Discovery of p-Terphenyl Metabolites as Potential Phosphodiesterase PDE4D Inhibitors from the Coral-Associated Fungus Aspergillus sp. ITBBc1
Abstract
1. Introduction
2. Results
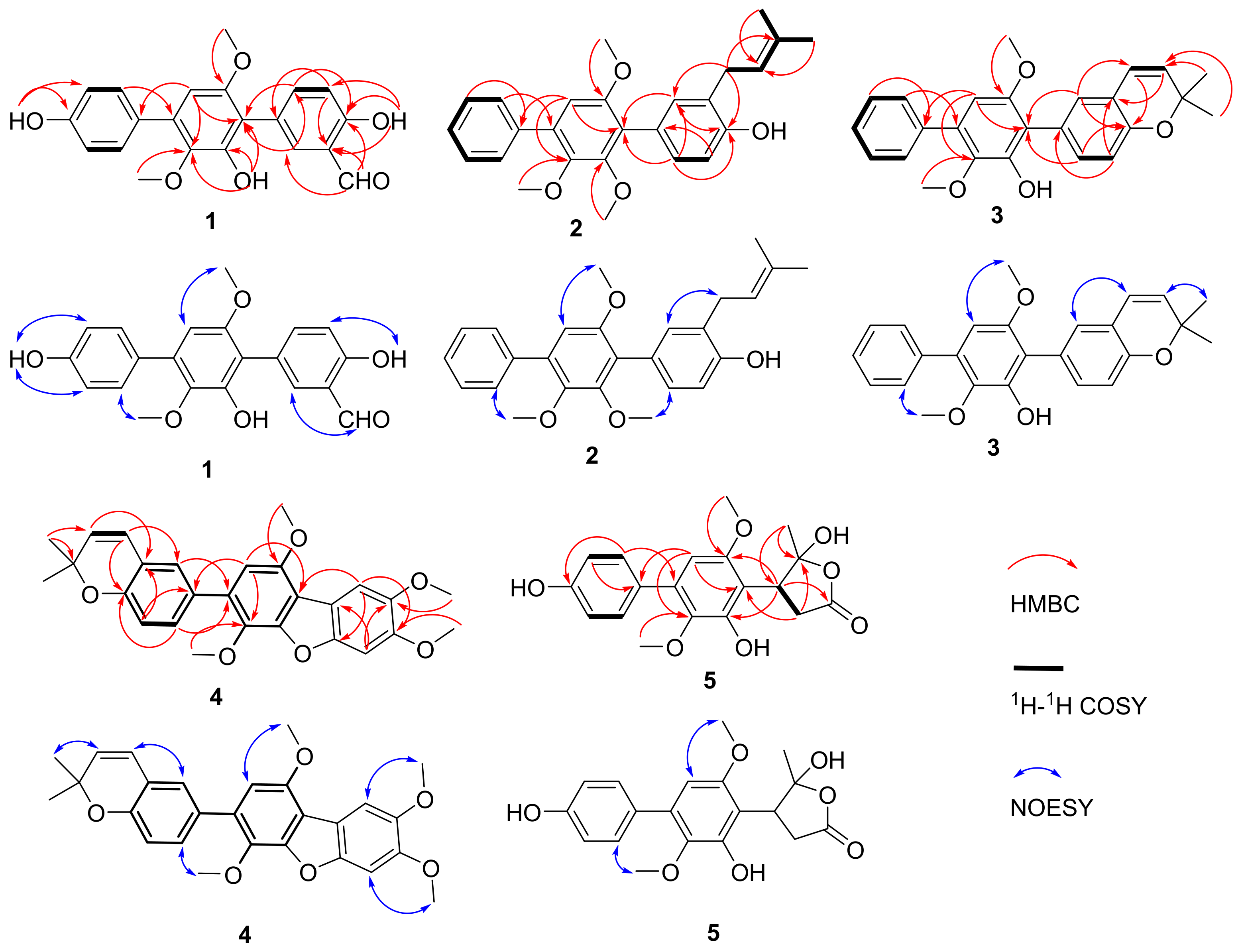
2.1. Structure Elucidation of New Compounds 1–5
2.2. In Vitro Evaluation of Type 4 Phosphodiesterase PDE4D Inhibitory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
- Sanshamycin E (5): white powder; 68 (c 0.10, MeOH); UV (MeOH) λmax (log ε): 231 (2.50), 266 (2.67) nm; IR(KBr) νmax: 3430, 2922, 1628, 1382, 1097, 638, 534 cm−1; ECD (MeOH) λmax (∆ε): 190 (−19.29), 199 (+21.27), 208 (+17.37), 234 (−0.33) nm; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS m/z 383.1080 [M + Na]+ (calculated for C19H20NaO7, 383.1101).
3.4. Type 4 Phosphodiesterase PDE4D Inhibitiory Screening Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Floresta, G.; Cilibrizzi, A.; Giovannoni, M.P. An overview of PDE4 inhibitors in clinical trials: 2010 to early 2022. Molecules 2022, 27, 4964. [Google Scholar] [CrossRef] [PubMed]
- Spina, D. PDE4 inhibitors: Current status. Brit. J. Pharmacol. 2008, 155, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-T.; Huang, Y.-Y.; Tang, G.-H.; Cheng, Z.-B.; Liu, X.; Luo, H.-B.; Yin, S. Prenylated coumarins: Natural phosphodiesterase-4 inhibitors from Toddalia asiatica. J. Nat. Prod. 2014, 77, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-B.; Deng, Y.-L.; Fan, C.-Q.; Han, Q.-H.; Lin, S.-L.; Tang, G.-H.; Luo, H.-B.; Yin, S. Prostaglandin derivatives: Nonaromatic phosphodiesterase-4 inhibitors from the soft coral Sarcophyton ehrenbergi. J. Nat. Prod. 2014, 77, 1928–1936. [Google Scholar] [CrossRef]
- Woo, S.; Kang, K.B.; Kim, J.; Sung, S.H. Molecular networking reveals the chemical diversity of selaginellin derivatives, natural phosphodiesterase-4 inhibitors from Selaginella tamariscina. J. Nat. Prod. 2019, 82, 1820–1830. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, X.; Zhang, X.; Shao, X.; Zhao, J.; Xu, Y.; Luo, X.; Zhao, W. Diterpenoids from the root bark of Pinus massoniana and evaluation of their phosphodiesterase type 4D inhibitory activity. J. Nat. Prod. 2020, 83, 1229–1237. [Google Scholar] [CrossRef]
- Marchelli, R.; Vining, L.C. Terphenyllin, a novel p-terphenyl metabolite from Aspergillus candidus. J. Antibiot. 1975, 28, 328–331. [Google Scholar] [CrossRef]
- Kurobane, I.; Vining, L.C.; Mcinnes, A.G.; Smith, D.G. 3-Hydroxyterphenyllin, a new metabolite of Aspergillus candidus. Structure elucidation by H and C nuclear magnetic resonance spectroscopy. J. Antibiot. 1979, 32, 559–564. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takemura, A.; Koshimizu, K.; Nagano, H.; Kawazu, K. Candidusin A and B: New p-terphenyls with cytotoxic effects on sea urchin embryos. Agric. Biol. Chem. 1982, 46, 585–589. [Google Scholar] [CrossRef]
- Guo, Z.K.; Yan, T.; Guo, Y.; Song, Y.C.; Tan, R.X.; Ge, H.M. p-Terphenyl and diterpenoid metabolites from endophytic Aspergillus sp. YXf3. J. Nat. Prod. 2012, 75, 15–21. [Google Scholar] [CrossRef]
- Rahbaek, L.; Frisvad, J.C.; Christophersen, C. An amendment of Aspergillus section Candidi based on chemotaxonomical evidence. Phytochemistry 2000, 53, 581–586. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, W.; Li, Y.-Y.; Deng, J.-J.; Zhu, D.-Y.; Duan, J.; Liu, Y.; Shi, G.-Y.; Xie, C.; Wang, H.-X.; et al. Identification and catalytic characterization of a nonribosomal peptide synthetase-like (NRPS-like) enzyme involved in the biosynthesis of echosides from Streptomyces sp. LZ35. Gene 2014, 546, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Biggins, J.B.; Liu, X.; Feng, Z.; Brady, S.F. Metabolites from the induced expression of cryptic single operons found in the genome of Burkholderia pseudomallei. J. Am. Chem. Soc. 2011, 133, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, F.; Enokita, R.; Naito, A.; Iijima, Y.; Yamazaki, M. Terferol, an inhibitor of cyclic adenosine 3′,5′-monophosphate phosphodiesterase. I. Isolation and characterization. J. Antibiot. 1984, 37, 6–9. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Figueroa, M.; Raja, H.A.; Graf, T.N.; Adcock, A.F.; Kroll, D.J.; Day, C.S.; Wani, M.C.; Pearce, C.J.; Oberlies, N.H. Benzoquinones and terphenyl compounds as phosphodiesterase-4B inhibitors from a fungus of the order Chaetothyriales (MSX47445). J. Nat. Prod. 2013, 76, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-K.; Zhu, W.-Y.; Zhao, L.-X.; Chen, Y.-C.; Li, S.-J.; Cheng, P.; Ge, H.-M.; Tan, R.-X.; Jiao, R.-H. New antibacterial depsidones from an ant-derived fungus Spiromastix sp. MY-1. Chin. J. Nat. Med. 2022, 20, 627–632. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Wu, W.C.; Chen, B.T.; Zhang, S.Q.; Wang, R.; Huang, J.; Guo, Z.K. Discovery of an unprecedented benz[α]anthraquinone-type heterodimer from a rare actinomycete Amycolatopsis sp. HCa1. Fitoterapia 2021, 155, 105039. [Google Scholar] [CrossRef]
- Tan, Y.-Z.; Guo, Z.-K.; Zhu, M.-Y.; Shi, J.; Li, W.; Jiao, R.-H.; Tan, R.-X.; Ge, H.-M. Anti-inflammatory spirobisnaphthalene natural products from a plant-derived endophytic fungus Edenia gomezpompae. Chin. Chem. Lett. 2020, 31, 1406–1409. [Google Scholar] [CrossRef]
- Han, H.; Guo, Z.-K.; Zhang, B.; Zhang, M.; Shi, J.; Li, W.; Jiao, R.-H.; Tan, R.-X.; Ge, H.-M. Bioactive phenazines from an earwig-associated Streptomyces sp. Chin. J. Nat. Med. 2019, 17, 475–480. [Google Scholar] [CrossRef]
- Ma, S.Y.; Xiao, Y.S.; Zhang, B.; Shao, F.L.; Guo, Z.K.; Zhang, J.J.; Jiao, R.H.; Sun, Y.; Xu, Q.; Tan, R.X.; et al. Amycolamycins A and B, two enediyne-derived compounds from a locust-associated actinomycete. Org. Lett. 2017, 19, 6208–6211. [Google Scholar] [CrossRef]
- Chen, B.; Wu, W.; Zhou, D.; Deng, X.; Zhang, S.; Yuan, J.; Xu, J.; Guo, Z. Bioactive components of two endophytic fungi from Hainan mangrove plants. J. Shenzhen Univ. Sci. Eng. 2022, 39, 245–252. [Google Scholar]
- Guo, Z.; Wang, R.; Wu, W.; Li, M.; He, W.; Zhou, S. Bioactive secondary metabolites from two sponge-derived actinomycetes. J. Shenzhen Univ. Sci. Eng. 2022, 39, 550–558. [Google Scholar]
- Guo, Z.; Ma, S.; Khan, S.; Zhu, H.; Zhang, B.; Zhang, S.; Jiao, R. Zhaoshumycins A and B, two unprecedented antimycin-type depsipeptides produced by a marine-derived Streptomyces sp. ITBB-ZKa6. Mar. Drugs 2021, 19, 624. [Google Scholar] [CrossRef]
- Guo, Z.-K.; Wang, R.; Chen, S.-Q.; Chen, F.-X.; Liu, T.-M.; Yang, M.-Q. Anthocidins A-D, new 5-hydroxyanthranilic acid related metabolites from the sea urchin-associated actinobacterium, Streptomyces sp. HDa1. Molecules 2018, 23, 1032. [Google Scholar] [CrossRef]
- Guo, Z.K.; Wang, R.; Chen, F.X.; Liu, T.M.; Yang, M.Q. Bioactive aromatic metabolites from the sea urchin-derived actinomycete Streptomyces spectabilis strain HDa1. Phytochem. Lett. 2018, 25, 132–135. [Google Scholar] [CrossRef]
- Guo, Z.-K.; Zhou, Y.-Q.; Han, H.; Wang, W.; Xiang, L.; Deng, X.-Z.; Ge, H.-M.; Jiao, R.-H. New antibacterial phenone derivatives asperphenone A-C from mangrove-derived fungus Aspergillus sp. YHZ-1. Mar. Drugs 2018, 16, 45. [Google Scholar] [CrossRef]
- Guo, Z.; Gai, C.; Cai, C.; Chen, L.; Liu, S.; Zeng, Y.; Yuan, J.; Mei, W.; Dai, H. Metabolites with insecticidal activity from Aspergillus fumigatus JRJ111048 isolated from mangrove plant Acrostichum specioum endemic to Hainan island. Mar. Drugs 2017, 15, 381. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Z.K.; Li, X.M.; Chen, F.X.; Zhan, X.F.; Shen, M.H. Spiculisporic acid analogues of the marine-derived fungus, Aspergillus candidus strain HDf2, and their antibacterial activity. Antonie Leeuwenhoek 2015, 108, 215–219. [Google Scholar] [CrossRef]
- Pan, T.; Xie, S.; Zhou, Y.; Hu, J.; Luo, H.; Li, X.; Huang, L. Dual functional cholinesterase and PDE4D inhibitors for the treatment of Alzheimer’s disease: Design, synthesis and evaluation of tacrine-pyrazolo[3,4-b]pyridine hybrids. Bioorg. Med. Chem. Lett. 2019, 29, 2150–2152. [Google Scholar] [CrossRef]
- Liu, X.; Luo, H.-B.; Huang, Y.-Y.; Bao, J.-M.; Tang, G.-H.; Chen, Y.-Y.; Wang, J.; Yin, S. Selaginpulvilins A-D, new phosphodiesterase-4 inhibitors with an unprecedented skeleton from Selaginella pulvinata. Org. Lett. 2014, 16, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-H.; Cai, Y.-H.; Fan, C.-Q.; Tang, G.-H.; Huo, H.-B.; Yin, S. Six new tetraprenylated alkaloids from the South China Sea gorgonian Echinogorgia pseudossapo. Mar. Drugs 2014, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]

| Position | 1 a | 2 b | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| 2 | 7.83, d (2.2) | 7.17, d (2.1) | 7.09, d (2.1) | 3.08, dd (6.4, 4.5) | |
| 3 | 7.17, s | 3.96, dd (6.4, 4.5) | |||
| 3-CHO | 10.06, s | ||||
| 4-OH | 11.02, s | ||||
| 4-OCH3 | 3.98, s | ||||
| 5 | 7.02, d (8.6) | 6.87, d (8.7) | 6.85, d (8.2) | 1.94, s | |
| 5-OCH3 | 4.02, s | ||||
| 6 | 7.68, dd (8.6, 2.2) | 7.17, overlapping | 7.22, dd (8.2, 2.1) | 7.56, s | |
| 2′-OH | 7.90, s | 5.92, (br s) | |||
| 2′-OCH3 | 3.66, s | ||||
| 3′-OCH3 | 3.40, s | 3.61, s | 3.44, s | 3.71, s | 3.66, s |
| 5′ | 6.55, s | 6.69, s | 6.48, s | 6.68, s | 6.43, s |
| 6′-OCH3 | 3.78, s | 3.74, s | 3.75, s | 4.03, s | 3.84, s |
| 2′′ | 7.55, d (8.6) | 7.59, d (7.5) | 7.64, m | 7.25, s | 7.41, d (8.5) |
| 3′′ | 6.96, d (8.6) | 7.45, t (7.5) | 7.46, t (7.5) | 6.90, d (8.5) | |
| 4′′ | 7.37, t (7.5) | 7.37, t (7.5) | |||
| 4′′-OH | 8.55, s | ||||
| 5′′ | 6.96, d (8.6) | 7.45, t (7.5) | 7.46, t (7.5) | 6.86, d (8.3) | 6.90, d (8.5) |
| 6′′ | 7.55, d (8.6) | 7.59, d (7.5) | 7.64, m | 7.38, d (8.3) | 7.41, d (8.5) |
| 1′′′ | 3.40, d (7.2) | 6.35, d (9.7) | 6.40, d (9.7) | ||
| 2′′′ | 5.40, dq (7.2, 1.4) | 5.60, d (9.7) | 5.66, d (9.7) | ||
| 4′′′ | 1.78, d (1.4) | 1.47, s | 1.49, s | ||
| 5′′′ | 1.79, br s | 1.47, s | 1.49, s |
| Position | 1 a | 2 b | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| 1 | 126.1, C | 126.1, C | 125.0, C | 115.3, C | 173.9, C |
| 2 | 136.2, CH | 132.1, CH | 128.7, CH | 150.6, C | 34.0, CH2 |
| 3 | 120.6, C | 125.9, C | 120.8, C | 95.4, CH | 45.7, CH |
| 3-CHO | 197.3, CH | ||||
| 4 | 160.1, C | 153.5, C | 152.3, C | 149.1, C | 118.3, C |
| 4-OCH3 | 56.3, CH3 | ||||
| 5 | 116.1, CH | 115.2, CH | 116.0, CH | 146.2, C | 23.9, CH3 |
| 5-OCH3 | 56.6, CH3 | ||||
| 6 | 140.0, CH | 130.0, CH | 131.5, CH | 104.3, CH | |
| 1′ | 114.8, C | 124.7, C | 116.7, C | 114.8, C | 114.6, C |
| 2′ | 148.3, C | 152.0, C | 147.2, C | 149.3, C | 149.9, C |
| 2′-OCH3 | 60.8, CH3 | ||||
| 3′ | 139.3, C | 144.8, C | 138.9, C | 136.7, C | 136.0, C |
| 3′-OCH3 | 59.8, CH3 | 60.9, CH3 | 60.9, CH3 | 61.1, CH3 | 60.7, CH3 |
| 4′ | 133.4, C | 134.7, C | 132.7, C | 131.1, C | 136.4, C |
| 5′ | 103.3, CH | 108.1, CH | 104.0, CH | 105.6, CH | 106.1, CH |
| 6′ | 153.5, C | 153.2, C | 153.5, C | 150.0, C | 151.5, C |
| 6′-OCH3 | 55.3, CH3 | 56.1, CH3 | 56.0, CH3 | 55.9, CH3 | 56.0, CH3 |
| 1′′ | 129.4, C | 138.5, C | 138.1, C | 131.0, C | 130.5, C |
| 2′′ | 129.9, CH | 129.2, CH | 128.8, CH | 127.4, CH | 130.5, CH |
| 3′′ | 115.2, CH | 128.2, CH | 128.5, CH | 121.0, C | 115.1, CH |
| 4′′ | 157.1, C | 127.3, CH | 127.5, CH | 152.3, C | 155.1, C |
| 5′′ | 115.2, CH | 128.2, CH | 128.5, CH | 116.1, CH | 115.1, CH |
| 6′′ | 129.9, CH | 129.2, CH | 128.8, CH | 130.2, CH | 130.5, CH |
| 1′′′ | 29.9, CH2 | 122.4, CH | 122.4, CH | ||
| 2′′′ | 122.0, CH | 130.3, CH | 130.8, CH | ||
| 3′′′ | 134.7, C | 76.4, C | 76.5, C | ||
| 4′′′ | 25.8, CH3 | 28.4, CH3 | 28.2, CH3 | ||
| 5′′′ | 17.9, CH3 | 28.4, CH3 | 28.2, CH3 |
| Compounds | PDE4D Inhibitory Percentage (100%) |
|---|---|
| Rolipram | 52.0 |
| 1 | 4.8 |
| 2 | 6.7 |
| 3 | 49.4 |
| 4 | 12.4 |
| 5 | 5.1 |
| 6 | 12.8 |
| 9 | 23.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Abulaizi, A.; Huang, L.; Xiong, Z.; Zhang, S.; Liu, T.; Wang, R. Discovery of p-Terphenyl Metabolites as Potential Phosphodiesterase PDE4D Inhibitors from the Coral-Associated Fungus Aspergillus sp. ITBBc1. Mar. Drugs 2022, 20, 679. https://doi.org/10.3390/md20110679
Guo Z, Abulaizi A, Huang L, Xiong Z, Zhang S, Liu T, Wang R. Discovery of p-Terphenyl Metabolites as Potential Phosphodiesterase PDE4D Inhibitors from the Coral-Associated Fungus Aspergillus sp. ITBBc1. Marine Drugs. 2022; 20(11):679. https://doi.org/10.3390/md20110679
Chicago/Turabian StyleGuo, Zhikai, Ailiman Abulaizi, Ling Huang, Zijun Xiong, Shiqing Zhang, Tianmi Liu, and Rong Wang. 2022. "Discovery of p-Terphenyl Metabolites as Potential Phosphodiesterase PDE4D Inhibitors from the Coral-Associated Fungus Aspergillus sp. ITBBc1" Marine Drugs 20, no. 11: 679. https://doi.org/10.3390/md20110679
APA StyleGuo, Z., Abulaizi, A., Huang, L., Xiong, Z., Zhang, S., Liu, T., & Wang, R. (2022). Discovery of p-Terphenyl Metabolites as Potential Phosphodiesterase PDE4D Inhibitors from the Coral-Associated Fungus Aspergillus sp. ITBBc1. Marine Drugs, 20(11), 679. https://doi.org/10.3390/md20110679





