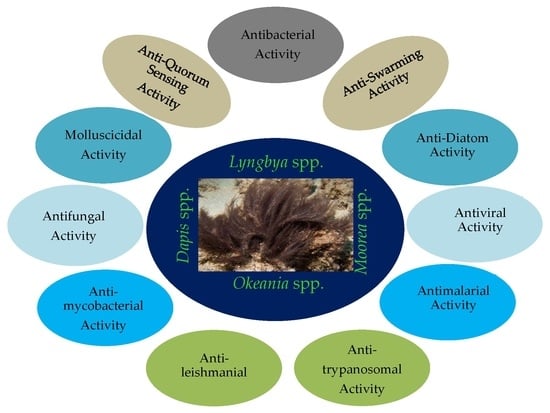
Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022
Abstract
1. Introduction
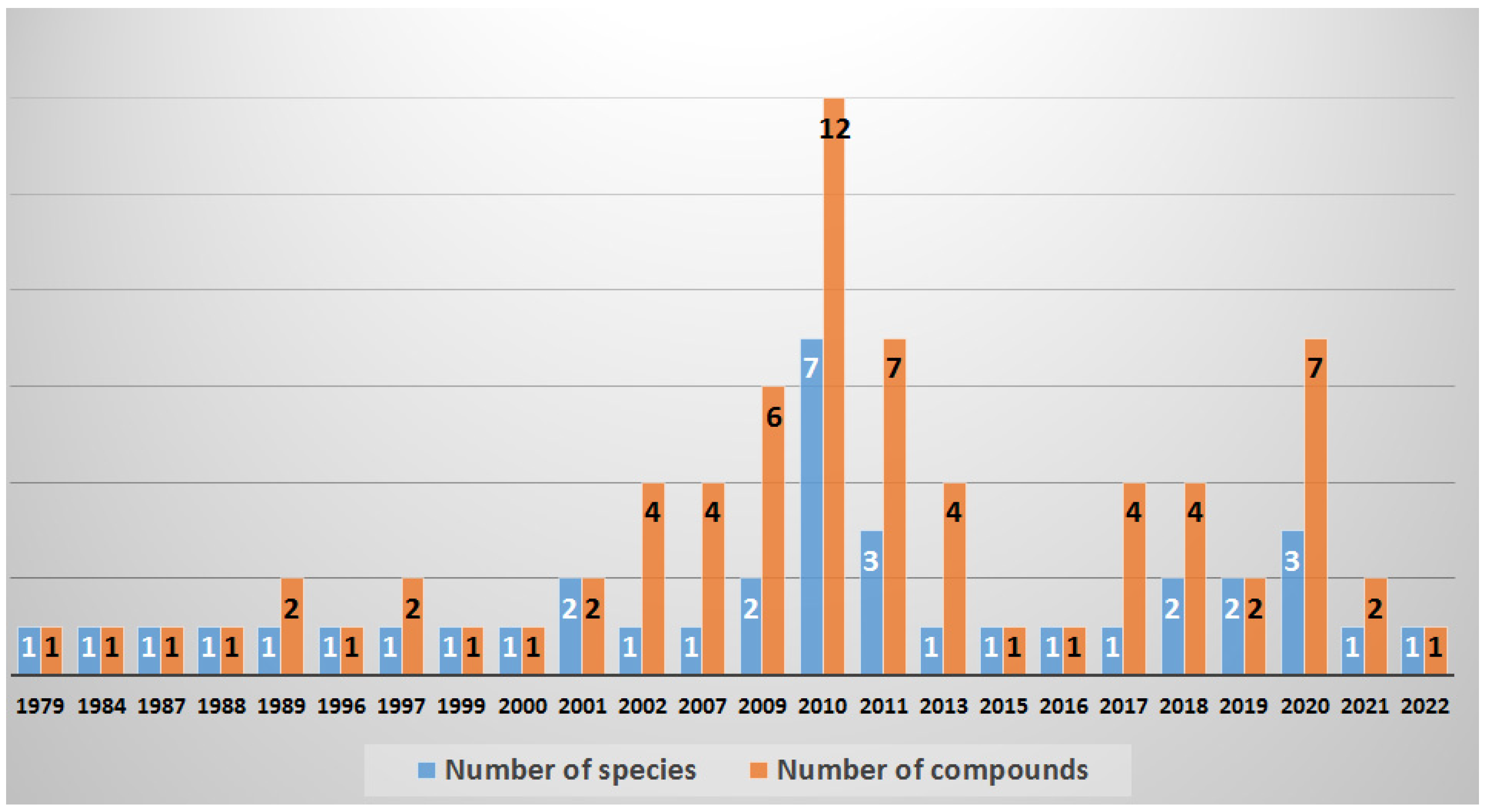
2. Collected Species and Geographical Locations
3. Compounds with Antibacterial Activities
4. Compounds with Anti-Swarming and Anti-Quorum Sensing Activities
5. Compounds with Antifungal Activities
6. Compounds with Antiparasitic Activities
8. Compounds with Molluscicidal Anti-Diatoms Activities (Table 7)
| Compound | Source Organism | Collection Site | Targeted Organism | LC50/LC100/LD50/% of Inhibition | Reference |
|---|---|---|---|---|---|
| Tanikolide (34) | L. majuscula | Madagascar | B. glabrata | LD50 = 9.0 µg/mL | [62] |
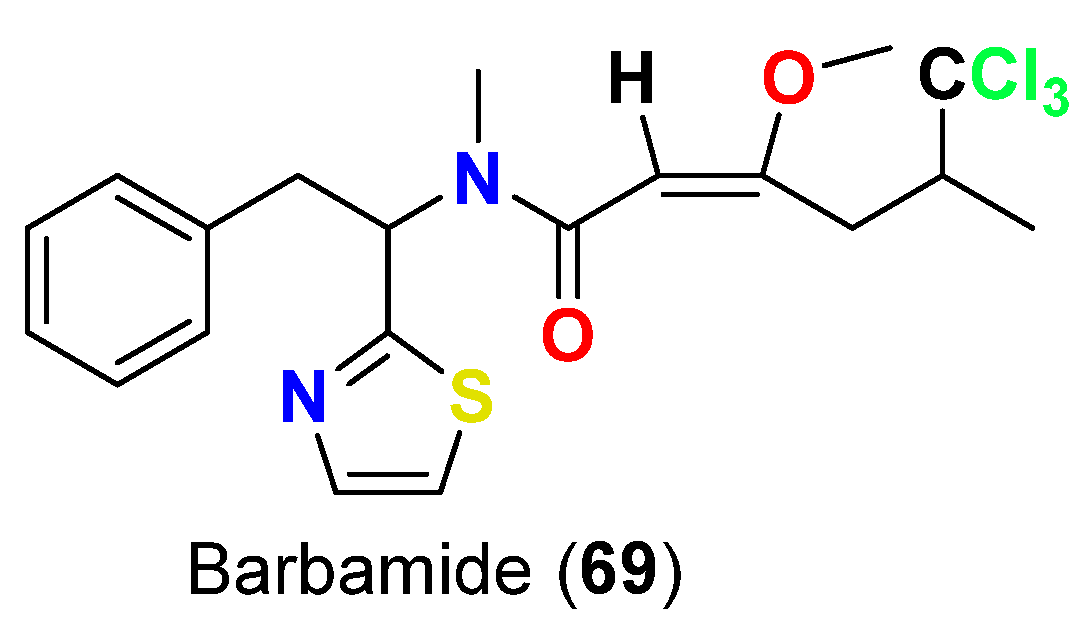
| Barbamide (69) | L. majuscula | Curaçao | B. glabrata | LC100 = 10 µg/mL | [82] |
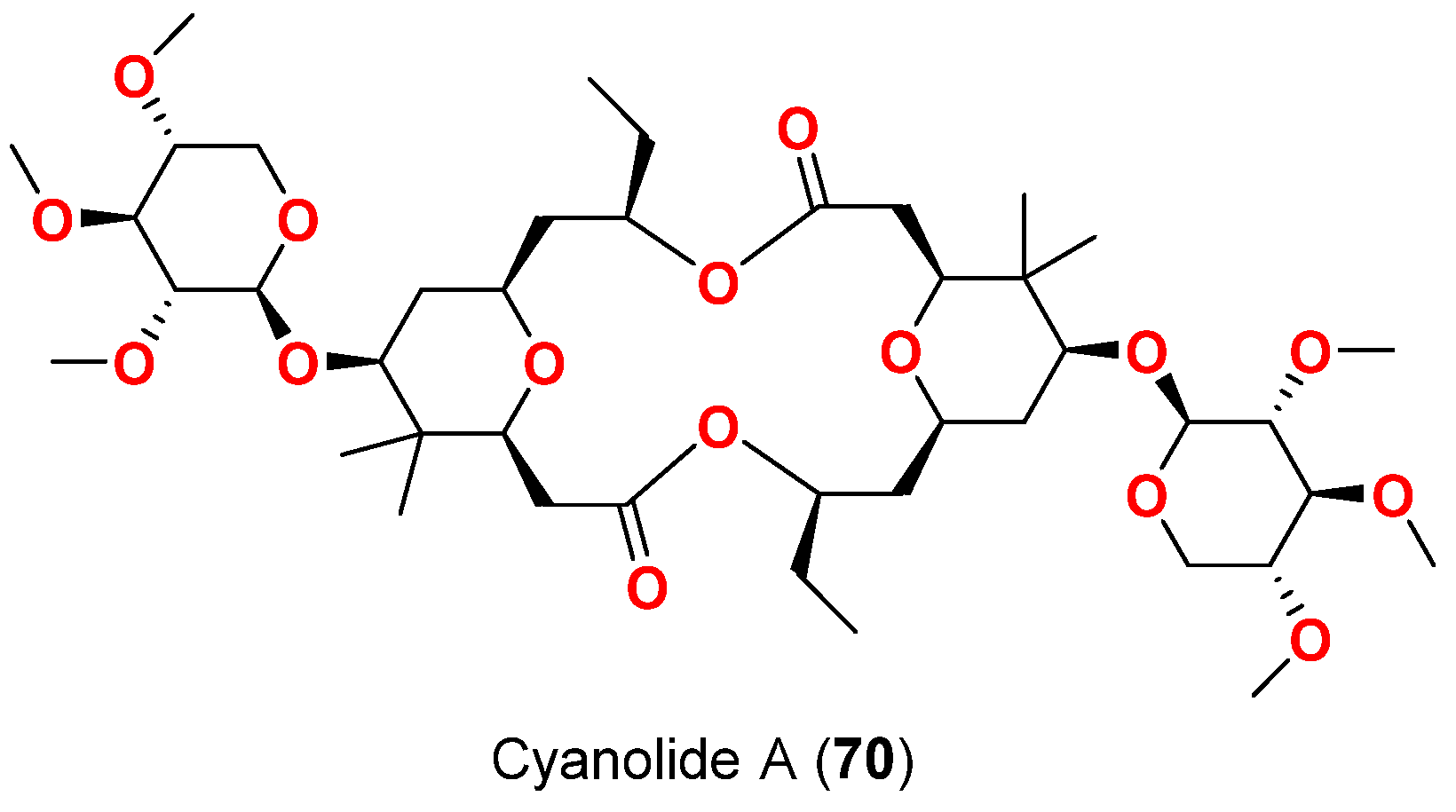
| Cyanolide A (70) | L. bouillonii | Papua New Guinea | B. glabrata | LC50 = 1.2 μM | [83] |
| Debromooscillatoxin G (71) | M. producens | Okinawa, Japan | N. amabilis | 30% at 10 μg/mL | [84] |
| Debromooscillatoxin I (72) | M. producens | Okinawa, Japan | N. amabilis | 30% at 10 μg/mL | [84] |
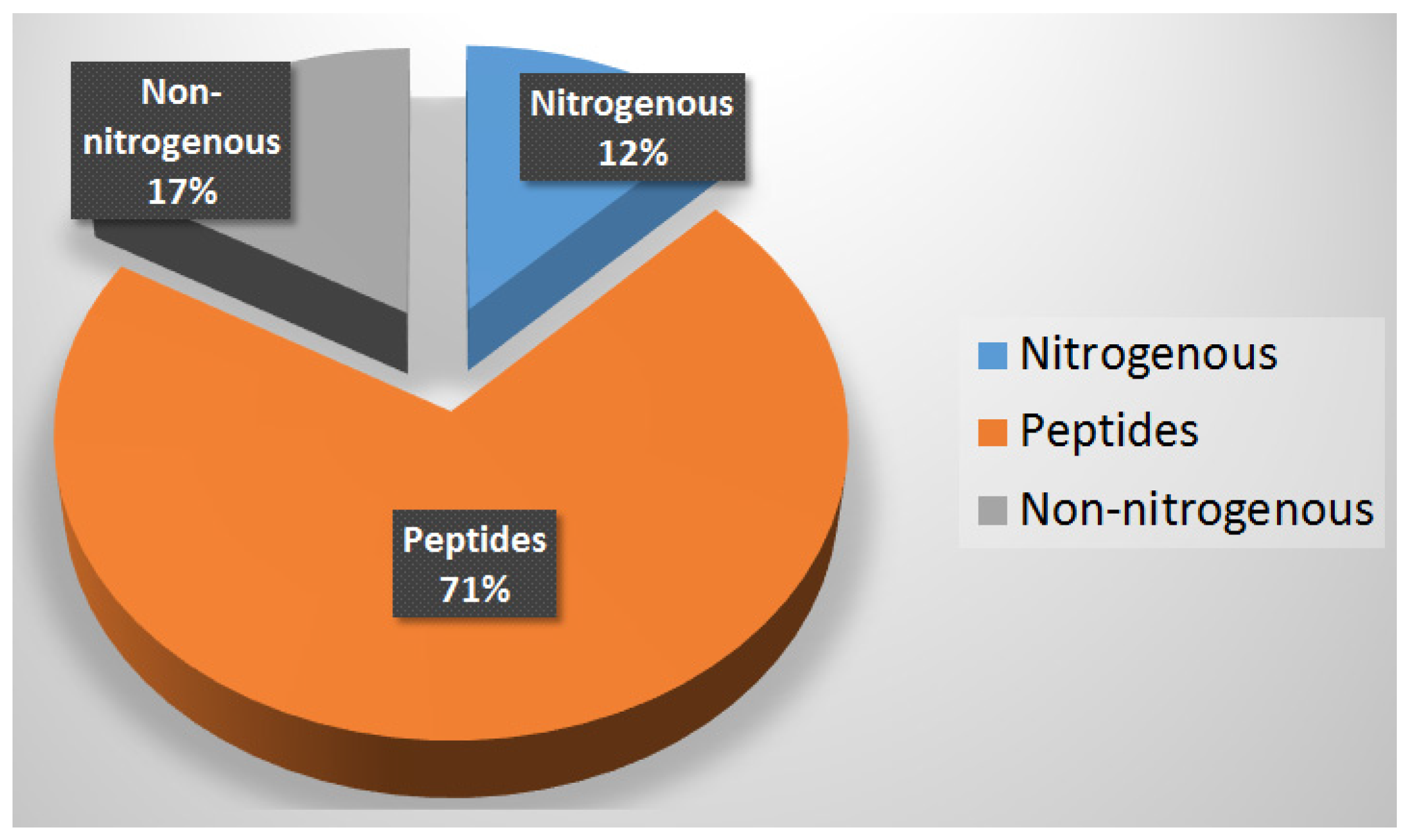
9. Summary
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, G.W.; Keating, C.L.; Newman, J.S. The golden anniversary of the silver bullet. JAMA 1993, 270, 1610–1611. [Google Scholar] [CrossRef]
- Kardos, N.; Demain, A.L. Penicillin: The medicine with the greatest impact on therapeutic outcomes. Appl. Microbiol. Biotechnol. 2011, 92, 677–687. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1334. [Google Scholar] [CrossRef]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; ECDC-EMA Working Group. Critical shortage of new antibiotics in development against multidrug-resistant bacteria-time to react is now. Drug Resist. Updat. 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 1, 36–42. [Google Scholar]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Resistance Threats in the United States; 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 25 October 2022).
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of infections due to MDR gram-negative bacteria. Front Med. 2019, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Poonawala, H.; Jain, Y. Drug-resistant tuberculosis: Is India ready for the challenge? BMJ Glob. Health 2018, 3, e000971. [Google Scholar] [CrossRef]
- Friedrich, M.J. Drug-resistant tuberculosis predicted to increase in high-burden countries. JAMA 2017, 318, 231. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Luo, G.D.; Gao, S.-J. Global health concerns stirred by emerging viral infections. Med. Virol. 2020, 92, 399–400. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Pandemics throughout history. Front. Microbiol. 2021, 11, 3594. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Garcia, M.A.; Gordon-Lipkin, E.; Parra, B.; Pardo, C.A. Emerging viral infections and their impact on the global burden of neurological disease. Semin. Neurol. 2018, 38, 163–175. [Google Scholar] [CrossRef]
- Bleibtreu, A.; Bertine, M.; Bertin, C.; Houhou-Fidouh, N.; Visseaux, B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med. Mal. Infect. 2020, 50, 243–251. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral pandemics of the last four decades: Pathophysiology, health impacts and perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef]
- Schaefer, T.J.; Panda, P.K.; Wolford, R.W. Dengue Fever; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Powell, J.R.; Human, M.-B. Viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 8, 1563–1565. [Google Scholar] [CrossRef]
- Singh, R.; Chauhan, N.; Kuddus, M. Exploring the therapeutic potential of marine-derived bioactive compounds against COVID-19. Environ. Sci. Pollut. Res. Int. 2021, 28, 52798–52809. [Google Scholar] [CrossRef]
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-yearold) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. The Ecology of Cyanobacteria: Their Diversity in Time and Space; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Nagle, D.G.; Paul, V.J. Production of secondary metabolites by filamentous tropical marine cyanobacteria: Ecological functions of the compounds. J. Phycol. 1999, 35, 1412–1421. [Google Scholar]
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F.G. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Engene, N.; Rottacker, E.C.; Kas, J.; Byrum, T.; Gerwick, W.H.; Choi, H.; Ellisman, M.H. Moorea producens Gen. Nov., Sp. Nov. and Moorea bouillonii Comb. Nov., Tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 5, 1171–1178. [Google Scholar]
- Engene, N.; Tronholm, A.; Paul, V.J. Uncovering cryptic diversity of Lyngbya: The new tropical marine cyanobacterial genus Dapis (Oscillatoriales). J. Phycol. 2018, 54, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Jiří, K.; Eliška, Z.; Jan, Š.; Jiří, K.; Jason, W.; Brett, A.N.; Jaroslava, K. Polyphasic evaluation of Limnoraphis robusta, a water—Bloom forming cyanobacterium from Lake Atitlán, Guatemala, with a description of Limnoraphis gen. nov. J. Czech Phycol. Soc. 2013, 13, 39–52. [Google Scholar]
- Tronholm, A.; Engene, N. Moorena Gen. Nov., a valid name for “Moorea Engene & Al.” Nom. Inval. (Oscillatoriaceae, Cyanobacteria). Not. Algarum 2019, 122, 1–2. [Google Scholar]
- Mcgregor, G.B.; Sendall, B.C. Phylogeny and toxicology of Lyngbya wollei (Cyanobacteria, Oscillatoriales) from North-Eastern Australia, with description of Microseira gen. nov. J. Phycol. 2015, 51, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Paul, V.J.; Byrum, T.; Gerwick, W.H.; Thor, A.; Ellisman, M.H. Five chemically rich species of tropical marine cyanobacteria of the genus Okeania gen. nov. (Oscillatoriales, Cyanoprokaryota). J. Phycol. 2013, 49, 1095–1106. [Google Scholar] [CrossRef]
- Marine Pharmacology. Available online: https://www.marinepharmacology.org/ (accessed on 1 November 2022).
- Cardllina, J.H.C.; Moore, R.E.; Arnold, E.V.; Clardy, J. Structure and absolute configuration of malyngolide, an antibiotic from the marine blue-green alga Lyngbya majuscula Gomont. J. Org. Chem. 1979, 44, 4039–4042. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; McPhail, K.L.; Elbandy, M. Malyngamide 4, a new lipopeptide from the Red Sea marine cyanobacterium Moorea producens (Formerly Lyngbya majuscula). Phytochem. Lett. 2013, 6, 183–188. [Google Scholar] [CrossRef]
- Gekwick, W.H.; Reyes, S.; Alvarado, B. Two malyngamides from the Caribbean cyanobacterium Lyngbya majuscula. Phytochemistry 1987, 26, 1701–1704. [Google Scholar] [CrossRef]
- Luesch, H.; Pangilinan, R.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Pitipeptolides A and B, new cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 304–307. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C-F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef]
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J. Nat. Prod. 2011, 74, 109–112. [Google Scholar] [CrossRef]
- Zainuddin, E.N.; Jansen, R.; Nimtz, M.; Wray, V.; Preisitsch, M.; Lalk, M.; Mundt, S. Lyngbyazothrins A-D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J. Nat. Prod. 2009, 72, 2080. [Google Scholar] [CrossRef]
- Levert, A.; Alvarin, R.; Bornancin, L.; Mansour, E.A.; Burja, A.M.; Genevie, A.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and activities of tiahuramides A−C, cyclic depsipeptides from a Tahitian collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310. [Google Scholar] [CrossRef]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation Sarah. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.Y.K.; Tan, L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2011, 72, 2369–2375. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Kwan, J.C.; Teplitski, M.; Gunasekera, S.P.; Paul, V.J.; Luesch, H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 463–466. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Alagely, A.; Gunasekera, S.P.; Paul, V.J. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ. Microbiol. Rep. 2010, 2, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “Tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol. Biosyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Matthew, S.; Chen, Q.Y.; Kwan, J.C.; Paul, V.J.; Luesch, H. Discovery and total synthesis of doscadenamide A: A quorum sensing signaling molecule from a marine cyanobacterium. Org. Lett. 2019, 21, 7274–7278. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Moore, R.E.; Mynderse, J.S.; Niemczura, W.P.; Todd, J.S. Structure of majusculamide C, a cyclic depsipeptide from Lyngbya majuscula. J. Org. Chem. 1984, 49, 236–241. [Google Scholar] [CrossRef]
- Mynderse, J.S.; Hunt, A.H.; Moore, R.E. 57-Normajusculamide C, a minor cyclic depsipeptide isolated from Lyngbya majuscula. J. Nat. Prod. 1988, 51, 1299–1301. [Google Scholar] [CrossRef]
- Meickle, T.; Matthew, S.; Ross, C.; Luesch, H.; Paul, V. Bioassay-guided isolation and identification of desacetylmicrocolin B from Lyngbya Cf. Polychroa. Planta Med. 2009, 75, 1427–1430. [Google Scholar] [CrossRef]
- Bonnard, I.; Rolland, M.; Francisco, C.; Banaigs, B. Total Structure and biological properties of laxaphycins A and B, cyclic lipopeptides from the marine cyanobacterium Lyngbya majuscula. Int. J. Pept. Res. Ther. 1997, 4, 289–292. [Google Scholar] [CrossRef]
- Singh, I.P.; Milligan, K.E.; Gerwick, W.H. Tanikolide, a toxic and antifungal lactone from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1999, 62, 1333–1335. [Google Scholar] [CrossRef]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya mojuscula. J. Nat. Prod. 2000, 63, 1440–1443. [Google Scholar] [CrossRef]
- Marquez, B.L.; Watts, K.S.; Yokochi, A.; Roberts, M.A.; Verdier-Pinard, P.; Jimenez, J.I.; Hamel, E.; Scheuer, P.J.; Gerwick, W.H. Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J. Nat. Prod. 2002, 65, 866–871. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Ernst-Russell, M.A.; De Ropp, J.S.; Molinski, T.F. Lobocyclamides A-C, lipopeptides from a cryptic cyanobacterial mat containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef]
- McPhail, K.L.; Correa, J.; Linington, R.G.; González, J.; Ortega-Barría, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef]
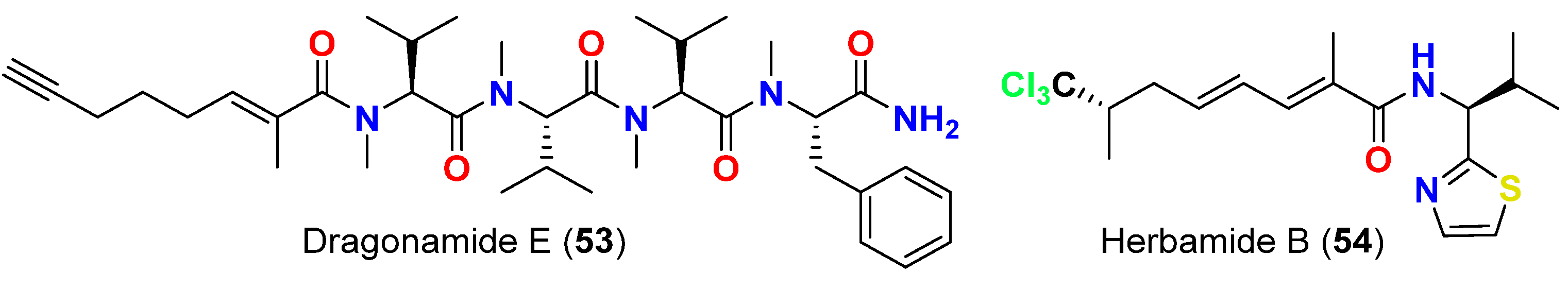
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.D.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Tidgewell, K.; Capson, T.L.; Engene, N.; Almanza, A.; Schemies, J.; Jung, M.; Gerwick, W.H. Malyngolide dimer, a bioactive symmetric cyclodepside from the Panamanian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 709–711. [Google Scholar] [CrossRef]
- Nozaki, T.; Saito-nakano, Y.; Suenaga, K. Ikoamide, an antimalarial lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2020, 83, 481–488. [Google Scholar]
- Ozaki, K.; Iwasaki, A.; Sezawa, D.; Fujimura, H.; Nozaki, T.; Saito-nakano, Y.; Suenaga, K.; Teruya, T. Isolation and total synthesis of mabuniamide, a lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2019, 82, 2907–2915. [Google Scholar] [CrossRef]
- Shao, C.; Mou, X.; Cao, F.; Spadafora, C.; Glukhov, E.; Gerwick, L.; Wang, C.; Gerwick, W.H. Bastimolide B, an antimalarial 24-membered marine macrolide possessing a tert-butyl group. J. Nat. Prod. 2018, 81, 211–215. [Google Scholar] [CrossRef]
- Shao, C.L.; Linington, R.G.; Balunas, M.J.; Centeno, A.; Boudreau, P.; Zhang, C.; Engene, N.; Spadafora, C.; Mutka, T.S.; Kyle, D.E.; et al. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta. J. Org. Chem. 2015, 80, 7849–7855. [Google Scholar] [CrossRef]
- Fathoni, I.; Petitbois, J.G.; Alarif, W.M.; Abdel-Lateff, A.; Al-lihaibi, S.S.; Yoshimura, E.; Nogata, Y.; Vairappan, C.S.; Sholikhah, E.N.; Okino, T. Bioactivities of lyngbyabellins from cyanobacteria of Moorea and Okeania genera. Molecules 2020, 5, 3986. [Google Scholar] [CrossRef]
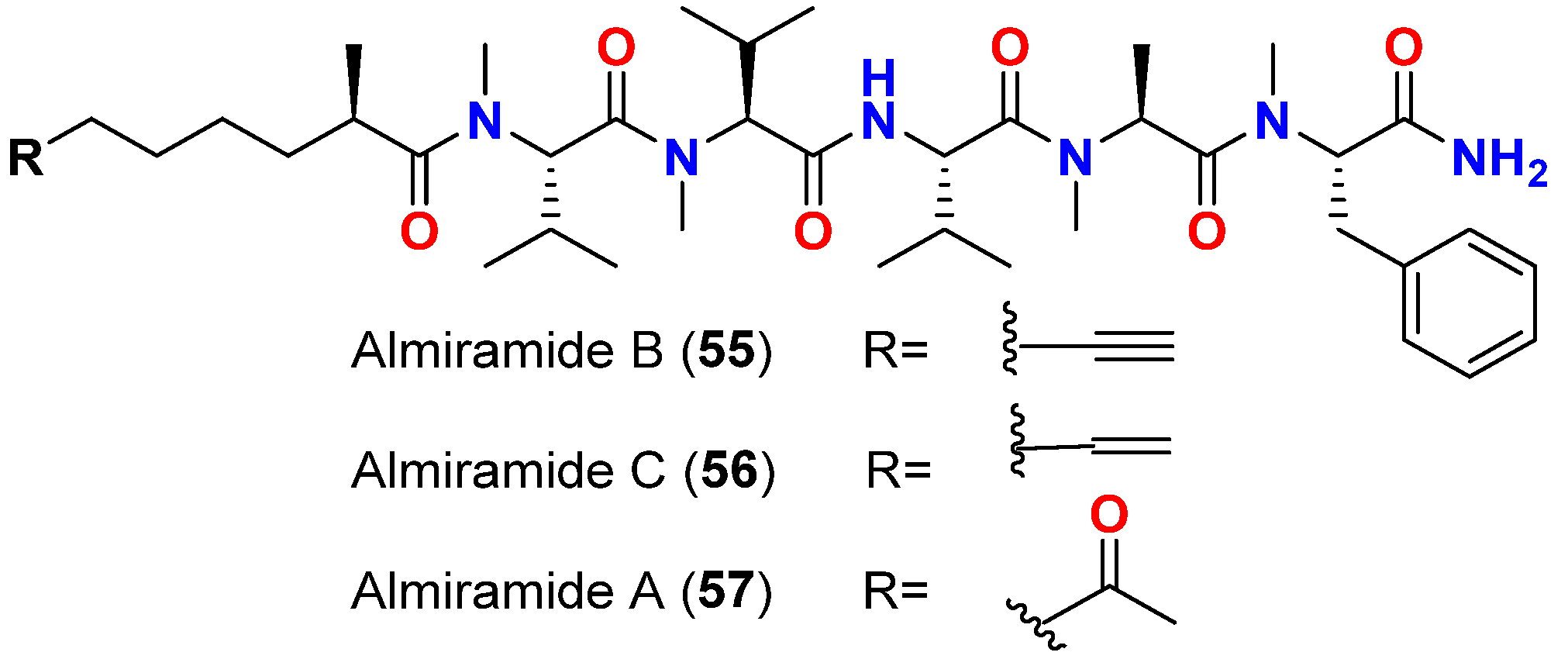
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Della Togna, G.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A-C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef]
- Almaliti, J.; Malloy, K.L.; Glukhov, E.; Spadafora, C.; Gutiérrez, M.; Gerwick, W.H. Dudawalamides A-D, antiparasitic cyclic depsipeptides from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2017, 80, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
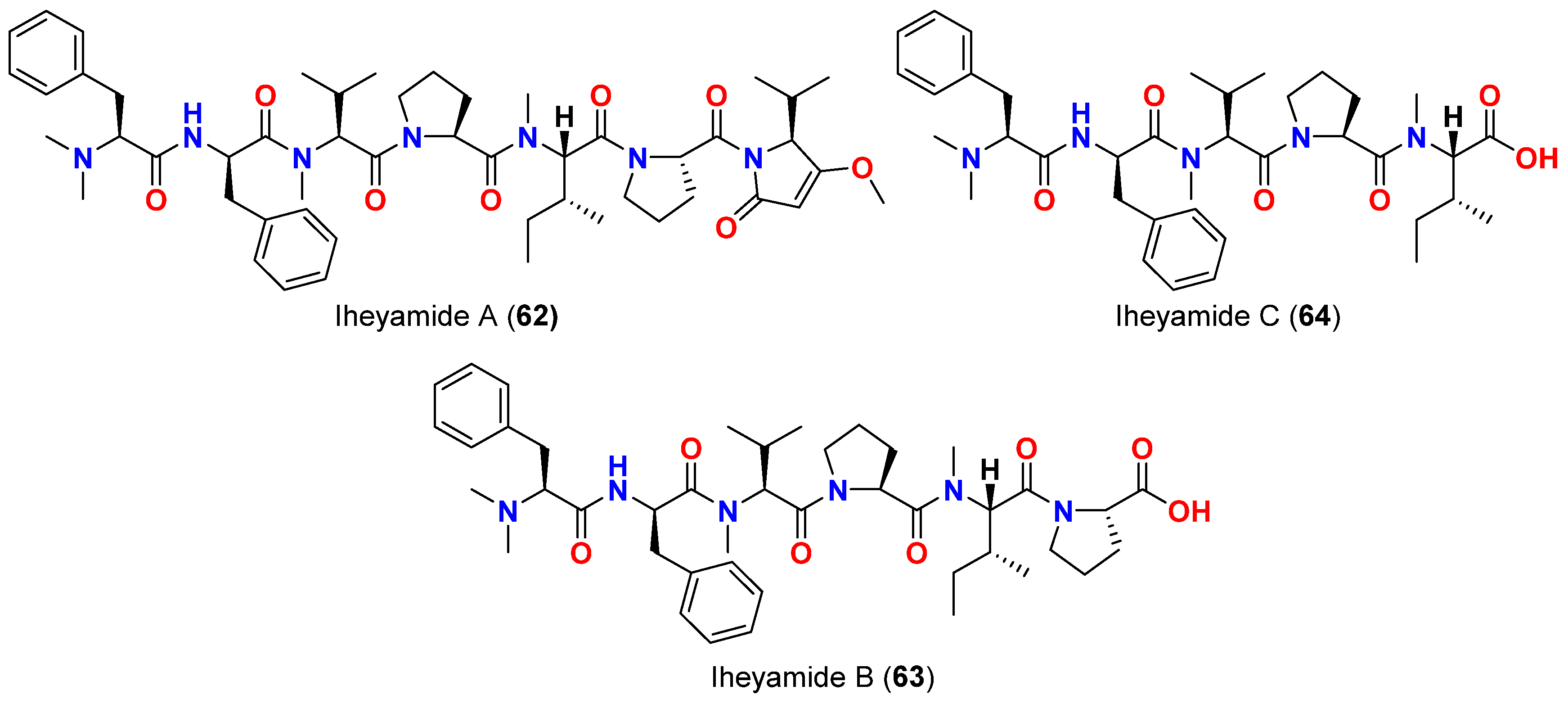
- Kurisawa, N.; Iwasaki, A.; Jeelani, G.; Nozaki, T.; Suenaga, K. Iheyamides A-C, antitrypanosomal linear peptides isolated from a marine Dapis sp. cyanobacterium. J. Nat. Prod. 2020, 83, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Kanamori, Y.; Ohno, O.; Iwatsuki, M.; Ishiyama, A.; Hokari, R.; Otoguro, K.; Omura, S.; et al. Janadolide, a cyclic polyketide-peptide hybrid possessing a tert-butyl group from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2016, 79, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R.; Iwasaki, A.; Ebihara, A.; Jeelani, G.; Nozaki, T.; Suenaga, K. Isolation and total synthesis of beru’amide, an antitrypanosomal polyketide from a marine cyanobacterium Okeania sp. Org. Lett. 2022, 24, 4710–4714. [Google Scholar] [CrossRef] [PubMed]
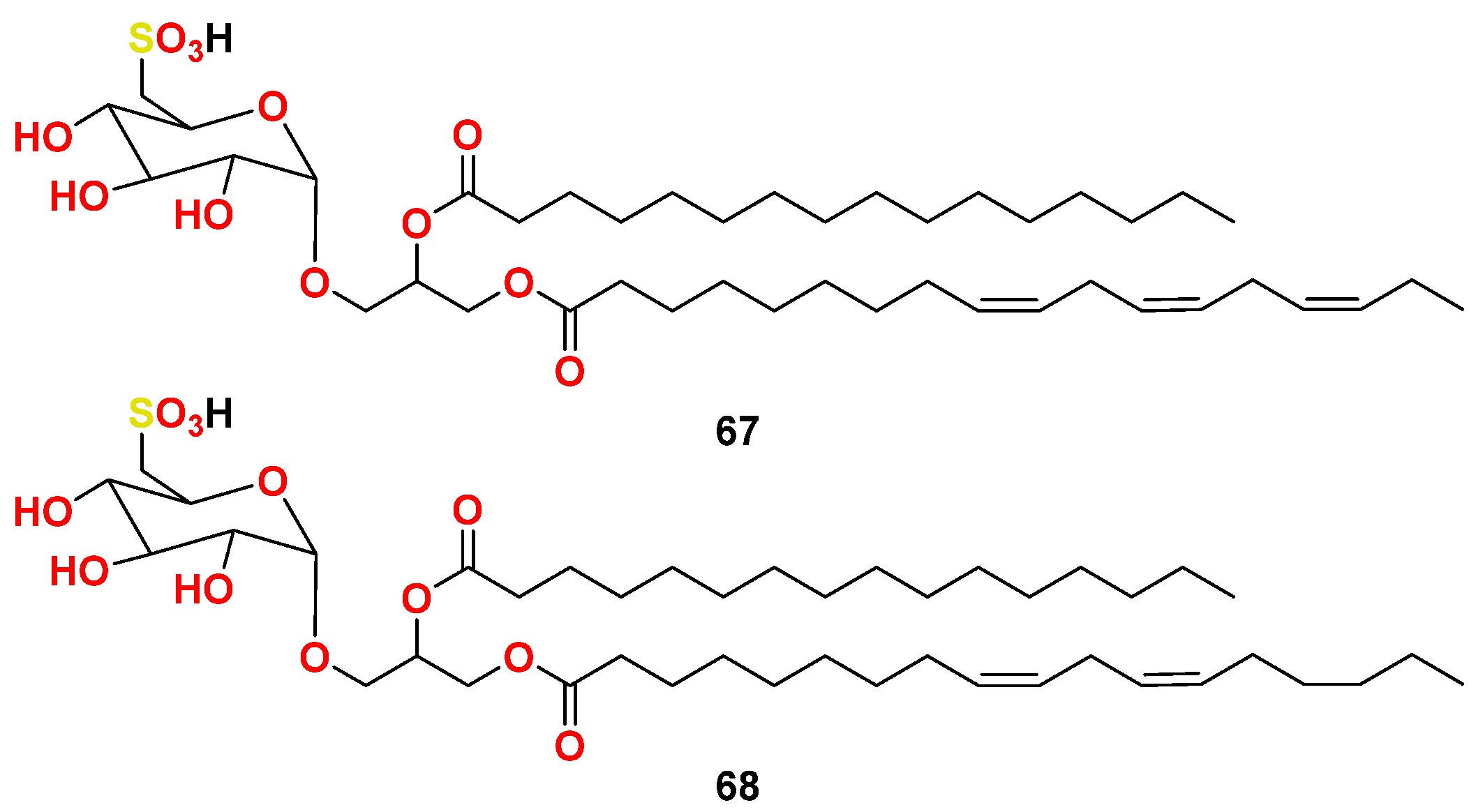
- Gustafson, K.R.; Cardellina, J.H., 2nd; Fuller, R.W.; Weislow, O.S.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.; Boyd, M.R. AIDS-antiviral sulfolipids from cyanobacteria (blue-green algae). J. Natl. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef]
- Reshef, V.; Mizrachi, E.; Maretzki, T.; Silberstein, C.; Loya, S.; Hizi, A.; Carmeli, S. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 1997, 60, 1251–1260. [Google Scholar] [CrossRef]
- Loya, S.; Reshef, V.; Mizrachi, E.; Silberstein, C.; Rachamim, Y.; Carmeli, S.; Hizi, A. The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from cyanobacteria: Contribution of different moieties to their high potency. J. Nat. Prod. 1998, 61, 891–895. [Google Scholar] [CrossRef]
- Orjala, J.; Gerwick, W.H. Barbamide, a chlorinated metabolite with molluscicidal activity from the Caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1996, 59, 427–430. [Google Scholar] [CrossRef]
- Pereira, A.R.; McCue, C.F.; Gerwick, W.H. Cyanolide A, a glycosidic macrolide with potent molluscicidal activity from the Papua New Guinea cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2010, 73, 217–220. [Google Scholar] [CrossRef]
- Iguchi, K.; Satake, M.; Nishio, Y.; Zhang, B.; Kawashima, K.; Uchida, H.; Nagai, H. Debromooscillatoxins G and I from the cyanobacterium Moorea Producens. Heterocycles 2021, 102, 1287–1293. [Google Scholar]
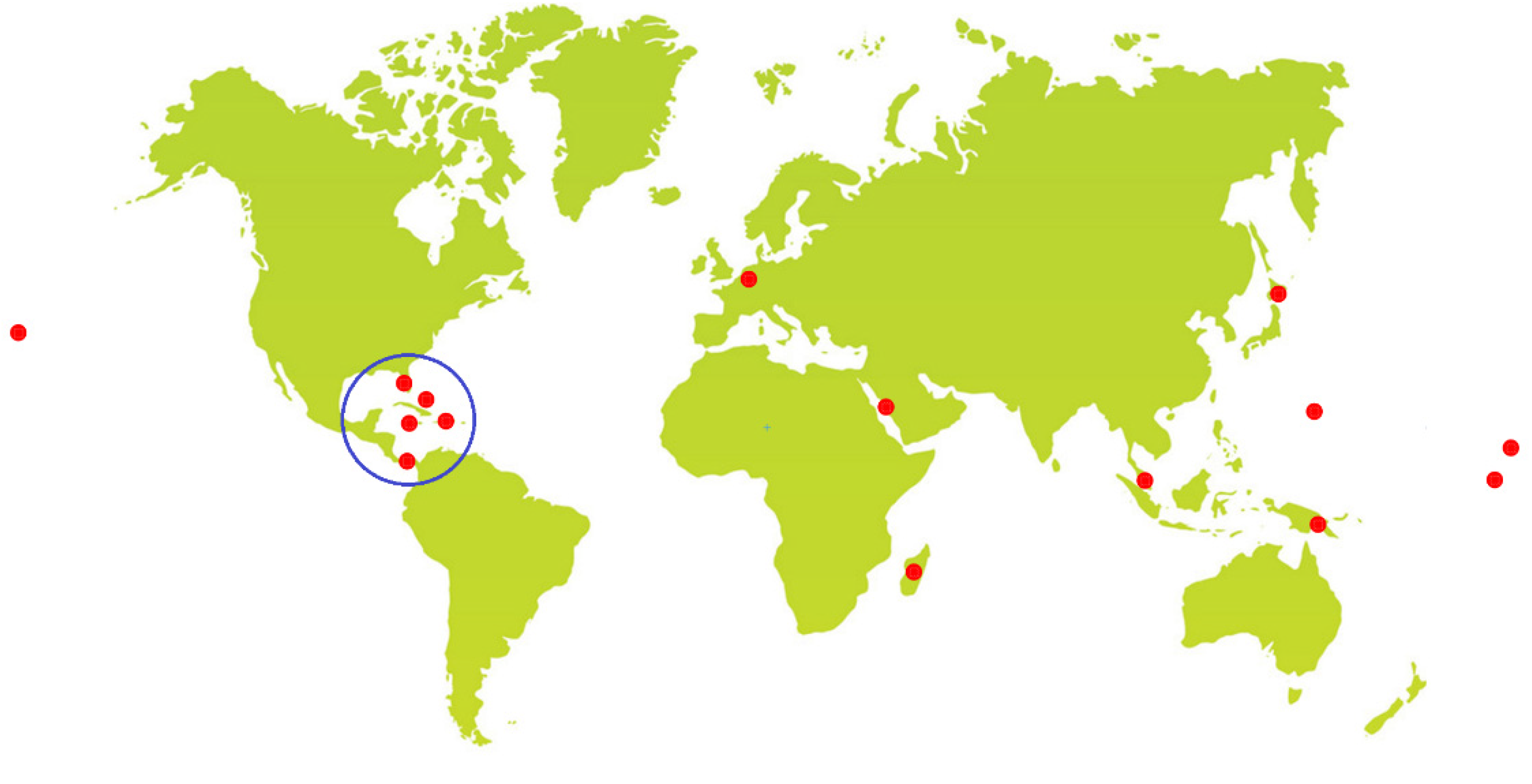
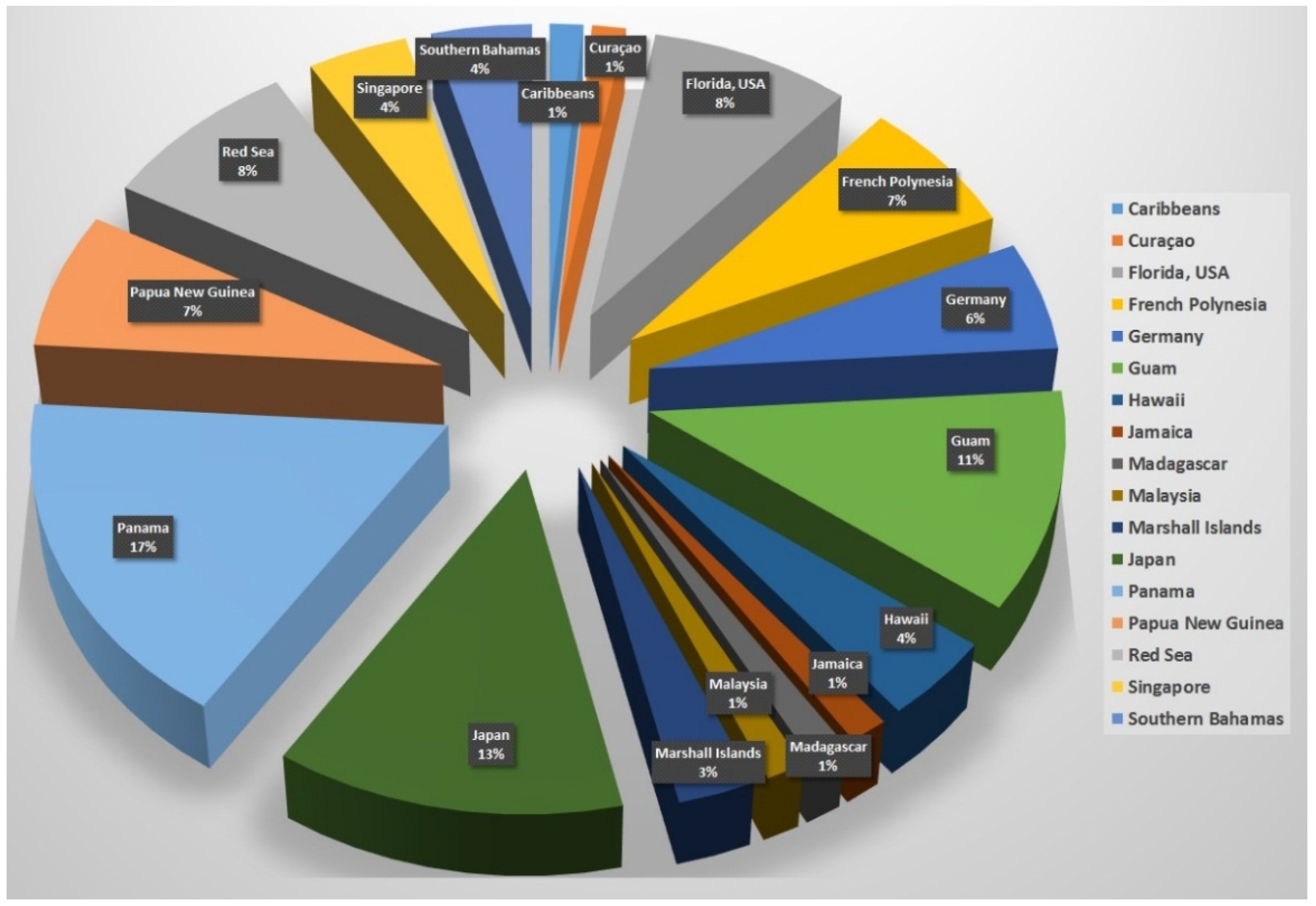
















| Species Name a | Number of Reported Compounds |
|---|---|
| Dapis sp. | 3 |
| Lyngbya sp. | 4 |
| Lyngbya bouillonii | 1 |
| Lyngbya confervoides | 3 |
| Lyngbya lagerheimii | 2 |
| Lyngbya majuscula | 35 |
| Lygnbya polychora | 2 |
| Moorea bouillonii | 4 |
| Moorea producens | 11 |
| Okeania sp. | 5 |
| Okeania hirsuta | 2 |
| Compound | Source Organism | Collection Site | Targeted Bacteria | MIC/Inhibition Zone/IC50 | Reference |
|---|---|---|---|---|---|
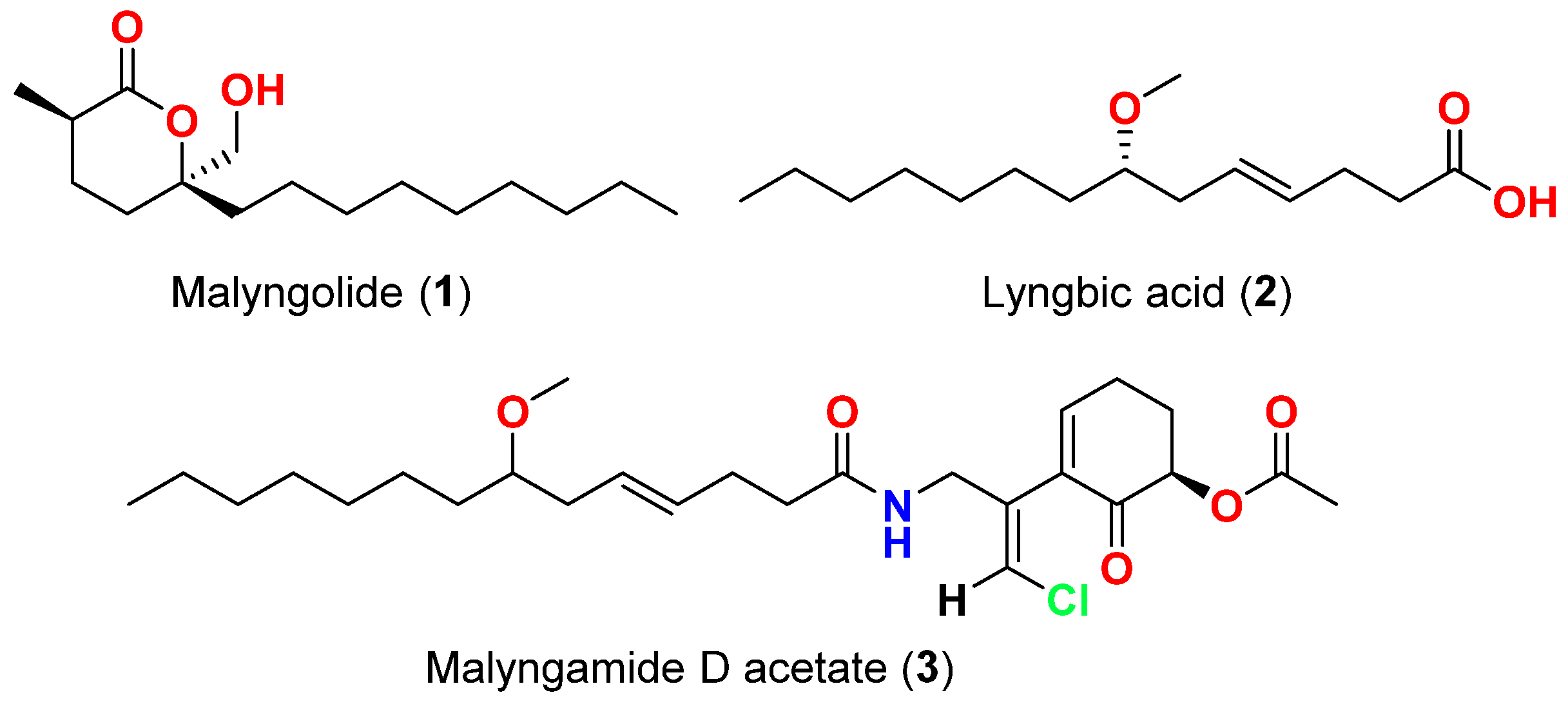
| Malyngolide (1) | L. majuscula | Hawaii, USA | M. smegmatis, S. pyogenes, S. aureus and B. subtilis | More active against M. smegmatis and S. pyogenes than S. aureus and B. subtilis | [40] |
| Lyngbic acid (2) | M. producens | Red Sea | M. tuberculosis H37Rv | 65% inhibition at 12.5 μg/mL | [41] |
| Lyngbic acid (2) | L. majuscula | Caribbean region | S. aureus and B. subtilis | Antibacterial activity | [42] |
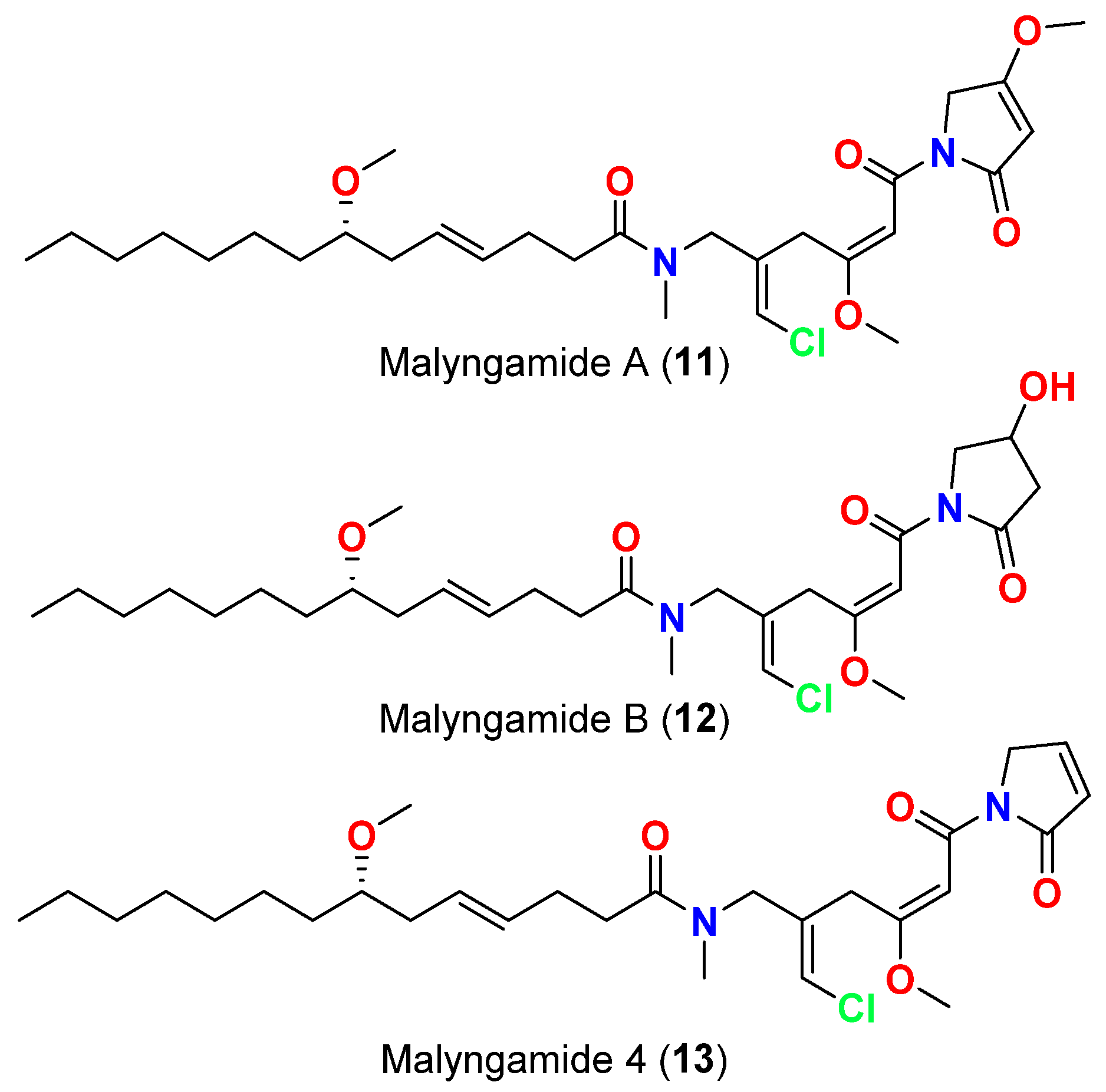
| Malyngamide D acetate (3) | L. majuscula | Caribbean region | S. aureus | Slight activity | [42] |
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 25 mm at 100 µg 10 mm at 25 µg | [43] |
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg 9 mm at 25 µg | [43] |
| Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 30 mm at 100 µg 15 mm at 25 µg | [43] |
| Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg 10 mm at 25 µg | [43] |
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 28 mm at 100 µg 23 mm at 50 µg 9 mm at 10 µg | [44] |
| Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 30 mm at 100 µg 24 mm at 50 µg 14 mm at 10 µg | [44] |
| Pitipeptolide C (6) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 26 mm at 100 µg 21 mm at 50 µg 18 mm at 10 µg | [44] |
| Pitipeptolide D (7) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 10 mm at 100 µg 0 mm at 50 µg 0 mm at 10 µg | [44] |
| Pitipeptolide E (8) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 21 mm at 100 µg 15 mm at 50 µg 0 mm at 10 µg | [44] |
| Pitipeptolide F (9) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 40 mm at 100 µg 30 mm at 50 µg 10 mm at 10 µg | [44] |
| Pitiprolamide (10) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 23 mm at 100 µg 13 mm at 50 µg 0 mm at 10 µg | [45] |
| Pitiprolamide (10) | L. majuscula | Guam | B. cereus ATCC 10987 | IC50 = 70 μM at 1 μM | [45] |
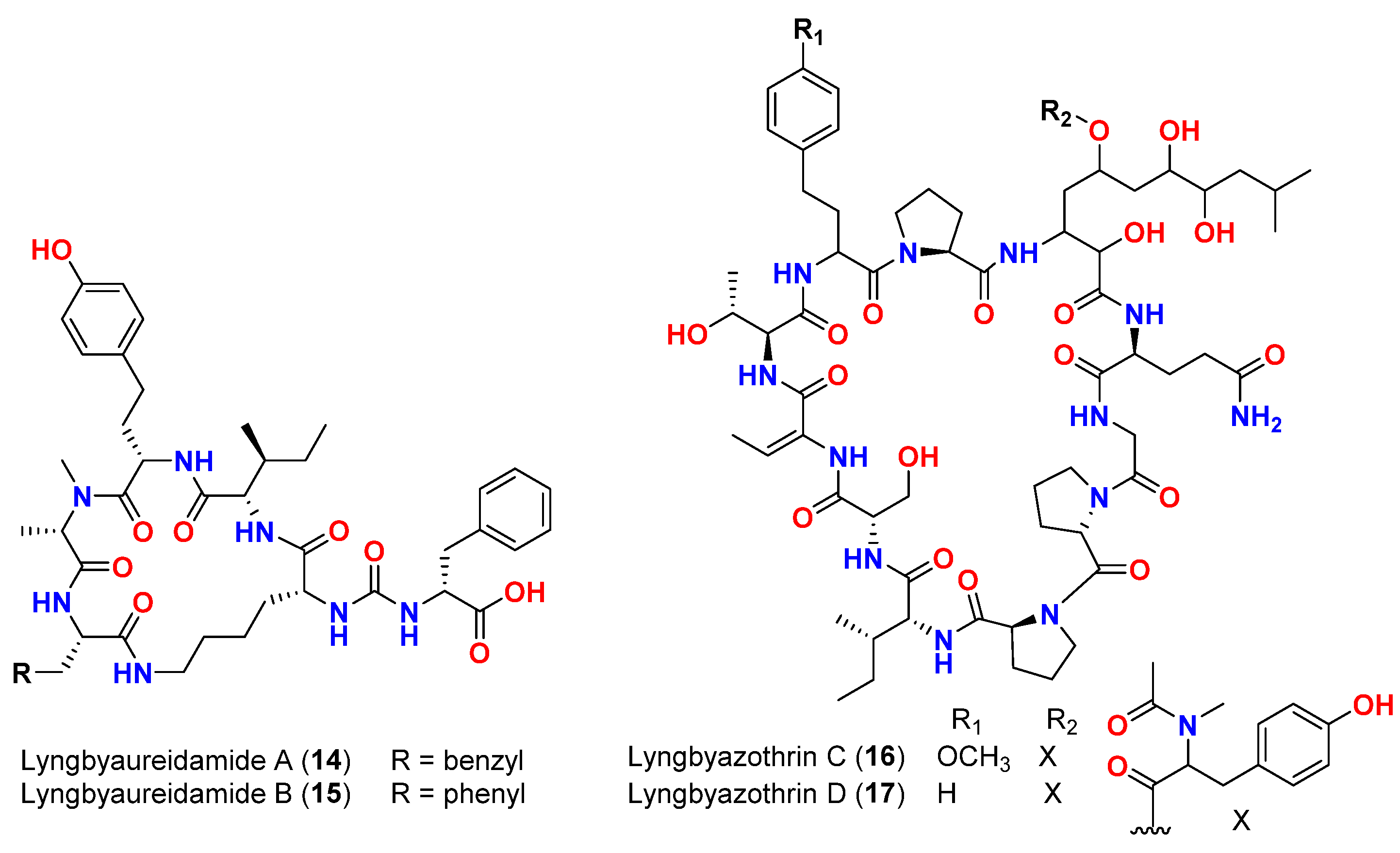
| Mixture of lyngbyazothrins A and B (14 and 15) | Lyngbya sp. | Germany (Culture) | M. flaVus SBUG 16 | 8 mm at 100 μg/disk | [46] |
| Mixture of lyngbyazothrins C (16) and D (17) | Lyngbya sp. | Germany (Culture) | B. subtilis SBUG 14 E. coli ATCC 11229 E. coli SBUG 13 P. aeruginosa ATCC 27853 S. marcescens SBUG 9 | 18 mm at 25 μg/disk 18 mm at 100 μg/disk 15 mm at 100 μg/disk 8 mm at 100 μg/disk 8 mm at 200 μg/disk | [46] |
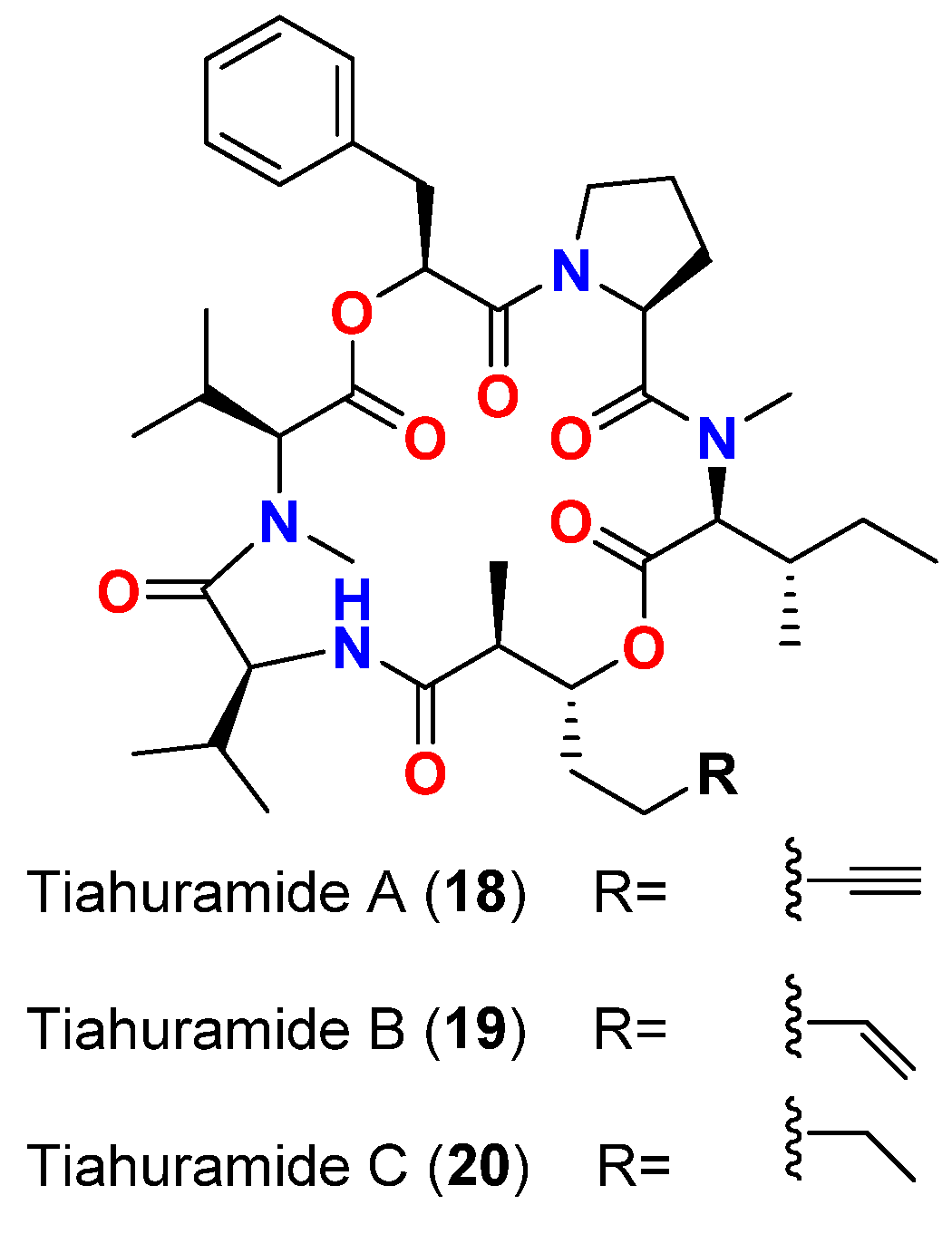
| Tiahuramide A (18) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 27, 33, >50, 35 and 47 μM, respectively | [47] |
| Tiahuramide B (19) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 9.4, 8.5, 22, 12 and 29 μM, respectively | [47] |
| Tiahuramide C (20) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 6.7, 7.4, 16, 14 and 17 μM, respectively | [47] |
| Compound | Source Organism | Collection Site | Targeted Bacteria/Receptor | Anti-Swarming/Anti-Quorum Sensing | Reference |
|---|---|---|---|---|---|
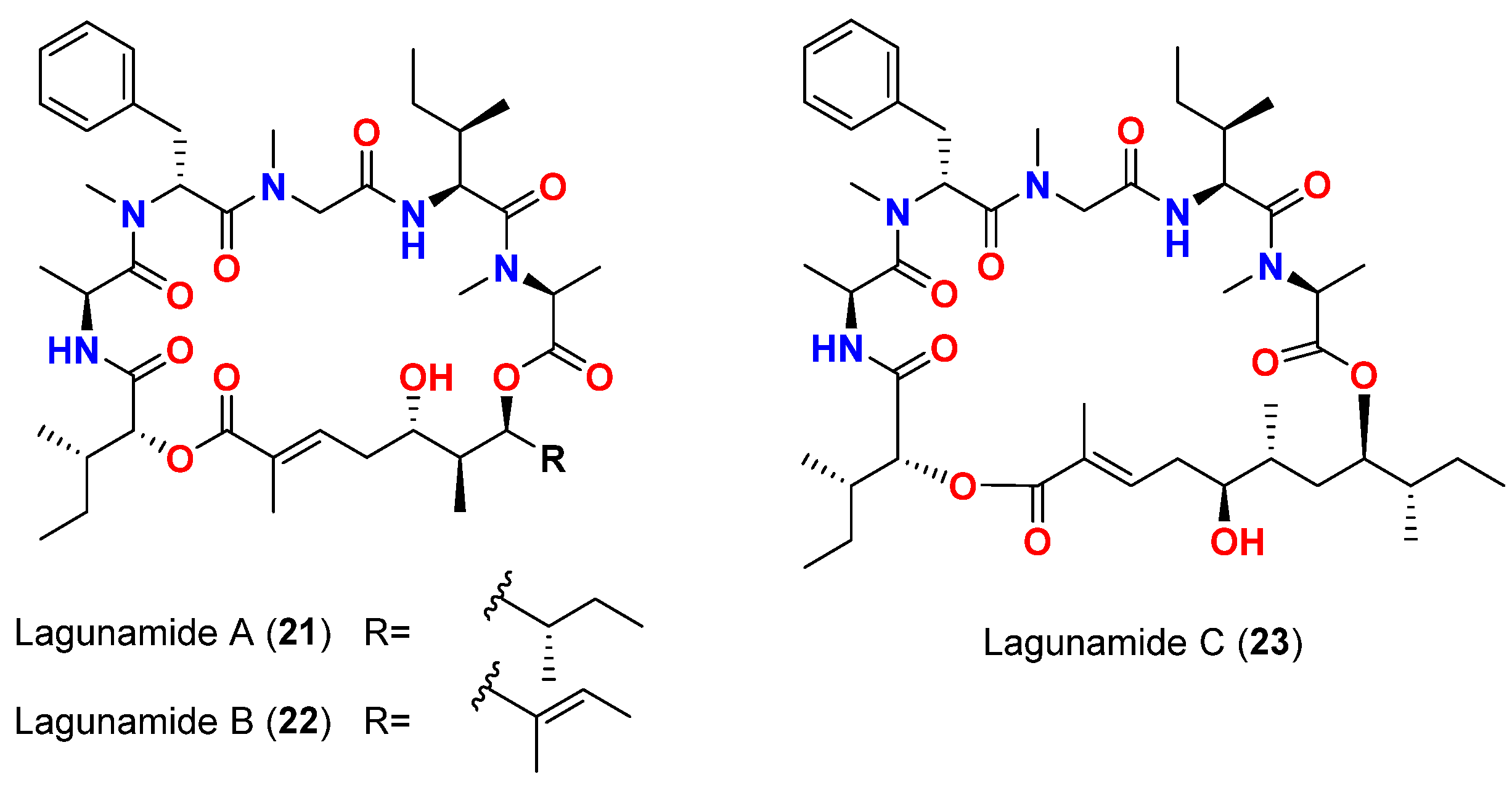
| Lagunamide A (21) | L. majuscula | Singapore | P. aeruginosa PA01 | Anti-swarming effect: 62% at 100 ppm | [50,51] |
| Lagunamide B (22) | L. majuscula | Singapore | P. aeruginosa PA01 | Anti-swarming effect: 56% at 100 ppm | [50,51] |
| Lagunamide C (23) | L. majuscula | Singapore | P. aeruginosa PA01 | Anti-swarming effect: 49%, at 100 ppm | [50,51] |
| Malyngamide C (24) | L. majuscula | Florida, USA | 3-oxo-C12-HSL (N-3-oxo-dodecanoyl-L-homoserine lactone) signaling in a LasR-based quorum sensing (QS) reporter pSB1075 | QS inhibitor reduction in 3-oxo-C12-HSL signaling at 10, 100 and 1000 µM | [54] |
| 8-epi-Malyngamide C (25) | L. majuscula | Florida, USA | 3-oxo-C12-HSL (N-3-oxo-dodecanoyl-L-homoserine lactone) signaling in a LasR-based quorum sensing (QS) reporter pSB1075 | QS inhibitor reduction in 3-oxo-C12-HSL signaling at 10, 100 and 1000 µM | [54] |
| Malyngolide (1) | L. majuscula | Florida, USA | Production of violacein pigment by C. violaceum CV017 in the QS bioassay | QS inhibitor inhibition of violacein production with effective concentrations ranged from 0.07 to 0.22 mM; EC50 = 0.11 mM | [55] |
| Responses of lasR+PlasI-luxCDABE reporter pSB1075 in the presence of 14 µM of 3-oxo-C12-HSL | Inhibition of responses of the lasR+PlasI-luxCDABE reporter pSB1075 with concentrations ranging from 3.57 to 57; EC50 = 12.2 µM | [55] | |||
| Production of elastase by P. aeruginosa PAO1 (an extracellular enzyme regulated by 3-oxo-C12-HSL and LasR) | Significant reduction in elastase production; EC50 = 10.6 µM, at higher concentrations of MAL, elastase production was inhibited to the level observed in the QS mutant of P. aeruginosa JP2 | [55] | |||
| Lyngbyoic acid (26) | L. majuscula | Florida, USA | Four reporters based on different acylhomoserine lactone (AHL) receptors acylhomoserine lactone (AHL) receptors (LuxR, AhyR, TraR and LasR) | QS inhibitor, most effective inhibition against LasR reporter | [56] |
| Production of pyocyanin and elastase (LasB) both on the protein and transcript level in wild-type P. aeruginosa. | Reduction in the production of pyocyanin and elastase (LasB) and direct inhibition of LasB enzymatic activity; Ki = 5.4 mM | ||||
| Doscadenamide A (27) | L. bouillonii | Guam | 3-Oxo-C12-HSL-responsive reporter plasmid pSB1075, which encodes LasR and contains a light-producing luxCDABE cassette expressed in E. coli | QS agonist in a LasR-dependent manner and activation of 3-oxo-C12-HSL-responsive reporter plasmid pSB1075 | [57] |
| Production of QS pigment pyocyanin in wild-type P. aeruginosa | Increase pyocyanin production at 10 µM |
| Compound | Source Organism | Collection Site | Targeted Fungi | MIC/Inhibition Zone/LD50 | Reference |
|---|---|---|---|---|---|
| Majusculamide C (28) | L. majuscula | Marshall Islands | P. infestans and P. viticola | Growth inhibition | [58] |
| 57-Normajusculamide C (29) | L. majuscula | Marshall Islands | S. pastorianus | Antimycotic activity | [59] |
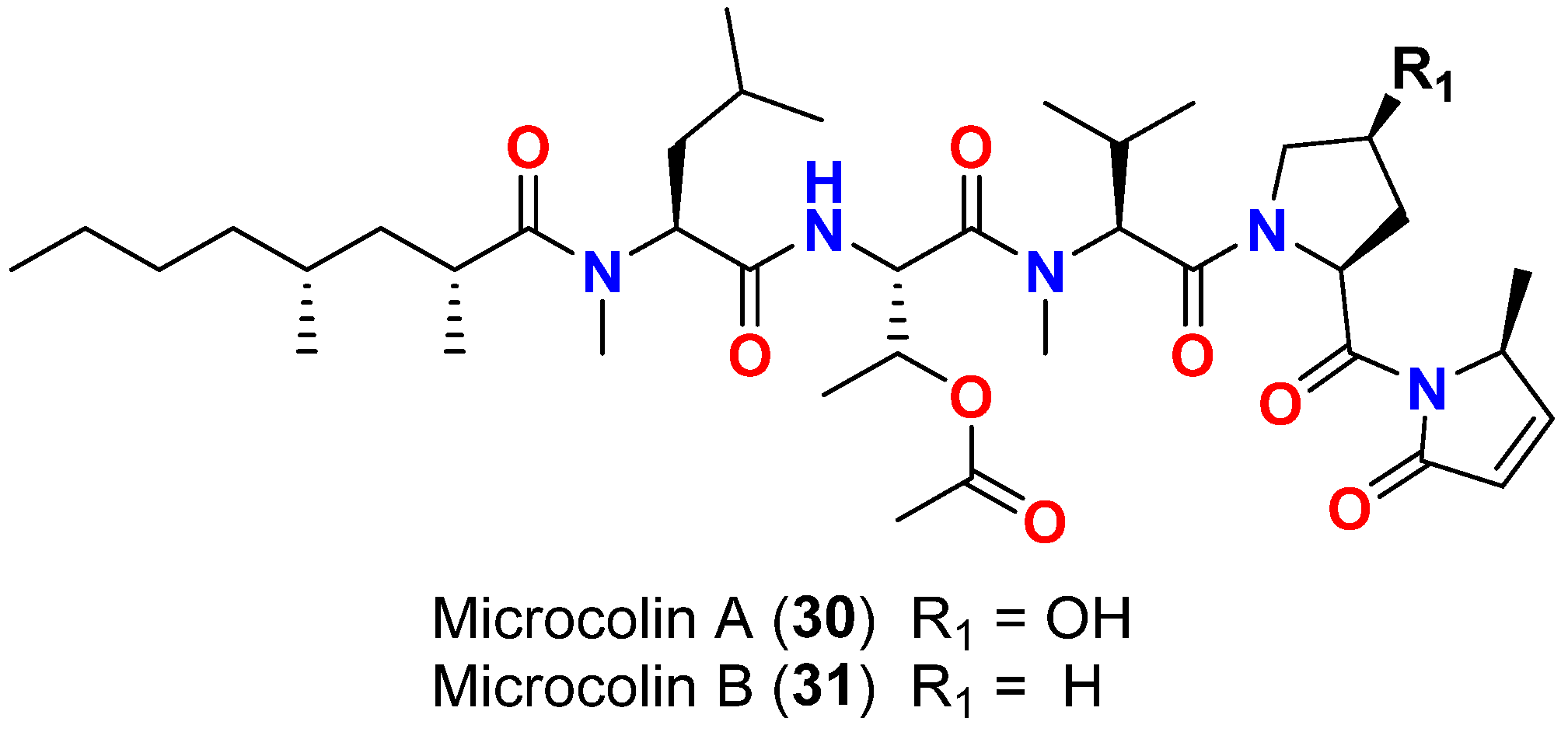
| Microcolin A (30) | L. polychroa | Marshall Islands | D. salina (SIO and EBGJ strains) | LD50 = >200 μg/mL | [60] |
| Microcolin B (31) | L. polychroa | Marshall Islands | D. salina (SIO and EBGJ strains) | LD50 = >200 μg/mL | [60] |
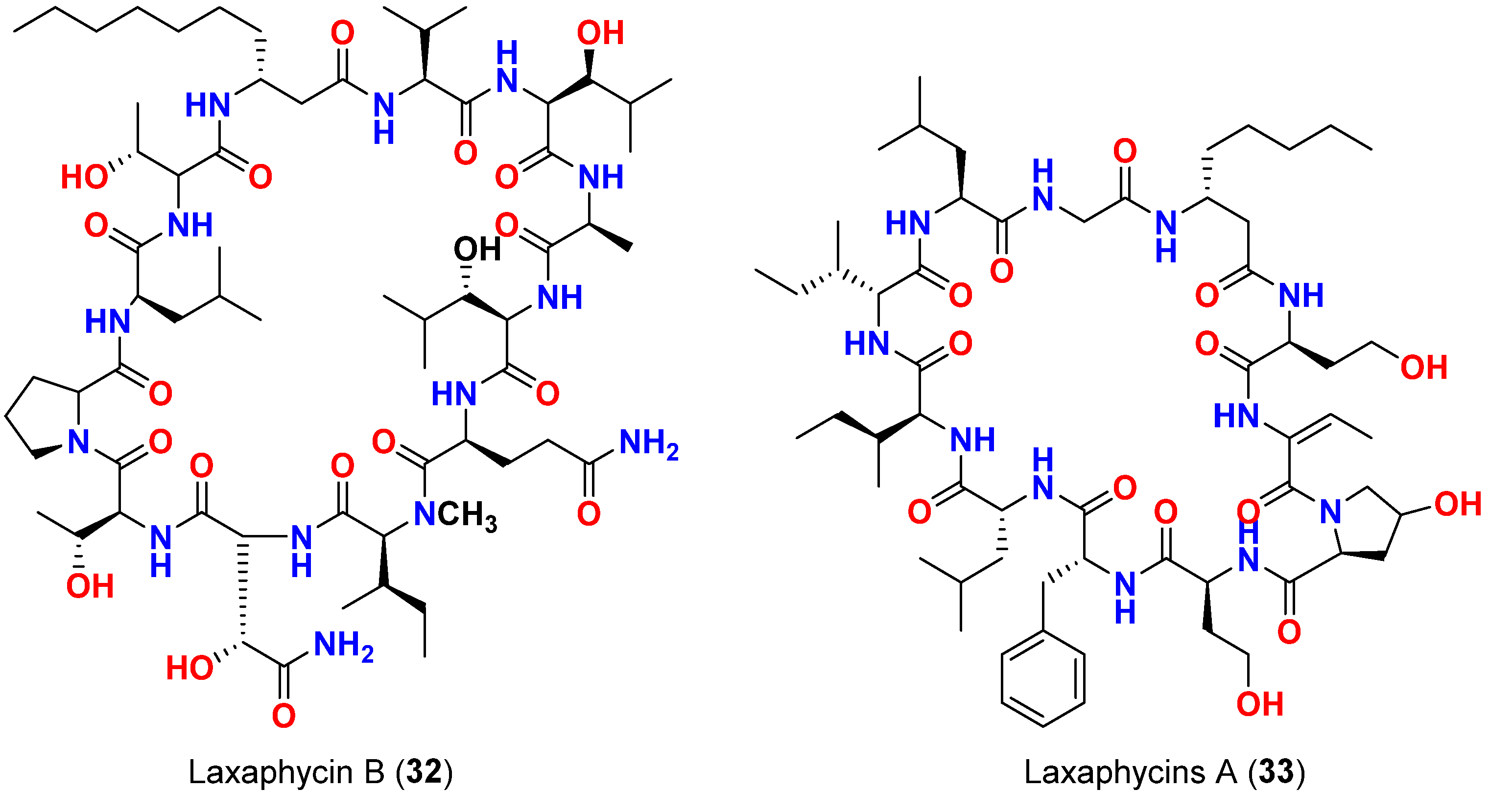
| Laxaphycin B (32) | L. majuscula | French Polynesia | C. albicans | Antifungal activity | [61] |
| Mixture of laxaphycins A (33) and B (32) | L. majuscula | French Polynesia | C. albicans | Laxaphycin B produces synergetic effect to the inactive laxaphycin A | |
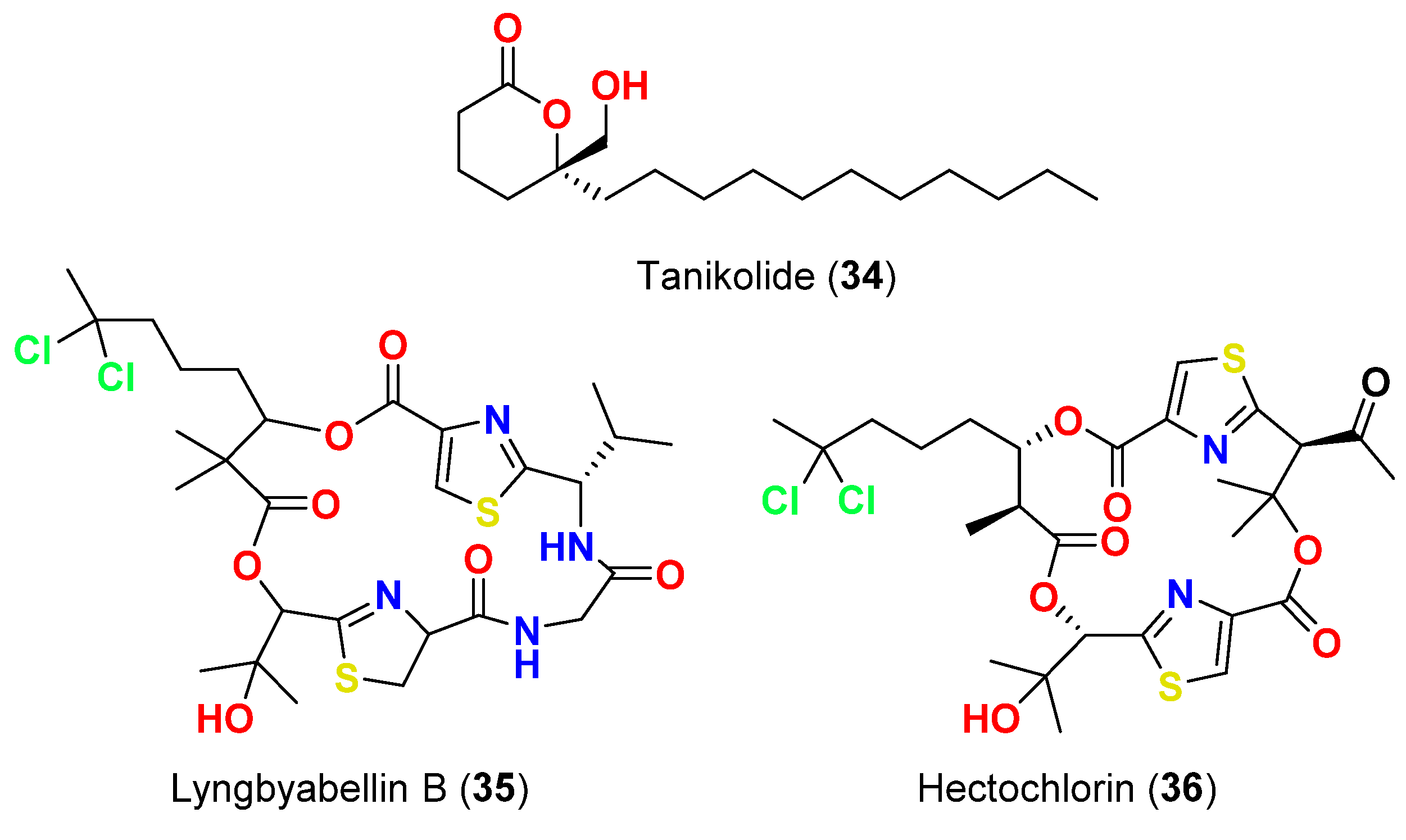
| Tanikolide (34) | L. majuscula | Madagascar | C. albicans | 13 mm at 100 µg/disk | [62] |
| Lyngbyabellin B (35) | L. majuscula | Florida, USA | C. albicans (ATCC 14053) | 10.5 mm at 100 µg/disk | [63] |
| Hectochlorin (36) | L. majuscula | Jamaica | C. albicans (ATCC 14053) | 16 mm at 100 µg/disk 11 mm at 10 µg/disk | [64] |
| Lobocyclamide A (37) | L. confervoides | Southern Bahamas | C. albicans 96–489 (Fluconazole-resistant) | 7 mm at 150 µg/disk and MIC = 100 µg/mL | [65] |
| Lobocyclamide B (38) | L. confervoides | Southern Bahamas | C. albicans 96–489 (Fluconazole-resistant) | 8 mm at 150 µg/disk and MIC = 30–100 µg/mL | [65] |
| Lobocyclamide B (38) | L. confervoides | Southern Bahamas | C. glabrata | 6 mm at 150 µg/disk | [65] |
| Mixture of lobocyclamides A and B (37 and 38) | L. confervoides | Southern Bahamas | - | MIC = 10–30 µg/mL | [65] |
| Lobocyclamide C (39) | L. confervoides | Southern Bahamas | C. albicans 96–489 (Fluconazole-resistant) | 10 mm at 150 µg/disk | [65] |
| Lobocyclamides C (39) | L. confervoides | Southern Bahamas | C. glabrata | 8 mm at 150 µg/disk | [65] |
| Compound | Source Organism | Collection Site | Targeted Microbe/Parasite | IC50/% of Inhibition | Reference |
|---|---|---|---|---|---|
| Lagunamide A (21) | L. majuscula | Singapore | P. falciparum (NF54 strain) | IC50 = 0.19 μM | [50,51] |
| Lagunamide B (22) | L. majuscula | Singapore | P. falciparum (NF54 strain) | IC50 = 0.91 μM | [50,51] |
| Lagunamide C (23) | L. majuscula | Singapore | P. falciparum (NF54 strain) | IC50 = 0.29 μM | [50,51] |
| Carmabin A (40) | L. majuscula | Panama | P. falciparum (Indochina W2 strain) | IC50 = 4.3 µM | [66,67] |
| Dragomabin (41) | L. majuscula | Panama | P. falciparum (Indochina W2 strain) | IC50 = 6.0 µM | [66,67] |
| Dragonamide A (42) | L. majuscula | Panama | P. falciparum (Indochina W2 strain) | IC50 = 7.7 µM | [66,67] |
| Dragonamide A (42) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 6.5 μM | [67] |
| Malyngolide dimer (44) | L. majuscula | Panama | P. falciparum (W2 strain) | IC50 = 19 μM | [68] |
| Dragonamide E (53) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 5.1 μM | [67] |
| Herbamide B (54) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 5.9 μM | [67] |
| Almiramide B (55) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 2.4 μM | [74] |
| Almiramide C (56) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 1.9 μM | [74] |
| Dudawalamide A (58) | M. producens | Papua New Guinea | P. falciparum | IC50 = 3.6 μM | [75] |
| Dudawalamide A (58) | M. producens | Papua New Guinea | T. cruzi | 12% inhibition at 10 μg/mL | [75] |
| Dudawalamide A (58) | M. producens | Papua New Guinea | L. donovani | IC50 = >10 μM | [75] |
| Dudawalamide B (59) | M. producens | Papua New Guinea | P. falciparum | IC50 = 10 μM | [75] |
| Dudawalamide B (59) | M. producens | Papua New Guinea | T. cruzi | 7% inhibition at 10 μg/mL | [75] |
| Dudawalamide B (59) | M. producens | Papua New Guinea | L. donovani | IC50 >10 μM | [75] |
| Dudawalamide C (60) | M. producens | Papua New Guinea | P. falciparum | IC50 = 3.5 μM | [75] |
| Dudawalamide D (61) | M. producens | Papua New Guinea | P. falciparum | IC50 = 8.0 μM | [75] |
| Dudawalamide D (61) | M. producens | Papua New Guinea | T. cruzi | 60% inhibition at 10 μg/mL | [75] |
| Dudawalamide D (61) | M. producens | Papua New Guinea | L. donovani | IC50 = 2.6 μM | [75] |
| Iheyamide A (62) | Dapis sp. | Okinawa, Japan | T. brucei rhodesiense T. bhurstuerusei brucei | IC50 = 1.5 μM IC50 = 1.5 μM | [76] |
| Janadolide (65) | Okeania sp. | Okinawa, Japan | T. brucei brucei | IC50 = 47 nM | [77] |
| Beru’amide (66) | Okeania sp. | Kagoshima, Japan | T. brucei rhodesiense | IC5 = 1.2 μM | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, D.T.A.; Mufti, S.J.; Badiab, A.A.; Shaala, L.A. Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022. Mar. Drugs 2022, 20, 768. https://doi.org/10.3390/md20120768
Youssef DTA, Mufti SJ, Badiab AA, Shaala LA. Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022. Marine Drugs. 2022; 20(12):768. https://doi.org/10.3390/md20120768
Chicago/Turabian StyleYoussef, Diaa T. A., Shatha J. Mufti, Abeer A. Badiab, and Lamiaa A. Shaala. 2022. "Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022" Marine Drugs 20, no. 12: 768. https://doi.org/10.3390/md20120768
APA StyleYoussef, D. T. A., Mufti, S. J., Badiab, A. A., & Shaala, L. A. (2022). Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022. Marine Drugs, 20(12), 768. https://doi.org/10.3390/md20120768