Overview of Phlorotannins’ Constituents in Fucales
Abstract
1. Introduction
2. Methods
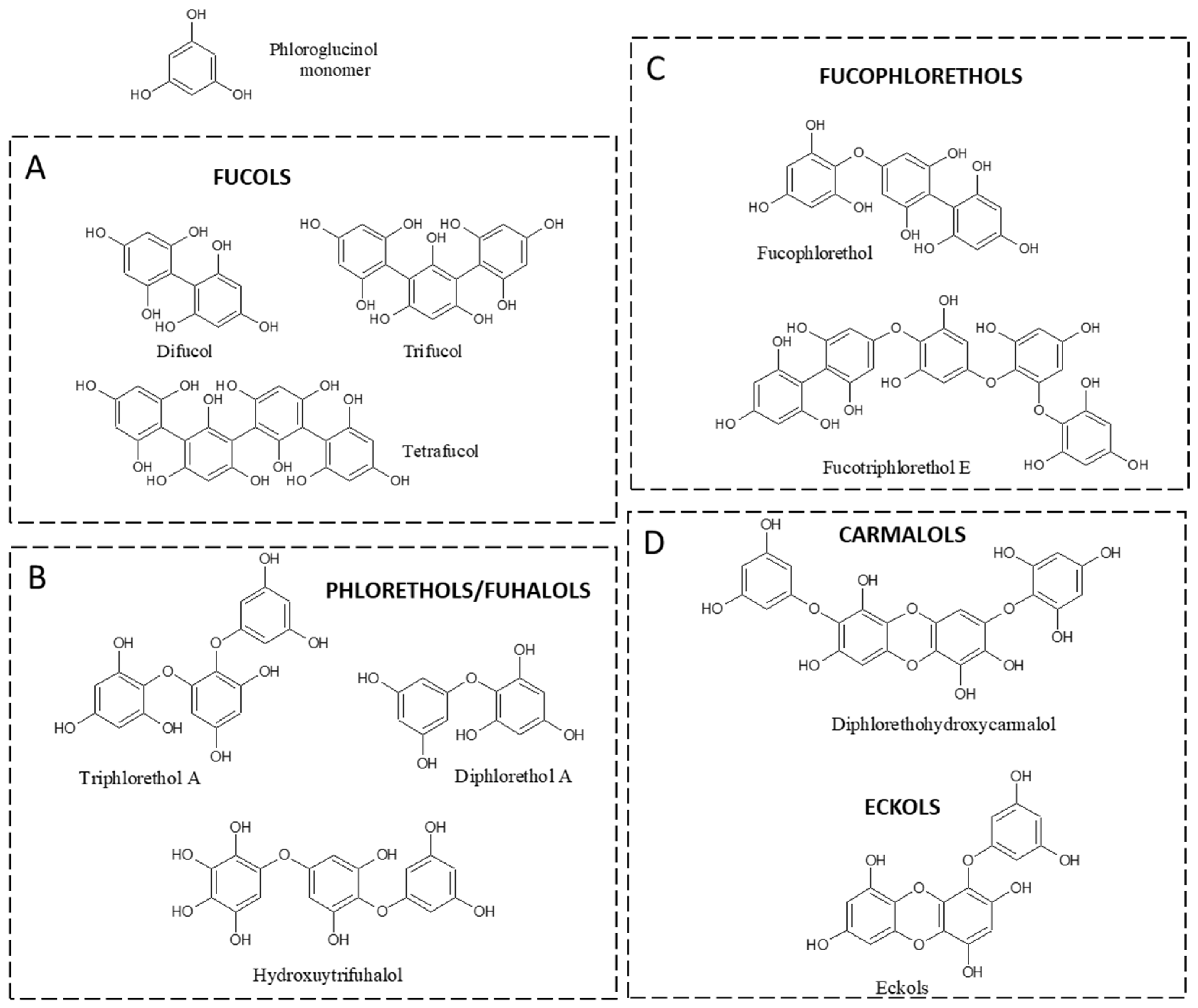
3. Phlorotannins and Main Classes in Fucale
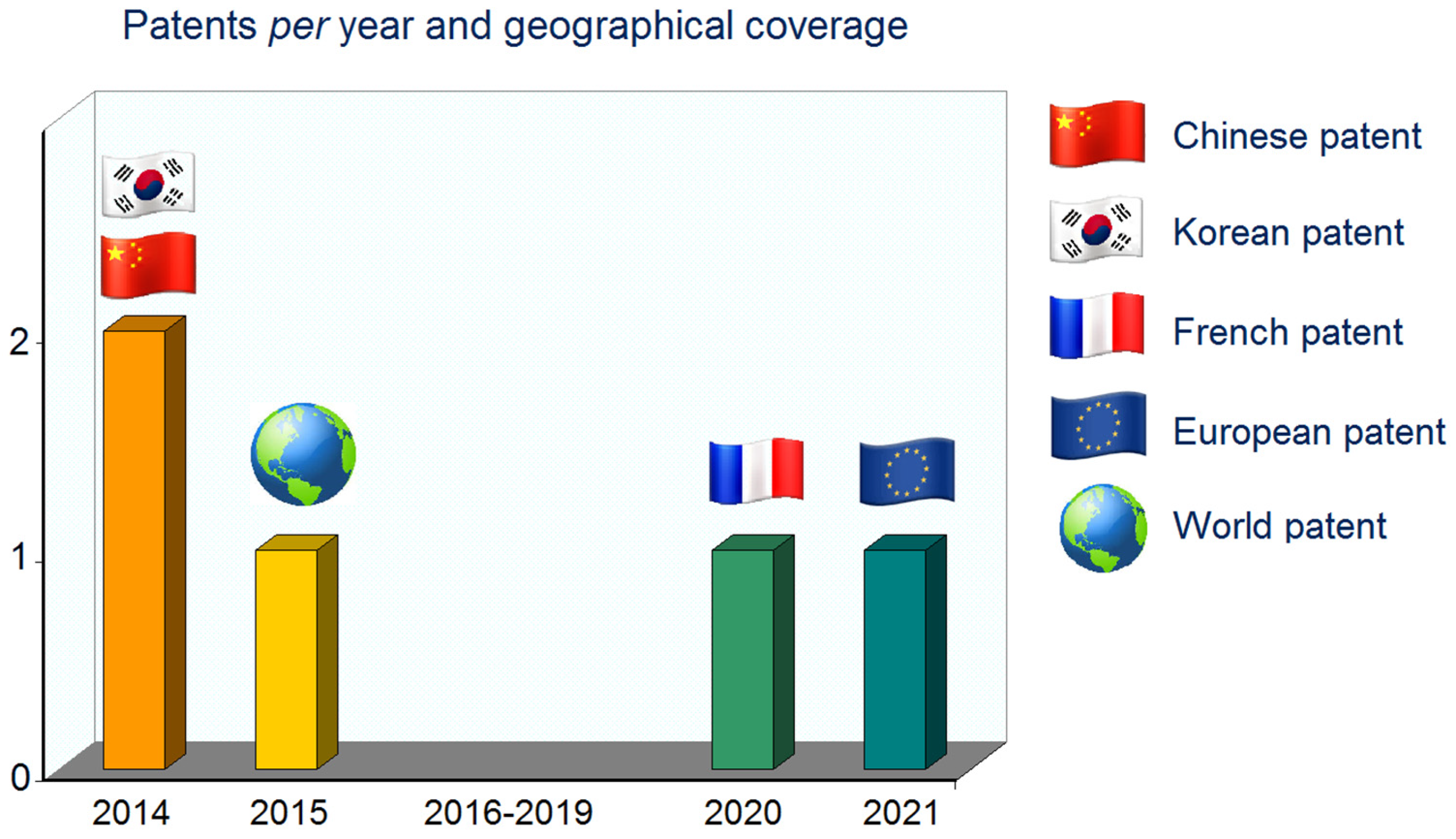
4. Extraction Processes and Patents
5. Methods for Characterization and Quantification
5.1. Spectrophotometric Quantification of Phlorotannins
5.2. Methods for Phlorotannins’ Separation and Identification
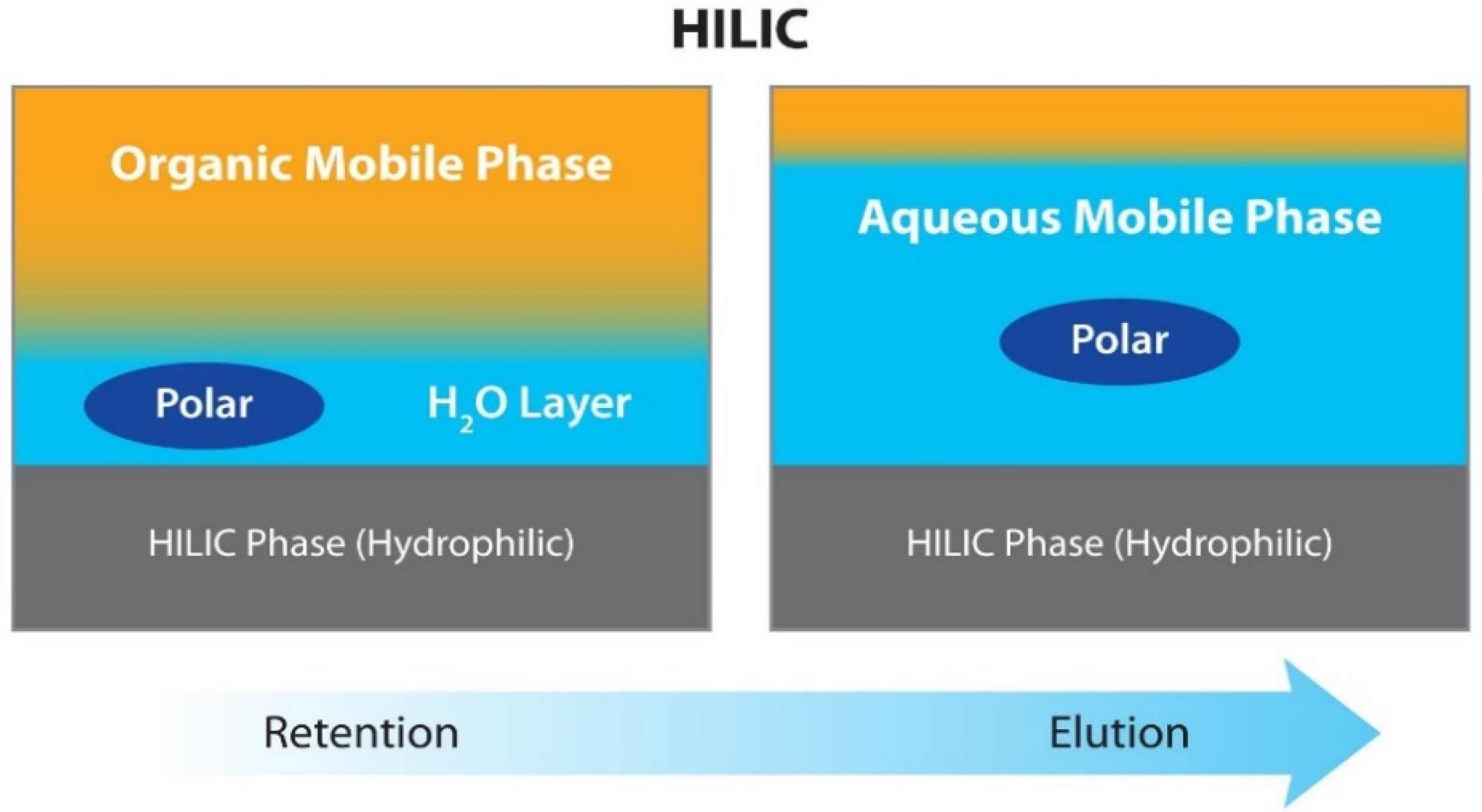
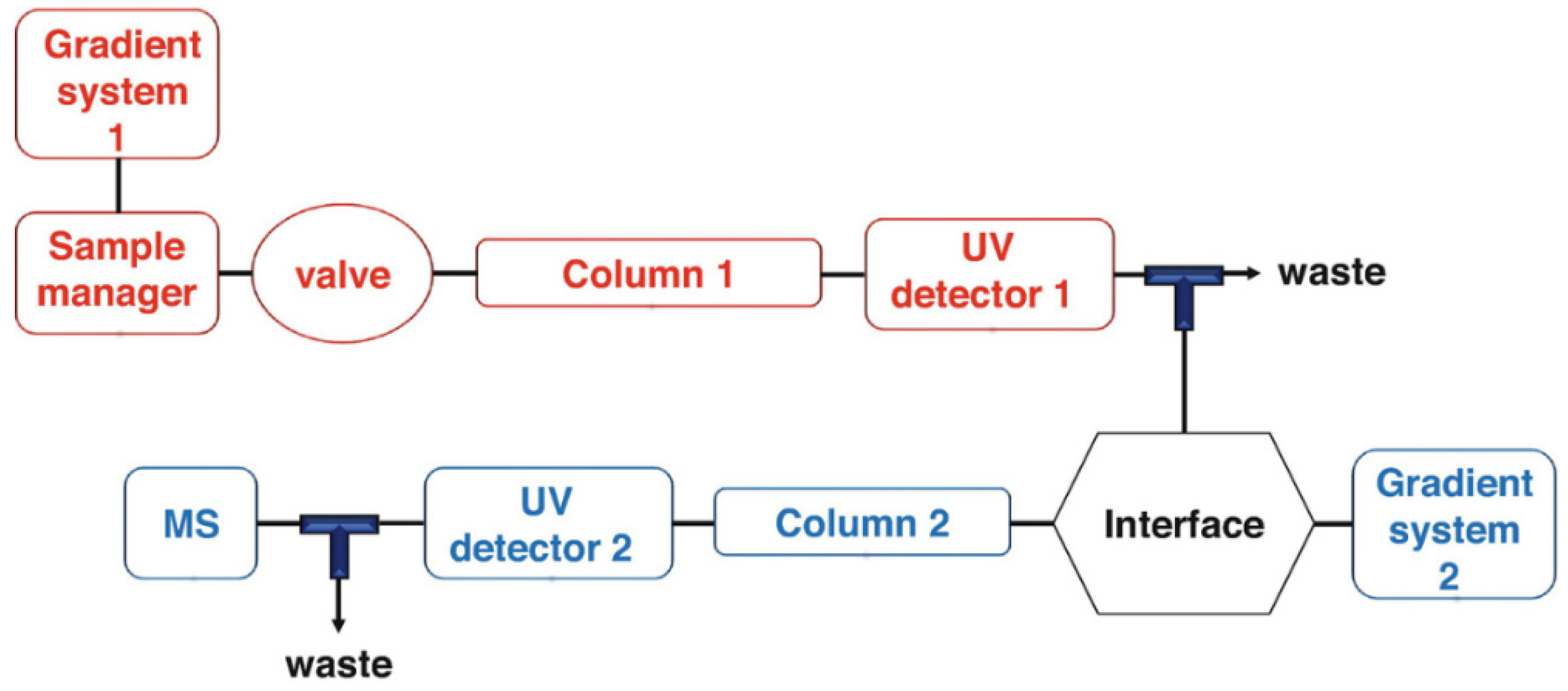
5.2.1. HPLC-DAD
5.2.2. Mass Spectrometry
5.2.3. NMR
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Cho, G.Y.; Rousseau, F.; de Reviers, B.; Boo, S.M.; Reviers, B.D.E.; Cho, G.Y.; Rousseau, F.; Reviers, B.D.E. Phylogenetic Relationships within the Fucales (Phaeophyceae) Assessed by the Photosystem I Coding PsaA Sequences. Phycologia 2006, 45, 512–519. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–106. [Google Scholar]
- Bermejo, R.; Chefaoui, R.M.; Engelen, A.H.; Buonomo, R.; Neiva, J.; Ferreira-Costa, J.; Pearson, G.A.; Marbà, N.; Duarte, C.M.; Airoldi, L.; et al. Marine Forests of the Mediterranean-Atlantic Cystoseira tamariscifolia Complex Show a Southern Iberian Genetic Hotspot and No Reproductive Isolation in Parapatry. Sci. Rep. 2018, 8, 10427. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Herrero, M.; Ibáñez, E.; Ibá, I.; Ibáñez, I.; Cifuentes, A. Separation and Characterization of Phlorotannins from Brown Algae Cystoseira abies-marina by Comprehensive Two-Dimensional Liquid Chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Jégou, C.; Connan, S.; Bihannic, I.; Cérantola, S.; Guérard, F.; Stiger-Pouvreau, V. Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology? Mar. Drugs 2021, 19, 504. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; Sargassum, C. Agardh, 1820—AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=77 (accessed on 4 November 2022).
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of Pelagic Sargassum Biomass into Sustainable Applications: Current Trends and Challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef]
- Daniel, S.L.; Kiril, B.; Leonel, P. Production of Bio-Fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Oceanol. Limnol. 2019, 37, 918–927. [Google Scholar] [CrossRef]
- Ghaffar Shahriari, A.; Mohkami, A.; Niazi, A.; Hamed Ghodoum Parizipour, M.; Habibi-Pirkoohi, M. Application of Brown Algae (Sargassum angustifolium) Extract for Improvement of Drought Tolerance in Canola (Brassica napus L.). Iran. J. Biotechnol. 2021, 19, e2775. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Optimization of Biogas Production from Sargassum Sp. Using a Design of Experiments to Assess the Co-Digestion with Glycerol and Waste Frying Oil. Bioresour. Technol. 2015, 175, 480–485. [Google Scholar] [CrossRef]
- Giovanna Lopresto, C.; Paletta, R.; Filippelli, P.; Galluccio, L.; de la Rosa, C.; Amaro, E.; Jáuregui-Haza, U.; Atilio de Frias, J. Sargassum Invasion in the Caribbean: An Opportunity for Coastal Communities to Produce Bioenergy Based on Biorefinery—An Overview. Waste Biomass Valorization 2022, 13, 2769–2793. [Google Scholar] [CrossRef]
- Luis Godínez-Ortega, J.; Cuatlán-Cortés, J.V.; López-Bautista, J.M.; van Tussenbroek, B.I. A Natural History of Floating Sargassum Species (Sargasso) from Mexico. In Natural History and Ecology of Mexico and Central America; IntechOpen: London, UK, 2021. [Google Scholar]
- Soleimani, S.; Yousefzadi, M.; Nezhad, S.B.M.; Pozharitskaya, O.N.; Shikov, A.N. Evaluation of Fractions Extracted from Polycladia Myrica: Biological Activities, UVR Protective Effect, and Stability of Cream Formulation Based on It. J. Appl. Phycol. 2022, 34, 1763–1777. [Google Scholar] [CrossRef]
- Serrão, E.A.; Alice, L.A.; Brawley, S.H. Evolution of the Fucaceae (Phaeophyceae) Infrred from NrDNA-ITS. J. Phycol. 1999, 35, 382–394. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling Phlorotannins from Fucus Spp. of the Northern Portuguese Coastline: Chemical Approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Stansbury, J.; Saunders, P.; Winston, D. Promoting Healthy Thyroid Function with Iodine, Bladderwrack, Guggul and Iris. J. Restor. Med. 2013, 1, 83–90. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. Fucus Linnaeus, 1753—AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=71 (accessed on 4 November 2022).
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a Seaweed Extract Strongly Improves Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Vodouhè, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.-F.; Jacques, H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs 2022, 20, 174. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Vel, M.; Nelson, W.A.; Macaya, E.C.; Hay, C.H.; Mccarthy, C.; Velásquez, M.; Nelson, W.A.; Macaya, E.C.; Hay, C.H. The Biogeographic Importance of Buoyancy in Macroalgae: A Case Study of the Southern Bull-Kelp Genus Durvillaea (Phaeophyceae), Including Descriptions of Two New Species. J. Phycol. 2007, 56, 23–36. [Google Scholar] [CrossRef]
- Capon, R.J.; Barrow, R.A.; Rochfort, S.; Jobliig, M.; Skene, C.; Lacey, E.; Gill, J.H.; Friedel, T.; Wadsworth, D.; Jobling, M.; et al. Marine Nematocides: Tetrahydrofurans from a Southern Australian Brown Alga, Notheia Anomaliz. Tetrahedron 1998, 54, 2227–2242. [Google Scholar] [CrossRef]
- Mueller, R.; Wright, J.T.; Bolch, C.J.S.S. Historical Demography and Colonization Pathways of the Widespread Intertidal Seaweed Hormosira banksii (Phaeophyceae) in Southeastern Australia. J. Phycol. 2018, 54, 56–65. [Google Scholar] [CrossRef]
- Clayton, M.N. Circumscription and Phylogenetic Relationships of the Southern Hemisphere Family Seirococcaceae (Phaeophyceae). Bot. Mar. 1994, 37, 213–220. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–Bioactivity and Extraction Perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Hermund, D.B.; Torsteinsen, H.; Vega, J.; Figueroa, F.L.; Jacobsen, C. Screening for New Cosmeceuticals from Brown Algae Fucus vesiculosus with Antioxidant and Photo-Protecting Properties. Marine Drugs 2022, 20, 687. [Google Scholar] [CrossRef]
- Lashika Blue Filter Sunscreen SPF 45 PA+++ with Brown Seaweed—30 mL. Available online: https://www.lashika.in/products/blue-filter (accessed on 16 November 2022).
- Hello Sunny Essence Sun Stick Glow SPF50+ Pa++++. Available online: https://incidecoder.com/products/banila-co-hello-sunny-essence-sun-stick-glow-spf50-pa (accessed on 16 November 2022).
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of Soluble, Cell-Wall-Bound and Exuded Phlorotannins in the Brown Alga Fucus vesiculosus, with Implications on Their Ecological Functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, S.W.; Jeon, J.-S.; Jung, Y.-J.; Kim, W.-R.; Kim, C.Y.; Um, B.-H. Determination of Major Phlorotannins in Eisenia bicyclis Using Hydrophilic Interaction Chromatography: Seasonal Variation and Extraction Characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and Temporal Variation in Phlorotannin Levels in an Assemblage of Brown Algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Pedersen, A. Studies on Phenol Content and Heavy Metal Uptake in Fucoids. In Eleventh International Seaweed Symposium. Developments in Hydrobiology; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 22, pp. 498–504. [Google Scholar]
- Connan, S.; Stengel, D.B. Impacts of Ambient Salinity and Copper on Brown Algae: 2. Interactive Effects on Phenolic Pool and Assessment of Metal Binding Capacity of Phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Nishio, T.; Yokoyama, A.; Yatsuya, K.; Nishigaki, T.; Yoshikawa, S.; Ohki, K. Seasonal Variation of Phlorotannin in Sargassacean Species from the Coast of the Sea of Japan. Phycol. Res. 2010, 58, 53–61. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative Studies on Brown Algal Phenols. II. Seasonal Variation in Polyphenol Content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B. Influence of Light and Nitrogen on the Phlorotannin Content of the Brown Seaweeds Ascophyllum nodosum and Fucus vesiculosus. Hydrobiologia 2000, 440, 299–305. [Google Scholar] [CrossRef]
- Pavia, H.; Brock, E. Extrinsic Factors Influencing Phlorotannin Production in the Brown Alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 2000, 193, 285–294. [Google Scholar] [CrossRef]
- Tala, F.; Velásquez, M.; Mansilla, A.; Macaya, E.C.; Thiel, M. Latitudinal and Seasonal Effects on Short-Term Acclimation of Floating Kelp Species from the South-East Pacific. J. Exp. Mar. Biol. Ecol. 2016, 483, 31–41. [Google Scholar] [CrossRef]
- Sardari, R.R.R.R.; Prothmann, J.; Gregersen, O.; Turner, C.; Karlsson, E.N. Identification of Phlorotannins in the Brown Algae, Saccharina Latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry. Molecules 2021, 26, 43. [Google Scholar] [CrossRef]
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS Profiling of Low Molecular Weight Phlorotannin Polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.A.M.S.; Cruz, M.T.; Mateus, N.; Silva, A.A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.J.J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algal Extracts: Technology and Advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B.; Mayer, A.M. Alternative and Efficient Extraction Methods for Marine-Derived Compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A Review of Extraction Methods, Structural Characteristics, Bioactivities, Bioavailability, and Future Trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to Control Hyperglycemia and Diabetes-Related Vascular Complications. J. Appl. Phycol. 2019, 31, 3143–3152. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-Assisted Extraction of Bioactive Compounds from Brown Seaweeds and Characterization. J. Appl. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Ank, G.; Antônio Perez Da Gama, B.; Pereira, R.C. Latitudinal Variation in Phlorotannin Contents from Southwestern Atlantic Brown Seaweeds. PeerJ 2019, 7, e7379. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal Variation of Chemical Composition and Biomethane Production from the Brown Seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef]
- Hermund, D.B.; Heung, S.Y.; Thomsen, B.R.; Akoh, C.C.; Jacobsen, C. Improving Oxidative Stability of Skin-Care Emulsions with Antioxidant Extracts from Brown Alga Fucus vesiculosus. J. Am. Oil Chem. Soc. 2018, 95, 1509–1520. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of Phenolic Antioxidants Extraction from Fucus vesiculosus by Pressurized Liquid Extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave Assisted Extraction of Phenolic Compounds from Four Economic Brown Macroalgae Species and Evaluation of Their Antioxidant Activities and Inhibitory Effects on α-Amylase, α-Glucosidase, Pancreatic Lipase and Tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Bian, C.; Gao, J.; Leng, X.; Sun, C.; Dai, L.; Xu, Z. Moisture-Retention Liquid and Method for Preparing the Same. Chinese Patent CN103520065A, 22 January 2014. [Google Scholar]
- da Silva, J.R.M.; Alves, C.M.M.; Pinteus, S.F.G.; Martins, A.I.M.; Freitas, R.P.F.; Pedrosa, R.F.P. Process of Obtaining a Phlorotannin-Enriched Extract Having Anti-Enzymatic Action for Use in Dermatology. European Patent EP3910064, 17 November 2021. [Google Scholar]
- Prigent, A. Method for Obtaining Extracts of Marine Algae. International Patent WO2015071477A1, 21 May 2015. [Google Scholar]
- Stiger-Poivreau, V.; Connan, S.; Gager, L.; Coiffard, L.; Couteau, C.; Decoster, S.; Gombault, L.N.; Cotterei, C.; Mahe, A. Brown Algae Extracts Including Phenolic Compounds and Their Cosmetic Uses. French Patent FR3095348A1, 30 October 2020. [Google Scholar]
- Tae, H.L.; Lee, J.M.; Park, S.Y. Process for Preparing Enzyme Treated Hizikia fusiforme Extracts Having Immune Enhancement Activity and a Functional Food and Pharmaceutical Composition Comprising the Same. Korean Patent KR20140032101A, 14 March 2014. [Google Scholar]
- Liu, X.; Yuan, W.; Sharma-shivappa, R.; Zanten, J. Van Antioxidant Activity of Phlorotannins from Brown Algae. Int. J. Agric. Biol. Eng. 2017, 10, 184–191. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Jouini, M.; Bel Haj Amor, H.; Mzoughi, Z.; Dridi, M.; Ben Said, R.; Bouraoui, A. Phytochemical Analysis and Evaluation of the Antioxidant, Anti-Inflammatory, and Antinociceptive Potential of Phlorotannin-Rich Fractions from Three Mediterranean Brown Seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M. Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 107–115. ISBN 9781119135388. [Google Scholar]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A New Assay for Quantifying Brown Algal Phlorotannins and Comparisons to Previous Methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef]
- Steevensz, A.J.; MacKinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling Phlorotannins in Brown Macroalgae by Liquid Chromatography-High Resolution Mass Spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic Compounds from Three Brown Seaweed Species Using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Schmidt, A. Trihydroxyphlorethols from the Brown Alga Carpophyllum angustifolium. Phytochemistry 1999, 51, 1095–1100. [Google Scholar] [CrossRef]
- Sailler, B.; Glombitza, K.W. Phlorethols and Fucophlorethols from the Brown Alga Cystophora retroflexa. Phytochemistry 1999, 50, 869–881. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Keusgen, M.; Hauperich, S. Fucophlorethols from the Brown Algae Sargassum spinuligerum and Cystophora torulosa. Phytochemistry 1997, 46, 1417–1422. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Keusgen, M. Fuhalols and Deshydroxyfuhalols from the Brown Alga Sargassum spinuligerum. Phytochemistry 1995, 38, 987–995. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Schmidt, A. Nonhalogenated and Halogenated Phlorotannins from the Brown Alga Carpophyllum angustifolium. J. Nat. Prod. 1999, 62, 1238–1240. [Google Scholar] [CrossRef]
- Koch, M.; Gregson, R.P. Brominated Phlorethols and Nonhalogenated Phlorotannins from the Brown Alga Cystophora congesta. Phytochemistry 1984, 23, 2633–2637. [Google Scholar] [CrossRef]
- Sailler, B.; Glombitza, K.W. Halogenated Phlorethols and Fucophlorethols from the Brown Alga Cystophora retroflexa. Nat. Toxins 1999, 7, 57–62. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-Performance Liquid Chromatographic Analysis of Phlorotannins from the Brown Alga Fucus vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal Modifications and Bioavailability of Brown Seaweed Phlorotannins and Effects on Inflammatory Markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzynska, K. HILIC Chromatography: Powerful Technique in the Analysis of Polyphenols. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 341–351. [Google Scholar] [CrossRef]
- Marrubini, G.; Appelblad, P.; Maietta, M.; Papetti, A. Hydrophilic Interaction Chromatography in Food Matrices Analysis: An Updated Review. Food Chem. 2018, 257, 53–66. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Recent Developments in the HPLC Separation of Phenolic Food Compounds. Crit. Rev. Anal. Chem. 2015, 45, 41–51. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-Proliferative Activity and Chemical Characterization by Comprehensive Two-Dimensional Liquid Chromatography Coupled to Mass Spectrometry of Phlorotannins from the Brown Macroalga Sargassum muticum Collected on North-Atlantic Coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Swartz, M. HPLC Detectors: A Brief Review. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1130–1150. [Google Scholar] [CrossRef]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.P.; Gruppen, H. Phlorotannin Composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Catarino, M.D.; Fernandes, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor Activity of Fucus vesiculosus-Derived Phlorotannins through Activation of Apoptotic Signals in Gastric and Colorectal Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef] [PubMed]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E.; Gall, E.A. Phenolic Compounds in the Brown Seaweed Ascophyllum nodosum: Distribution and Radical-Scavenging Activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The Chemical and Antioxidant Stability of Isolated Low Molecular Weight Phlorotannins. Food Chem. 2017, 221, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuen, A.K.L.; Magnusson, M.; Wright, J.T.; de Nys, R.; Masters, A.F.; Maschmeyer, T. A Comparative Assessment of the Activity and Structure of Phlorotannins from the Brown Seaweed Carpophyllum flexuosum. Algal Res. 2018, 29, 130–141. [Google Scholar] [CrossRef]
- Allwood, J.W.; Evans, H.; Austin, C.; McDougall, G.J. Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum. Mar. Drugs 2020, 18, 448. [Google Scholar] [CrossRef]
- Koivikko, R.; Eränen, J.K.; Loponen, J.; Jormalainen, V. Variation of Phlorotannins among Three Populations of Fucus vesiculosus as Revealed by HPLC and Colorimetric Quantification. J. Chem. Ecol. 2008, 34, 57–64. [Google Scholar] [CrossRef]
- Kirke, D.A.; Rai, D.K.; Smyth, T.J.; Stengel, D.B. An Assessment of Temporal Variation in the Low Molecular Weight Phlorotannin Profiles in Four Intertidal Brown Macroalgae. Algal Res. 2019, 41, 101550. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.W.; Carmeli, S.; Klimo, K.; Gerhäuser, C.; König, G.M. In Vitro Chemopreventive Potential of Fucophlorethols from the Brown Alga Fucus vesiculosus L. by Anti-Oxidant Activity and Inhibition of Selected Cytochrome P450 Enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure Dependent Antioxidant Capacity of Phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, D.V.; Bogolitsyn, K.G.; Druzhinina, A.S.; Kaplitsin, P.A.; Parshina, A.E.; Pikovskoi, I.I.; Khoroshev, O.Y.; Turova, P.N.; Stavrianidi, A.N.; Shpigun, O.A. Study of Polyphenol Components in Extracts of Arctic Brown Algae of Fucus vesiculosus Type by Liquid Chromatography and Mass-Spectrometry. J. Anal. Chem. 2020, 75, 633–639. [Google Scholar] [CrossRef]
- Kellogg, J.; Grace, M.H.; Lila, M.A. Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity. Mar. Drugs 2014, 12, 5277–5294. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.; Esposito, D.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan Seaweeds Lower Inflammation in RAW 264.7 Macrophages and Decrease Lipid Accumulation in 3T3-L1 Adipocytes. J. Funct. Foods 2015, 15, 396–407. [Google Scholar] [CrossRef]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (Poly)Phenol-Rich Extract from the Brown Algae Ascophyllum nodosum on DNA Damage and Antioxidant Activity in an Overweight or Obese Population: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Santos-Zea, L.; Cruz-Suárez, L.E. Ultrasound-Assisted Extraction of Phlorotannins and Polysaccharides from Silvetia compressa (Phaeophyceae). J. Appl. Phycol. 2020, 32, 1441–1453. [Google Scholar] [CrossRef]
- Keusgen, M.; Glombitza, K.W. Phlorethols, Fuhalols and Their Derivatives from the Brown Alga Sargassum spinuligerum. Phytochemistry 1995, 38, 975–985. [Google Scholar] [CrossRef]
- Keusgen, M.; Glombitza, K.W. Pseudofuhalols from the Brown Alga Sargassum spinuligerum. Phytochemistry 1997, 46, 1403–1415. [Google Scholar] [CrossRef]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring Bioactive Fraction of Sargassum wightii: In Vitro Elucidation of Angiotensin-i-Converting Enzyme Inhibition and Antioxidant Potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef]
- Kord, A.; Foudil-Cherif, Y.; Amiali, M.; Boumechhour, A.; Benfares, R. Phlorotannins Composition, Radical Scavenging Capacity and Reducing Power of Phenolics from the Brown Alga Cystoseira sauvageauana. J. Aquat. Food Prod. Technol. 2021, 30, 426–438. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and Antioxidant Activity of Phlorotannins Extracted from the Brown Seaweed Cystoseira compressa in Streptozotocin-Induced Diabetic Rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Vasincu, A.; Luca, S.V.; Neophytou, C.; Wolfram, E.; Opitz, S.E.W.; Sava, D.; Bucur, L.; Cioroiu, B.I.; Miron, A.; et al. Unravelling the Potential of Seaweeds from the Black Sea Coast of Romania as Bioactive Compounds Sources. Part I: Cystoseira barbata (Stackhouse) C. Agardh. Food Chem. Toxicol. 2019, 134, 110820. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Fernández, C.; Sineiro, J.; Moreira, R.; Gualillo, O. Extraction and Characterization of Phlorotannin-Enriched Fractions from the Atlantic Seaweed Bifurcaria bifurcata and Evaluation of Their Cytotoxic Activity in Murine Cell Line. J. Appl. Phycol. 2019, 31, 2573–2583. [Google Scholar] [CrossRef]
- Catarino, M.D.; Alves-Silva, J.M.; Falcão, S.I.; Vilas-Boas, M.; Jordão, M.; Cardoso, S.M. Chromatography as a Tool for Identification of Bioactive Compounds in Honeybee Products of Botanical Origin. In Chemistry, Biology and Potential Applications of Honeybee Plant-Derived Products; Cardoso, S.M., Silva, A.M.S., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; pp. 89–149. ISBN 9781681082370. [Google Scholar]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A Critical Review of Analytical Methods Used for the Chemical Characterisation and Quantification of Phlorotannin Compounds in Brown Seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef]
- Rajauria, G. Optimization and Validation of Reverse Phase HPLC Method for Qualitative and Quantitative Assessment of Polyphenols in Seaweed. J. Pharm. Biomed. Anal. 2018, 148, 230–237. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound Assisted Extraction of Selected Edible Macroalgae: Effect on Antioxidant Activity and Quantitative Assessment of Polyphenols by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Catarino, D.M.; Silva, M.A.; Cardoso, M.S. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Pantidos, N.; Boath, A.; Lund, V.; Conner, S.; McDougall, G.J. Phenolic-Rich Extracts from the Edible Seaweed, Ascophyllum nodosum, Inhibit α-Amylase and α-Glucosidase: Potential Anti-Hyperglycemic Effects. J. Funct. Foods 2014, 10, 201–209. [Google Scholar] [CrossRef]
- Karthik, R.; Manigandan, V.; Sheeba, R.; Saravanan, R.; Rajesh, P.R. Structural Characterization and Comparative Biomedical Properties of Phloroglucinol from Indian Brown Seaweeds. J. Appl. Phycol. 2016, 28, 3561–3573. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Pete, R.; Küpper, F.C.; Glombitza, K.-W.; König, G.M. Seasonal Variation of Polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 2009, 44, 331–338. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From Isolation and Structural Characterization, to the Evaluation of Their Antidiabetic and Anticancer Potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef] [PubMed]
- Jégou, C.; Kervarec, N.; Cérantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR Use to Quantify Phlorotannins: The Case of Cystoseira tamariscifolia, a Phloroglucinol-Producing Brown Macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef]
- Jégou, C.; Culioli, G.; Kervarec, N.; Simon, G.; Stiger-Pouvreau, V. LC/ESI-MSn and 1H HR-MAS NMR Analytical Methods as Useful Taxonomical Tools within the Genus Cystoseira C. Agardh (Fucales; Phaeophyceae). Talanta 2010, 83, 613–622. [Google Scholar] [CrossRef]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Rosener, H.U.; Müller, D. Bifuhalol Und Diphlorethol Aus Cystoseira tamariscifolia. Phytochemistry 1975, 14, 1115–1116. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Hauperich, S.; Keusgen, M. Phlorotannins from the Brown Algae Cystophora torulosa and Sargassum spinuligerum. Nat. Toxins 1997, 5, 58–63. [Google Scholar] [CrossRef]
- Koch, M.; Glombitza, K.W.; Rösener, H.U. Polyhydroxyphenyl Ethers from Bifurcaria bifurcata. Phytochemistry 1981, 20, 1373–1379. [Google Scholar] [CrossRef]
- Cérantola, S.; Breton, F.; Gall, E.A.; Deslandes, E. Co-Occurrence and Antioxidant Activities of Fucol and Fucophlorethol Classes of Polymeric Phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- McInnes, A.G.; Ragan, M.A.; Smith, D.G.; Walter, J.A. The High Molecular Weight Polyphloroglucinols of the Marine Brown Alga Fucus vesiculosus L. 1H and 13C Nuclear Magnetic Resonance Spectroscopy. Can. J. Chem. 1985, 63, 304–313. [Google Scholar] [CrossRef]
- le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, Antioxidant, and Bactericide Capacities of Phlorotannins from the Brown Macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef]

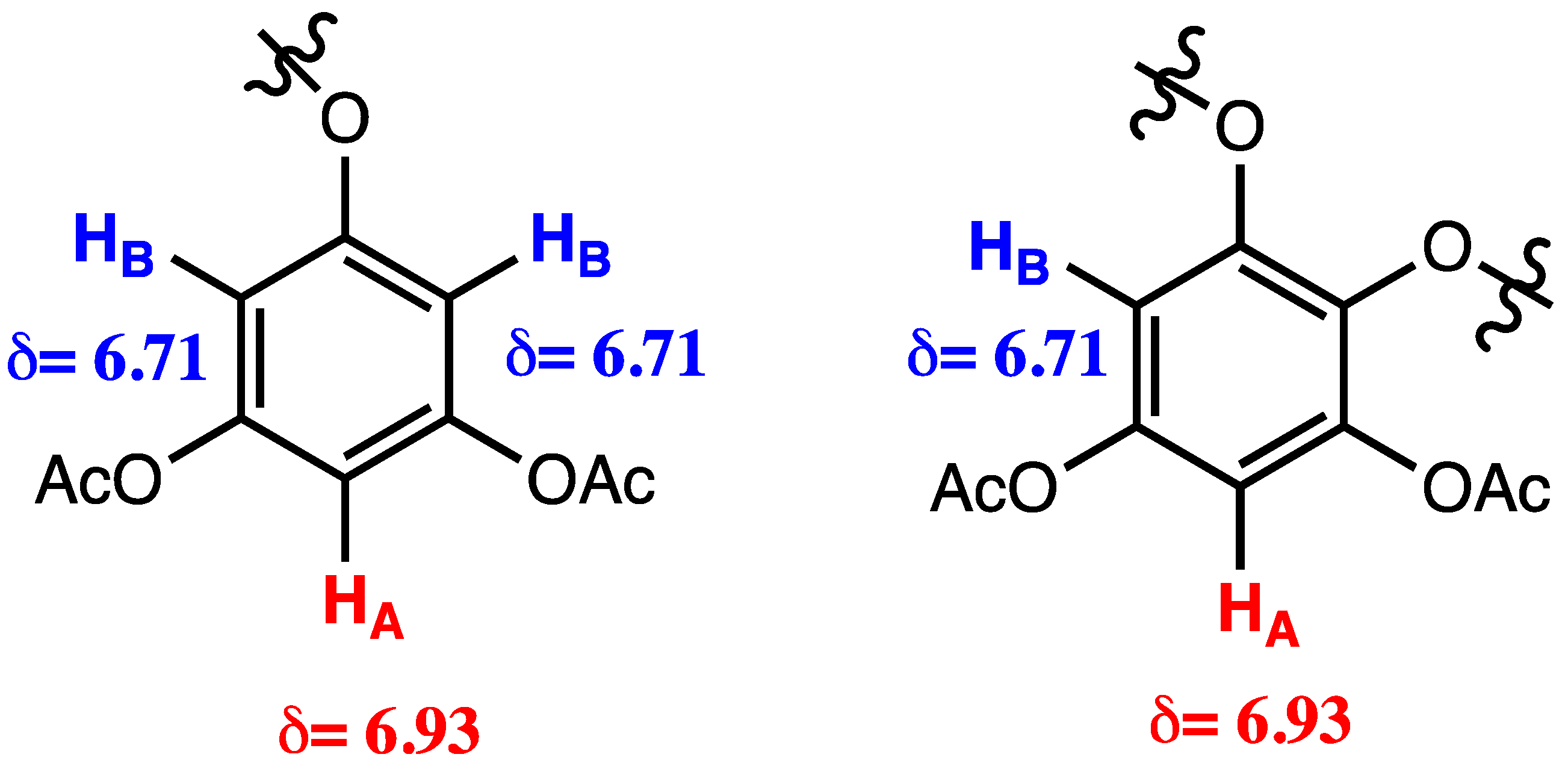
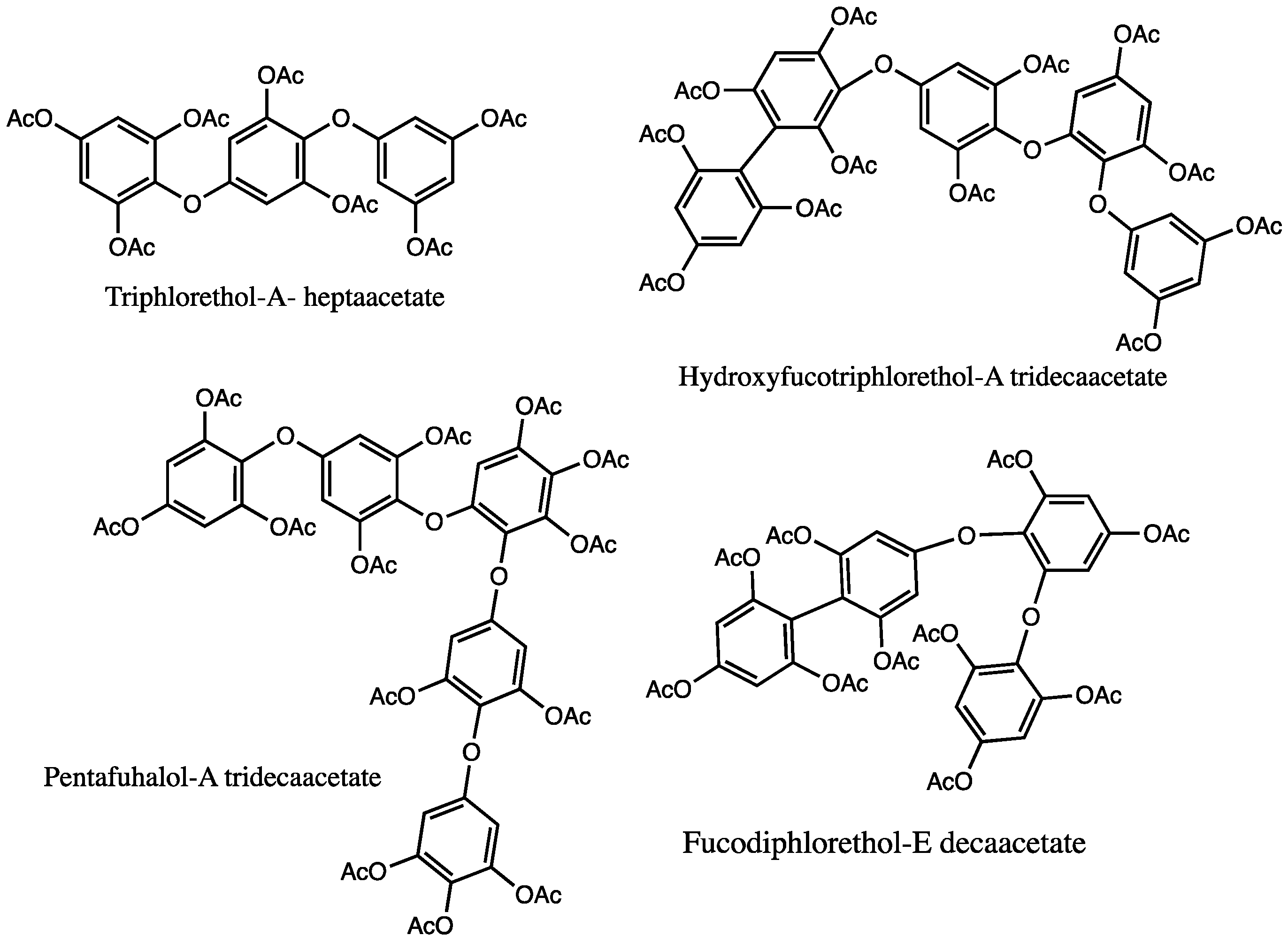
| Raw Material | Preparation of the Crude Extract | Secondary Treatment of the Crude Extract | Phlorotannin Content | Ref. | |
|---|---|---|---|---|---|
| Solvent | Conditions | ||||
| Bifurcaria bifurcata dry powder | 5% algae in mixture of water and glycerin (60:40) | RT time = 30 min pH = 8 | setting pH at 4–5, filtering (nylon, 0.2 μm mesh) | 0.23 ± 0.03 g/L | [69] |
| Sargassum fusiforme dry powder | water (96% wt) | temp = 55 °C time = 48 h enzyme is added | filtering and extracting solid residue with 40 L of ethanol (50% vol) for 12 h; concentrating both filtrate and secondary extract (6 h vacuum), spray-drying them and mixing them to obtain solid extract | — | [71] |
| Fucus vesiculosus dry powder | water (95% wt) | pre-soak (1 h) temp = 50–60 °C time = 120 min stirring | centrifuging and extracting solid pellet with 1 L of water (90 min, 50–60 °C); merging two liquid extracts, adding activated carbon (0.5%), stirring (15 min, 100 °C), filtering | — | [67] |
| Fucus spirallis dry powder | ethanol/water (70%) | soxhlet extraction followed by drying in rotating evaporator at 40 °C | re-suspending in water at 80 °C, filtering (paper), extracting liquid with ethyl ether and ethyl acetate | — | [68] |
| Ascophyllum nodosum dry powder | mixture of water and ethanol (pref. 75:25) | accelerated solvent extraction in a 10 mL-chamber, added with Fonteinebleau sand temp = 150 °C time = 2 × 5 min pressure = 106 Pa | drying liquid extract using rotating evaporator and freeze-drier sequentially | 150 g/kg | [70] |
| Halidrys siliquosa dry powder | mixture of water and ethanol (pref. 75:25) | accelerated solvent extraction in a 10 mL-chamber, added with Fonteinebleau sand temp = 150 °C time = 2 × 5 min pressure = 106 Pa | drying liquid extract using, sequentially, rotating evaporator and freeze-drier | 119 g/kg | [70] |
| Species [Ref] | Configuration | Column | Mobile Phase | Gradient |
|---|---|---|---|---|
| Durvillaea antarctica [95] | RP-HPLC-DAD-ESI-MS/MS (+/−) | Unspecified C18 (5 μm, 4.6 mm ID × 25 cm) | A: 1% FA; B: ACN; flow rate: 1 mL/min | %B (min): 5 (0–5); 5–30 (60); 30–60 (70); 60 (80) |
| Ascophyllum nodosum [44] | RP-UHPLC-DAD-ESI-MS/MS (−) | CSH Phenyl-hexyl (2.1 mm ID × 100 mm, 1.7 µm) | A: H2O; B: ACN 95%; flow rate: 0.3 mL/min | %B (min): 5 (0–15); 5–30 (30); 30–100 (35); 100/47) |
| A. nodosum [88] | RP-UHPLC-DAD-ESI-MS/MS (−) | Zorbax SB C18 (2.1 mm ID × 100 mm, 1.8 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 0.2 mL/min | %B (min): 10 (0–3); 40 (15); 70 (40); 70 (50) |
| Pelvetia canaliculata, Fucus spiralis, F. vesiculosus, A. nodosum [78] | HILIC-UHPLC-DAD-ESI-HRMS (−) | BEH Amide (2.1 ID × 100 mm, 1.7 μm) | A: 10 mM ammonium acetate; B: ACN; flow rate: 0.4 mL/min | %A (min): 5 (0–1); 35 (17) |
| A. nodosum, P. canaliculata, F. spiralis [45] | RP-UHPLC-DAD-ESI-MS (−) | HSS PFP (2.1 ID × 100 mm, 1.8 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 0.5 mL/min | %B (min): 0.5 (0–10); 30 (26); 90 (28) |
| F. vesiculosus [46,48,66,96] | RP-UHPLC-DAD-ESI-MS (−) | Hypersil Gold C18 (2.1 ID × 100 mm, 1.9 μm) | A: 0.1% FA; B: ACN; flow rate: 0.2 mL/min | %A (min): 5 (0); 40 (14.7); 100 (16.6); 100 (18.8) |
| A. nodosum [97] | RP-HPLC-DAD | Sun Fire C18 (4.6 ID × 250 mm, 5 μm) | A: 0.05% orthophosphoric acid; B: ACN; flow rate: 0.8 mL/min | %B (min): 0 (0–4); 30 (8); 60 (16); 70 (20) |
| F. serratus, F. vesiculosus, Cystoseira nodicaulis, H. elongata [98] | RP-UHPLC-DAD-ESI-MS (−) | HSS PFP (2.1 ID × 100 mm, 1.8 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 0.5 mL/min | %B (min): 0.5 (0–10); 30 (26); 90 (28) |
| F. vesiculosus [99] P-UHPLC-DAD-ESI-MS/MS (−) | HSS PFP (2.1 ID x 100 mm, 1.8 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 0.5 mL/min | %B (min): 0.5 (0–10); 30 (26); 90 (28) | |
| Carpophyllum flexuosum [100] | RP-HPLC-DAD-ESI-MS (+) | Phenomenex C18 (4.6 ID × 250 mm, 5 μm) | A: 0.1% FA; B: ACN; flow rate: 0.8 mL/min | %B (min): 0 (0); 60 (60) |
| F. vesiculosus [50] | RP-HPLC-DAD-ESI-MS/MS (−) | Zorbax SB-C18 (4.6 mm ID × 250 mm, 5 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 1 mL/min | %B (min): 10 (0–5); 26 (15); 30 (30); 44 (32); 60 (42) |
| A. nodosum, B. bifurcata, F. vesiculosus [79] | RP-HPLC-DAD–ESI-MS/MS (−) | Zorbax SB-C18 (3.0 mm ID × 150 mm, 3.5 μm) | A: 2.5% HOAc; B: 2.5% HOAc in MeOH; flow rate: 1 mL/min | %B (min): 5 (0); 15 (15); 30 (35); 40 (40); 60 (50); 90 (55); 100 (55.01), 100 (75) |
| A. nodosum [101] | RP-HPLC-DAD-ESI-MS/MS (−) | Synergi Hydro C18 (2.0 ID × 150 mm, 4 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 0.3 mL/min | %B (min): 2 (0–2); 5 (5); 45 (25); 100 (26); 100 (29) |
| F. vesiculosus [87,102] | NP-HPLC-ESI-MS (−) | LiChrospher Si 60 (4.0 mm ID × 250 mm, 4 μm) | A: 82:14:2:2 DCM:MeOH:H2O:HOAc; B: 96:2:2 MeOH:H2O:HOAc; flow rate: 1 mL/min | %A (min): 100 (0–30); 17.6 (45); 30.7 (50); 87.8 (60) |
| P. canaliculata, F. vesiculosus, A. Nodosum, H. elongata [103] | RP-UHPLC-DAD-ESI-MS (−) | HSS PFP (2.1 ID × 100 mm, 1.8 μm) | A: 0.1% FA; B: 0.1% FA in ACN; flow rate: 0.5 mL/min | %B (min): 0.5 (0–10); 30 (26); 90 (28) |
| F. vesiculosus [104] | RP-HPLC-DAD-ESI-MS (−) | Nucleodur 100 (2 mm ID × 100 mm, 5 μm) | A: 2mM NH4Ac; B: 2mM NH4Ac in MeOH | %B (min): 10 (0); 100 (20) |
| F. vesiculosus [16] | RP-HPLC-DAD-ESI-MS/MS (−) | Kinetex (4.6 mm ID × 150 mm, 5 μm) | A: 0.1% FA; B: ACN; flow rate: 0.8 mL/min | %B (min): 1 (0); 10 (12); 30 (25); 50 (27); 50 (28) |
| F. vesiculosus [105] | RP-UHPLC-DAD-ESI-MS/MS (+/−) | Phenomenex Prodigy (2 mm ID × 150 mm, 3 μm) | A: 20 mM FA, 60 mM NH4HCOO/FA in water; B: ACN; flow rate: 0.3 mL/min | %B (min): 2 (0–2); 40 (16); 100 (18) |
| F. spiralis, C. usneoides, C. tamariscifolia, C. nodicaulis [47] | RP-HPLC-DAD-ESI-MS/MS (+) | Luna C18 (4.6 mm ID × 250 mm, 5 μm) | A: 0.1% FA; B: ACN; flow rate: 1 mL/min | %B (min): 0 (0–10); 30 (30); 80 (35); 80 (40) |
| F. vesiculosus [106] | RP-HPLC-DAD-ESI-MS/MS (−) | Acclaim RSLC 120 (2.1 mm ID × 150 mm, 2.2 μm) | A: 0.1% FA; B: ACN; flow rate: 0.4 mL/min | %B (min): 10 (0–2); 95 (17) |
| F. distichus [107,108] | NP-HPLC-DAD-ESI-MS/MS (+) | Develosil Diol (4.6 mm ID × 250 mm, 5 μm) | A: 0.2% HOAc in ACN; B: MeOH:H2O:HOAc (97:3:0.2); flow rate: 0.8 mL/min | %B (min): 0 (0); 40 (35); 100 (40); 100 (45) |
| A. nodosum [109] | RP-UHPLC-DAD-ESI-HRMS (−) | Zorbax SB C18 (2.1 mm ID × 100 mm, 1.8 μm) | A: 0.1% FA in MeOH; B: 0.1% FA; flow rate: 0.2 mL/min | %A (min): 3 (50); 70 (50) |
| Silvetia compressa [110] | RP-HPLC-DAD-ESI-MS (+) | Luna C18 (4.6 mm ID × 250 mm, 5 μm) | A: 0.1% FA; B: MeOH; flow rate: 1 mL/min | %B (min): 10 (0–5); 60 (30); 60 (35) |
| Sargassum fusiforme [49] | RP-UHPLC-DAD-ESI-MS/MS (+/−) | Poroshell 120 EC-C18 (2.1 mm ID × 50 mm, 2.7 μm) | A: H2O; B: ACN; flow rate: 0.4 mL/min | %B (min): 5 (0–4); 10 (10); 20 (15); 20 (20); 40 (40); 45 (52); 75 (55) |
| Sargassum muticum, Cystoseira abies-marina [4,92] | HILIC x RP-2D-HPLC-DAD-ESI-MS/MS (−) | D1–Lichrospher diol-5 (1.0 mm ID × 150 mm, 5 μm); D2–Ascentis Express C18 (4.6 mm ID × 50 mm, 2.7 μm) | D1–A: 1% HOAc in ACN; B: 95:3:2 MeOH:H2O:HOAc; flow rate: 0.015 mL/min D2–A: 0.1% FA; B: ACN; flow rate: 3 mL/min | D1–%B (min): 0 (0–3); 7 (5); 15 (30); 15 (70); 25 (75); 25 (85) D2–%B (min): 0 (0–0.1); 5 (0.3); 70 (0.8); 90 (0.9) |
| Sargassum spinuligerum, Cystophora torulosa [82,83,111,112] | NP-HPLC-DAD | LiChrosorb Si-60 (16 mm ID × 250 mm, 7 μm and 8 mm ID × 250 mm, 5 μm) | A: CHCl3; B: EtOH; flow rate: unspecified | Unspecified |
| Sargassum wightii [113] | RP-UHPLC-DAD-ESI-MS (+) | Not specified | A: 0.1% FA; B: ACN; flow rate: 0.3 mL/min | %B (min): 60 (isocratic) |
| Cystoseira sauvageauana [114] | RP-HPLC-DAD-ESI-MS/MS (−) | Eurospher II 100–2 C18 (2.0 mm ID × 250 mm, 2 μm) | A: 5 mM NH4CH3CO2; B: ACN; flow rate: 0.6 mL/min | %B (min): 5 (0–15); 30 (30); 80 (31) |
| C. compressa [115] | RP-UHPLC-DAD-ESI-MS/MS (−) | ACQUITY UPLC-BEH C18 (2.1 mm ID × 50 mm, 1.7 μm) | A: 0.1% FA; B: 0.1% FA in MeOH; flow rate: 0.2 mL/min | Unspecified |
| Cystoseira barbata [116] | RP-UHPLC-DAD-ESI-MS/MS (−) | Zorbax Rapid Resolution High Definition Eclipse Plus C18 (2.1 mm ID × 50 mm, 1.8 μm) | A: 0.1% FA; B: ACN; flow rate: 0.3 mL/min | %B (min): 0.5 (0–1); 30 (7); 95 (8); 95 (10) |
| Bifurcaria bifurcata [117] | RP-HPLC-DAD-ESI-MS/MS (+) | Kromasil 100 C18 (0.46 mm ID × 25 mm, 5 μm) | A: H2O; B: EtOH; flow rate: 0.7 mL/min | %B (min): 1 (0–30); 95 (32) |
| [M-H]−/+ ([M-H]2−/+), m/z | Compound/Group of Compounds | Species (No. Isomers Detected) [Ref] |
|---|---|---|
| DP1 | ||
| 125− | Phloroglucinol | H. elongata (1) [121]; S. wightii (1) [122] |
| DP2 | ||
| 247− | Dibenzodioxin-1,3,6,8-tetraol | A. nodosum (1) [101], (2) [88]; F. vesiculosus (1) [66], (1) [48] |
| 249−/251+ | Difucol/Diphlorethol | A. nodosum (2) [88]; S. fusiforme (1) [49] |
| 263− | Carmalol | S. fusiforme (1) [49] |
| 265−/267+ | Bifuhalol | S. fusiforme (1) [49]; C. flexuosum (2) [100] |
| 273+ (+Na) | Diphlorethol | H. siliquosa (1) [123] |
| DP3 | ||
| 369−/371+ | Eckstolonol | F. guiryi (1) [16]; F. spiralis (1) [16]; F. vesiculosus (1) [96]; S. compressa (1) [110]; S. fusiforme (1) [49] |
| 371− | Eckol | S. fusiforme (1) [49] |
| 373−/375+ | Fucol/Phlorethol/Fucophlorethol | C. barbata (3) [116]; F. vesiculosus (1) [16], (3) [105]; F. serratus (1) [16]; F. spiralis (1) [16]; C. usneoides (2) [47]; D. antarctica (3) [95]; F. vesiculosus (4) [50]; S. compressa (4) [110] |
| 373− | Trifucol | F. vesiculosus [48] |
| 375+ | Triphlorethol | H. siliquosa [123] |
| 389−/391+ | Trifuhalol | A. nodosum (1) [44], (1) [79]; C. flexuosum (1) [110]; S. fusiforme (1) [16]; S. muticum (3) [92] |
| 405−/407+ | Hydroxytrifuhalol A | A. nodosum (1) [88]; C. flexuosum (1) [100]; S. compressa (1) [110] |
| 387− | 7-Hydroxyeckol | A. nodosum (1) [88] |
| 387− | Phlorethoxycarmalol | S. fusiforme (1) [49] |
| 413+ (+Na) | Trifuhalol | H. siliquosa (1) [123] |
| DP4 | ||
| 493− | Hydroxyfucofuroeckol | F. guiryi (1) [16] |
| 479− | Fucofurodiphlorethol | F. vesiculosus (1) [66], (2) [48], (1) [96] |
| 495−/497+ | Phloroeckol | C. tamariscifolia (1) [47]; C. nodicaulis (2) [47]; D. antarctica (1) [95]; A. nodosum (2) [124] |
| 497+ | 7-Phloroeckol | S. compressa (1) [110] |
| 497−/499+ | Fucol/Phlorethol/Fucophlorethol | C. barbata (4) [116]; F. spiralis (1) [47], (2) [16]; F. guiryi (5) [16]; A. nodosum (1) [44], (1) [88]; F. serratus (3) [16]; D. antarctica (1) [95]; C. nodicaulis (3) [47]; C. tamariscifolia (1) [47]; C. usneoides (1) [47]; F. vesiculosus(4) [50], (2) [106], (2) [16] |
| 497− | Tetrafucol | F. vesiculosus (2) [66], (1) [48], (1) [46] |
| 497− | Fucophlorethol | F. vesiculosus (1) [46] |
| 511− | Diphlorethohydroxycarmalol | A. nodosum (1) [101]; F. vesiculosus (1) [46], S. fusiforme (1) [49] |
| 513−/515+ | Tetrafuhalol | C. flexuosum (1) [100]; A. nodosum (1) [79]; B. bifurcata (1) [79]; S. fusiforme (1) [49]; S. muticum (5) [93] |
| 527− | Hydroxydiphlorethoxycarmalol | S. fusiforme (1) [49] |
| 529−/531+ | Hydroxytetrafuhalol | F. vesiculosus (1) [66]; C. flexuosum (2) [101]; F. vesiculosus (1) [48], (1) [79], (1) [46]; B. bifurcata (1) [79]; S. fusiforme (1) [49]; S. muticum (6) [92] |
| 545−/547+ | Dihydroxytetrafuhalol | B. bifurcata (1) [79]; F. vesiculosus (1) [79]; S. compressa (1) [110]; S. muticum (1) [92] |
| 537+ (+Na) | Tetrafuhalol | H. siliquosa (1) [123] |
| 591− | Diphlorethohydroxycarmalol sulphate | A. nodosum (1) [101] |
| DP5 | ||
| 601−/603+ | Phlorofucofuroeckol | C. barbata (1) [116]; C. tamariscifolia (2) [47] |
| 603+ | Phlorofucofuroeckol A | S. compressa (1) [110] |
| 603− | Fucofurotriphlorethol | F. vesiculosus (1) [48]; F. vesiculosus (1) [66] |
| 621−/623+ | Fucol/Phlorethol/Fucophlorethol | C. barbata (4) [116]; C. usneoides (1) [47]; C. abies-marina (8) [4]; D. antarctica (4) [96]; A. nodosum (1) [44]; S. fusiforme (1) [49]; F. vesiculosus (4) [50], (7) [16], (3) [105]; |
| 621− | Pentafucol | F. vesiculosus (1) [66] |
| 621− | Trifucophlorethol | F. vesiculosus (1) [48], (1) [96], |
| 621− | Pentaphlorethol | S. muticum (1) [92] |
| 635− | Triphlorethoxycarmalol | S. fusiforme (1) [49] |
| 637− | Pentafuhalol | F. vesiculosus (1) [66], (2) [48]; A. nodosum (1) [79], S. fusiforme (1) [49], S. muticum (5) [92] |
| 639+ | Pentafuhalol | S. compressa (1) [110] |
| 651− | Hydroxytriphlorethoxycarmalol | S. fusiforme (1) [49] |
| 653− | Hydroxypentafuhalol | S. muticum (2) [92]; S. fusiforme (1) [49] |
| 669− | Dihydroxypentafuhalol | S. muticum (3) [92] |
| 671+ | Dihydroxypentafuhalol | S. compressa (1) [110] |
| DP6 | ||
| 741−/743+ | Dieckol | C. tamariscifolia (1) [47]; S. compressa (1) [110]; S. fusiforme (1) [49] |
| 745−/747+ | Fucol/Phlorethol/Fucophlorethol | D. antarctica (6) [95]; C. abies-marina (2) [4]; C. barbata (3) [116]; F. spiralis (1) [47]; A. nodosum (1) [44]; F. vesiculosus (3) [50], (2) [16], (3) [105]; S. fusiforme (1) [49] |
| 745− | Hexafucol | F. vesiculosus (1) [66], (1) [48], (1) [96] |
| 745− | Tetrafucophlorethol | F. vesiculosus (1) [96] |
| 745− | Hexaphlorethol | S. muticum (1) [92] |
| 759− | Tetraphlorethoxycarmalol | S. fusiforme (1) [49] |
| 761− | Hexafuhalol | S. fusiforme (1) [49]; S. muticum (3) [92] |
| 777−/779+ | Hydroxyhexafuhalol | C. flexuosum [100]; F. vesiculosus (2) [79]; S. fusiforme (1) [49]; S. muticum (2) [92] |
| 791− | Dihydroxytetraphlorethoxycarmalol | S. fusiforme (1) [49] |
| 793− | Dihydroxyhexafuhalol | S. fusiforme (1) [49]; C. flexuosum (3) [100]; S. muticum (6) [92] |
| 809− | Trihydroxyhexafuhalol | S. muticum (1) [92] |
| DP7 | ||
| 851− | Fucofuropentaphlorethol | F. vesiculosus (1) [48] |
| 869−/871+ | Fucol/Phlorethol/Fucophlorethol | D. antarctica (3) [95]; C. abies-marina (4) [4]; C. barbata (3) [116]; F. spiralis (3) [47]; F. vesiculosus (2) [50], (2) [105] |
| 869− | Heptafucol | F. vesiculosus (1) [66] |
| 869− | Trifucotriphlorethol | F. vesiculosus (1) [48] |
| 869− | Difucotetraphlorethol | F. vesiculosus (1) [48] |
| 869− | Heptaphlorethol | S. muticum (1) [92] |
| 883− | Pentaphlorethoxycarmalol | S. fusiforme (1) [49] |
| 887− | Heptafuhalol | S. fusiforme (1) [49] |
| 901− | Hydroxyheptafuhalol | S. muticum (1) [93]; S. fusiforme (1) [49] |
| 915− | Dihydroxypentaphlorethoxycarmalol | S. fusiforme (1) [49] |
| 917− | Dihydroxyheptafuhalol | S. fusiforme (1) [49]; S. muticum (5) [92] |
| 933− | Trihydroxyheptafuhalol | B. bifurcata (1) [79]; S. muticum (2) [92] |
| DP8 | ||
| 993−/995+ | Fucol/Phlorethol/Fucophlorethol | D. antarctica (3) [95]; C. abies-marina (4) [4]; F. spiralis (3) [47]; A. nodosum (1) [79]; S. fusiforme (1) [49] |
| 993− | Pentafucodiphlorethol | F. vesiculosus (1) [48] |
| 993− | Hexafucophlorethol | F. vesiculosus (1) [48] |
| 993− | Tetrafucotetraphlorethol | F. vesiculosus (2) [48] |
| 1007− | Hexaphlorethoxycarmalol | S. fusiforme (1) [49] |
| 1009− | Octafuhalol | S. muticum (1) [92] |
| 1041− | Dihydroxynonafuhalol | S. fusiforme (1) [49]; S. muticum (1) [92] |
| 1043+ | Hydroxyoctafuhalol | C. flexuosum (1) [100] |
| 1055− | Trihydroxyhexaphlorethoxycarmalol | S. fusiforme (1) [49] |
| 1057− | Trihydroxyoctafuhalol | S. fusiforme (1) [49]; S. muticum (2) [92] |
| DP9 | ||
| 1117−/1119+ | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (4) [4]; F. vesiculosus (3) [50]; F. distichus (1) [108]; S. fusiforme (1) [49] |
| 1133− | Nonafuhalol | S. muticum (2) [92] |
| 1165− | Dihydroxinonafuhalol | S. fusiforme (1) [49]; S. muticum (1) [93] |
| 1181− | Trihydroxynonafuhalol | S. fusiforme (1) [49] |
| DP10 | ||
| 1241−/1243+ | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (4) [4]; F. vesiculosus (2) [50]; A. nodosum (2) [122]; F. distichus (1) [108]; S. fusiforme (1) [49] |
| 1257− | Decafuhalol | S. muticum (1) [92] |
| 1305− | Trihydroxydecafuhalol | S. fusiforme (1) [49] |
| 1321− | Tetrahydroxydecafuhalol | S. fusiforme (1) [49] |
| DP11 | ||
| 1365− | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (4) [4]; A. nodosum (1) [124]; F. distichus (1) [108]; S. fusiforme (1) [49] |
| 1445− | Tetrahydroxyfuhalol | S. fusiforme (1) [49] |
| DP12 | ||
| 1489− (744−) | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (3) [4]; F. distichus (1) [108] |
| 1585− | Pentahydroxyfuhalol | S. fusiforme (1) [49] |
| DP13 | ||
| (806−) | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (2) [4] |
| (806−) | Fucol/Phlorethol/Fucophlorethol | F. distichus (1) [108] |
| DP14 | ||
| 1737− (868−) | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (2) [4]; A. nodosum (2) [124]; F. distichus (1) [108] |
| DP15 | ||
| (930−) | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (1) [4]; F. distichus (1) [108] |
| DP16 | ||
| (992−) | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (4) [4]; F. distichus (1) [108] |
| DP17 | ||
| (1054−) | Fucol/Phlorethol/Fucophlorethol | C. abies-marina (1) [4]; F. distichus (1) [108] |
| DP18 | ||
| (1116−) | Fucol/Phlorethol/Fucophlorethol | F. distichus (1) [108] |
| DP19 | ||
| (1178−) | Fucol/Phlorethol/Fucophlorethol | F. distichus (1) [108] |
| DP20 | ||
| (1240−) | Fucol/Phlorethol/Fucophlorethol | F. distichus (1) [108] |
| δ (ppm) | Bound Type | Conclusion |
|---|---|---|
| 100 | -C-C- | - |
| 120–150 | -C-O- | Presence of phlorethols |
| 145–155 | Signals for hydroxy groups additional to the monomer -OH | Presence of fuhalols |
| 166–168 | -C=O | From the acetyl groups |
| Species | Identified Phlorotanins Using NMR | Ref. |
|---|---|---|
| Cystoseira tamariscifolia | Fuhalols: bifuhalol hexaacetate Phlorethols: diphlorethol pentaacetate | [136] |
| Fucus vesiculosus | Fucophlorethols: trifucotriphlorethol A, fucotriphlorethol E Fucols: tetrafucol A, tetrafucol B | [138] |
| Fucus spiralis | Groups fucol and fucophlorethol | [137] |
| Bifurcaria bifurcata | Fuhalols: tetrafuhalol-B-undecacetate, desacetoxyheptafuhalol- heptadecacetate, heptafuhaloloctadecacetate, nonafuhaloltricosacetate, undecafuhaloloctacosacetate, tridecafuhaloltritriacontacetate | [136] |
| Halidrys siliquosa | Fuhalols: trifuhalol, tetrafuhalol Phlorethols: diphlorethol, triphlorethol | [139] |
| Sargassum spinuligerum | Fuhalols: hydroxytrifuhalol-A nonaacetate, hydroxytrifuhalol-B nonaacetate, hydroxypentafuhalol-B tetradecaacetate, deshydroxytetrafuhalol-B decaacetate, deshydroxyhexafuhalol-B pentadecaacetate, hydroxyheptafuhalol-A nonadecaacetate, hydroxyheptafuhalol-B nonadecaacetate, pentafuhalol-A tridecaacetate, heptafuhalol-A octadecaacetate, pentafuhalol-B tridecaacetate, heptafuhalol-B octadecaacetate, nonafuhalol-B tricosaacetate, trifuhalol-B octaacetate, tetrafuhalol-B undecaacetate, hexafuhalol-B hexadecaacetate Phlorethols: diphlorethol, triphlorethol, diphloretholpentaacetate, triphlorethol-A heptaacetate, triphlorethol- B heptaacetate, tetraphlorethol-C nonaacetate, dihydroxytetraphlorethol-A undecaacetate, dihydroxytetraphlorethol-B undecaacetate, pentaphlorethol-A undecaacetate Fucophlorethols: fucophlorethol-B octaacetate, fucodiphlorethol-B decaacetate, fucodiphlorethol-D decaacetate, fucodiphlorethol-F decaacetate, bisfucotriphlorethol-A pentadecaacetate, bisfucotetraphlorethol-A heptadecaacetate, chlorobisfucopentaphlorethol nonadecaacetate, difucodiphlorethol-A tridecaacetate, fucodifucotetraphlorethol-A icosaacetate Hydroxyfucophlorethols: hydroxyfucophlorethol-A undecaacetate, hydroxybisfucophlorethol-A hexadecaacetate, dihydroxyfucotriphlorethol-A tetradecaacetate, dihydroxyfucotriphlorethol-B tetradecaacetate, bisfucopentaphlorethol-B nonadecaacetate, chlorobisfucopentaphlorethol-A nonadecaacetate | [82,83] |
| Cystophora torulosa | Fucols: difucol hexaacetate, trifucol nonaacetate Phlorethols: diphlorethol pentaacetate, triphlorethol-A- heptaacetate, chlorobisfucopentaphlorethol-A nonadeca-acetate Fuhalols: trifuhalol-A octaacetate, tetrafuhalol-A undecaacetate, pentafuhalol-A tridecaacetate, hexafuhalol-A hexadecaacetate Fucophlorethols: fucophlorethol-B octaacetate, fucophlorethol-B decaacetate, fucodiphlorethol-D decaacetate, difucophlorethol-A undecaacetate, fucotriphlorethol-B dodecaacetate, fucotetraphlorethol-B tetradecaacetate, bisfucotriphlorethol-A pentadecaacetate, bisfucotetraphlorethol-A heptadecaacetate, bisfucopentaphlorethol-A nonadecaacetate, fucophlorethol-C octaacetate, fucodiphlorethol-E decaacetate, fucodifucotetraphlorethol-A eicosaacetate, bisfucotriphlorethol-B pentadecaacetate Hydroxyfucophlorethols: hydroxyfucophlorethol-A nonaacetate, hydroxyfucodiphlorethol-A undecaacetate, hydroxyfucotriphlorethol-A tridecaacetate, hydroxyfucotetraphlorethol-A pentadecaacetate, hydroxyfucopentaphlorethol-A heptadecaacetate, hihydroxyfucotriphlorethol-A tetradecaacetate, hihydroxyfucotetraphlorethol-A hexadecaacetate, trihydroxyfucopentaphlorethol-A nonadecaacetate, hydroxyfucodiphlorethol-B undecaacetate, hydroxyfucotriphlorethol-B tridecaacetate, hydroxybisfucopentaphlorethol-A eicosaacetate | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catarino, M.D.; Pires, S.M.G.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.C.G.A.; Silva, A.M.S.; Cardoso, S.M. Overview of Phlorotannins’ Constituents in Fucales. Mar. Drugs 2022, 20, 754. https://doi.org/10.3390/md20120754
Catarino MD, Pires SMG, Silva S, Costa F, Braga SS, Pinto DCGA, Silva AMS, Cardoso SM. Overview of Phlorotannins’ Constituents in Fucales. Marine Drugs. 2022; 20(12):754. https://doi.org/10.3390/md20120754
Chicago/Turabian StyleCatarino, Marcelo D., Sónia M. G. Pires, Sónia Silva, Filipa Costa, Susana S. Braga, Diana C. G. A. Pinto, Artur M. S. Silva, and Susana M. Cardoso. 2022. "Overview of Phlorotannins’ Constituents in Fucales" Marine Drugs 20, no. 12: 754. https://doi.org/10.3390/md20120754
APA StyleCatarino, M. D., Pires, S. M. G., Silva, S., Costa, F., Braga, S. S., Pinto, D. C. G. A., Silva, A. M. S., & Cardoso, S. M. (2022). Overview of Phlorotannins’ Constituents in Fucales. Marine Drugs, 20(12), 754. https://doi.org/10.3390/md20120754











