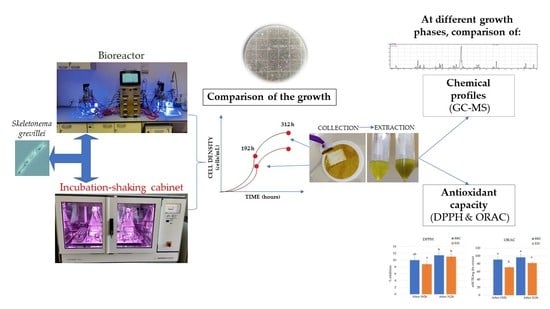
Comparison of Growth and Chemical Profile of Diatom Skeletonema grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases
Abstract
1. Introduction
2. Results and Discussion
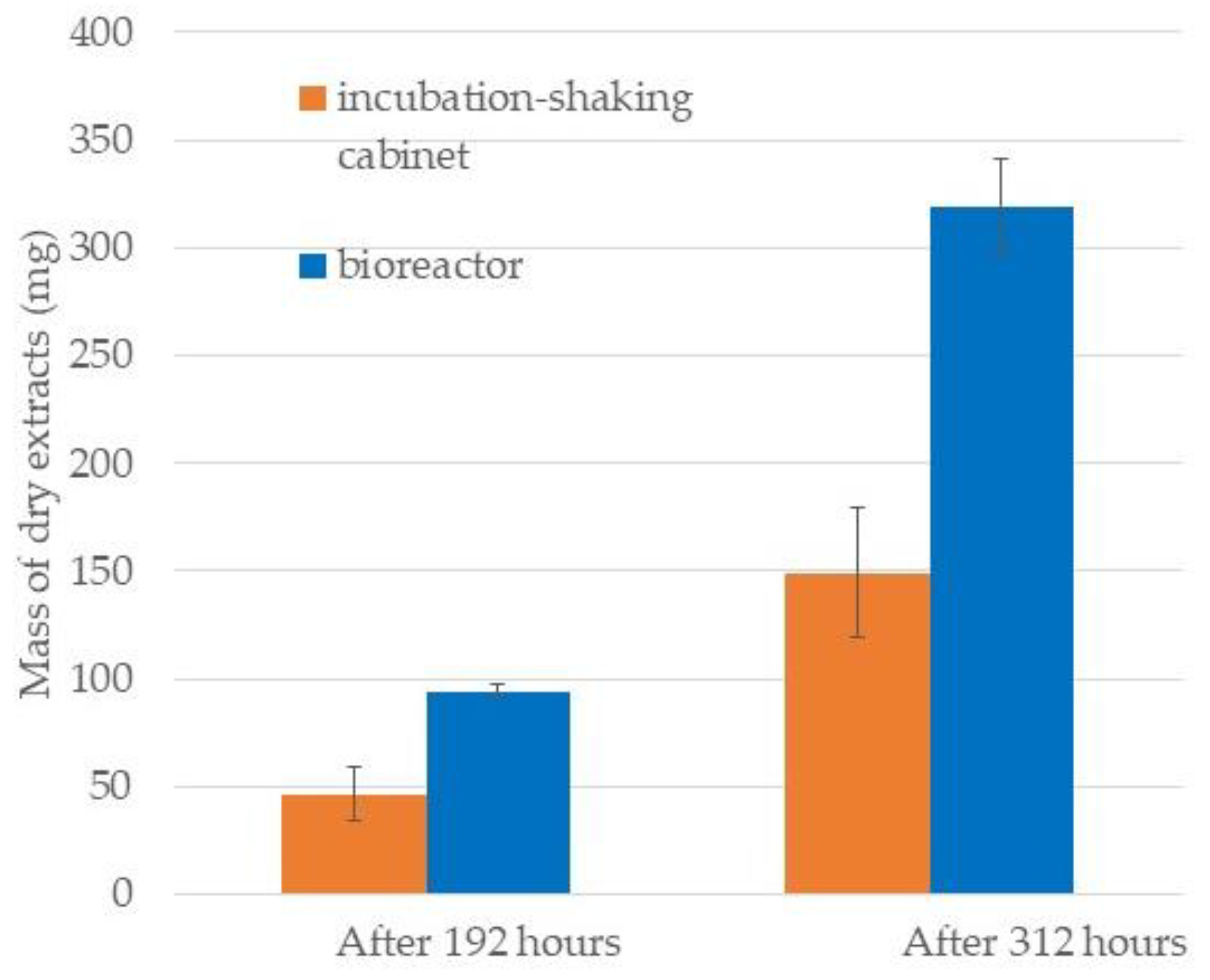
2.1. Comparison of Growth Curves in BRC and EIS
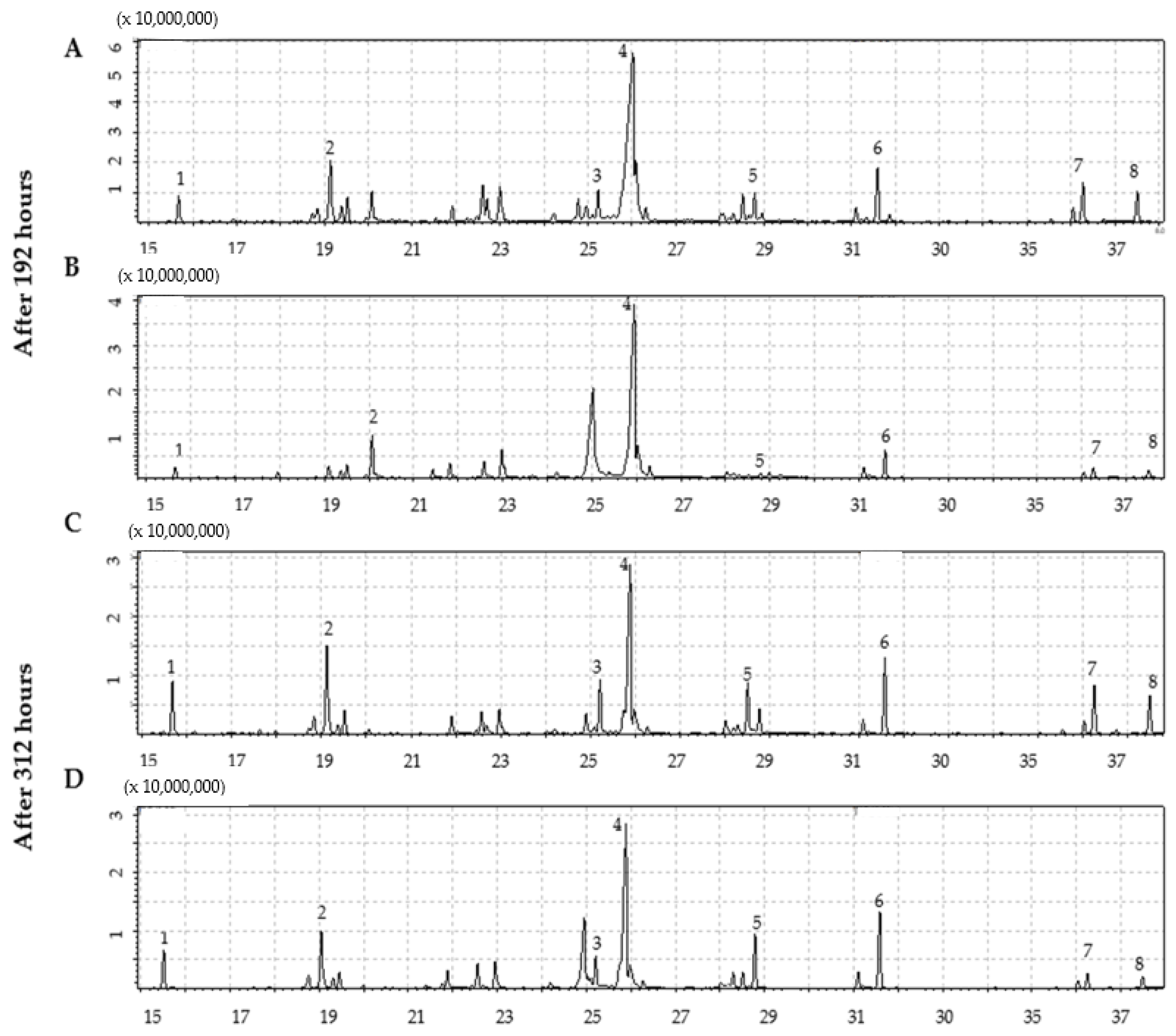
2.2. Identification of Compounds by GC-MS
2.3. Antioxidation Activity Assays
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Design
3.3. The Growth Curve Determination
3.4. Collection and Extraction of Diatom Biomass
3.5. Identification of Compounds by GC/MS
3.6. Antioxidant Activity of Diatom Extracts
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae Metabolites: A Rich Source for Food and Medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Sirohi, R.; Udayan, A.; Yadav, P.; Raj, A.; Sim, S.J.; Pandey, A. Sustainable Microalgal Biomass Production in Food Industry Wastewater for Low-Cost Biorefinery Products: A Review. Phytochem. Rev. 2022, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, S.A.; Potapova, M.G.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.Org: Supporting Taxonomists, Connecting Communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.S.; Stonik, I. Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Valdés, J.R.; Méndez-Zavala, A.; Hernández-López, I.; Carreón-González, B.A.; Velázquez-Arellano, M.E.; Morales-Oyervides, L.; Montañez-Saénz, J.C. Unconventional Microalgae Species and Potential for Their Use in the Food Industry. Cultured Microalgae for the Food Industry; Current and Potential Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 49–71. [Google Scholar] [CrossRef]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Gallo, C.; Landi, S.; D’Ippolito, G.; Nuzzo, G.; Manzo, E.; Sardo, A.; Fontana, A. Diatoms Synthesize Sterols by Inclusion of Animal and Fungal Genes in the Plant Pathway. Sci. Rep. 2020, 10, 4204. [Google Scholar] [CrossRef]
- Vidoudez, C.; Pohnert, G. Comparative Metabolomics of the Diatom Skeletonema Marinoi in Different Growth Phases. Metabolomics 2012, 8, 654–669. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Muñoz, R. Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Gao, G.; Wu, M.; Fu, Q.; Li, X.; Xu, J. A Two-Stage Model with Nitrogen and Silicon Limitation Enhances Lipid Productivity and Biodiesel Features of the Marine Bloom-Forming Diatom Skeletonema Costatum. Bioresour. Technol. 2019, 289, 121717. [Google Scholar] [CrossRef]
- Yuan, W.; Gao, G.; Shi, Q.; Xu, Z.; Wu, H. Combined Effects of Ocean Acidification and Warming on Physiological Response of the Diatom Thalassiosira Pseudonana to Light Challenges. Mar. Environ. Res. 2018, 135, 63–69. [Google Scholar] [CrossRef]
- Souffreau, C.; Vanormelingen, P.; Verleyen, E.; Sabbe, K.; Vyverman, W. Tolerance of Benthic Diatoms from Temperate Aquatic and Terrestrial Habitats to Experimental Desiccation and Temperature Stress. Phycologia 2010, 49, 309–324. [Google Scholar] [CrossRef]
- Fukami, K.; Nishimura, S.; Ogusa, M.; Asada, M.; Nishijima, T. Continuous Culture with Deep Seawater of a Benthic Food Diatom Nitzschia Sp. Hydrobiologia 1997, 358, 245–249. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae Cultivation and Metabolites Production: A Comprehensive Review. Biofuels Bioprod. Biorefining 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Fagundes, M.B.; Vendruscolo, R.G.; Wagner, R. Sterols from Microalgae; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Iglesias, S.; Míguez, C.; Sánchez, A.; Cancela, A.; Álvarez, X. Thalassiosira Pseudonana and Skeletonema Costatum Biomass Optimization: Cultivation, Harvesting, Extraction of Oils and Biodiesel and Pelletization of the Residue. J. Sea Res. 2022, 187, 102243. [Google Scholar] [CrossRef]
- Sharmin, T.; Monirul Hasan, C.M.; Aftabuddin, S.; Rahman, M.A.; Khan, M. Growth, Fatty Acid, and Lipid Composition of Marine Microalgae Skeletonema Costatum Available in Bangladesh Coast: Consideration as Biodiesel Feedstock. J. Mar. Biol. 2016, 2016, 6832847. [Google Scholar] [CrossRef]
- Thangaraj, S.; Sun, J. The Biotechnological Potential of the Marine Diatom Skeletonema Dohrnii to the Elevated Temperature and PCO2. Mar. Drugs 2020, 18, 259. [Google Scholar] [CrossRef]
- Jiang, X.; Han, Q.; Gao, X.; Gao, G. Conditions Optimising on the Yield of Biomass, Total Lipid, and Valuable Fatty Acids in Two Strains of Skeletonema Menzelii. Food Chem. 2016, 194, 723–732. [Google Scholar] [CrossRef]
- Raniello, R.; Iannicelli, M.M.; Nappo, M.; Avila, C.; Zupo, V. Production of Cocconeis Neothumensis (Bacillariophyceae) Biomass in Batch Cultures and Bioreactors for Biotechnological Applications: Light and Nutrient Requirements. J. Appl. Phycol. 2007, 19, 383–391. [Google Scholar] [CrossRef]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and Temperature Effects on Bioactivity in Diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef]
- Bondoc, K.G.V.; Heuschele, J.; Gillard, J.; Vyverman, W.; Pohnert, G. Selective Silicate-Directed Motility in Diatoms. Nat. Commun. 2016, 7, 10540. [Google Scholar] [CrossRef]
- Leynaert, A.; Fardel, C.; Beker, B.; Soler, C.; Delebecq, G.; Lemercier, A.; Pondaven, P.; Durand, P.E.; Heggarty, K. Diatom Frustules Nanostructure in Pelagic and Benthic Environments. Silicon 2018, 10, 2701–2709. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Cao, X.; Yu, Z. Responses of Photosynthetic Characters of Skeletonema Costatum to Different Nutrient Conditions. J. Plankton Res. 2013, 35, 165–176. [Google Scholar] [CrossRef]
- Gao, G.; Shi, Q.; Xu, Z.; Xu, J.; Campbell, D.A.; Wu, H. Global Warming Interacts with Ocean Acidification to Alter PSII Function and Protection in the Diatom Thalassiosira Weissflogii. Environ. Exp. Bot. 2018, 147, 95–103. [Google Scholar] [CrossRef]
- Ramirez, E.E.; Gonzales, M.A.; Cifuentes, A.S.; Inostroza, I.; Urrutia, R.E. Culture and Growth of Two Benthic Diatoms Species Isolated from the Salar Del Huasco (North of Chile, 20° S) at Different Conditions of Temperature, Light and Nutrient. Gayana Bot. 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Marzec, M.; Dąbek, P.; Witkowski, A.; Monedeiro, F.; Pomastowski, P.; Buszewski, B.; Nowak, I. Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles. Pharmaceutics 2022, 14, 1171. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Zhang, Y. Engineering of Saccharomyces Cerevisiae for 24-Methylene-Cholesterol Production. Biomolecules 2021, 11, 1710. [Google Scholar] [CrossRef] [PubMed]
- Ameamsri, U.; Chaveerach, A.; Sudmoon, R.; Tanee, T.; Peigneur, S.; Tytgat, J. Oleamide in Ipomoea and Dillenia Species and Inflammatory Activity Investigated through Ion Channel Inhibition. Curr. Pharm. Biotechnol. 2020, 22, 254–261. [Google Scholar] [CrossRef]
- Shao, J.; He, Y.; Li, F.; Zhang, H.; Chen, A.; Luo, S.; Gu, J.D. Growth Inhibition and Possible Mechanism of Oleamide against the Toxin-Producing Cyanobacterium Microcystis Aeruginosa NIES-843. Ecotoxicology 2016, 25, 225–233. [Google Scholar] [CrossRef]
- Leitner, P.D.; Jakschitz, T.; Gstir, R.; Stuppner, S.; Perkams, S.; Kruus, M.; Trockenbacher, A.; Griesbeck, C.; Bonn, G.K.; Huber, L.A.; et al. Anti-Inflammatory Extract from Soil Algae Chromochloris Zofingiensis Targeting TNFR/NF-ΚB Signaling at Different Levels. Cells 2022, 11, 1407. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Hong, J.H.; Kim, J.S.; Kim, D.K.; Kim, C.S. Oleamide Suppresses Inflammatory Responses in LPS-Induced RAW264.7 Murine Macrophages and Alleviates Paw Edema in a Carrageenan-Induced Inflammatory Rat Model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef]
- Premjanu, N.; Jaynthy, C. Identification and Characterization of Antimicrobial Metabolite from an Endophytic Fungus, Colletotrichum Gloeosporioides Isolated from Lannea Corammendalica. Int. J. ChemTech Res. 2015, 7, 369–374. [Google Scholar]
- Dos Reis, C.M.; da Rosa, B.V.; da Rosa, G.P.; do Carmo, G.; Morandini, L.M.B.; Ugalde, G.A.; Kuhn, K.R.; Morel, A.F.; Jahn, S.L.; Kuhn, R.C. Antifungal and Antibacterial Activity of Extracts Produced from Diaporthe Schini. J. Biotechnol. 2019, 294, 30–37. [Google Scholar] [CrossRef]
- Hameed, I.H.; Altameme, H.J.; Mohammed, G.J. Evaluation of Antifungal and Antibacterial Activity and Analysis of Bioactive Phytochemical Compounds of Cinnamomum Zeylanicum (Cinnamon Bark) Using Gas Chromatography-Mass Spectrometry. Orient. J. Chem. 2016, 32, 1769–1788. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; El-Sheekh, M.M. Induction of the Synthesis of Bioactive Compounds of the Marine Alga Tetraselmis Tetrathele (West) Butcher Grown under Salinity Stress. Egypt. J. Aquat. Res. 2016, 42, 385–391. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus Aureus and Other Gram-Positive Bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Xie, J. Diatoms as Cell Factories for High-Value Products: Chrysolaminarin, Eicosapentaenoic Acid, and Fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef]
- Zulu, N.N.; Zienkiewicz, K.; Vollheyde, K.; Feussner, I. Current Trends to Comprehend Lipid Metabolism in Diatoms. Prog. Lipid Res. 2018, 70, 1–16. [Google Scholar] [CrossRef]
- Javid, S.; Purohit, M.N.; Yogish Kumar, H.; Ramya, K.; Mithuna, N.F.A.; Salahuddin, M.D.; Prashantha Kumar, B.R. Semisynthesis of Myristic Acid Derivatives and Their Biological Activities: A Critical Insight. J. Biol. Act. Prod. Nat. 2020, 10, 455–472. [Google Scholar] [CrossRef]
- Chama, M.A.; Dziwornu, G.A.; Waibel, R.; Osei-Safo, D.; Addae-Mensah, I.; Otchere, J.; Wilson, M. Isolation, Characterization, and Anthelminthic Activities of a Novel Dichapetalin and Other Constituents of Dichapetalum Filicaule. Pharm. Biol. 2016, 54, 1179–1188. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Li, T.; Shan, X.; Lu, R.; Zhang, S.; Xu, H. Cholesterol: Bioactivities, Structural Modification, Mechanisms of Action, and Structure-Activity Relationships. Mini-Rev. Med. Chem. 2021, 21, 1830–1848. [Google Scholar] [CrossRef]
- Ceccarelli, V.; Ronchetti, S.; Marchetti, M.C.; Calvitti, M.; Riccardi, C.; Grignani, F.; Vecchini, A. Molecular Mechanisms Underlying Eicosapentaenoic Acid Inhibition of HDAC1 and DNMT Expression and Activity in Carcinoma Cells. Biochim. Biophys. Acta—Gene Regul. Mech. 2020, 1863, 194481. [Google Scholar] [CrossRef]
- Bie, N.; Han, L.; Meng, M.; Zhang, Y.; Guo, M.; Wang, C. Anti-Tumor Mechanism of Eicosapentaenoic Acid (EPA) on Ovarian Tumor Model by Improving the Immunomodulatory Activity in F344 Rats. J. Funct. Foods 2020, 65, 103739. [Google Scholar] [CrossRef]
- Ferreira, M.; Teixeira, C.; Abreu, H.; Silva, J.; Costas, B.; Kiron, V.; Valente, L.M.P. Nutritional Value, Antimicrobial and Antioxidant Activities of Micro- and Macroalgae, Single or Blended, Unravel Their Potential Use for Aquafeeds. J. Appl. Phycol. 2021, 33, 3507–3518. [Google Scholar] [CrossRef]
- Ribeiro-Vidal, H.; Sánchez, M.C.; Alonso-Español, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of Epa and Dha against Oral Pathogenic Bacteria Using an in Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Liaw, C.-C.; Lin, M.-K.; Chen, C.-J.; Chao, C.-L.; Chao, C.-H.; Kuo, Y.-H.; Chiu, Y.-P.; Peng, Y.-S.; Hui-Chi, H. Anti-Influenza Virus Activity and Chemical. Molecules 2020, 25, 4427. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Conte, M.; Tirino, V.; del Vecchio, V.; de Angelis, R.; Nebbioso, A.; Altucci, L.; Romano, G. Cytotoxic Potential of the Marine Diatom Thalassiosira Rotula: Insights into Bioactivity of 24-Methylene Cholesterol. Mar. Drugs 2022, 20, 595. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Z.; Li, Y.; Cao, X.; Yang, L.; Xu, Y.; Li, Z.; He, N. Function of ORFC of the Polyketide Synthase Gene Cluster on Fatty Acid Accumulation in Schizochytrium Limacinum SR21. Biotechnol. Biofuels 2021, 14, 163. [Google Scholar] [CrossRef]
- Rampen, S.W.; Abbas, B.A.; Schouten, S.; Damsté, J.S.S. A Comprehensive Study of Sterols in Marine Diatoms (Bacillariophyta): Implications for Their Use as Tracers for Diatom Productivity. Limnol. Oceanogr. 2010, 55, 91–105. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Cole, W.J.; McIntyre, H.B.; Smith, A.G. Selective Reactions in the Analysis and Characterization of Steroids by Gas Chromatography-Mass Spectrometry. Lipids 1980, 15, 745–755. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal Lipids, Fatty Acids and Sterols; Woodhead Publishing Ltd.: Sawston, UK, 2013. [Google Scholar] [CrossRef]
- Barrett, S.M.; Volkman, J.K.; Dunstan, G.A.; LeRoi, J.-M. Sterols of 14 Species of Marine Diatoms (Bacillariophyta). J. Phycol. 1995, 31, 360–369. [Google Scholar] [CrossRef]
- Bank, B.G.; Schauss, A. Antioxidant Testing: An ORAC Update. Nutraceuticals World 2004, 7, 68–71. [Google Scholar]
- Xiao, B.; Li, Y.; Lin, Y.; Lin, J.; Zhang, L.; Wu, D.; Zeng, J.; Li, J.; Liu, J.w.; Li, G. Eicosapentaenoic Acid (EPA) Exhibits Antioxidant Activity via Mitochondrial Modulation. Food Chem. 2022, 373, 131389. [Google Scholar] [CrossRef]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid Composition and Some Bioactivities of 3 Newly Isolated Microalgae (Tetraselmis Sp. IMP3, Tetraselmis Sp. CTP4, and Skeletonema Sp.). Aquac. Int. 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Karlson, B.; Caroline, C.; Bresnan, E. Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; United Nations Educational, Scientific and Cultural Organization (UNESCO): Paris, France, 2010. [Google Scholar]
- Torras-Claveria, L.; Berkov, S.; Jáuregui, O.; Caujapé, J.; Viladomat, F.; Codina, C.; Bastida, J. Metabolic Profiling of Bioactive Pancratium Canariense Extracts by GC-MS. Phytochem. Anal. 2010, 21, 80–88. [Google Scholar] [CrossRef]
- NIST. NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 11 October 2022).
- Oberacher, H. Wiley Registry of Tandem Mass Spectral Data, MS for ID; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Čagalj, M.; Skroza, D.; del Carmen Razola-Díaz, M.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira Compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase Inhibiting, Antioxidant, and Anti-Inflammatory Activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Generalić Mekinić, I.; Čagalj, M.; Hamed, I.; Skroza, D. Production and Characterization of Crude Oils from Seafood Processing By-Products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]


| No. | Retention Index | Similarty (%) | Proportion (%) | Identified Compound | Molar Weight | |||
|---|---|---|---|---|---|---|---|---|
| BRC192 | BRC312 | BRC192 | BRC312 | BRC192 | BRC312 | |||
| 1 | 1242 | n.d. | 96 | n.d. | 0.05 | n.d. | Benzoic acid, TMS derivative | 194 |
| 2 | 1253 | n.d. | 86 | n.d. | 0.02 | n.d. | Octanoic acid, TMS derivative | 216 |
| 3 | 1269 | 1265 | 94 | 92 | 0.13 | 0.21 | Glycerol, 3TMS derivative | 308 |
| 4 | 1348 | 1345 | 95 | 92 | 0.08 | 0.05 | Nonanoic acid, TMS derivative | 230 |
| 5 | 1451 | 1450 | 95 | 91 | 0.02 | 0.02 | Decanoic acid, TMS derivative | 244 |
| 6 | n.d. | 1499 | n.d. | 94 | n.d. | 0.13 | Malic acid, 3TMS derivative | 350 |
| 7 | 1534 | n.d. | 91 | n.d. | 0.01 | n.d. | 1-Dodecanethiol | 202 |
| 8 | 1653 | 1499 | 97 | 93 | 0.05 | 0.03 | Dodecanoic acid, TMS derivative | 272 |
| 9 | 1691 | n.d. | 85 | n.d. | 0.03 | n.d. | Tetradecanenitrile | 326 |
| 10 | 1753 | 1752 | 94 | 94 | 0.10 | 0.14 | Tridecanoic acid, TMS derivative | 286 |
| 11 | 1767 | n.d. | 91 | n.d. | 0.01 | n.d. | 1-Tetradecanol, TMS derivative | 286 |
| 12 | 1788 | 1787 | 93 | 93 | 0.06 | 0.17 | Loliolide, TMS | 268 |
| 13 | 1853 | 1852 | 95 | 96 | 2.55 | 6.50 | Myristic acid, TMS derivative | 300 |
| 14 | n.d. | 1877 | n.d. | 90 | n.d. | 0.28 | (Z)-3-Hexenyl β -glucopyranoside, 4TMS derivative | 550 |
| 15 | 1879 | n.d. | 94 | n.d. | 0.11 | n.d. | Palmitoleonitrile | 235 |
| 16 | 1930 | n.d. | 86 | n.d. | 0.05 | n.d. | (E)-13-Methyltetradec-9-enoic acid, TMS derivative | 312 |
| 17 | 1952 | 1951 | 94 | 85 | 0.17 | 0.49 | Pentadecanoic acid, TMS derivative | 314 |
| 18 | 2009 | 2008 | 89 | 88 | 0.97 | 0.63 | Eicosapentaenoic acid, TMS derivative | 374 |
| 19 | 2031 | 2030 | 96 | 97 | 7.37 | 12.15 | Palmitelaidic acid, TMS | 326 |
| 20 | 2053 | 2052 | 96 | 95 | 2.31 | 2.73 | Palmitic acid, TMS derivative | 328 |
| 21 | 2076 | n.d. | 89 | n.d. | 0.43 | n.d. | 9-Octadecynenitrile | 261 |
| 22 | 2083 | 2082 | 97 | 97 | 2.96 | 0.38 | (Z)-9-Octadecenenitrile | 263 |
| 23 | 2151 | 2094 | 92 | 83 | 0.04 | 0.03 | Heptadecanoic acid, TMS derivative | 342 |
| 24 | n.d. | 2157 | n.d. | 93 | n.d. | 0.04 | Palmitoleamide | 253 |
| 25 | n.d. | 2163 | n.d. | 87 | n.d. | 0.01 | 1-Octadecanol, TMS derivative | 342 |
| 26 | n.d. | 2178 | n.d. | 85 | n.d. | 0.03 | Octadecanamide | 319 |
| 27 | 2184 | 2184 | 92 | 97 | 1.48 | 2.09 | Phytol, TMS derivative | 368 |
| 28 | 2216 | 2215 | 88 | 92 | 0.47 | 0.44 | Linoleic acid, TMS derivate | 352 |
| 29 | 2230 | 2229 | 94 | 94 | 2.36 | 0.90 | (Z)-Oleic acid, TMS derivative | 354 |
| 30 | 2251 | 2250 | 97 | 95 | 0.88 | 0.65 | Stearic acid, TMS derivative | 356 |
| 31 | n.d. | 2256 | n.d. | 86 | n.d. | 0.05 | 11-Methyloctadec-12-enoic acid, TMS derivative | 368 |
| 32 | n.d. | 2331 | n.d. | 90 | n.d. | 0.16 | (all-Z)-5,8,11,14,17-Eicosapentaenoic acid, methyl ester | 330 |
| 33 | 2337 | n.d. | 92 | n.d. | 0.10 | n.d. | (Z)-10-Nonadecenoic acid, TMS | 368 |
| 34 | 2365 | 2362 | 95 | 96 | 2.30 | 2.50 | 9-Octadecenamide | 281 |
| 35 | n.d. | 2373 | n.d. | 90 | n.d. | 0.68 | (Z)-5,8,11-Eicosatrienoic acid, TMS derivative | 378 |
| 36 | 2381 | 2380 | 94 | 96 | 3.29 | 6.00 | Eicosapentaenoic acid, TMS derivative | 374 |
| 37 | 2430 | 2422 | 83 | 82 | 49.84 | 33.65 | Oleamide, TMS derivative | 353 |
| 38 | 2450 | 2448 | 95 | 95 | 1.24 | 0.70 | Steramide, TMS derivative | 355 |
| 39 | 2564 | 2564 | 97 | 89 | 0.54 | 0.19 | Doconexent, TMS derivative | 400 |
| 40 | 2578 | 2578 | 93 | 87 | 1.39 | 1.09 | 2-Palmitoylglycerol, 2TMS derivative | 474 |
| 41 | 2610 | 2610 | 96 | 96 | 2.91 | 2.89 | 1-Monopalmitin, 2TMS derivative | 474 |
| 42 | 2771 | 2770 | 90 | 90 | 1.46 | 1.59 | 2-Monostearin, 2TMS derivative | 502 |
| 43 | 2787 | 2787 | 93 | 90 | 0.34 | 0.10 | 1-Monooleoylglycerol, 2TMS derivative | 500 |
| 44 | 2805 | 2804 | 97 | 96 | 5.92 | 9.30 | Glycerol monostearate, 2TMS derivative | 502 |
| 45 | 2826 | 2825 | 88 | 81 | 0.57 | 0.10 | (Z)-Docos-13-enamide, TMS | 409 |
| 46 | n.d. | 2997 | n.d. | 89 | n.d. | 0.15 | Eicosanoic acid, 2,3-bis-(OTMS) propyl ester | 530 |
| 47 | 2998 | n.d. | 93 | n.d. | 0.09 | n.d. | 2,3-Dihydroxypropyl icosanoate, 2TMS derivative | 530 |
| 48 | 3066 | 3066 | 89 | 88 | 0.09 | 0.10 | 2-Arachidonoylglycerol, 2TMS derivative | 522 |
| 49 | 3163 | 3162 | 96 | 96 | 3.97 | 6.21 | Cholesterol, TMS derivative | 458 |
| 50 | n.d. | 3201 | n.d. | 90 | n.d. | 0.40 | Desmosterol, TMS derivative | 456 |
| 51 | 3261 | 3260 | 90 * | 88 * | 3.03 | 5.09 | 24-Methylene cholesterol * | 398 * |
| 52 | 3380 | 3380 | 88 | 81 | 0.15 | 0.20 | Isofucosterol, TMS | 484 |
| 53 | 3672 | 3672 | 90 | 92 | 0.06 | 0.80 | Oleanolic acid 2TMS | 600 |
| No. | Retention Index | Similarity (%) | Proportion(%) | Identified Compound | Molar Weight | |||
|---|---|---|---|---|---|---|---|---|
| EIS192 | EIS312 | EIS192 | EIS312 | EIS192 | EIS312 | |||
| 1 | 1335 | n.d. | 95 | n.d. | 0.10 | n.d. | Nonanoic acid, TMS derivative | 230 |
| 2 | 1444 | 1379 | 94 | 92 | 0.03 | 0.03 | Decanoic acid, TMS derivative | 244 |
| 3 | n.d. | 1448 | n.d. | 75 | n.d. | 0.01 | Butanedioic acid, TMS derivate | 350 |
| 4 | 1650 | 1630 | 93 | 95 | 0.04 | 0.05 | Dodecanoic acid, TMS derivative | 272 |
| 5 | 1691 | n.d. | 93 | n.d. | 0.02 | n.d. | Tetradecanenitrile | 209 |
| 6 | 1751 | 1739 | 88 | 93 | 0.03 | 0.13 | Tridecanoic acid, TMS derivative | 286 |
| 7 | 1787 | 1773 | 86 | 92 | 0.01 | 0.22 | Loliolide, TMS | 268 |
| 8 | 1805 | 1796 | 88 | 80 | 0.05 | 0.02 | Azelaic acid, 2TMS derivative | 332 |
| 9 | n.d. | 1831 | n.d. | 86 | n.d. | 0.06 | Myristoleic acid, trimethylsilyl ester | 298 |
| 10 | 1852 | 1845 | 95 | 96 | 1.59 | 5.12 | Myristic acid, TMS derivative | 300 |
| 11 | 1877 | n.d. | 94 | n.d. | 0.08 | n.d. | Palmitoleonitrile | 235 |
| 12 | 1898 | n.d. | 96 | n.d. | 0.19 | n.d. | Heptadecanenitrile | 251 |
| 13 | n.d. | 1956 | n.d. | 82 | n.d. | 0.03 | Hexadecanenitrile | 251 |
| 14 | 1951 | 1947 | 93 | 94 | 0.10 | 0.23 | Pentadecanoic acid, TMS derivative | 314 |
| 15 | n.d. | 1960 | n.d. | 93 | n.d. | 0.05 | 1-Hexadecanol, TMS derivative | 314 |
| 16 | 1968 | 1964 | 96 | 95 | 0.66 | 0.25 | Tetradecanamide | 227 |
| 17 | 2031 | 2026 | 88 | 96 | 1.63 | 7.89 | Palmitelaidic acid, TMS | 326 |
| 18 | 2052 | 2049 | 96 | 96 | 1.65 | 2.07 | Palmitic acid, TMS derivative | 328 |
| 19 | 2076 | n.d. | 89 | n.d. | 0.43 | n.d. | 9-Octadecynenitrile | 261 |
| 20 | 2082 | 2078 | 97 | 97 | 6.10 | 0.37 | (Z)-9-Octadecenenitrile | 263 |
| 21 | 2151 | 2149 | 91 | 80 | 0.04 | 0.02 | Heptadecanoic acid, trimethylsilyl ester | 342 |
| 22 | 2158 | 2155 | 97 | 96 | 0.97 | 0.36 | Palmitoleamide | 253 |
| 23 | n.d. | 2161 | n.d. | 86 | n.d. | 0.05 | 1-Octadecanol, TMS derivative | 342 |
| 24 | 2178 | 2176 | 98 | 97 | 1.93 | 0.52 | Hexadecanamide | 255 |
| 25 | 2184 | 2183 | 93 | 97 | 0.20 | 2.06 | Phytol, TMS derivative | 368 |
| 26 | n.d. | 2213 | n.d. | 88 | n.d. | 0.24 | Linoleic, TMS derivative | 352 |
| 27 | 2251 | 2250 | 95 | 95 | 0.75 | 0.53 | Stearic acid, TMS derivative | 356 |
| 28 | n.d. | 2364 | n.d. | 96 | n.d. | 13,97 | 9-Octadecenamide | 281 |
| 29 | n.d. | 2380 | n.d. | 96 | n.d. | 3.78 | Eicosapentaenoic acid, TMS derivative | 374 |
| 30 | 2427 | 2422 | 84 | 82 | 73.31 | 35,53 | Oleamide, TMS derivative | 353 |
| 31 | 2449 | 2419 | 95 | 95 | 1.37 | 0.75 | Octadecanamide, N-TMS derivate | 355 |
| 32 | 2571 | n.d. | 93 | n.d. | 0.81 | n.d. | 13-Docosenamide, (Z)- | 337 |
| 33 | 2578 | 2577 | 93 | 94 | 0.32 | 1.88 | 2-Palmitoylglycerol, 2TMS derivative | 474 |
| 34 | 2610 | 2610 | 94 | 96 | 0.47 | 6.92 | 1-Monopalmitin, 2TMS derivative | 474 |
| 35 | 2771 | 2771 | 89 | 91 | 1.28 | 2.20 | 2-Monostearin, 2TMS derivative | 502 |
| 36 | 2804 | 2804 | 95 | 96 | 3.52 | 10,64 | Glycerol monostearate, 2TMS derivative | 502 |
| 37 | 2998 | 2997 | 87 | 80 | 0.04 | 0.10 | 2,3-Dihydroxypropyl icosanoate, 2TMS derivative | 530 |
| 38 | 3162 | 3160 | 96 | 96 | 1.21 | 1.97 | Cholesterol, TMS derivative | 458 |
| 39 | 3260 | 3529 | 88 * | 89 * | 1.05 | 1.59 | 24-Methylene cholesterol * | 398 * |
| 40 | n.d. | 3379 | n.d. | 88 | n.d. | 0.13 | Isofucosterol, O-TMS | 484 |
| 41 | n.d. | 3672 | n.d. | 90 | n.d. | 0.22 | Oleanolic acid 2TMS | 600 |
| No. | Compounds | Molecular formula | Structure | Properties |
|---|---|---|---|---|
| 1. | Oleamide | C18H35NO |  | Anti-inflammatory, antialgal, antimicrobial and antifungal [30,31,34,35,36] |
| 2. | Palmitelaidic acid | C16H30O2 | 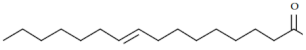 | Antibiofilm activity [37] |
| 3. | Glycerol monostearate | C21H42O4 | 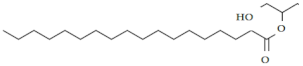 | Anthelmintic [41] |
| 4. | Myristic acid | C14H28O2 |  | Antifungal, antiviral, anticancer and antiparasitic [40] |
| 5. | Cholesterol | C27H46O | 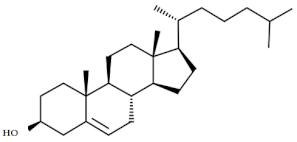 | Anticancer, anticardiac, anti-inflammatory, antimicrobial, anti-psychotic, antioxidative [42] |
| 6. | Eicosapentaenoic acid | C20H30O2 | 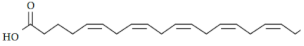 | Cardioprotective, neuroprotective, anti-inflammatory, anticancer, antimicrobial and antioxidative [43,44,45,46] |
| 7. | 1-monopalmitin | C19H38O4 |  | Antiviral [47] |
| 8. | 24-methylene cholesterol | C28H46O |  | Anticancer [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frleta, R.; Popović, M.; Smital, T.; Šimat, V. Comparison of Growth and Chemical Profile of Diatom Skeletonema grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases. Mar. Drugs 2022, 20, 697. https://doi.org/10.3390/md20110697
Frleta R, Popović M, Smital T, Šimat V. Comparison of Growth and Chemical Profile of Diatom Skeletonema grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases. Marine Drugs. 2022; 20(11):697. https://doi.org/10.3390/md20110697
Chicago/Turabian StyleFrleta, Roberta, Marijana Popović, Tvrtko Smital, and Vida Šimat. 2022. "Comparison of Growth and Chemical Profile of Diatom Skeletonema grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases" Marine Drugs 20, no. 11: 697. https://doi.org/10.3390/md20110697
APA StyleFrleta, R., Popović, M., Smital, T., & Šimat, V. (2022). Comparison of Growth and Chemical Profile of Diatom Skeletonema grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases. Marine Drugs, 20(11), 697. https://doi.org/10.3390/md20110697