Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value
Abstract
1. Introduction
2. Results and Discussion
2.1. Biomasses Characterization
2.1.1. Macroscopic Characteristics
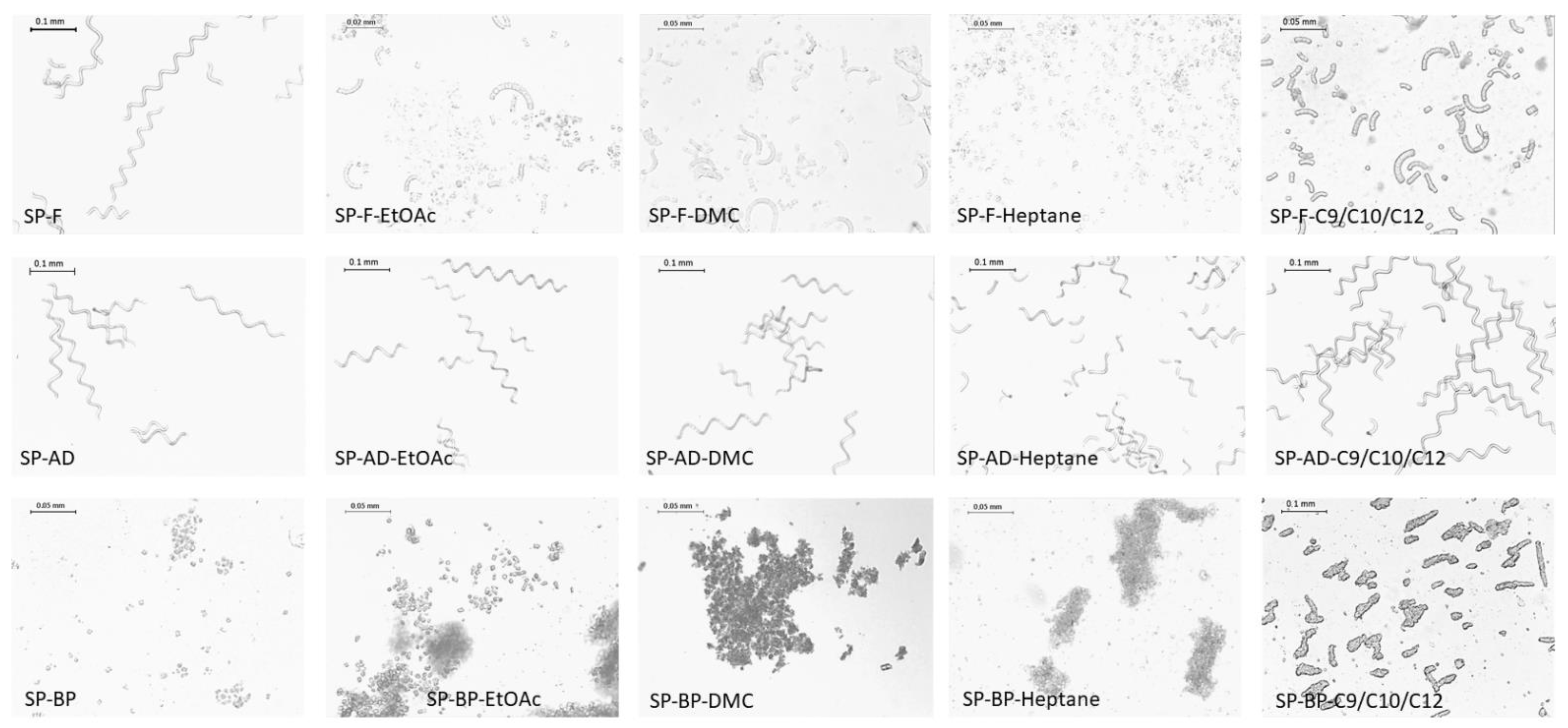
2.1.2. Cell Integrity
2.1.3. Dye Content and Profile
2.1.4. Lipid Content
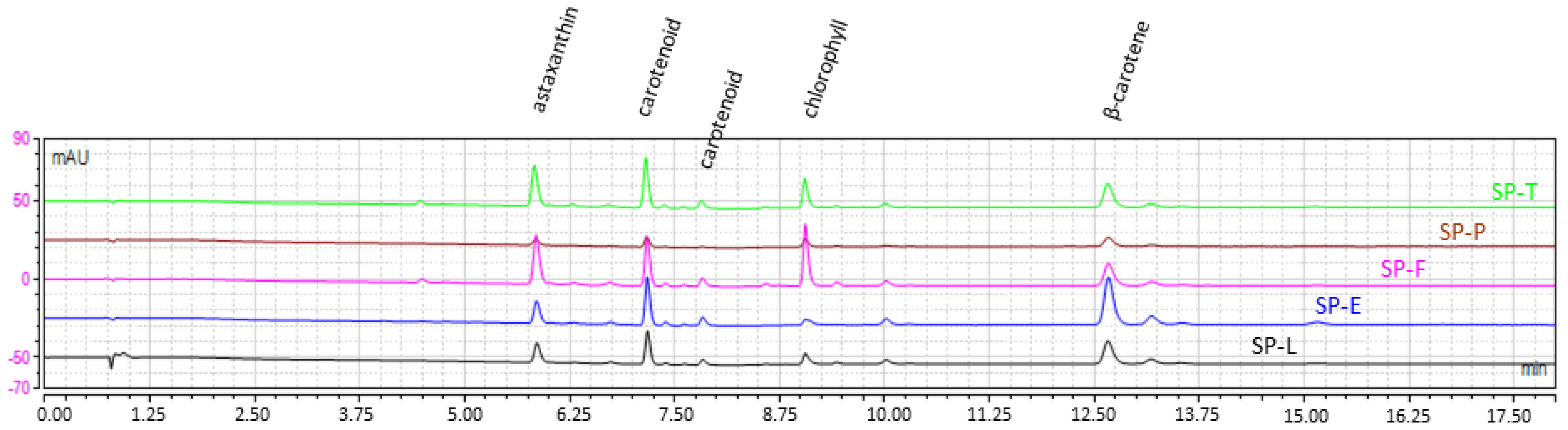
2.1.5. FFA Profile
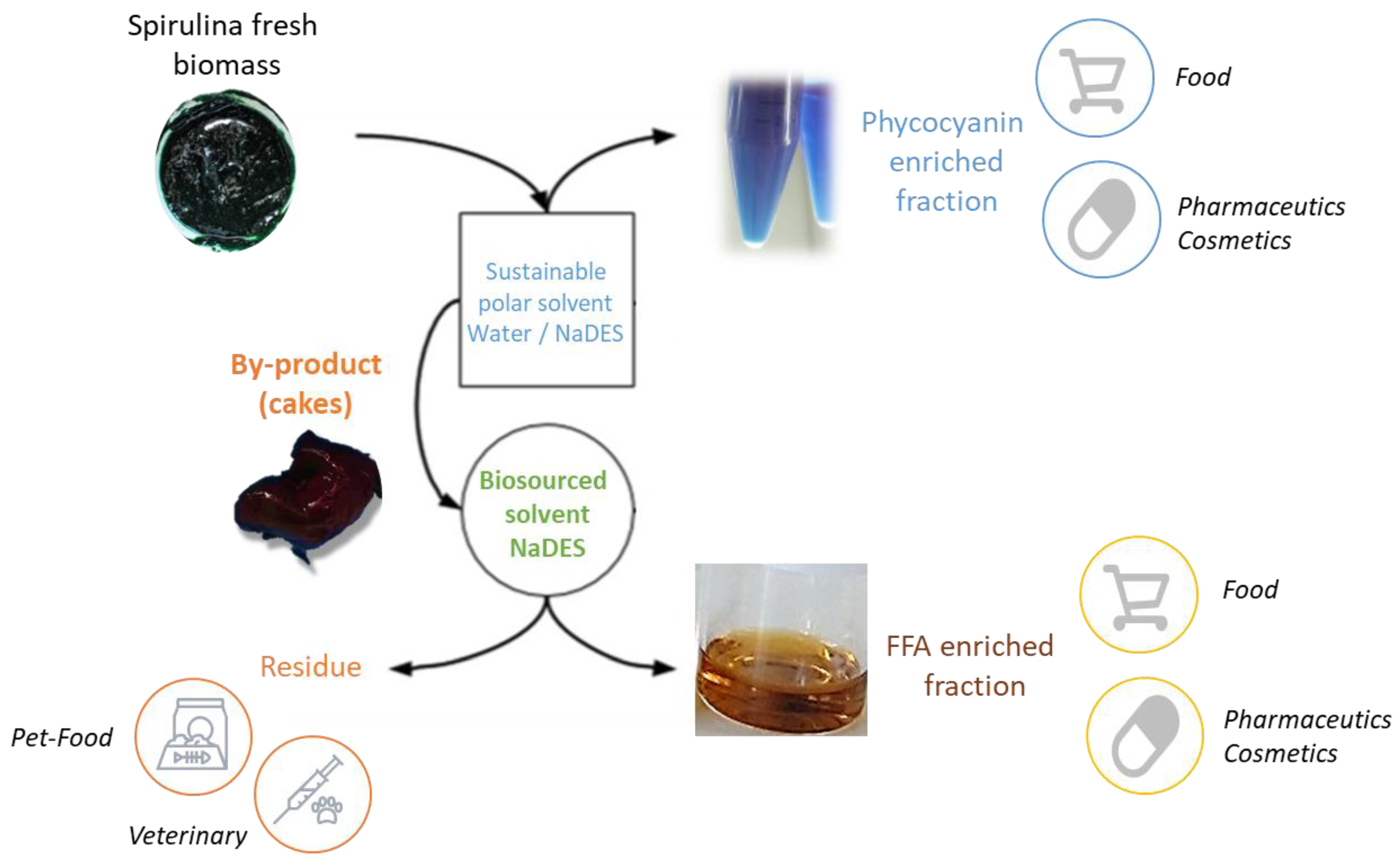
2.2. Extraction
2.2.1. Cell Integrity
2.2.2. Pigment and Lipid Content
2.2.3. FFA Profile
3. Materials and Methods
3.1. Raw Material
3.2. Chemicals
3.3. NaDES Preparation
3.4. Extraction Process
3.5. Analytical Protocols
3.5.1. Microscopy
3.5.2. Dye Content
3.5.3. Dye Profile
3.5.4. Lipid Content
3.5.5. FFA Profile
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Irineudo Magalhães, A.; Vinicius de Melo Pereira, G.; Bianchi Pedroni Medeiros, A.; Bittencourt Sydney, E.; Rodrigues, C.; Molina Aulestia, D.T.; Porto de Souza Vandenberghe, L.; Thomaz Soccol, V.; Soccol, C.R. Microalgal biomass pretreatment for integrated processing into biofuels, food, and feed. Bioresour. Technol. 2020, 300, 122719. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Siva, C.J. The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J. Appl. Phycol. 2007, 19, 567–590. [Google Scholar] [CrossRef]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Van Eykelenburg, C. On the morphology and ultrastructure of the cell wall of Spirulina platensis. Antonie Van Leeuwenhoek 1977, 43, 89–99. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- Global Market Study on Spirulina: Powder Product form Segment Anticipated to Dominate the Global Market in Terms of both Value and Volume during 2016–2026. Available online: https://www.persistencemarketresearch.com/market-research/spirulina-market.asp (accessed on 11 September 2022).
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Ruffell, S.E.; Müller, K.M.; McConkey, B.J. Comparative Assessment of Microalgal Fatty Acids as Topical Antibiotics. J. Appl. Phycol. 2016, 28, 1695–1704. [Google Scholar] [CrossRef]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Menezes, C.R.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Bioactive pigments from microalgae Phormidium autumnale. Food Res. Int. 2015, 77, 273–279. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-Based Solvents for Green Extraction of Lipids from Oleaginous Yeast Biomass for Sustainable Aviation Biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiang, W.; Sun, X.; Wu, H.; Li, T.; Long, L. A Novel Lipid Extraction Method from Wet Microalga Picochlorum sp. at Room Temperature. Mar. Drugs 2014, 12, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.R.P.; da Costa, E.; Silva, J. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Boutin, R.; Munnier, E.; Renaudeau, N.; Girardot, M.; Pinault, M.; Chevalier, S.; Chourpa, I.; Clément-Larosière, B.; Imbert, C.; Boudesocque-Delaye, L. Spirulina platensis Sustainable Lipid Extracts in Alginate-Based Nanocarriers: An Algal Approach against Biofilms. Algal Res. 2019, 37, 160–168. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosière, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Van Annaland, M.S.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid–Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal Degradation Kinetics of Chlorophyll Pigments in Virgin Olive Oils. 1. Compounds of Series a. J. Agric. Food Chem. 2010, 58, 6200–6208. [Google Scholar] [CrossRef] [PubMed]
- Mühling, M.; Belay, A.; Whitton, B.A. Variation in fatty acid composition of Arthrospira (Spirulina) strains. J. Appl. Phycol. 2005, 17, 137–146. [Google Scholar] [CrossRef]
- Esquivel, B.C.; Lobina, D.V.; Sandoval, F.C. The biochemical composition of two diatoms after different preservation techniques. Biochem. Physiol. Comp. 1993, 105B, 369–373. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Duarte, J.H.; Moraes, K.; Crexi, V.T.; Pinto, L.A. A Optimisation of Spirulina platensis convective drying: Evaluation of phycocyanin loss and lipid oxidation. Int. J. Food Sci. Technol. 2010, 45, 1572–1578. [Google Scholar] [CrossRef]
- Pan-utai, W.; Kahapana, W.; Iamtham, S. Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid. J. Appl. Phycol. 2017, 30, 231–242. [Google Scholar] [CrossRef]
- Ehimen, E.A.; Sun, Z.F.; Carrington, C.G. Variables affecting the in situ transesterification of microalgae lipids. Fuel 2010, 89, 677–684. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Jacob-Lopes, E.; Goldbeck, R.; Queiroz, M.I. Production and biochemical profile of the microalgae Aphanothece microscopica Nägeli submitted to different drying conditions. Chem. Engin. Process. 2008, 47, 1305–1310. [Google Scholar] [CrossRef]
- Widjaja, A.; Chao-Chang, C.; Yi-Hsu, J. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Hilali, S.; Wils, L.; Chevalley, A.; Clément-Larosière, B.; Boudesocque-Delaye, L. Glycerol-based NaDES as green solvents for ultrasound-assisted extraction of phycocyanin from Arthrospira platensis—RSM optimization and ANN modeling. Biomass Conv. Bioref. 2022, 12, 157–170. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green algae. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.; Silva, A.; Rocha, J. Extraction and quantification of pigments from a marine microalga: A simple and reproducible method. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2007; Volume 1, pp. 586–593. [Google Scholar]
- Jaime, L.; Mendiola, J.A.; Herrero, M.; Soler-Rivas, C.; Santoyo, S.; Señorans, F.J.; Cifuentes, A.; Ibáñez, E. Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J. Sep. Sci. 2005, 28, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, H.J.; Yoon, E.Y.; Moon, S.J.; Park, J.; Jeong, H.J.; Yoon, E.Y.; Moon, S.J. Easy and rapid quantification of lipid contents of marine dinoflagellates using the sulpho-phospho-vanillin method. Algae 2016, 31, 391–401. [Google Scholar] [CrossRef]




| Microalgae | Pretreatment (Code) | DM (%) | Phycocyanin (mg/g DM) n = 3 | Chlorophylls (mg/g DM) n = 3 | Carotenoids (mg/g DM) n = 3 | Total Lipid (mg eq Castor Oil/g DM) n = 3 |
|---|---|---|---|---|---|---|
| A. platensis | By-product SP-BP | 17.2 | 84.8 ± 0.4 | 4.4 ± 0.1 | 1.6 ± 0.1 | 37.3 ± 2.7 |
| Frozen SP-F | 25.8 | 120.9 ± 0.9 | 13.3 ± 0.5 | 4.8 ± 0.2 | 22.4 ± 2.0 | |
| Oven-dried SP-OD | 77.6 | 94.3 ± 1.3 | 2.7 ± 0.1 | 1.5 ± 0.0 | 26.5 ± 3.2 | |
| Air-dried SP-AD | 93.1 | 82.2 ± 2.9 | 4.3 ± 0.1 | 1.3 ± 0.0 | 13.4 ± 0.5 | |
| Freeze-dried SP-FD | 99.3 | 98.9 ± 0.7 | 5.9 ± 0.6 | 1.7 ± 0.2 | 20.7 ± 1.7 |
| Pretreatment | Solvent | Chlorophylls (mg/g DM) n = 3 | Carotenoids (mg/g DM) n = 3 | Total Lipid (mg eq Castor Oil/g DM) n = 3 |
|---|---|---|---|---|
| SP-BP | EtOAc | 19.0 ± 0.52 | 6.21 ± 0.16 | 308.4 ± 38.6 |
| DMC | 8.86 ± 0.52 | 4.76 ± 0.24 | 414.5 ± 35.2 | |
| Heptane | 0.02 ± 0.00 | 0.00 ± 0.00 | 131.2 ± 25.6 | |
| C9/C10/C12 | 5.75 ± 0.09 | 0.15 ± 0.06 | / | |
| SP-F | EtOAc | 1.25 ± 0.00 | 1.25 ± 0.00 | 256.8 ± 23.3 |
| DMC | 0.19 ± 0.00 | 0.14 ± 0.00 | 324.6 ± 33.2 | |
| Heptane | 0.02 ± 0.00 | 0.00 ± 0.00 | 188.8 ± 9.8 | |
| C9/C10/C12 | 0.27 ± 0.09 | 0.03 ± 0.02 | / | |
| SP-OD | EtOAc | 0.45 | 0.40 | 323.3 ± 22.2 |
| DMC | 0.24 ± 0.00 | 0.17 ± 0.02 | 417.4 ± 31.37 | |
| Heptane | 0.03 ± 0.00 | 0.07 ± 0.00 | 242.9 ± 34.4 | |
| C9/C10/C12 | 2.01 ± 0.12 | 1.05 ± 0.08 | / | |
| SP-AD | EtOAc | 0.50 ± 0.14 | 0.26 ± 0.01 | 255.0 ± 41.2 |
| DMC | 0.02 ± 0.00 | 0.01 ± 0.00 | 225.0 ± 12.0 | |
| Heptane | 0.01 ± 0.00 | 0.02 ± 0.00 | 217.6 ± 35.5 | |
| C9/C10/C12 | 0.16 ± 0.03 | 0.01 ± 0.00 | / | |
| SP-FD | EtOAc | 1.42 | 0.06 | 303.5 ± 9.8 |
| DMC | 0.58 | 0.31 | 173.9 ± 12.7 | |
| Heptane | 0.04 ± 0.00 | 0.2 ± 0.00 | 264.9 ± 21.3 | |
| C9/C10/C12 | 1.02 ± 0.15 | 0.73 ± 0.09 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wils, L.; Yagmur, M.; Phelippe, M.; Montigny, B.; Clément-Larosière, B.; Jacquemin, J.; Boudesocque-Delaye, L. Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value. Mar. Drugs 2022, 20, 600. https://doi.org/10.3390/md20100600
Wils L, Yagmur M, Phelippe M, Montigny B, Clément-Larosière B, Jacquemin J, Boudesocque-Delaye L. Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value. Marine Drugs. 2022; 20(10):600. https://doi.org/10.3390/md20100600
Chicago/Turabian StyleWils, Laura, Mervé Yagmur, Myriam Phelippe, Bénédicte Montigny, Barbara Clément-Larosière, Johan Jacquemin, and Leslie Boudesocque-Delaye. 2022. "Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value" Marine Drugs 20, no. 10: 600. https://doi.org/10.3390/md20100600
APA StyleWils, L., Yagmur, M., Phelippe, M., Montigny, B., Clément-Larosière, B., Jacquemin, J., & Boudesocque-Delaye, L. (2022). Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value. Marine Drugs, 20(10), 600. https://doi.org/10.3390/md20100600







