutrition; Fratamico, P.M., Annous, B.A., Gunther, N.W., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 396–431. [Google Scholar] [CrossRef]
- Burgess, S.A.; Flint, S.H.; Lindsay, D.; Cox, M.P.; Biggs, P.J. Insights into the Geobacillus stearothermophilus species based on phylogenomic principles. BMC Microbiol. 2017, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Gironés, R.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e4524. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Schraft, H. Chapter 20—Bacillus cereus Food Poisoning. In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 395–405. [Google Scholar] [CrossRef]
- Kimura, K.; Yokoyama, S. Trends in the application of Bacillus in fermented foods. Curr. Opin. Biotechnol. 2019, 56, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pavic, S.; Brett, M.; Petric, N.; Lastre, D.; Smoljanovic, M.; Atkinson, M. An outbreak of food poisoning in a kindergarten caused by milk powder containing toxigenic Bacillus subtilis and Bacillus licheniformis. Arch. Lebensmittelhygiene 2005, 56, 20–22. [Google Scholar]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Fayzi, L.; Askarne, L.; Cherifi, O.; Boufous, E.H.; Cherifi, K. Comparative antibacterial activity of some selected seaweed extracts from Agadir coastal regions in Morocco. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 390–399. [Google Scholar] [CrossRef]
- Salvador, N.; Garreta, A.G.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Götz, F.; Bannerman, T.; Schleifer, K.H. The genera Staphylococcus and Macrococcus. Prokaryotes 2006, 4, 5–75. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Karima, S.; Fatiha, B. Study of the antimicrobial activity of four algerian marin algae species. In Microbes in Applied Research; World Scientific: Singapore, 2012; pp. 578–581. [Google Scholar]
- Johnsi-Christobel, G.; Lipton, A.P.; Aishwarya, M.S.; Sarika, A.R.; Udayakumar, A. Antibacterial activity of aqueous extract from selected macroalgae of Southwest coast of India. Seaweed Res. Util. 2011, 33, 67–75. [Google Scholar] [CrossRef]
- Zouaoui, B.; Ghalem, B.R. The phenolic contents and antimicrobial activities of some marine algae from the Mediterranean Sea (Algeria). Russ. J. Mar. Biol. 2017, 43, 491–495. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Cabral, E.M.; Mondala, J.M.R.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Major lipophilic pigments in Atlantic seaweeds as valuable food ingredients: Analysis and assessment of quantification methods. Food Res. Int. 2022, 159, 111609. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.d.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the composition, antioxidant and antimicrobial activities of Cystoseira compressa during seasonal growth. Mar. Drugs. 2022, 20, 64. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghassempour, A.R. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.H.; Li, D.Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth) International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile; Koeltz Botanical Books: Glashütten, Germany, 2018; Volume 159. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 141, 3–11. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A Simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J. Limit of detection. A closer look at the IUPAC definition. In Statistics for Environmental Engineers, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1983; Volume 55, pp. 712A–724A. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically, 11th ed.; Approved Standard, CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).