Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents
Abstract
:1. Introduction
2. Activities of Alkaloids Discovered in Marine Organisms
2.1. Inhibition of Aβ Production
2.1.1. Derivatives of Tryptophan
2.1.2. Derivatives of Tyrosine
2.2. Inhibition of NFTs Formation
2.2.1. Inhibition of GSK3β
Derivatives of Tryptophan
Derivatives of Tyrosine
Derivatives of 3,4-Dihydroxyphenylalanine (DOPA)
Derivatives of Glycine
Derivatives of Proline
Derivatives of Phenylalanine
2.2.2. Inhibition of CKlδ
2.2.3. Inhibition of DyrklA
2.2.4. Inhibition of CLKl
2.2.5. MT-Stabilizing
2.3. Inhibition of Pro-Inflammatory Factors
2.4. Inhibition of Acetylcholinesterase (AChE)
2.4.1. Inhibitors from Bacteria
Derivatives of Tryptophan
Derivatives of Anthranilic Acid
2.4.2. Inhibitors from Fungi
2.4.3. Inhibitors from Animals: Jellyfish, Ascidian, and Molluscs
2.4.4. Inhibitors from Animals: Sponges and Corals
Derivatives of Tryptophan
Derivatives of Phenylalanine or Tyrosine
Derivatives of Nicotinic Acid
Derivatives of Ornithine
Derivatives of l-Proline
Derivatives of Glycine
2.5. Stabilization of Nicotinic Acetylcholine Receptors (nAChRs)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New drugs from marine organisms in Alzheimer’s disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar]
- Isik, A.T. Late onset Alzheimer’s diseasae in older people. Clin. Interv. Aging 2010, 5, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenberghe, C.; van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Hauser, P.S.; Narayanaswami, V.; Ryan, R.O. Apolipoprotein E: From lipid transport to neurobiology. Prog. Lipid Res. 2011, 50, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Kanekiyo, T.; Xu, H.; Bu, G. Apoliprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Ver. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apoliprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhan, L.; Lun, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoEε4 markedly exacerbates tau-mediated neurodegeneration in a mouse mode tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef]
- Bekris, L.M.; Yu, C.; Bird, T.D.; Tsuang, T.W. Genetics of Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.; Silva, R.; MM Pinto, M.; Sousa, E. Marine natural products, multitarget therapy and repurposed agents in Alzheimer’s disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Macauley, S.L.; Holtzman, D.M. Recent advances from the bench toward the bedside in Alzheimer’s disease. EBioMedicine 2015, 2, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Takashima, A. Tau aggregation is a therapeutic target for Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 665–669. [Google Scholar] [CrossRef]
- Coman, H.; Nemes, B. New therapeutic targets in Alzheimer’s disease. Int. J. Gerontol. 2017, 11, 2–6. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinatoirs for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [Green Version]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorgan. Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [Green Version]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Ballatore, C.; Brunden, K.R.; Huryn, D.M.; Trojanowski, J.Q.; Lee, V.M.-Y.; Smith, A.B., III. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.A., II; Banerjee, R.; Gunawardena, S. Axonal transport and neurodegeneration: How marine drugs can be used for the development of therapeutics. Mar. Drugs 2016, 14, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [Green Version]
- Naini, S.; Soussi-Yanicostas, N. Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxidative Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripava, D.; Ripava, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers Dis. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terra, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Li, G.; Yin, H.; Kuret, J. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J. Biol. Chem. 2004, 279, 15938–15945. [Google Scholar] [CrossRef] [Green Version]
- Llorach-Pares, L.; Nonell-Canals, A.; Avila, C.; Sanchez-Martinez, M. Kororamides, convolutamines, and índole derivatives as possible tau and dual-specifity kinase inhibitors for Alzheimer’s disease: A computational study. Mar. Drugs 2018, 16, 386. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.; Karthikeyan, C.; Moorthy, H.N.; Waiker, D.K.; Jain, A.K.; Trivedi, P. Human CDC2-Like Kinase 1 (CLK1): A Novel Target for Alzheimer’s Disease. Curr. Drug Targets 2014, 15, 539–550. [Google Scholar] [CrossRef]
- Tell, V.; Hilgeroth, A. Recent developments of protein kinase inhibitors as potential AD therapeutics. Front. Cell Neurosci. 2013, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Dolan, P.J.; Johnson, G.V.W. The role of tau kinases in Alzheimer’s disease. Curr. Opin. Drug Discov. Devel. 2010, 13, 595–603. [Google Scholar] [PubMed]
- Stotani, S.; Giordanetto, F.; Medda, F. DYRKlA inhibition as potential treatment for Alzheimer’s disease. Future Med. Chem. 2016, 8, 681–696. [Google Scholar] [CrossRef]
- Branca, C.; Shaw, D.M.; Belfiore, R.; Gokhale, V.; Shaw, A.Y.; Foley, C.; Smith, B.; Hulme, C.; Dunckley, T.; Meechoovet, B.; et al. Dyrkl inhibition improves Alzheimer’s disease-like pathology. Aging Cell 2017, 16, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Llorens-Martín, M.; Jurado, J.; Hemández, F.; Avila, J. GSK-3, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Hemández, F.; Gómez de Barreda, E.; Fuster-Matanzo, A.; Lucas, J.J.; Avila, J. GSK3: A possible link between beta amyloid peptide and tau protein. Exp. Neurol. 2010, 223, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Hemandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2012, 33, S141–S144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Kummer, M.P. Innate immune activation in neurodegenerative disease. Nat. Rev. lmmunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in neurodegenerative disorders—A review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [Green Version]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A. Microglia: Immune regulators of neurodevelopment. Front. Lmmunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell. Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 44–468. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, X.; Cheng, J.; Hou, L. Drug development for Alzheimer’s disease: Microglia induced neuroinflammation as a target? Int. J. Mol. Sci. 2019, 20, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.Y.; Wang, X.; Liu, C.; Zhang, H.L. Pharmacological targeting of microglial activation: New therapeutic approach. Front. Cell. Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef] [Green Version]
- Anglister, L.; Stiles, J.R.; Salpetert, M.M. Acetylcholinesterase density and turnover number at frog neuromuscular–junctions, with modeling of their role in synaptic function. Neuron 1994, 12, 783–794. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Houghton, P.J.; Ren, Y.; Howes, M. Acetylcholinesterase inhibitors from plants and fungi. J. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, A.; Alarcón, R.; Opazo, C.; Campos, E.O.; Muñoz, F.J.; Calderón, F.H.; Dajas, F.; Gentry, M.K.; Doctor, B.P.; De Mello, F.G.; et al. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J. Neurosci. 1998, 18, 3213–3223. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Genereux, J.C.; Wiseman, R.L. Endoplasmic reticulum quality control and systemic amyloid disease: Impacting protein stability from the inside out. IUBMB Life 2015, 67, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Freitas Silva, M.; Dias, K.S.T.; Gontijo, V.S.; Ortiz, C.J.C.; Viegas, C., Jr. Multi-target directed drugs as a modem approach for drug design towards Alzheimer’s disease: An update. Curr. Med. Chem. 2018, 25, 349–3525. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.H.; Guan, J.; Ge, S.; Wu, M.B.; Lin, J.P. Advancement of multi-target drug discoveries and promising applications in the field of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 169, 200–223. [Google Scholar] [CrossRef]
- Koslow, T. The Silent Deep: The Discovery, Ecology and Consertvation of the Deep Sea; University Chicago Press: Chicago, IL, USA, 2007; pp. 1–288. [Google Scholar]
- Russo, P.; del Bufalo, A.; Fini, A. Deep sea as a source of novel anticancer drugs: Update on discovery and preclinical/clinical evaluation in a systems medicine perspective. EXCLI J. 2015, 14, 228–236. [Google Scholar] [PubMed]
- Catassi, A.; Cesario, A.; Arzani, D.; Menichini, P.; Alama, A.; Bruzzo, C.; Imperatori, A.; Rotolo, N.; Granone, P.; Russo, P. Characterization of apoptosis induced by marine natural products in non small cell lung cancer A549 cells. Cell Mol. Life Sci. 2006, 63, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Nastrucci, C.; Cesario, A. From the sea to anticancer therapy. Curr. Med. Chem. 2011, 18, 3551–3562. [Google Scholar] [CrossRef]
- Nastrucci, C.; Cesario, A.; Russo, P. Anticancer drug discovery from the marine environment. Recent Pat. Anticancer Drug Discov. 2012, 7, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Cesario, A. New anticancer drugs from marine cyanobacteria. Curr. Drug Targets 2012, 13, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.; Castro, A.; Martinez, A. Marine compounds for the therapeutic treatment of neurological disorders. Expert Opin. Ther. Pat. 2005, 15, 10. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.; Facchini, P.J. Alkaloids biosynthesis: Methods and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [Green Version]
- Waterman, P.G. Chemical taxonomy of alkaloids. In Alkaloids, Biochemistry, Ecology and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 87–107. [Google Scholar]
- Zhang, H.; Conte, M.M.; Khalil, Z.; Huang, X.-C.; Capon, R.J. New dictyodendrins as BACE inhibitors from a Southern Australian marine sponge Ianthella sp. RSC Adv. 2012, 2, 4209–4214. [Google Scholar] [CrossRef]
- Zhang, H.; Conte, M.M.; Huang, X.-C.; Khalil, Z.; Capon, R.J. A search for BACE inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern Australian marine sponge Ianthella sp. Org. Biomol. Chem. 2012, 10, 2656–2663. [Google Scholar] [CrossRef]
- Meijer, L.; Skaltsounis, A.L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R.; et al. GSK-3-selective inhibitors derived from tyrian purple indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Raot, K.V.; Doniat, M.S.; Pengt, J.; Garcia-Palomero, E.; Alonso, D.; Martinez, A.; Medina, M.; Franzblau, S.G.; Tekwanit, B.L.; Khan, S.I.; et al. Manzamine B and E and ircinal A related alkaloids from an indonesian Acanthostrongylophora sponge and their activity against infectious, tropical parasitic, and Alzheimer’s diseases. J. Nat. Prod. 2006, 69, 1034–1040. [Google Scholar]
- Gompel, M.; Leost, M.; de Kier Joffe, E.B.; Puricelli, L.; Franco, L.H.; Puricelli, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef]
- Echalier, A.K.; Bettayeb, K.; Ferandin, B.Y.; Lozach, O.; Clément, M.; Valette, A.; Liger, F.; Marquet, B.; Morris, J.C.; Endicott, J.A.; et al. Meriolins (3-(pyrimidin-4-yl)-7-azaindoles): Synthesis, kinase inhibitory activity, cellular effects, and structure of a CDK2/cyclin A/meriolin complex. J. Med. Chem. 2008, 51, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Klein-Junior, L.; Passos, C.S.; Moraes, A.; Wakui, V.; Konrath, E.; Nurisso, A.; Carrupt, P.-A.; Alves de Oliveira, C.; Kato, L.; Henriques, A. Indole alkaloids and semisynthetic indole derivatives asmultifunctional scaffolds aiming the inhibition of enzymes related to neurodegenerative diseases—A focus on Psychotria L. Genus. Curr. Top. Med. Chem. 2014, 14, 1056–1075. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Asal, B.A.; Mudit, M.; Kaddoumi, A.; EI Sayed, K.A. The marine natural-derived inhibitors of glycogen synthase kinase-3 beta- phenylmethylene hydantoins: Ln vitro and in vivo activities and pharmacophore modeling. Bioorg. Med. Chem. 2009, 17, 6032–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plisson, F.; Conte, M.; Khalil, Z.; Huang, X.-C.; Piggott, A.M.; Capon, R.J. Kinase inhibitor scaffolds against neurodegenerative diseases from a Southern Australian ascidian, Didemnum sp. Chem. Med. Chem. 2012, 7, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Aβ and hyperphosphorylated tau in vitro. Neurochem. Int. 2011, 59, 1056–1065. [Google Scholar] [CrossRef]
- Zhang, H.; Khalil, Z.; Conte, M.M.; Plisson, F.; Capon, R.J. A search for kinase inhibitors and antibacterial agents: Bromopyrrolo-2- amino-imidazoles from a deep-water Great Australian Bight sponge Axinella sp. Tetrahedron Lett. 2012, 53, 3784–3787. [Google Scholar] [CrossRef]
- Fedorov, O.; Huber, K.; Eisenreich, A.; Filippakopoulos, P.; King, O.; Bullock, A.N.; Szklarczyk, D.; Jensen, L.J.; Fabbro, D.; Trappe, J.; et al. Specific CLK inhibitors from a novel chemotype for regulation of alternative splicing. Chem. Biol. 2011, 18, 67–76. [Google Scholar] [CrossRef] [Green Version]
- De Souza, É.T.; Pereira de Lira, D.; Cavalcanti de Queiroz, A.; Costa da Silva, D.J.; Bezerra de Aquino, A.; Campessato Mella, E.; Prates Lorenzo, V.; de Miranda, G.E.; de Araújo-Júnior, J.X.; de Oliveira Chaves, M.C.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.E.; Jung, I.; Na, J.Y.; Lee, Y.; Lee, J.; Lee, J.S.; Lee, J.S. Pseudane-VII regulates LPS-induced neuroinflammation in brain microglia cells through the inhibition of iNOS expression. Molecules 2018, 23, 3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef]
- Kim, W.G.; Song, N.K.; Yoo, I.D. Quinolactacins A1 and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. J. Antibiot. 2001, 54, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hu, L.; Liu, D.; Huang, J.; Lin, W. Circumdatin D exerts neuroprotective effects by attenuating LPS-induced pro inflammatory responses and downregulating acetylcholinesterase activity in vitro and in vivo. Front. Pharmacol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Tadokoro, Y.; Nishikawa, T.; Ichimori, T.; Matsunaga, S.; Fujita, M.J.; Sakai, R. N-Methyl-beta-carbolinium Salts and an N-Methylated 8-oxoisoguanine as acetylcholinesterase inhibitors from a solitary ascidian, Cnemidocarpa irene. ACS Omega 2017, 2, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Kigoshi, H.; Kanematsu, K.; Yokota, K.; Uemura, D. Turbotoxins A and B, novel diiodotyramine derivatives from the Japanese gastropod Turbo marmorata. Tetrahedron 2000, 56, 9063–9070. [Google Scholar] [CrossRef]
- Tadesse, M.; Svenson, J.; Sepčić, K.; Trembleau, L.; Engqvist, M.; Andersen, J.H.; Jaspars, M.; Stensvåg, K.; Haug, T. Isolation and Synthesis of Pulmonarins A and B, acetylcholinesterase inhibitors from the colonial ascidian Synoicum pulmonaria. J. Nat. Prod. 2014, 77, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Botić, T.; Defant, A.; Zanini, P.; Žužek, M.C.; Frangež, R.; Janussen, D.; Kersken, D.; Knez, Ž.; Mancin, I.; Sepčić, K. Discorhabdin alkaloids from Antarctic Latrunculia spp. sponges as a new class of cholinesterase inhibitors. Eur. J. Med. Chem. 2017, 136, 294–304. [Google Scholar] [CrossRef]
- Nukoolkarn, V.S.; Saen-oon, S.; Rungrotmongkol, T.; Hannongbua, S.; Ingkaninan, K.; Suwanborirux, K. Petrosamine, a potent anticholinesterase pyridoacridine alkaloid from a Thai marine sponge Petrosia n. sp. Bioorg. Med. Chem. 2008, 16, 6560–6567. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.; Žužek, M.C.; Frangež, R.; Andersen, J.H.; Hansen, E.; Olsen, E.K.; Cergolj, M.; Sepčić, K.; Hansen, K.Ø.; Svenson, J. Synthetic analogs of stryphnusin isolated from the marine sponge Stryphnus fortis inhibit acetylcholinesterase with no effect on muscle function or neuromuscular transmission. Org. Biomol. Chem. 2016, 14, 11220–11229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xynas, R.; Capon, R. 2 Bromotyrosine-derived metabolites from an australian marine sponge Aplysina sp. Aust. J. Chem. 1989, 42, 1427–1433. [Google Scholar] [CrossRef]
- Sepcić, K.; Marcel, V.; Klaebe, A.; Turk, T.; Suput, D.; Fournier, D. Inhibition of acetylcholinesterase by an alkylpyridinium polymer from the marine sponge, Reniera sarai. Biochim. Biophys. Acta 1998, 1387, 217–225. [Google Scholar] [CrossRef]
- Defant, A.; Mancini, I.; Raspor, L.; Guella, G.; Turk, T.; Sepcic, K. New structural insights into saraines A, B, and C, macrocyclic alkaloids from the Mediterranean sponge Reniera (Haliclona) sarai. Eur. J. Org. Chem. 2011, 2011, 3761–3767. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.E.; Ozcelik, B.; Konuklugil, B.; Putz, A.; Kaban, U.G.; Proksch, P. Bioactivity screening of selected Turkish marine sponges and three compounds from Agelas oroides. Rec. Nat. Prod. 2012, 6, 356–367. [Google Scholar]
- Kem, W.R.; Mahnir, V.M.; Papke, R.L.; Lingle, C.J. Anabaseine is a potent agonist on muscle and neuronal alphabungarotoxin-sensitive nicotinic receptors. J. Pharmacol. Exp. Ther. 1997, 283, 979–992. [Google Scholar] [PubMed]
- Bharate, S.B.; Manda, S.; Joshi, P.; Singh, B.; Vishwakarma, R.A. Total synthesis and anti-cholinesterase activity of marine-derived bis-indole alkaloid fascaplysin. Med. Chem. Comm. 2012, 3, 1098–1103. [Google Scholar] [CrossRef]
- Muralidharan, A.; Josyula, V.R.; Hariharapura, R.C. Exploring the potential of marine microbes in clinical management of Alzheimer’s disease: A road map for bioprospecting and identifying promising isolates. Life Sci. 2018, 208, 149–160. [Google Scholar] [CrossRef]
- Choi, D.-Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015, 38, 139–170. [Google Scholar] [CrossRef]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousaf, M.; Hammond, N.L.; Peng, J.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.S.; Hamann, M.T. New manzamine alkaloids from an indo-pacific sponge. Pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory diseases. J. Med. Chem. 2004, 47, 3512–3517. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Hu, J.F.; Kazi, A.B.; Li, Z.; Avery, M.; Peraud, O.; Hill, R.T.; Franzblau, S.G.; Zhang, F.; Schinazi, R.F.; et al. Manadomanzamines A and B: A novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J. Am. Chem. Soc. 2003, 125, 13382–13386. [Google Scholar] [CrossRef] [Green Version]
- Bharate, S.B.; Yadav, R.R.; Battula, S.; Vishwakarma, R.A. Meridianins: Marine-derived potent kinase inhibitors. Mini Rev. Med. Chem. 2012, 12, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.H.; Joffe, E.B.D.K.; Puricelli, L.; Tatian, M.; Seldes, A.M.; Palermo, J.A. Indole alkaloids from the tunicate Aplidium meridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Lebar, M.D.; Baker, B.J. Synthesis and structure reassessment of psammopemmin A. Aust. J. Chem. 2010, 63, 862–866. [Google Scholar] [CrossRef]
- Giraud, F.; Alves, G.; Debiton, E.; Nauton, L.; Théry, V.; Durieu, E.; Ferandin, Y.; Lozach, O.; Meijer, L.; Anizon, F.; et al. Synthesis, protein kinase inhibitory potencies, and in vitro antiproliferative activities of meridianin derivatives. J. Med. Chem. 2011, 54, 4474–4489. [Google Scholar] [CrossRef]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.S.; Lo, D.C.; Delhommel, F.; et al. Selectivity, cocrystal structures, and neuroprotective properties of leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid leucettamine B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef]
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.G.; Parkin, S.; Hope, H. Alkaloids from the Antarctic sponge Kirkpatrickia varialosa. Part 1: Variolin B, a new antitumour and antiviral compound. Tetrahedron 1994, 50, 3987–3992. [Google Scholar] [CrossRef]
- Carroll, A.R.; Wild, S.J.; Duffy, S.; Avery, V.M. Kororamide A, a new tribrominated índole alkaloid from australian bryozoan Amathia tortuosa. Tetrahedron Lett. 2012, 53, 2873–2875. [Google Scholar] [CrossRef] [Green Version]
- Dashti, Y.; Vial, M.L.; Wood, S.A.; Mellick, G.D.; Roullier, C.; Quinn, R.J. Korforamide B, a brominated alkloid from the bryozoan Amathia tortuosa and its effects on Parkinson’s disease cells. Tetrahedron 2015, 71, 7879–7884. [Google Scholar] [CrossRef]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The phylum bryozoa: From biology to biomedical potential. Mar. Drugs 2020, 18, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Fenical, W. Ningalins A-D: Novel aromatic alkaloids from a Western Australian ascidian of the genus Didemnum. J. Org. Chem. 1997, 62, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Burton, I.W.; Hu, T.; Walter, J.A.; Wright, J.L.C. Assignment of the relative stereochemistry of the spirolides, macrocyclic toxins isolated from shellfish and from the cultured dinoflagellate Alexandrium ostenfeldii. Tetrahedron 2001, 57, 8659–8665. [Google Scholar] [CrossRef]
- Supriyono, A.; Schwarz, B.; Wray, V.; Witte, L.; Müller, W.E.; van Soest, R.; Sumaryono, W.; Proksch, P. Bioactive alkaloids from the tropical marine sponge Axinella carteri. Z. Für Nat. C 1995, 50, 669–674. [Google Scholar] [CrossRef]
- Meijer, L.; Thunnissen, A.M.W.H.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.Z.; et al. Inhibition of cyclin-dependent kinases, GSK3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef]
- Sharma, G.M.; Buyer, S.S.; Pomerantz, M.W. Characterization of a yellow compound isolated from the marine sponge Phakellia flabellate. J. Chem. Soc. Chem. Comm. 1980, 10, 435–436. [Google Scholar] [CrossRef]
- Lindel, T.; Jensen, P.R.; Penicai, W.; Long, B.H.; Casazza, A.M.; Carboni, J.; Pairchild, C.R. Eleutherobin, a new cytotoxin that mimics paclitaxel (Taxol) by stabilizing microtubules. J. Am. Chem. Soc. 1997, 119, 8744–8745. [Google Scholar] [CrossRef]
- Long, B.; Carboni, J.M.; Wasserman, A.J.; Comell, L.A.; Casazza, A.M.; Jensen, P.R.; Lindel, T.; Fenical, W.; Fairchild, C.R. Eleutherobin, a novel cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol (R)). Cancer Res. 1998, 58, 1111–1115. [Google Scholar]
- D’Ambrosio, M.; Guerriero, A.; Pietra, P.; Sarcodictyin, A.; Sarcodictyin, B. Novel diterpenoidic alcohols esterified by (E)-N(l)-methylurocanic acid. Isolation from the Mediterranean stolonifer Sarcodictyon roseum. Helv. Chim. Acta 1987, 70, 2019–2027. [Google Scholar] [CrossRef]
- D’Anbrosio, M.; Guerriero, A.; Pietra, P. lsolation from the Mediterranean stolonifern coral Sarcodictyon roseum of sarcodictyin C, D, E, and P, novel diterpenoidic alcohols esterified by (E)- or (Z)-N(1)-methylurocanic acid. Failure of the carbon-skeleton type as a classification criterion. Helv. Chim. Acta. 1988, 71, 964–976. [Google Scholar] [CrossRef]
- Ciomei, M.; Albanese, C.; Pastori, W.; Grandi, M.; Pietra, P.; D’Ambrosio, M.; Guerriero, A.; Battistini, C. Sarcodictyins: A new class of marine derivatives with mode of action similar to Taxol. Abstract 30. Proc. Am. Ass. Canc. Res. 1997, 38, 5. [Google Scholar]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Mar. Drugs. 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Kim, M.E.; Jung, I.; Lee, J.S.; Na, J.Y.; Kim, W.J.; Kim, Y.O.; Park, Y.D. Pseudane-VII isolated from Pseudoalteromonas sp. M2 ameliorates LPS-induced inflammatory response in vitro and in vivo. Mar. Drugs. 2017, 15, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.L.; Huang, L.; Liu, J.; Song, Y.; Gao, J.; Jung, J.H.; Liu, Y.; Chen, G. Acetylcholinesterase inhibitory dimeric indole derivatives from the marine actinomycetes Rubrobacter radiotolerans. Fitoterapia 2015, 102, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Sangnoi, Y.; Sakulkeo, O.; Yuenyongsawad, S.; Kanjana-Opas, A.; Ingkaninan, K.; Plubrukarn, A.; Suwanborirux, K. Acetylcholinesterase-inhibiting activity of pyrrole derivatives from a novel marine gliding bacterium, Rapidithrix thailandica. Mar. Drugs 2008, 6, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Beedessee, G.; Ramanjooloo, A.; Surnam-Boodhun, R.; van Soest, R.W.M.; Marie, D.E.P. Acethylcholinesterase-inhibitory activities of the extracts from sponges collected in Mauritius waters. Chem. Biodivers. 2013, 10, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Ebel, R.; Edrada, R.; Riebe, F.; Liu, H.; Diesel, A.; Bayer, M.; Li, X.; Lin, W.H.; Grebenyuk, V. Sponge-associated fungi and their bioactive compounds: The Suberites case. Bot. Mar. 2008, 51, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.Q.; Song, J.L.; Zhu, K.; Zhang, J.; Jiang, C.S.; Zhang, H. Total synthesis of pulmonarin B and design of brominated phenylacetic acid/tacrine hybrids: Marine pharmacophore inspired discovery of neu Che and Aβ aggregation inhibitors. Mar. Drugs 2018, 16, 293. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Baker, B.J.; Grimwade, J.; Leonard, A.; McClintock, J.B. Discorhabdin alkaloids from the Antarctic sponge Latrunculia apicalis. J. Nat. Prod. 1995, 58, 1596–1599. [Google Scholar] [CrossRef]
- Antunes, E.M.; Beukes, D.R.; Kelly, M.; Samaai, T.; Barrows, L.R.; Marshall, K.M.; Sincich, C.; Davies-Coleman, M.T. Cytotoxic pyrroloiminoquinones from four new species of south African latrunculide sponges. J. Nat. Prod. 2004, 67, 1268–1276. [Google Scholar] [CrossRef]
- Perry, N.B.; Blunt, J.W.; Munro, M.H. Cytotoxic pigments from New Zealand sponges of the genus Latrunculia: Discorhabdin-A, Discorhabdin-B and Discorhabdin-C. Tetrahedron 1988, 44, 1727–1734. [Google Scholar] [CrossRef]
- Reyes, F.; Martín, R.; Rueda, A.; Fernández, R.; Montalvo, D.; Gómez, C.; Sánchez-Puelles, J.M. Discorhabdins I and L, cytotoxic alkaloids from the sponge Latrunculia brevis. J. Nat. Prod. 2004, 67, 463–465. [Google Scholar] [CrossRef]
- Lidgren, G.; Bohlin, L.; Bergman, J. Studies of Swedish marine organisms 7. A novel biologically active indole alkaloid from the sponge Geodia baretti. Tetrahedron Lett. 1986, 27, 3283–3284. [Google Scholar] [CrossRef]
- Sölter, S.; Dieckmann, R.; Blumenberg, M.; Francke, W. Barettin, revisited? Tetrahedron Lett. 2002, 43, 3385–3386. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Ogundajo, A.L.; Oladosu, I.A.; Changwichit, K.; Ingkanina, K.; Yuenyongsawad, S.; Plubrukarn, A. Non-competitive inhibition of acetylcholinesterase by bromotyrosine alkaloids. Nat. Prod. Commun. 2014, 9, 1559–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabudravu, J.N.; Jaspars, M. Purealidin S and purpuramine J, bromotyrosine alkaloids from the Fijian marine sponge Druinella sp. J. Nat. Prod. 2002, 65, 1798–1801. [Google Scholar] [CrossRef]
- Jurek, J.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borges, M. 3 New bromotyrosine-derived metabolites of the sponge Psammaplysilla purpurea. J. Nat. Prod. 1993, 56, 1609–1612. [Google Scholar] [CrossRef]
- Sepčić, K.; Mancin, I.; Vidic, I.; Franssanito, R.; Pietra, F.; Macek, P.; Turk, T. Antibacterial and anticholinesterase activities of aplysamine-4, a bromotyrosine-derived metabolite of a Red Sea marine sponge. J. Nat. Toxins 2001, 10, 181–191. [Google Scholar] [PubMed]
- Moody, K.; Thomson, R.; Fattorusso, E.; Minale, L.; Sodano, G. Aerothionin and homoaerothionin—2 tetrabromo spirocyclohexadienylisoxazoles from Verongia sponges. JCS Perkin I. 1972, 1, 18–24. [Google Scholar] [CrossRef]
- Sirimangkalakitti, N.; Olatunji, O.J.; Changwichit, K.; Saesong, T.; Chamni, S.; Chanvorachote, P.; Ingkaninan, K.; Plubrukarn, A.; Suwanborirux, K. Bromotyrosine alkaloids with acetylcholinesterase inhibitory activity from the Thai sponge Acanthodendrilla sp. Nat. Prod. Commun. 2015, 10, 1945–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopichand, Y.; Schmitz, F. Marine natural-products—Fistularin-1, fistularin 2 and fistularin-3 from the sponge Aplysina fistularis forma fluva. Tetrahedron Lett. 1979, 20, 3921–3924. [Google Scholar] [CrossRef]
- Cimino, G.; de Rosa, S.; de Stefano, S.; Sodano, G. Marine natural products: New results from Mediterranean invertebrates. Pure Appl. Chem. 1986, 58, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Cimino, G.; de Stefano, S.; Scognamiglio, G.; Sodano, G.; Trivellone, E. Sarains A new class of alkaloids from the marine sponge Reniera sarai. Bull. Soc. Chim. Belg. 1986, 95, 783–800. [Google Scholar] [CrossRef]
- Cimino, G.; Mattia, C.A.; Mazzarella, L.; Puliti, R.; Scognamiglio, G.; Spinella, A.; Trivellone, E. Unprecedented alkaloid skeleton from the Mediterranean sponge Reniera sarai: X-ray structure of an acetate derivative of sarain-A. Tetrahedron 1989, 45, 3863–3872. [Google Scholar] [CrossRef]
- Cimino, G.; Spinella, A.; Trivellone, E. Isosarain-1: A new alkaloid from the Mediterranean sponge Reniera sarai. Tetrahedron Lett. 1989, 30, 133–136. [Google Scholar] [CrossRef]
- Guo, Y.; Madaio, E.; Trivellone, E.; Scognamiglio, G.; Cimino, G. Further studies of alkaloids from Reniera sarai: Structures of saraine-3 and isosaraine-3, absolute stereochemistry of saraine-1 and saraine-2. Tetrahedron 1996, 52, 14961–14974. [Google Scholar] [CrossRef]
- Guo, Y.; Trivellone, E.; Scognamiglio, G.; Cimino, G. Absolute stereochemistry of isosaraine-1 and isosaraine-2. Tetrahedron Lett. 1998, 39, 463–466. [Google Scholar] [CrossRef]
- Langjae, R.; Bussarawit, S.; Yuenyongsawad, S.; Ingkaninan, K.; Plubrukarn, A. Acetylcholinesterase-inhibiting steroidal alkaloid from the sponge Corticium sp. Steroids 2007, 72, 682–685. [Google Scholar] [CrossRef]
- Forenza, S.; Minale, L.; Riccio, R.; Fattorusso, E. New bromo-pyrrole derivatives from the sponge Agelas oroides. J. Chem. Soc. D Chem. Commun. 1971, 18, 1129–1130. [Google Scholar] [CrossRef]
- Garcia, E.E.; Benjamin, L.E.; Fryer, R.I. Reinvestigation into the structure of oroidin, a bromopyrrole derivative from a marine sponge. J. Chem. Soc. Chem. Commun. 1973, 33, 78–79. [Google Scholar] [CrossRef]
- Turk, T.; Macek, P.; Suput, D. Inhibition of acetylcholinesterase by a pseudozoanthoxanthin-like compound isolated from the zoanthid Parazoanthus axinellae (O. Schmidt). Toxicon 1995, 33, 133–142. [Google Scholar] [CrossRef]
- Šuput, J.S.; Turk, T.; Maček, P.; Šuput, D. Pseudozo-anthoxantin-like compound from Parazoanthus axinellae Adriaticus inhibits acetylcholinesterase. Pflugers Arch. 1996, 431, R315–R316. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Yunker, M.B.; Scheuer, P.J.; Ottersen, T. Pseudo-zoanthoxanthins from gold coral. Can. J. Chem. 1979, 57, 1707–1711. [Google Scholar] [CrossRef] [Green Version]
- Vitale, R.M.; Rispoli, V.; Desiderio, D.; Sgammato, R.; Thellung, S.; Canale, C.; Vassalli, M.; Carbone, M.; Ciavatta, M.L.; Mollo, E.; et al. In silico identification and experimental validation of novel anti-Alzheimer’s multitargeted ligands from a marine source featuring a “2-aminoimidazole plus aromatic group” scaffold. ACS Chem. Neurosci. 2018, 9, 1290–2130. [Google Scholar] [CrossRef]
- Albizati, K.F.; Faulkner, D.J. Stevensine, a novel alkaloid of an unidentified sponge. J. Org. Chem. 1985, 50, 4163–4164. [Google Scholar] [CrossRef]
- Coates, R.M.; Kem, W.R.; Abbott, B.C. Isolation and structure of a hoplonemertine toxin. Toxicon 1971, 9, 15–22. [Google Scholar]
- Papke, R.L.; Meyer, E.M.; Lavieri, S.; Bollampally, S.R.; Papke, T.A.S.; Horenstein, N.A.; Itoh, Y.; Papke, J.K.P. Effects at a distance in α7 nAChR selective agonists: Benzylidene substitutions that regulate potency and efficacy. Neuropharmacology 2004, 46, 1023–1038. [Google Scholar] [CrossRef]
- Wheeler, J.W.; Olubajo, O.; Storm, C.B.; Duffield, R.M. Anabaseine: Venom alkaloid of Aphaenogaster ants. Science 1981, 211, 1051–1052. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.-L.; Kornprobst, J.M. Marine pharmacology: Potentialities in the treatment of infectious diseases, osteoporosis and Alzheimer’s disease. Adv. Biochem. Engin/Biotechnol. 2005, 97, 105–131. [Google Scholar]
- Hentschel, J.; Piel, S.; Degnan, M.; Taylor, M.W. Genomic insights into the marine sponge microbiome. Nat. Rev. Microbiol. 2012, 10, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Manda, S.; Sharma, S.; Wani, A.; Josi, P.; Kumar, V.; Guru, S.K.; Bharate, S.S.; Bhushan, S.; Vishwakarma, R.A.; Kumar, A.; et al. Discovery of a marine-derived bis-indole alkaloid fascaplysin, as a new class of potent P-glycoprotein inducer and establishement of its structure–activity relationship. Eur. J. Med. Chem. 2016, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, F.; Sang, J.; Lin, M.; Ma, J.; Xiao, X.; Yan, S.; Naman, C.B.; Wang, N.; He, S.; et al. 9-Methyl -fascaplysin is a more potent Aβ aggregation inhibitor than the marine-derived alkaloid, fascaplysin, and produces nanomolar neuroprotective effects in SH-SY5Y cells. Mar. Drugs 2019, 17, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Qiu, H.; Zhang, K.; Zhang, P.; Liang, W.; Yang, M.; Mou, C.; Lin, M.; He, M.; Xiao, X.; et al. Fascaplysin derivatives are potent multitarget agents against Alzheimer’s disease: In vitro and in vivo evidence. ACS Chem. Neurosci. 2019, 10, 4741–4756. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodeghenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational design of multitarget-directed ligands: Strategies and emerging paradigms. J. Med. Chem. 2019, 62, 888–8914. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uliassi, E.; Bolognesi, M. Two disease, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. Med. Chem. Comm. 2014, 5, 853–861. [Google Scholar] [CrossRef]
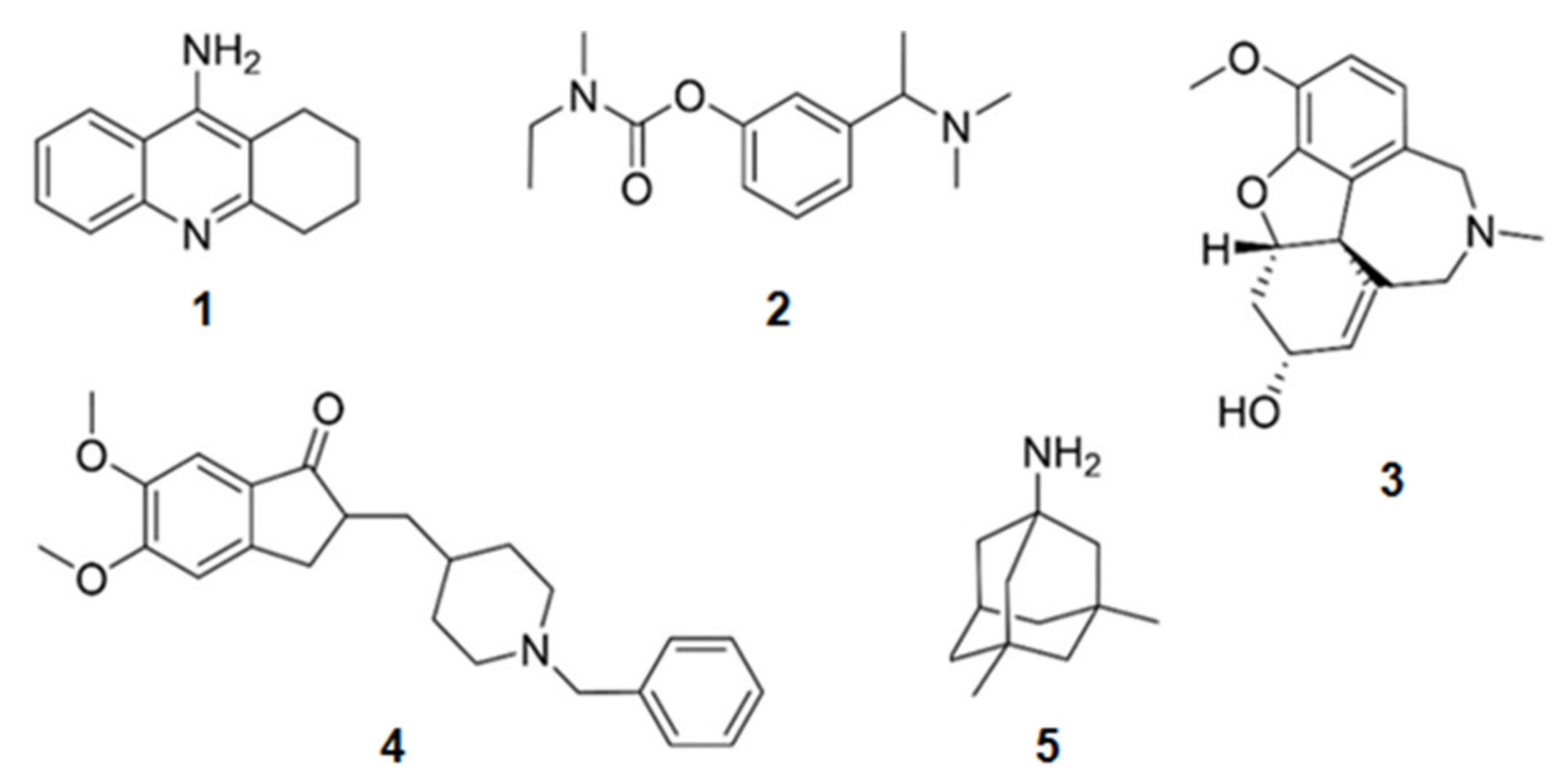

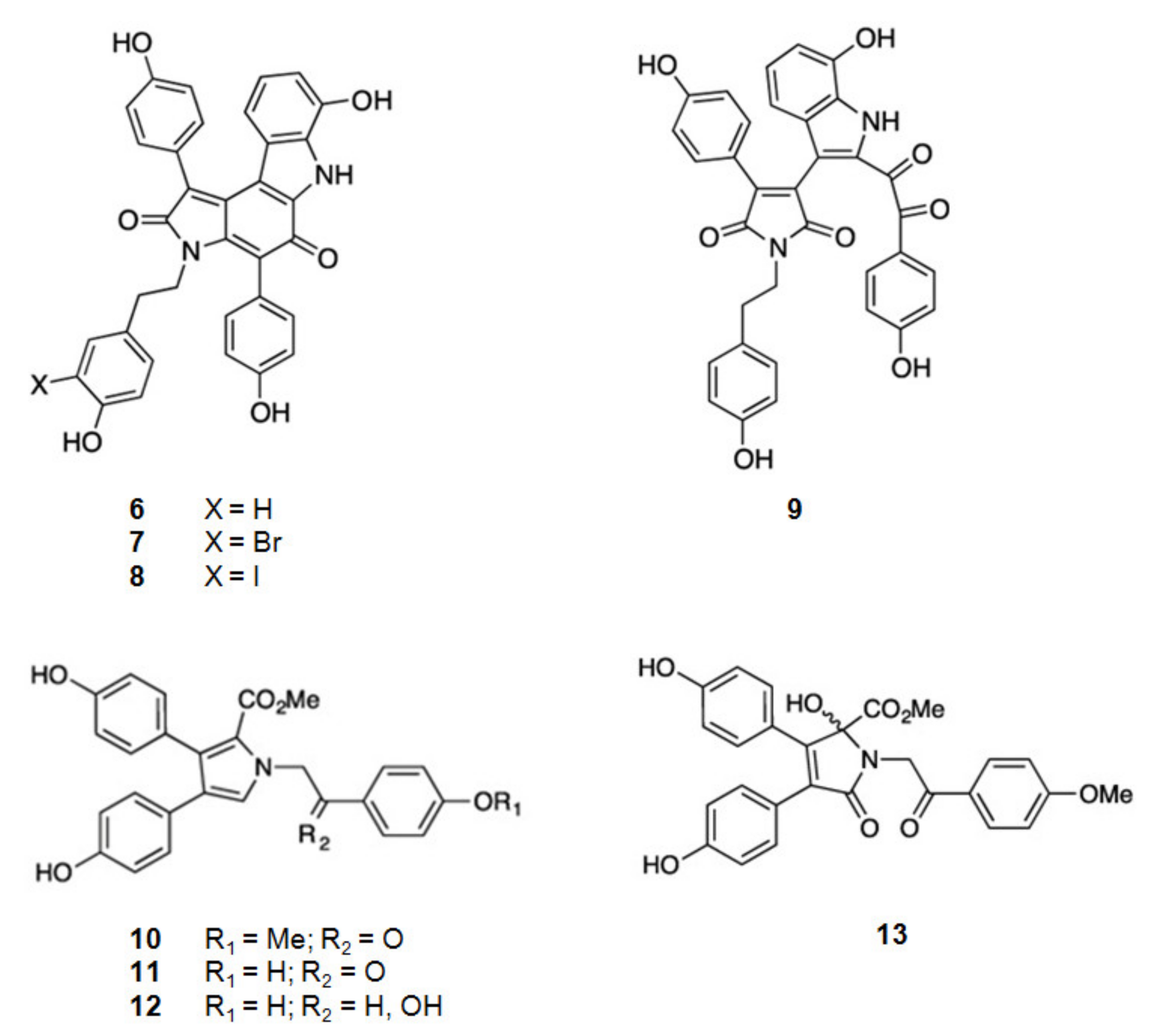
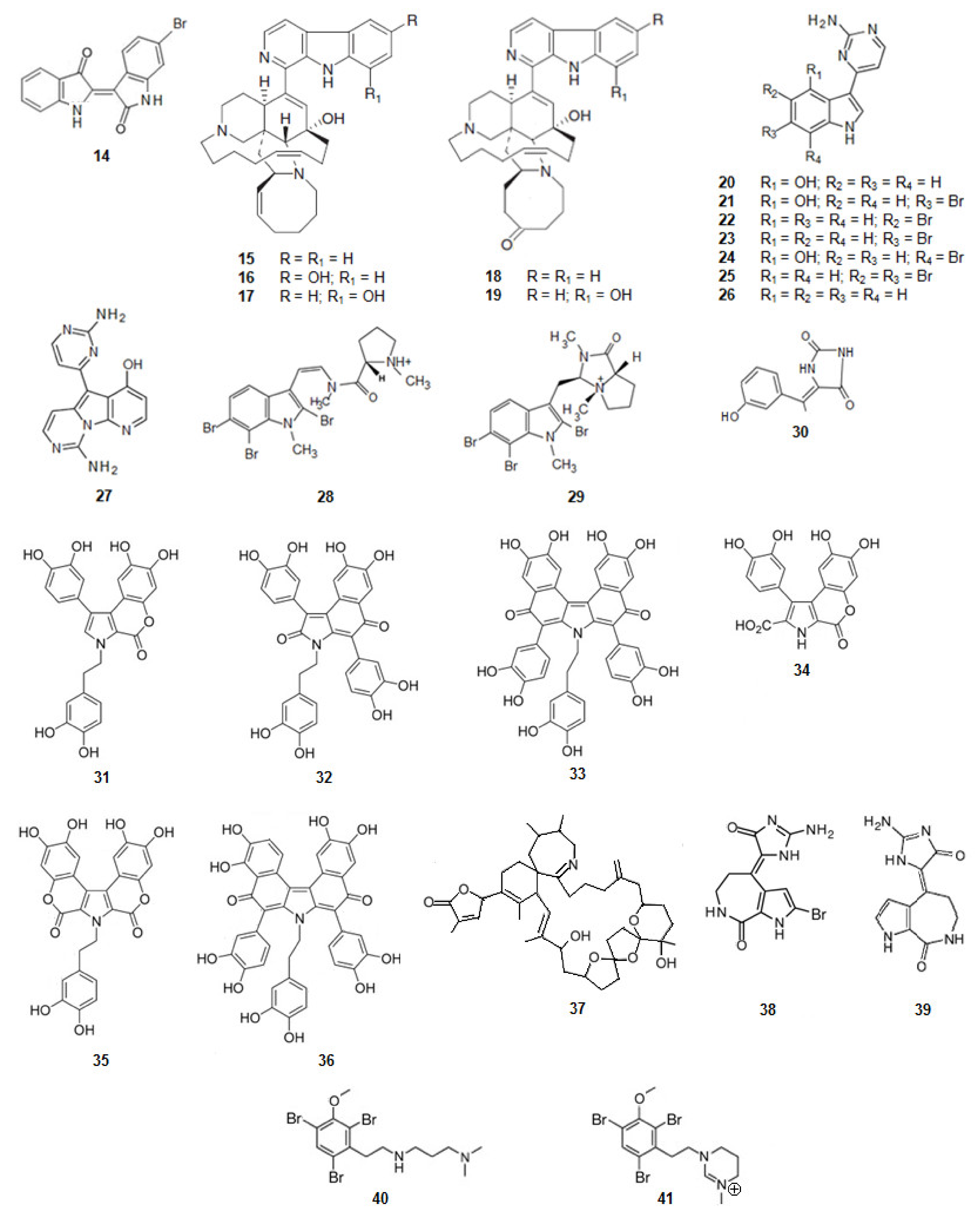


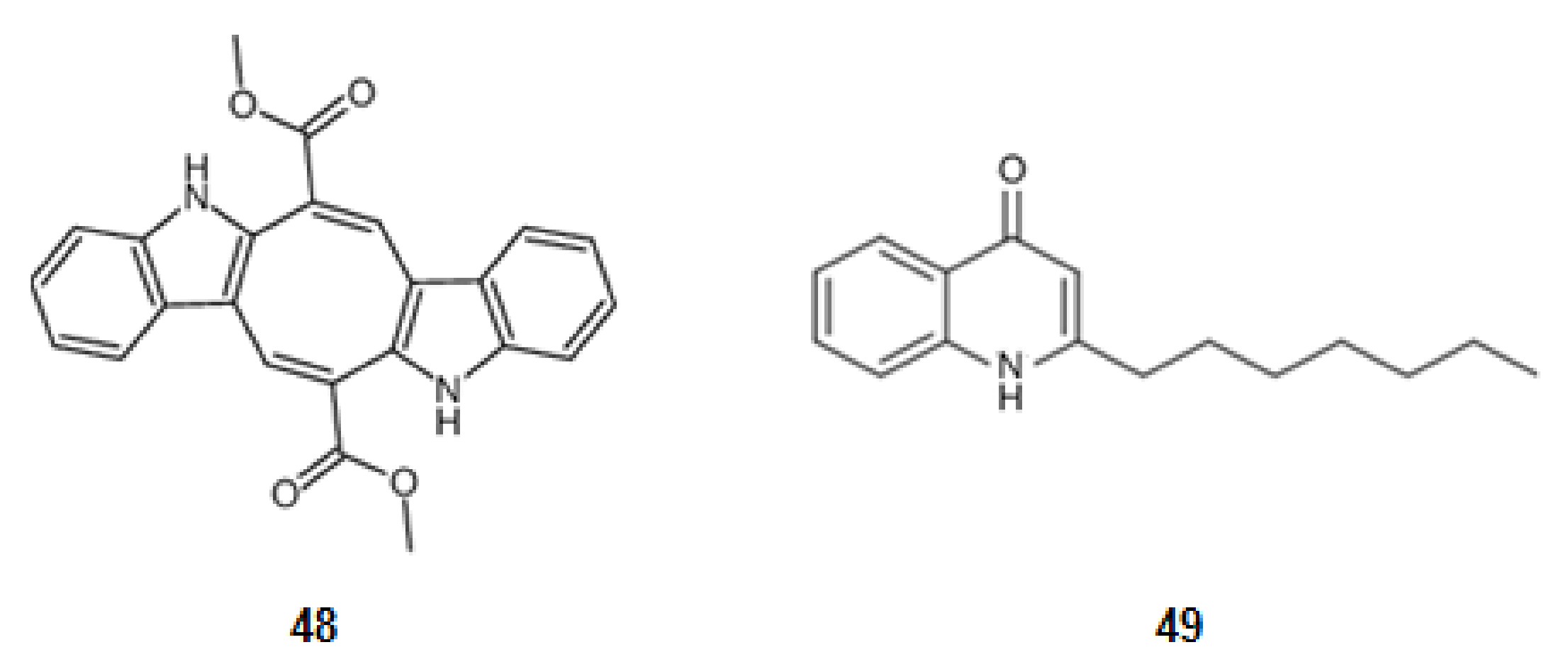


| Compound 1 | Mechanism of Action | IC50 (μM) | Ref. | |
|---|---|---|---|---|
| 6 | Dictyodendrin F | Inhibition of Aβ production | 1.5 | [66] |
| 7 | Dictyodendrin H | 1.0 | [66] | |
| 8 | Dictyodendrin I | 2.0 | [66] | |
| 9 | Dictyodendrin J | 2.0 | [66] | |
| 10 | Lamellarin O | >10 | [67] | |
| 11 | Lamellarin O1 | <10 | [67] | |
| 12 | Lamellarin O2 | >10 | [67] | |
| 13 | Iianthellidone F | >10 | [67] | |
| 14 | 6-Bromoindirubin | Inhibition of GSK3β | 0.045 | [68] |
| 15 | Manzamine A | 10.0 | [69] | |
| 16 | Manzamine Y | <25 | [69] | |
| 17 | 8-Hydroxymanzamine A | <25 | [69] | |
| 18 | Manzamine E | <25 | [69] | |
| 19 | Manzamine F | >25 | [69] | |
| 20 | Meridianin A | Inhibition of GSK3β | 1.3 | [70] |
| Inhibition of CKlδ | NE | |||
| 21 | Meridianin B | Inhibition of GSK3β | 0.5 | [70] |
| Inhibition of CKlδ | 1.0 | |||
| 22 | Meridianin C | Inhibition of GSK3β | 2.0 | [70] |
| Inhibition of CKlδ | 30.0 | |||
| 23 | Meridianin D | Inhibition of GSK3β | 2.5 | [70] |
| Inhibition of CKlδ | 100.0 | |||
| 24 | Meridianin E | Inhibition of GSK3β | 2.5 | [70] |
| Inhibition of CKlδ | 0.4 | |||
| 25 | Meridianin F | Inhibition of GSK3β | 2.0 | [70] |
| Inhibition of CKlδ | NE | |||
| 26 | Meridian G | Inhibition of GSK3β | 350 | [70] |
| Inhibition of CKlδ | NE | |||
| 27 | Variolin B | Inhibition of GSK3β | 0.07 | [71] |
| Inhibition of CKlδ | 0.005 | |||
| 28 | Kororamide A | Inhibition of GSK3β | NE | [72] |
| Inhibition of CKlδ | ||||
| Inhibition of DyrklA | ||||
| Inhibition of CLK1 | ||||
| 29 | Kororamide B | Inhibition of GSK3β | NE | [72] |
| Inhibition of CKlδ | ||||
| 30 | (Z)-5-(4-Hydroxybenzylidene)-hydantoin | Inhibition of GSK3β | 13.7 | [73] |
| 31 | Ningalin B | 0.8 | [74] | |
| 32 | Ningalin C | <0.2 | [74] | |
| 33 | Ningalin D | <0.2 | [74] | |
| 34 | Ningalin E | 1.6 | [74] | |
| 35 | Ningalin F | 3.1 | [74] | |
| 36 | Ningalin G | <0.5 | [74] | |
| 37 | 13-Desmethyl spirolide C | NE | [75] | |
| 38 | Hymenaldisine | Inhibition of GSK3β | 0.07 | [76] |
| Inhibition of CKlδ | 0.03 | |||
| 39 | Debromohymenialdisine | Inhibition of GSK3β | 0.2 | |
| Inhibition of CKlδ | 0.1 | |||
| 40 | Convolutamine I | Inhibition of GSK3β | NE | [72] |
| Inhibition of CKlδ | ||||
| 41 | Convolutamine J | Inhibition of GSK3β | ||
| Inhibition of CKlδ | ||||
| 42 | KH-CB 19 | Inhibition of DyrklA | 0.06 | [77] |
| Inhibition of CLK1 | 0.02 | |||
| 43 | Eleutherobin | MT-stabilizing | NE | [20] |
| 44 | Sarcodyctin A | |||
| 45 | Sarcodyctin B | |||
| 46 | Sarcodyctin C | |||
| 47 | Sarcodyctin D | |||
| 48 | Caulerpin | Inhibition of pro-inflammatory factors | NE | [78] |
| 49 | Pseudane-VII | [79] | ||
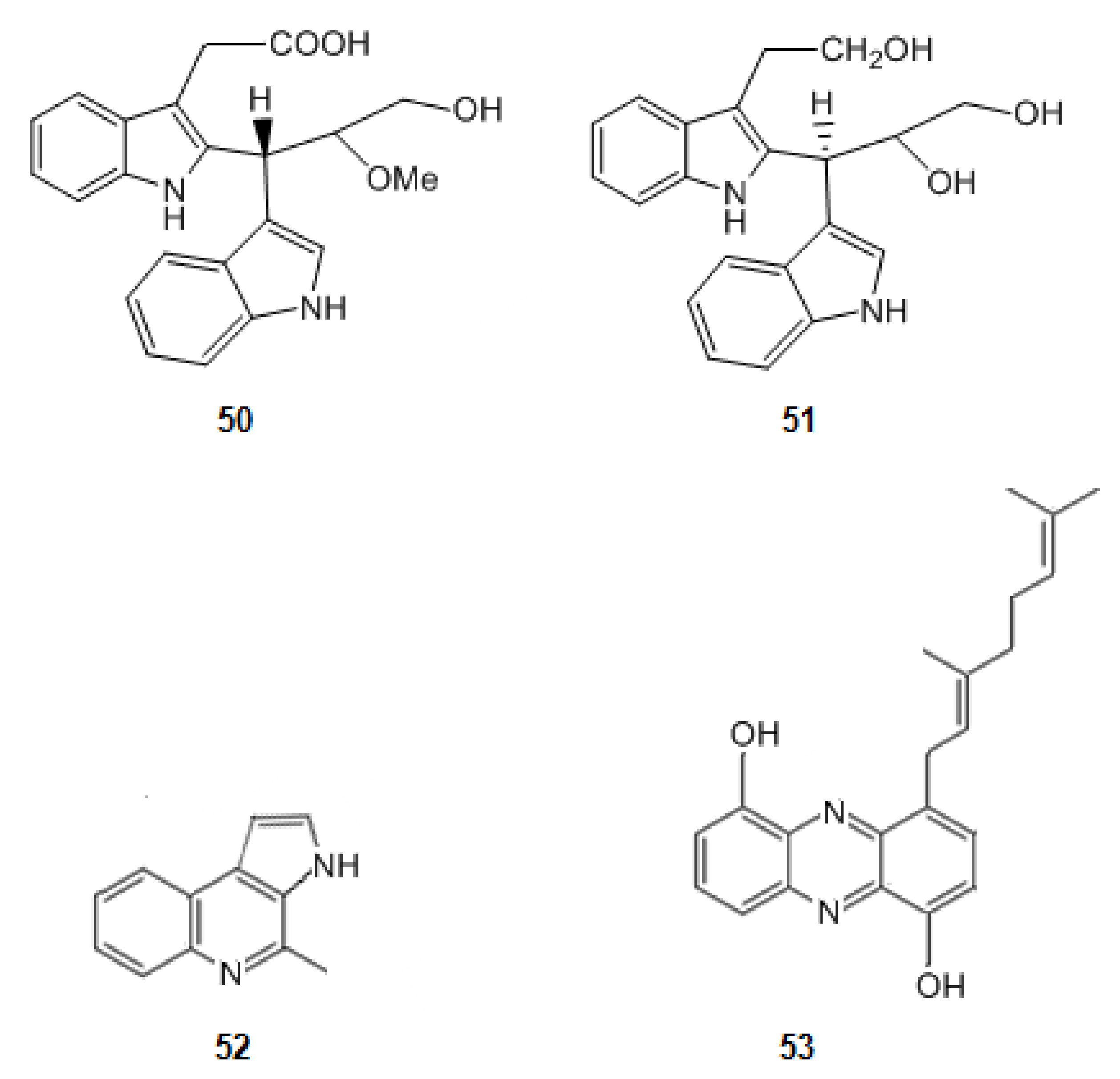
| 50 | 2-{2-[(1R)-3-Hydroxy-1-(1H-indol-3-yl)-2-methoxypropyl]-1H-indol-3-yl}acetic acid | Inhibition of AChE | 11.8 | [80] |
| 51 | (3S)-3-[3-(2-hydroxyethyl)-1H-indol-2-yl]-3-(1H-indol-3-yl)propane-1,2-diol | 13.5 | [80] | |
| 52 | Marinoquinoline | 4.9 | [80] | |
| 53 | Geranylphenazinediol | 2.6 | [81] | |
| 34 | Quinolactacin A1 | 280 | [82] | |
| 55 | Quinolactacin A2 | 19.8 | [82] | |
| 56 | Circumdantin C | 15.6 | [83] | |
| 57 | Circumdantin D | 8.7 | [83] | |
| 58 | Circumdantin F | 11.8 | [83] | |
| 59 | Circumdantin G | 18.9 | [83] | |
| 60 | Circumdantin H | 33.3 | [83] | |
| 61 | Circumdantin I | 18.6 | [83] | |
| 62 | 2-Hydroxycircumdantin C | 16.5 | [83] | |
| 63 | Irene-carboline A | 0.7 | [84] | |
| 64 | Irene-carboline B | 0.5 | [84] | |
| 65 | Turbotoxin A | 90.0 | [85] | |
| 66 | Pulmonarin B | 20.0 | [86] | |
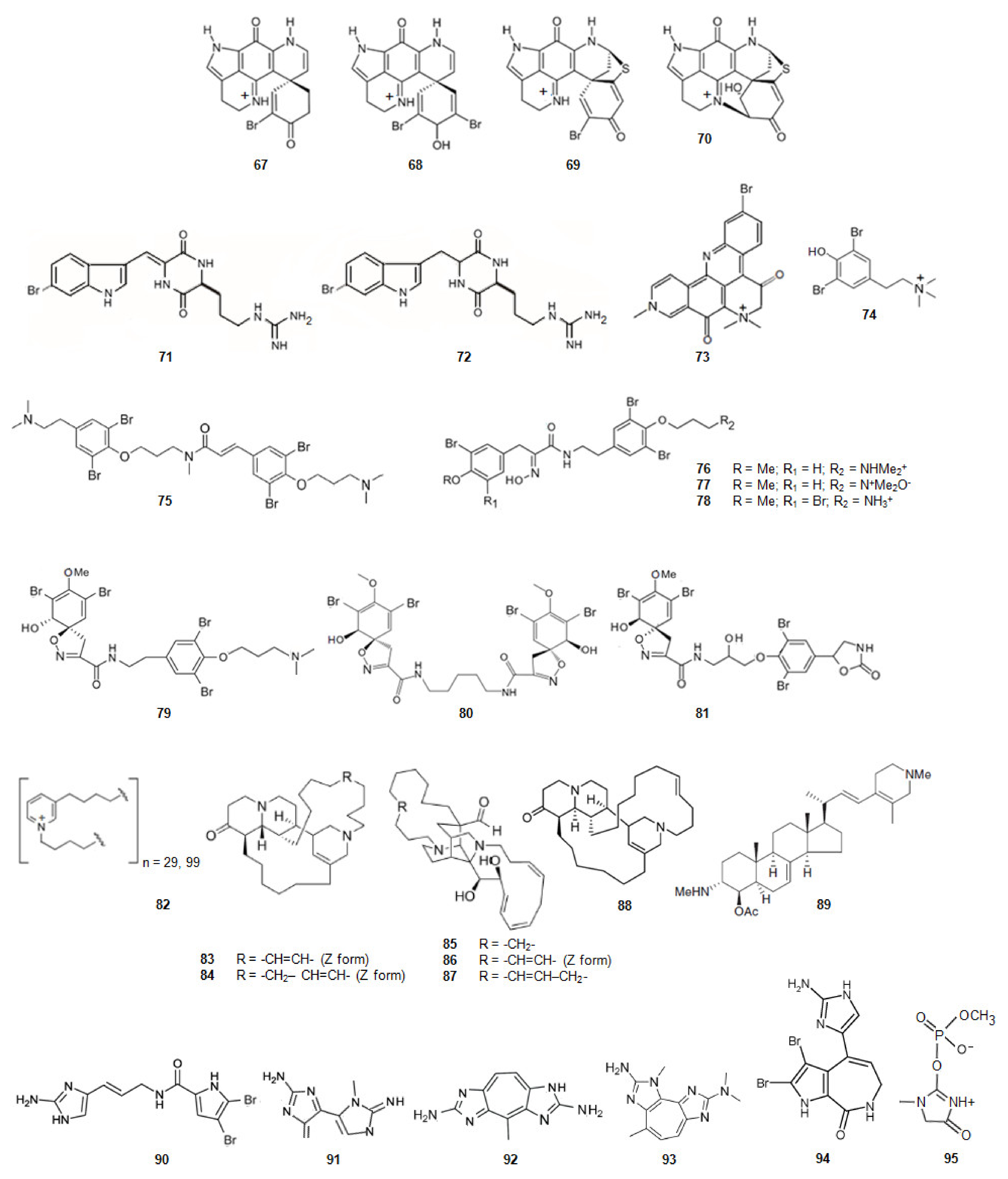
| 67 | (+)-Discorhabdin G | Inhibition of hAChE | 116.0 | [87] |
| 68 | (-)-3-Dihydro-7,8-dehydrodiscorhabdin C | 152.0 | ||
| 69 | (+)-Discorhabdin B | 49.4 | ||
| 70 | (-)-Discorhabdin L | 158.2 | ||
| 71 | Barettin | Inhibition of AChE | 36.0 | [80] |
| 72 | 8,9-Dihydrobarettin | 29.0 | [80] | |
| 73 | Petrosamine | 0.1 | [88] | |
| 74 | stryphnusin | 232.0 | [89] | |
| 75 | Psammaplysene D | 1.3 | [90] | |
| 76 | Aplysamine-2 | 1.3 | [80] | |
| 77 | Purpuramine J | NA | [80] | |
| 78 | Aplysamine-4 | 16.0 | [80] | |
| 79 | Purealidin Q | 1.2 | [80] | |
| 80 | Homoaerothioin | Inhibition of hAChE | 4.5 | [80] |
| 81 | Fistularin 1 | 47.5 | ||
| 82 | N-butyl(3-butyl-pyridinium)n | Inhibition of AChE | 0.1 | [91] |
| 83 | Saraine 1 | 6.4 | [92] | |
| 84 | Saraine 3 | 6.3 | [92] | |
| 85 | Saraine A | 4.4 | [92] | |
| 86 | Saraine B | 4.4 | [92] | |
| 87 | Saraine C | 8.4 | [92] | |
| 88 | Isosaraine 1 | 10.7 | [92] | |
| 89 | 4-Acetoxy-plakinamine B | 3.8 | [80] | |
| 90 | Oroidin | <0.5 | [93] | |
| 91 | PZT | 4.0 | [80] | |
| 92 | Parazoanthoxanthin A | 26.0 | [80] | |
| 93 | Pseudozoanthoxanthin | 12.2 | [80] | |
| 94 | Stevensine | Inhibition of hAChE | 14.6 | [80] |
| 95 | Ulosantoin | Inhibition of AChE | <0.1 | [80] |
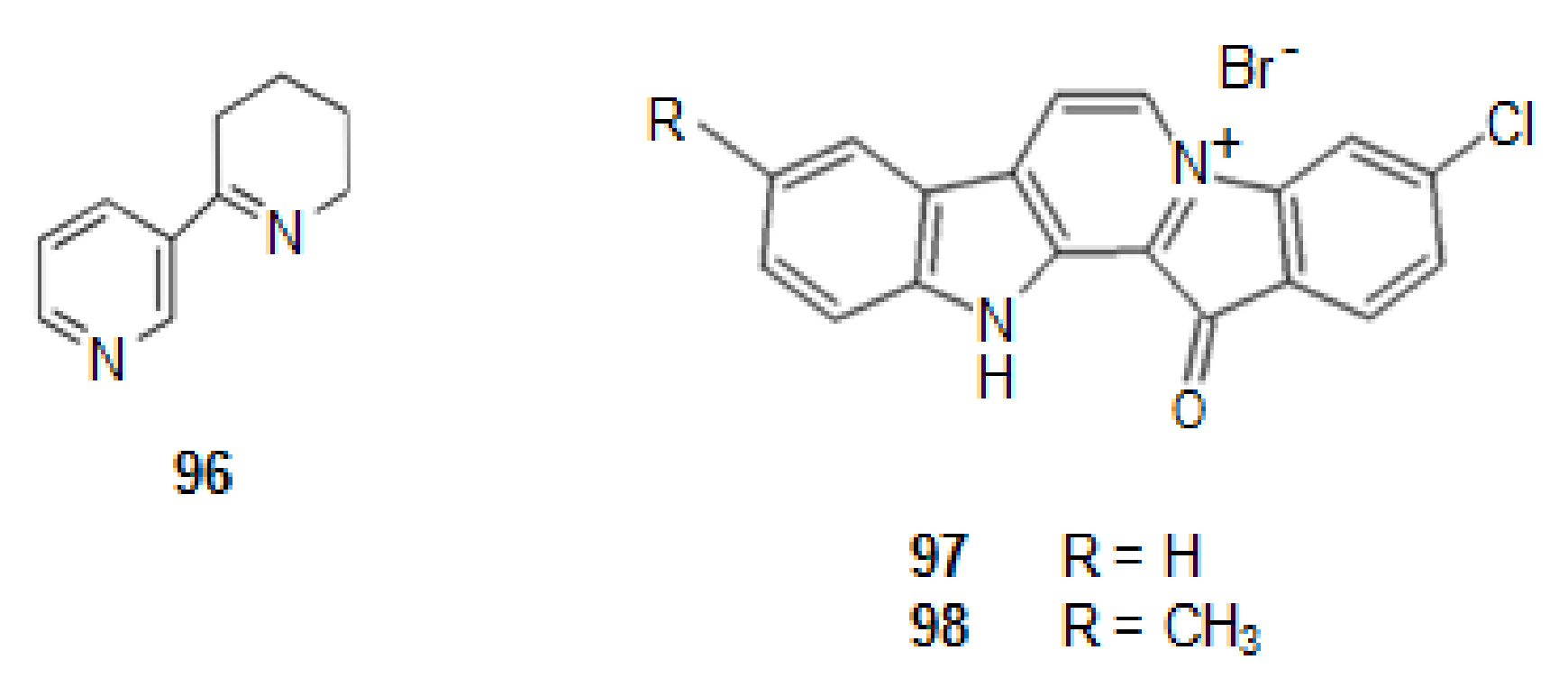
| 96 | Anabaseine | Stabilization of nAChRs | NE | [94] |
| 97 | Fascaplysin | Stabilization of nAChRs | NE | [95] |
| Inhibition of AChE | 1.49 | [80] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.; Medeiros, J. Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents. Mar. Drugs 2022, 20, 75. https://doi.org/10.3390/md20010075
Lima E, Medeiros J. Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents. Marine Drugs. 2022; 20(1):75. https://doi.org/10.3390/md20010075
Chicago/Turabian StyleLima, Elisabete, and Jorge Medeiros. 2022. "Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents" Marine Drugs 20, no. 1: 75. https://doi.org/10.3390/md20010075
APA StyleLima, E., & Medeiros, J. (2022). Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents. Marine Drugs, 20(1), 75. https://doi.org/10.3390/md20010075







