Comparative Study of Docosahexaenoic Acid with Different Molecular Forms for Promoting Apoptosis of the 95D Non-Small-Cell Lung Cancer Cells in a PPARγ-Dependent Manner
Abstract
1. Introduction
2. Results
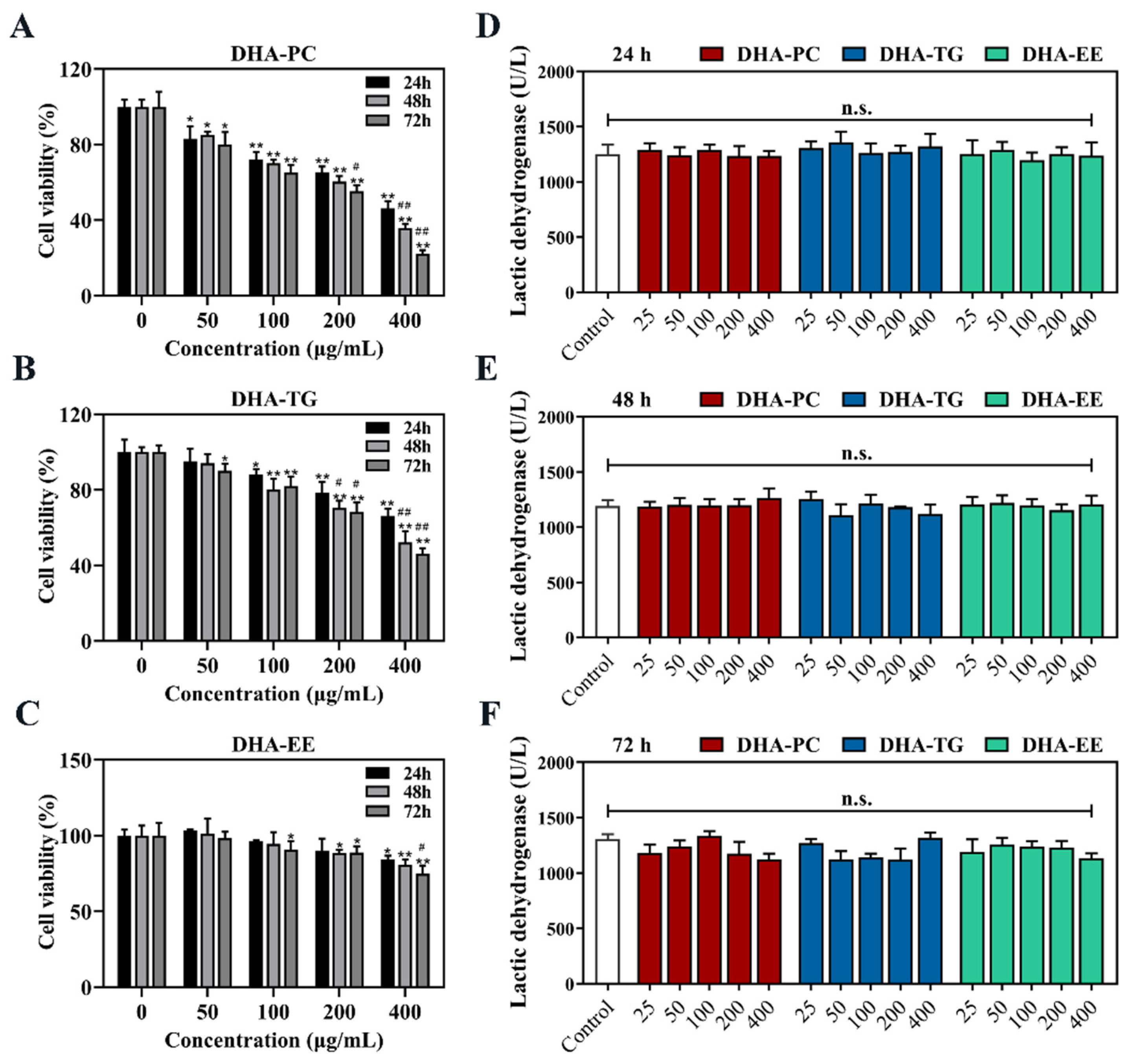
2.1. Effect of DHA with Different Molecular Forms on the Cell Viability of 95D Cells
2.2. Effect of DHA with Different Molecular Forms on the Morphology of 95D Cells
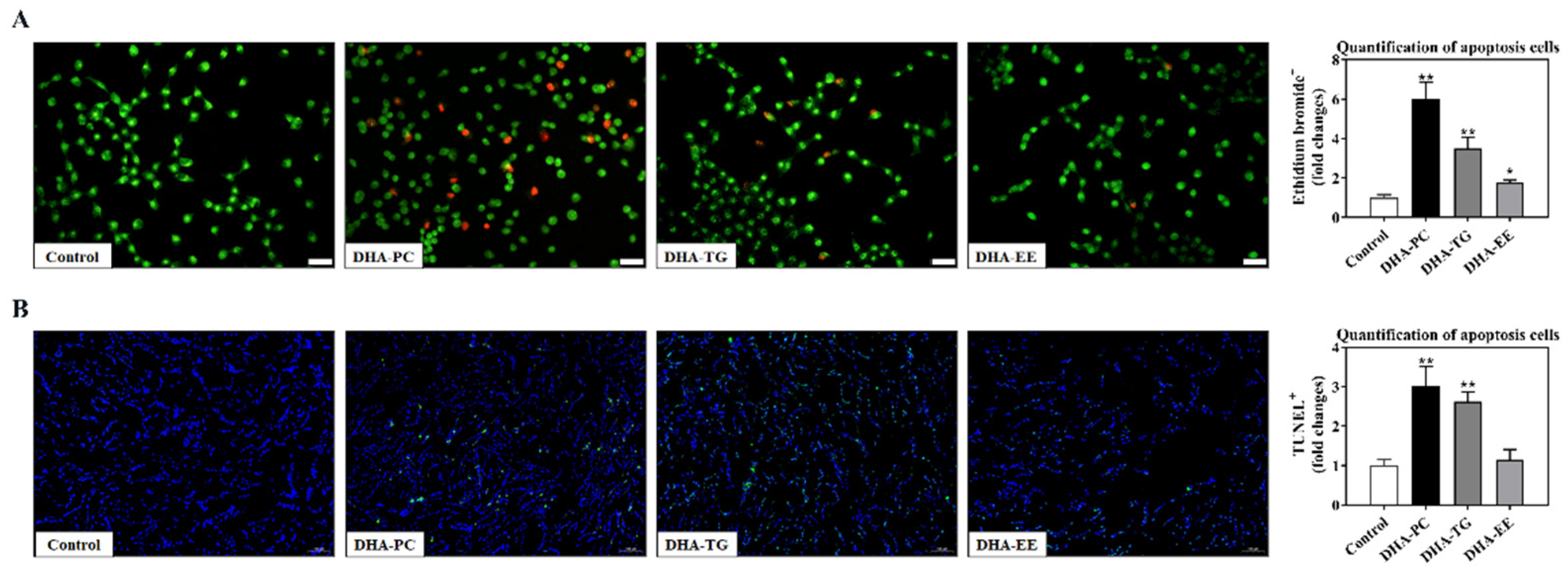
2.3. Effect of DHA with Different Molecular Forms on Apoptosis in 95D Cells
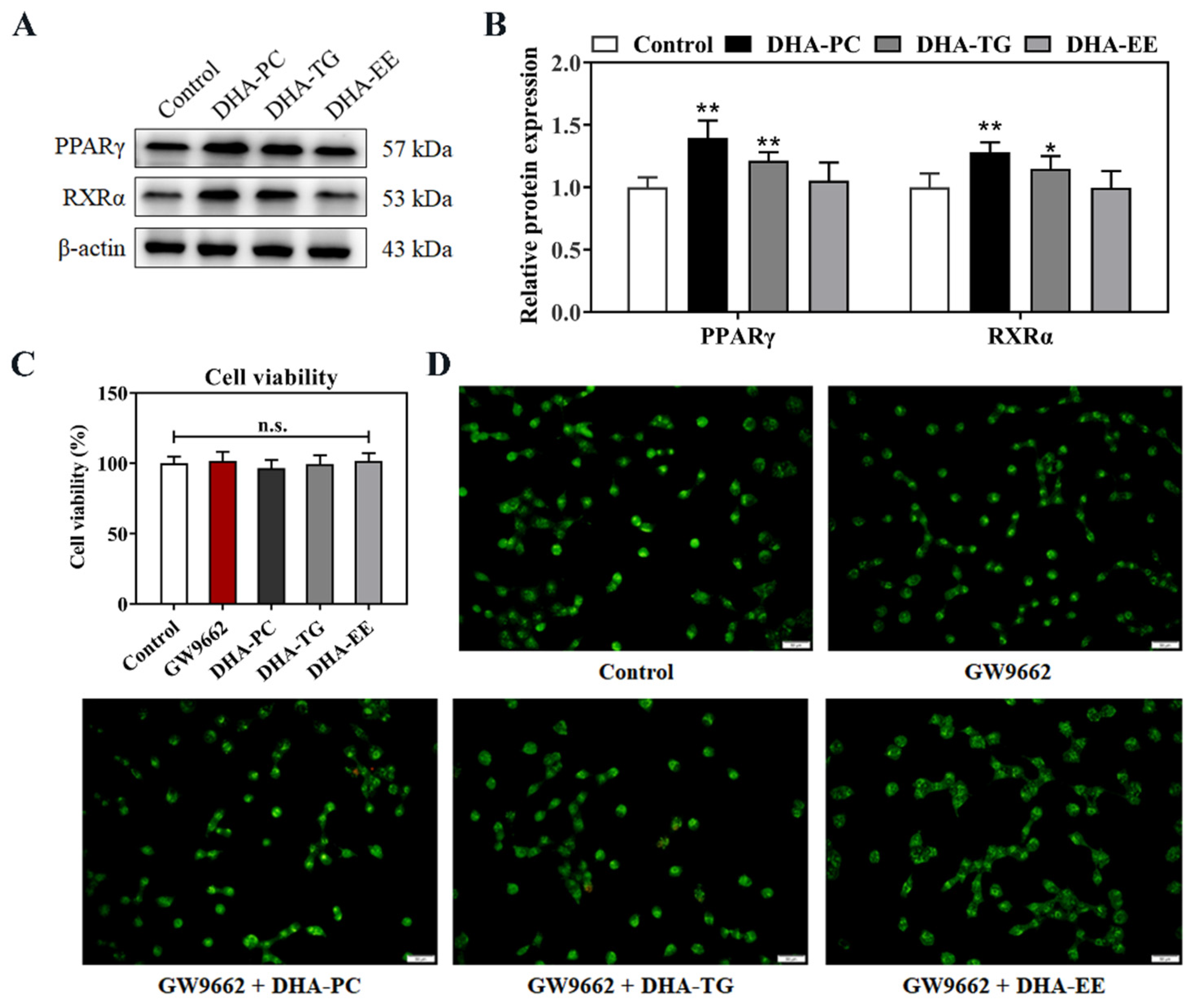
2.4. Effect of DHA with Different Molecular Forms on the PPARγ Expression in 95D Cells
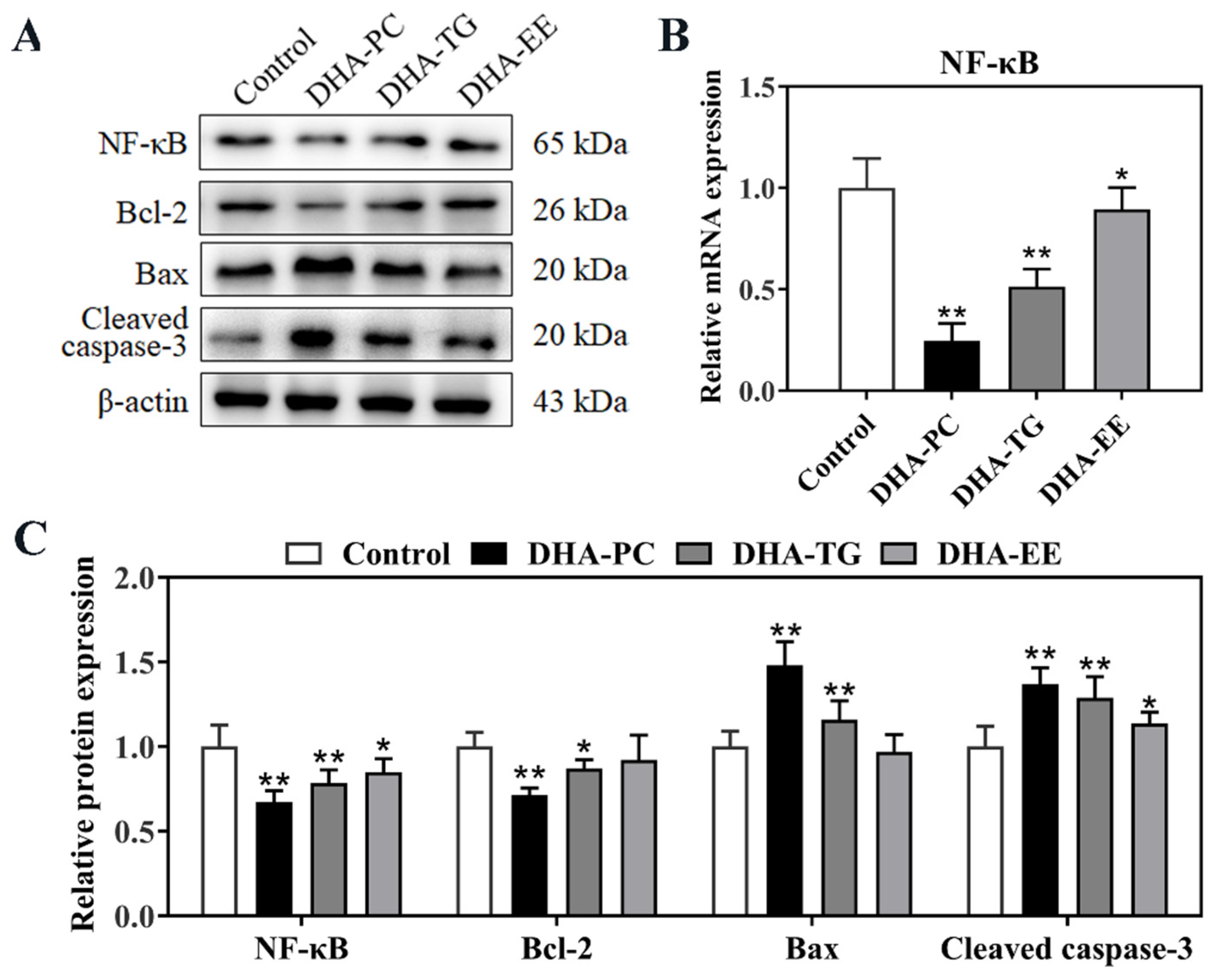
2.5. Effect of DHA with Different Molecular Forms on the PPARγ/NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Preparation of DHA
4.2. Liposome Preparation
4.3. Cell Culture and Viability Assay
4.4. Hematoxylin and Acridine Orange/Ethidium Bromide (AO/EB) Staining
4.5. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Staining
4.6. Transmission Electron Microscopy (TEM)
4.7. Quantitative Real-Time PCR Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lu, G.; Lee, M.J.; Hu, J.; Ju, J.; Yang, C.S. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol. Nutr. Food Res. 2009, 53, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. (Lausanne) 2021, 12, 624112. [Google Scholar] [CrossRef]
- Kim, T.W.; Hong, D.W.; Park, J.W.; Hong, S.H. CB11, a novel purine-based PPARɣ ligand, overcomes radio-resistance by regulating ATM signalling and EMT in human non-small-cell lung cancer cells. Br. J. Cancer 2020, 123, 1737–1748. [Google Scholar] [CrossRef]
- Skelhorne-Gross, G.; Nicol, C.J. The Key to Unlocking the Chemotherapeutic Potential of PPARγ Ligands: Having the Right Combination. PPAR Res. 2012, 2012, 946943. [Google Scholar] [CrossRef][Green Version]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-κB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Zand, H.; Rhimipour, A.; Bakhshayesh, M.; Shafiee, M.; Nour Mohammadi, I.; Salimi, S. Involvement of PPAR-gamma and p53 in DHA-induced apoptosis in Reh cells. Mol. Cell Biochem. 2007, 304, 71–77. [Google Scholar] [CrossRef]
- Barkett, M.; Gilmore, T.D. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-kappaB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Li, Y.; Ren, X.; Zhou, Y.; Hu, W.; Zhou, C.; Jing, Q.; Yang, C.; Wang, L.; et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021, 12, 705. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, Y.; Wang, Q.; Fu, M.; Xue, C.; Wang, J. Comparative Study of DHA with Different Molecular Forms for Ameliorating Osteoporosis by Promoting Chondrocyte-to-Osteoblast Transdifferentiation in the Growth Plate of Ovariectomized Mice. J. Agric. Food Chem. 2021, 69, 10562–10571. [Google Scholar] [CrossRef]
- Ichihara, H.; Zako, K.; Komizu, Y.; Goto, K.; Ueoka, R. Therapeutic effects of hybrid liposomes composed of phosphatidylcholine and docosahexaenoic acid on the hepatic metastasis of colon carcinoma along with apoptosis in vivo. Biol. Pharm. Bull. 2011, 34, 901–905. [Google Scholar] [CrossRef]
- Hossain, Z.; Kurihara, H.; Hosokawa, M.; Takahashi, K. Docosahexaenoic acid and eicosapentaenoic acid-enriched phosphatidylcholine liposomes enhance the permeability, transportation and uptake of phospholipids in Caco-2 cells. Mol. Cell Biochem. 2006, 285, 155–163. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Yu, X.; Yang, R.; Han, Z.C. Peroxisome proliferator-activated receptor gamma in malignant diseases. Crit. Rev. Oncol. Hematol. 2006, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhao, B.; Ni, C.; Ni, H.; Xu, L.; He, Q.; Xu, M.; Xu, C.; Luo, G.; Zhu, J.; et al. Rosiglitazone Alleviates Mechanical Allodynia of Rats with Bone Cancer Pain through the Activation of PPAR-γ to Inhibit the NF-κB/NLRP3 Inflammatory Axis in Spinal Cord Neurons. PPAR Res. 2021, 2021, 6086265. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef]
- Zick, S.M.; Snyder, D.; Abrams, D.I. Pros and Cons of Dietary Strategies Popular Among Cancer Patients. Oncology (Williston Park) 2018, 32, 542–547. [Google Scholar]
- Vega, O.M.; Abkenari, S.; Tong, Z.; Tedman, A.; Huerta-Yepez, S. Omega-3 Polyunsaturated Fatty Acids and Lung Cancer: Nutrition or Pharmacology? Nutr. Cancer 2021, 73, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Rastinejad, F.; Huang, P.; Chandra, V.; Khorasanizadeh, S. Understanding nuclear receptor form and function using structural biology. J. Mol. Endocrinol. 2013, 51, T1–T21. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Wang, M.; Wang, X.; Yang, K.; Xie, F.; Liao, Z.; Wei, P. PPAR-γ Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 737776. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Guo, Y.; Yan, Z.; Xue, C.; Wang, J. DHA-enriched phosphatidylcholine suppressed angiogenesis by activating PPARγ and modulating the VEGFR2/Ras/ERK pathway in human umbilical vein endothelial cells. Food Sci. Biotechnol. 2021, 30, 1543–1553. [Google Scholar] [CrossRef]
- Ghnaimawi, S.; Rebello, L.; Baum, J.; Huang, Y. DHA but not EPA induces the trans-differentiation of C2C12 cells into white-like adipocytes phenotype. PLoS ONE 2021, 16, e0249438. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Cai, W.; Guo, Y.; Xue, C.; Wang, J. DHA/EPA-Enriched Phosphatidylcholine Suppresses Tumor Growth and Metastasis via Activating Peroxisome Proliferator-Activated Receptor γ in Lewis Lung Cancer Mice. J. Agric. Food Chem. 2021, 69, 676–685. [Google Scholar] [CrossRef]
- Rasmi, R.R.; Sakthivel, K.M.; Guruvayoorappan, C. NF-κB inhibitors in treatment and prevention of lung cancer. Biomed. Pharmacother. 2020, 130, 110569. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Glass, C.K. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta 2007, 1771, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gomez, J.A.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Miranda-Roblero, H.O.; Lucano-Landeros, S.; Santos, A.; Monroy-Ramirez, H.C.; Armendariz-Borunda, J. Hepatocarcinogenesis Prevention by Pirfenidone Is PPARγ Mediated and Involves Modification of Nuclear NF-κB p65/p50 Ratio. Int. J. Mol. Sci. 2021, 22, 11360. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Moreau, F.; Chadee, K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef]
- Lee, N.J.; Oh, J.H.; Ban, J.O.; Shim, J.H.; Lee, H.P.; Jung, J.K.; Ahn, B.W.; Yoon, D.Y.; Han, S.B.; Ham, Y.W.; et al. 4-O-methylhonokiol, a PPARγ agonist, inhibits prostate tumour growth: p21-mediated suppression of NF-κB activity. Br. J. Pharmacol. 2013, 168, 1133–1145. [Google Scholar] [CrossRef]
- Fahy, B.N.; Schlieman, M.G.; Mortenson, M.M.; Virudachalam, S.; Bold, R.J. Targeting BCL-2 overexpression in various human malignancies through NF-kappaB inhibition by the proteasome inhibitor bortezomib. Cancer Chemother. Pharmacol. 2005, 56, 46–54. [Google Scholar] [CrossRef]
- Li, L.; Wu, W.; Huang, W.; Hu, G.; Yuan, W.; Li, W. NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm. Res. 2013, 62, 387–397. [Google Scholar] [CrossRef]
- Farrell, S.W.; DeFina, L.F.; Tintle, N.L.; Leonard, D.; Cooper, K.H.; Barlow, C.E.; Haskell, W.L.; Pavlovic, A.; Harris, W.S. Association of the Omega-3 Index with Incident Prostate Cancer with Updated Meta-Analysis: The Cooper Center Longitudinal Study. Nutrients 2021, 13, 384. [Google Scholar] [CrossRef]
- Glatz, J.F.; Luiken, J.J.; van Nieuwenhoven, F.A.; Van der Vusse, G.J. Molecular mechanism of cellular uptake and intracellular translocation of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 1997, 57, 3–9. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hughes, B.G. Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem. Bioph. Res. Commun. 1988, 152, 328–335. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Cao, W.; Yang, B.; Mu, Y.; Dong, Y.; Xiu, Z. E-configuration structures of EPA and DHA derived from Euphausia superba and their significant inhibitive effects on growth of human cancer cell lines in vitro. Prostaglandins Leukot. Essent. Fatty Acids 2017, 117, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, H.; Tian, Y.; Zhao, Z.; Bo, Y.; Guo, Y.; Wang, J. Comparative Study of Docosahexaenoic Acid with Different Molecular Forms for Promoting Apoptosis of the 95D Non-Small-Cell Lung Cancer Cells in a PPARγ-Dependent Manner. Mar. Drugs 2022, 20, 599. https://doi.org/10.3390/md20100599
Yue H, Tian Y, Zhao Z, Bo Y, Guo Y, Wang J. Comparative Study of Docosahexaenoic Acid with Different Molecular Forms for Promoting Apoptosis of the 95D Non-Small-Cell Lung Cancer Cells in a PPARγ-Dependent Manner. Marine Drugs. 2022; 20(10):599. https://doi.org/10.3390/md20100599
Chicago/Turabian StyleYue, Hao, Yingying Tian, Zifang Zhao, Yuying Bo, Yao Guo, and Jingfeng Wang. 2022. "Comparative Study of Docosahexaenoic Acid with Different Molecular Forms for Promoting Apoptosis of the 95D Non-Small-Cell Lung Cancer Cells in a PPARγ-Dependent Manner" Marine Drugs 20, no. 10: 599. https://doi.org/10.3390/md20100599
APA StyleYue, H., Tian, Y., Zhao, Z., Bo, Y., Guo, Y., & Wang, J. (2022). Comparative Study of Docosahexaenoic Acid with Different Molecular Forms for Promoting Apoptosis of the 95D Non-Small-Cell Lung Cancer Cells in a PPARγ-Dependent Manner. Marine Drugs, 20(10), 599. https://doi.org/10.3390/md20100599




