Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties
Abstract
:1. Introduction
2. Results
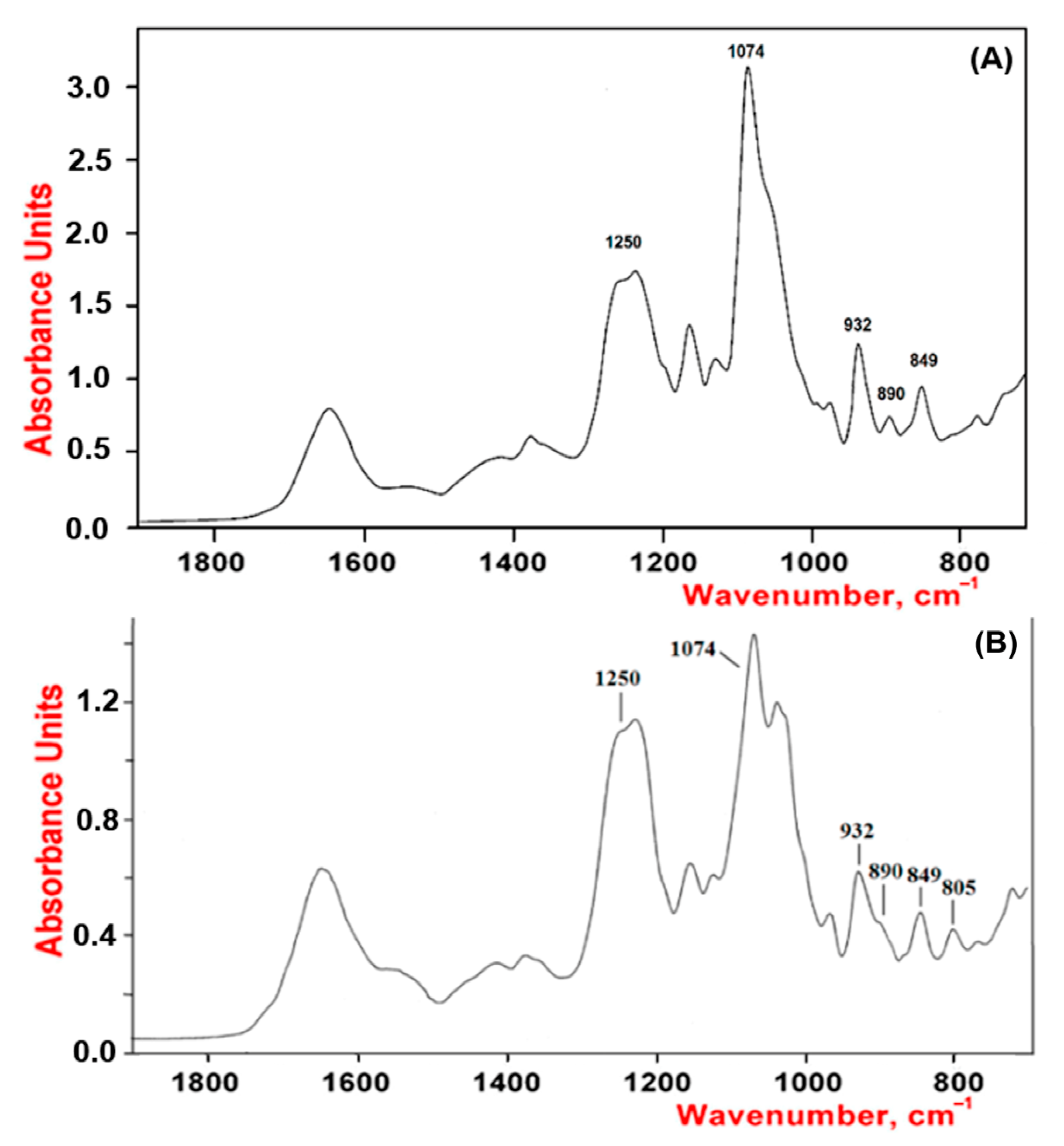
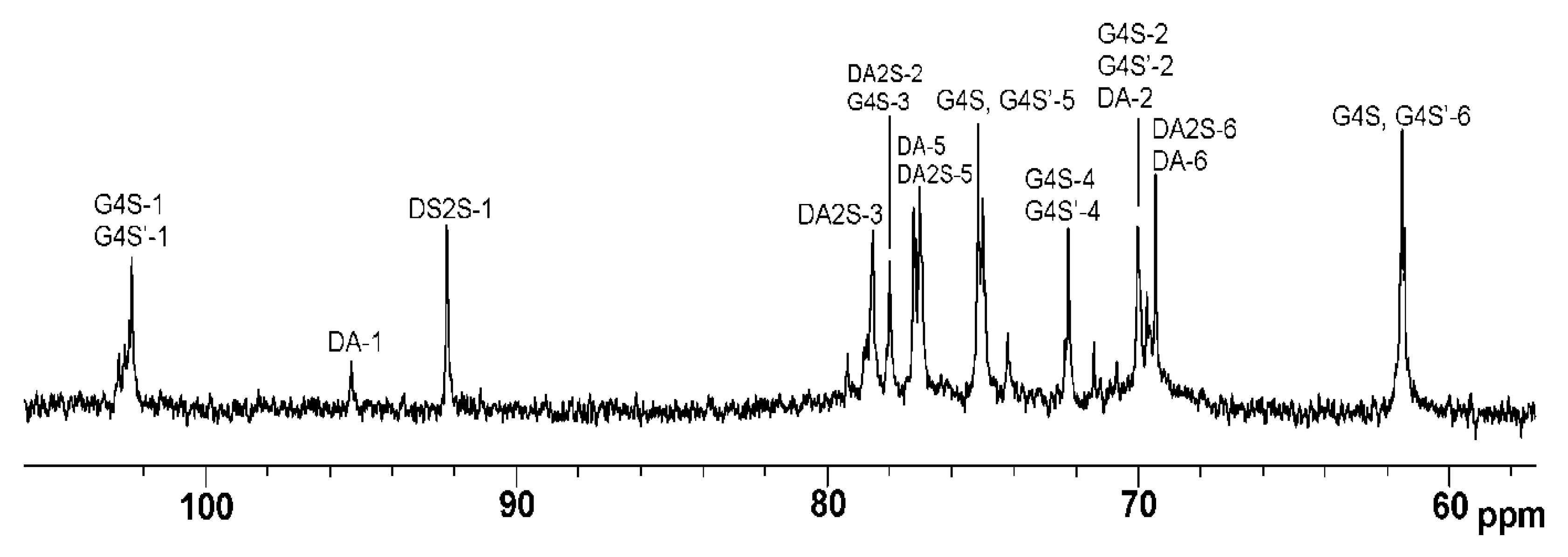
2.1. Characteristics of CRGs
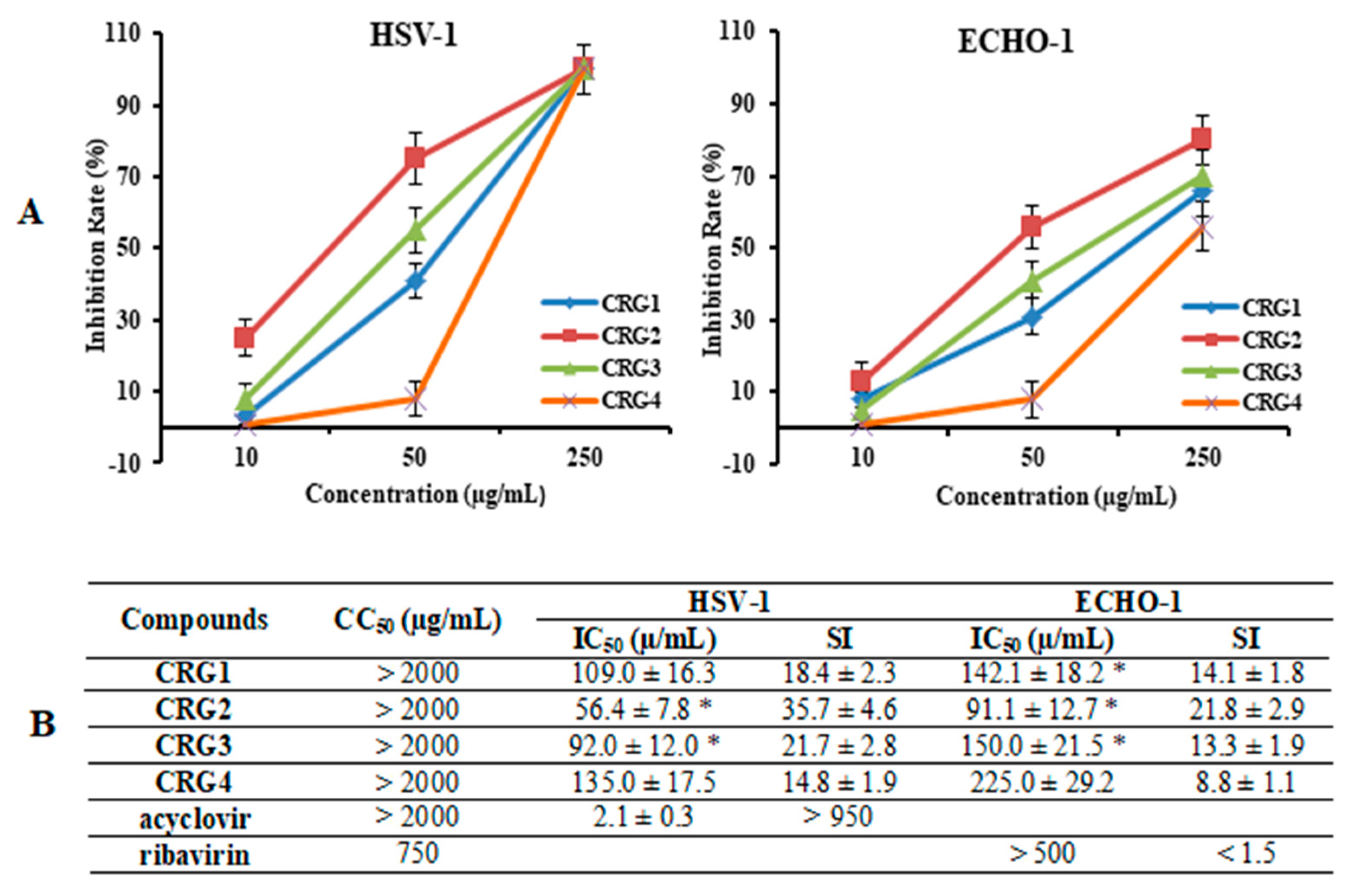
2.2. Cytotoxicity and Antiviral Activity of CRGs
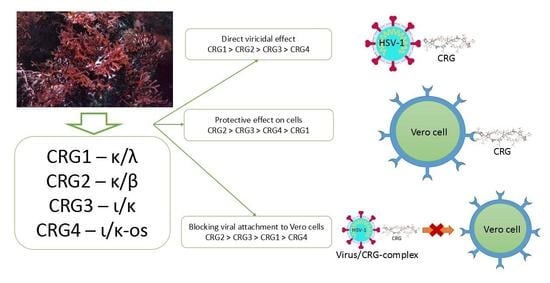
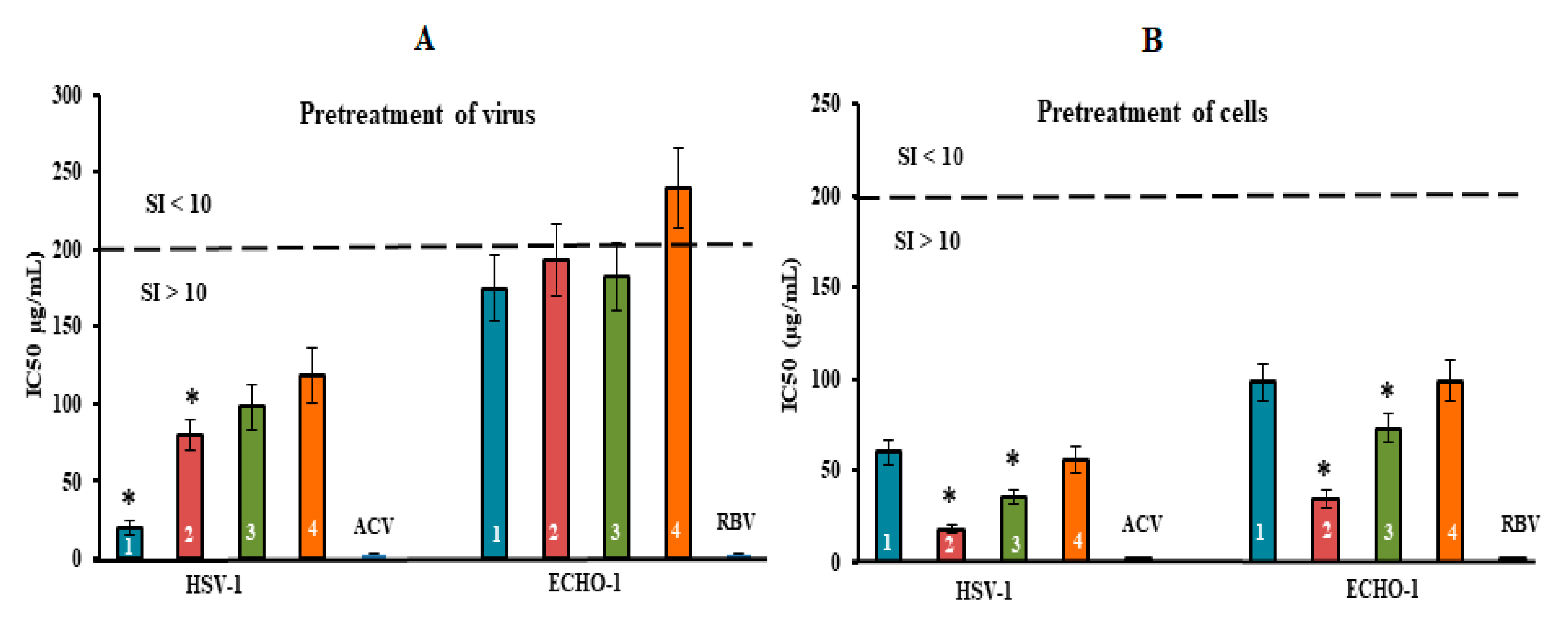
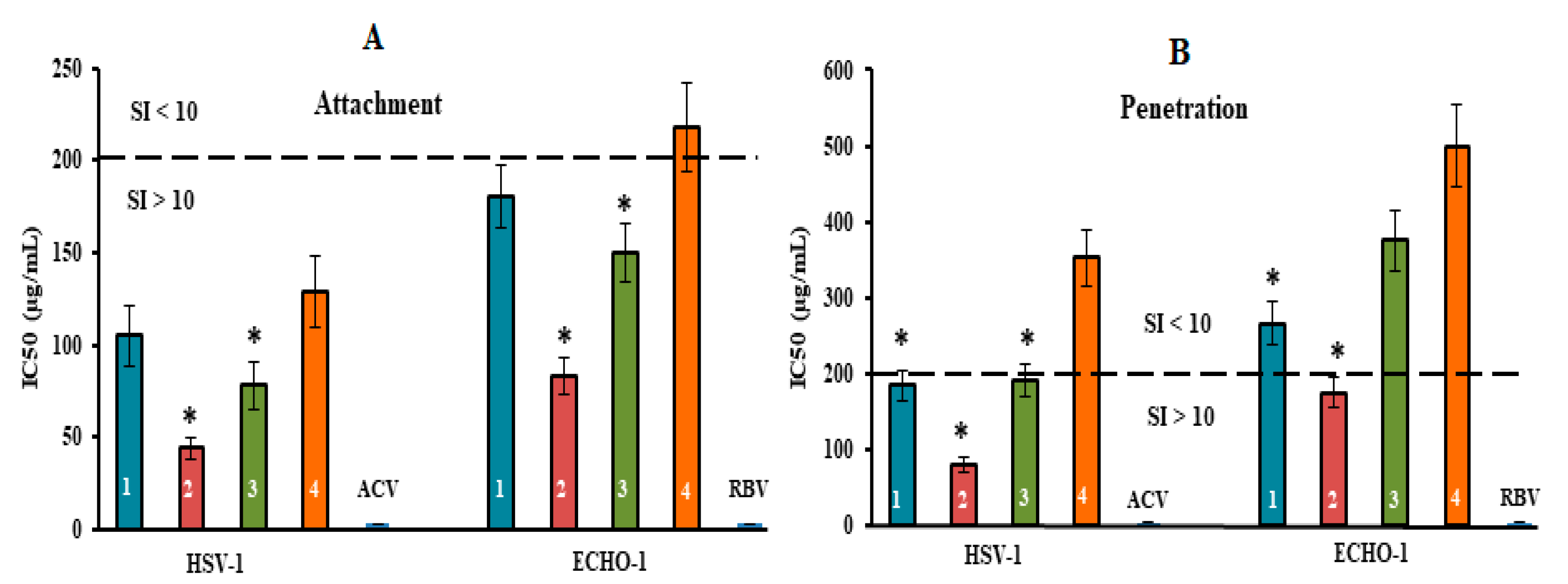
2.3. Antiviral Mode of Action of Investigated CRGs
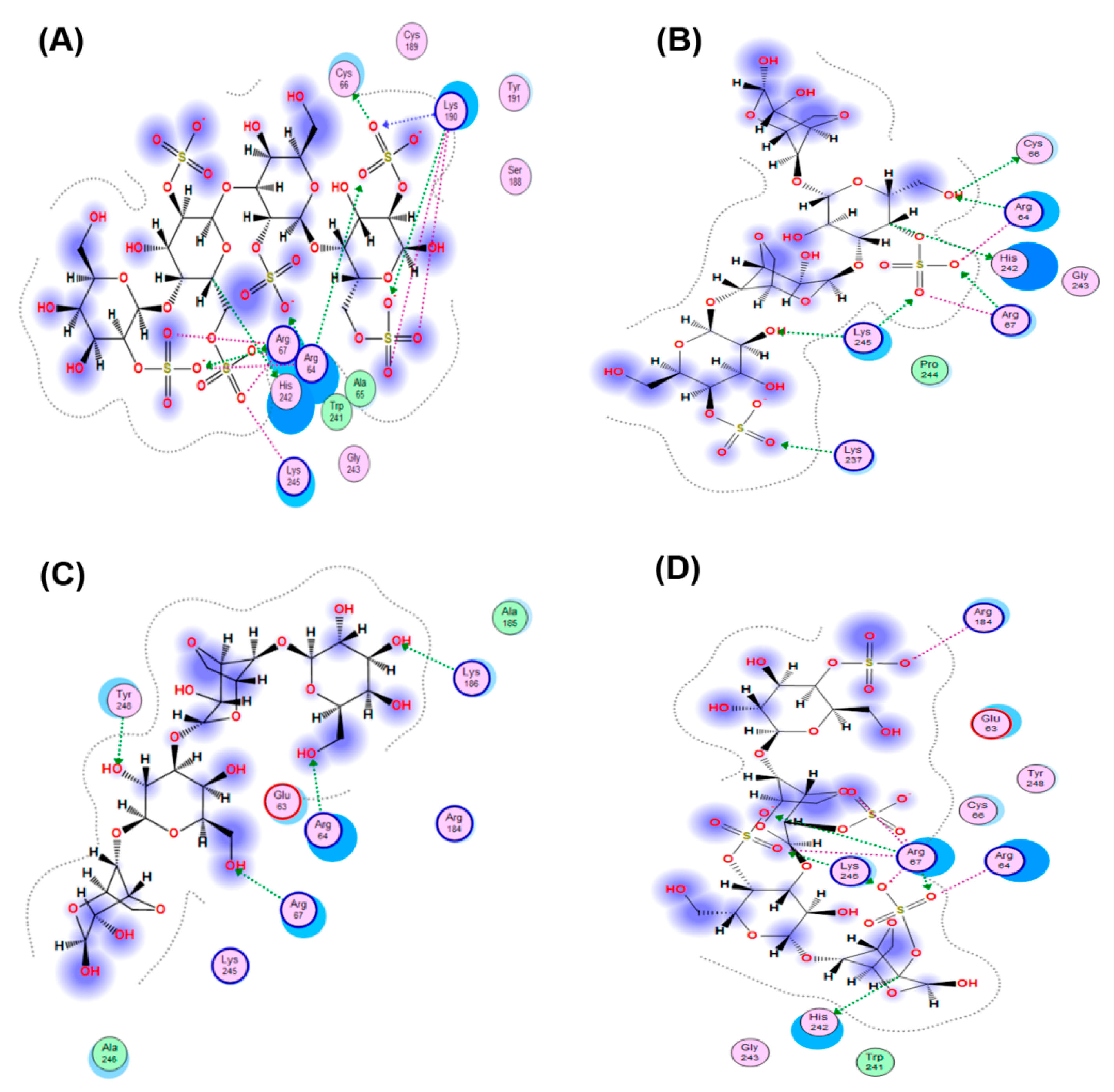
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Virus and Cell Culture
4.2. Algal Material
4.3. Extraction of Carrageenans
4.4. Obtaining Oligosaccharides by Mild Acid Hydrolysis
4.5. Analytical Methods
4.6. Molecular Weight Estimation of CRGs
4.7. Virological Methods
4.7.1. Cytotoxicity of the CRGs
4.7.2. Antiviral Activity of the CRGs
4.7.3. Time of Compound Addition Assay
- Pre-treatment of virus with compounds. The virus was mixed with compounds in a ratio of 1:1 (v/v), pre-incubated for 1 h at 37 °C, and then the mixture was used to infect cellular monolayers. After viral adsorption for 1 h at 37 °C, the cells were washed with PBS to remove unabsorbed virus and incubated with maintenance medium (DMEM) containing 1% CMC.
- Pre-treatment of cells with compounds. A monolayer of cells was pre-treated with compounds for 1 h at 37 °C before infection. The cells were washed with PBS to remove the compounds and infected with the virus for 1 h at 37 °C. Then, unabsorbed virus was removed by washing with PBS, and the cells were incubated with DMEM with 1% CMC.
- The attachment assay. The monolayer of cells was pre-chilled at 4 °C for 1 h and then treated with a mixture of virus and compound (1:1). After incubation at 4 °C for 3 h, the compounds and unabsorbed virus were removed by washing with cold PBS and the cells were incubated with DMEM with 1% CMC.
- The penetration assay. The monolayer of cells, pre-chilled at 4 °C for 1 h, was infected with the virus and incubated at 4 °C for 3 h. The unbound virus was removed with cold PBS and the infected cells were treated with the compounds and incubated for 1 h at 37 °C. Then, the unpenetrated virus was inactivated with citrate buffer (pH 3.0) and the cells were washed with PBS, and incubated with DMEM with 1% CMC.
- Treatment of infected cells. The monolayer of cells was infected with the virus at 37 °C for 1 h, then washed and overlaid with DMEM with 1% CMC containing different concentrations of the tested compounds.
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luescher-Mattli, M. Algae, A Possible Source for New Drugs in the Treatment of HIV and Other Viral Diseases. Curr. Med. Chem.-Anti-Infect. Agents 2003, 2, 219–225. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. Biomed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bourgougnon, N. Anti-HIV compounds from red seaweeds. In Recent Advances in Marine Biotechnology; Fingerman, M., Nagabhushanam, R., Eds.; Science Publisher Inc.: Enfield, NH, USA; Plymouth, UK, 2003; Volume 9, pp. 165–206. [Google Scholar]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral activity of carrageenans and processing implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, K.; Hahn, F.; Wangen, C.; Strojan, H.; Müller, R.; Anand, N.; Ali, I.; Bera, K.; Ray, B.; et al. Chemically sulfated polysaccharides from natural sources: Assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int. J. Biol. Macromol. 2019, 136, 521–530. [Google Scholar] [CrossRef]
- Zeng, K.; Groth, T.; Zhang, K. Recent Advances in Artificially Sulfated Polysaccharides for Applications in Cell Growth and Differentiation, Drug Delivery, and Tissue Engineering. ChemBioChem 2019, 20, 737–746. [Google Scholar] [CrossRef]
- Van De Velde, F.; Lourenço, N.D.; Pinheiro, H.M.; Bakker, M. Carrageenan: A Food-Grade and Biocompatible Support for Immobilisation Techniques. Adv. Synth. Catal. 2002, 344, 815–835. [Google Scholar] [CrossRef]
- Yermak, I.; Khotimchenko, Y. Chemical properties, biological activities and the applications of carrageenan from red algae. In Recent Advances in Marine Biotechnology; Fingerman, M., Nagabhushanam, R., Eds.; Science Publisher Inc.: Enfield, NH, USA; Plymouth, UK, 2003; Volume 9, pp. 207–255. [Google Scholar]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Carlucci, M.; Scolaro, L.; Noseda, M.; Cerezo, A.; Damonte, E. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Ogamo, A.; Saito, T.; Uchiyama, H.; Nakagawa, Y. Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohydr. Polym. 2000, 41, 115–120. [Google Scholar] [CrossRef]
- Haaland, R.E.; Chaowanachan, T.; Evans-Strickfaden, T.; van de Wijgert, J.H.; Kilmarx, P.H.; McLean, C.A.; Hart, C.E. Carrageenan-Based Gel Retains Limited Anti-HIV-1 Activity 8–24 Hours After Vaginal Application by HIV-Infected Thai Women Enrolled in a Phase I Safety Trial. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 61, e71–e73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T.; et al. Iota-carrageenan is a potent inhibitor of influenza a virus infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, P.; Hao, C.; Zhang, X.E.; Cui, Z.Q.; Guan, H.S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talarico, L.B.; Noseda, M.D.; Ducatti, D.R.B.; Duarte, M.E.R.; Damonte, E.B. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J. Gen. Virol. 2011, 92, 1332–1342. [Google Scholar] [CrossRef]
- Klimyte, E.M.; Smith, S.E.; Oreste, P.; Lembo, D.; Dutch, R.E. Inhibition of Human Metapneumovirus Binding to Heparan Sulfate Blocks Infection in Human Lung Cells and Airway Tissues. J. Virol. 2016, 90, 9237–9250. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Tian, D.; Zhou, M.; Xiao, W.; Zhang, Y.; Li, M.; Sui, B.; Wang, W.; Guan, H.; Chen, H.; et al. λ-Carrageenan P32 Is a Potent Inhibitor of Rabies Virus Infection. PLoS ONE 2015, 10, e0140586. [Google Scholar] [CrossRef]
- Frediansyah, A. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review. Clin. Epidemiol. Glob. Heal. 2021, 12, 100826. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chan, Y.L.; Tsai, L.W.; Li, T.L.; Wu, C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012, 95, 128–134. [Google Scholar] [CrossRef]
- Laurie, C.; El-Zein, M.; Coutlée, F.; De Pokomandy, A.; Franco, E.L. Carrageenan as a Preventive Agent against Human Papillomavirus Infection: A Narrative Review. Sex. Transm. Dis. 2021, 48, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef] [Green Version]
- Eccles, R.; Winther, B.; Johnston, S.L.; Robinson, P.; Trampisch, M.; Koelsch, S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial. Respir. Res. 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matloub, A.A.; Elsouda, S.S.M.; El-Senousy, W.M.; Hamed, M.; Aly, H.; Ali, S.A.; Mohammed, R.S.; Mahmoud, K.; El-Hallouty, S.; Ibrahim, N.A.; et al. In vitro antiviral, cytotoxic, antioxidant and hypolipidemic activites of polysaccharide isolated from marine algae. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1099–1111. [Google Scholar]
- Jabeen, M.; Dutot, M.; Fagon, R.; Verrier, B.; Monge, C. Seaweed sulfated polysaccharides against respiratory viral infections. Pharmaceutics 2021, 13, 733. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef]
- Reunov, A.; Nagorskaya, V.; Lapshina, L.; Yermak, I.; Barabanova, A. Effect of κ/β-Carrageenan from red alga Tichocarpus crinitus (Tichocarpaceae) on infection of detached tobacco leaves with tobacco mosaic virus. J. Plant Dis. Prot. 2004, 111, 165–172. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Yermak, I.M.; Kim, Y.H.; Titlynov, E.A.; Isakov, V.V.; Solov’eva, T.F. Chemical structure and gel properties of carrageenans from algae belonging to the Gigartinaceae and Tichocarpaceae, collected from the Russian Pacific Coast. J. Appl. Phycol. 1999, 11, 41–48. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Anastyuk, S.D.; Isakov, V.V.; Sokolova, E.V.; Glazunov, V.P.; Yermak, I.M. Structural peculiarities of polysaccharide from sterile form of Far Eastern red alga Ahnfeltiopsis flabelliformis. Carbohydr. Polym. 2014, 111, 1–9. [Google Scholar] [CrossRef]
- Barabanova, A.O.; Yermak, I.M.; Glazunov, V.P.; Isakov, V.V.; Titlyanov, E.A.; Solov’eva, T.F. Comparative study of carrageenans from reproductive and sterile forms of Tichocarpus crinitus (Gmel.) Rupr (Rhodophyta, Tichocarpaceae). Biochemistry 2005, 70, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Correc, G.; Barabanova, A.; Tuvikene, R.; Truus, K.; Yermak, I.; Helbert, W. Comparison of the structures of hybrid κ/β-carrageenans extracted from Furcellaria lumbricalis and Tichocarpus crinitus. Carbohydr. Polym. 2012, 88, 31–36. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Anastyuk, S.D.; Sokolova, E.V.; Isakov, V.V.; Glazunov, V.P.; Helbert, W.; Yermak, I.M. Structural analysis and cytokine-induced activity of gelling sulfated polysaccharide from the cystocarpic plants of Ahnfeltiopsis flabelliformis. Carbohydr. Polym. 2016, 151, 523–534. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Arii, J.; Kawaguchi, Y. The Role of HSV Glycoproteins in Mediating Cell Entry; Springer: Singapore, 2018; pp. 3–21. [Google Scholar]
- O’Donnell, C.D.; Kovacs, M.; Akhtar, J.; Valyi-Nagy, T.; Shukla, D. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology 2010, 397, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishchenko, N.P.; Krylova, N.V.; Iunikhina, O.V.; Vasileva, E.A.; Likhatskaya, G.N.; Pislyagin, E.A.; Tarbeeva, D.V.; Dmitrenok, P.S.; Fedoreyev, S.A. Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1. Mar. Drugs 2020, 18, 550. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocoll. 2008, 22, 727–734. [Google Scholar] [CrossRef]
- Stortz, C.A. Carrageenans: Structural and conformational studies. In Handbook of Carbohydrate Engineering; CRC Press: Boca Raton, FL, USA, 2005; pp. 211–246. [Google Scholar]
- Sokolova, E.V.; Barabanova, A.O.; Homenko, V.A.; Solov’eva, T.F.; Bogdanovich, R.N.; Yermak, I.M. In Vitro and Ex Vivo Studies of Antioxidant Activity of Carrageenans, Sulfated Polysaccharides from Red Algae. Bull. Exp. Biol. Med. 2011, 150, 426–428. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Barabanova, A.O.; Correc, G.; Nazarenko, E.L.; Davydova, V.N.; Helbert, W.; Dmitrenok, P.S.; Yermak, I.M. Analysis of structural heterogeneity of κ/β-carrageenan oligosaccharides from Tichocarpus crinitus by negative-ion ESI and tandem MALDI mass spectrometry. Carbohydr. Polym. 2011, 86, 546–554. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Kravchenko, A.O.; Chistyulin, D.A.; Pimenova, E.A.; Glazunov, V.P. Mucoadhesive properties of sulphated polysaccharides carrageenans from red seaweed families Gigartinaceae and Tichocarpaceae. Int. J. Biol. Macromol. 2020, 142, 634–642. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.; Faria, P.C.; Scolaro, L.A.; Duarte, M.E.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Van de Velde, F.; Peppelman, H.A.; Rollema, H.S.; Tromp, R.H. On the structure of κ/ι-hybrid carrageenans. Carbohydr. Res. 2001, 331, 271–283. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: Correlation between structure and biological activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef]
- Usov, A.I.; Elashvili, M.Y. Investigation of Sulfated Galactan from Laurencia nipponica Yamada (Rhodophyta, Rhodomelaceae) Using Partial Reductive Hydrolysis. Bot. Mar. 1991, 34, 553–560. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Anastyuk, S.D.; Glazunov, V.P.; Sokolova, E.V.; Isakov, V.V.; Yermak, I.M. Structural characteristics of carrageenans of red alga Mastocarpus pacificus from sea of Japan. Carbohydr. Polym. 2020, 229, 115518. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochas, C.; Rinaudo, M.; Landry, S. Role of the molecular weight on the mechanical properties of kappa carrageenan gels. Carbohydr. Polym. 1990, 12, 255–266. [Google Scholar] [CrossRef]
- Park, J.T.; Johnson, M.J. A submicrodetermination of glucose. J. Biol. Chem. 1949, 181, 149–151. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pott, A.B.; Krylova, N.V.; Kravchenko, A.O.; Yermak, I.M.; Lavrov, V.F. Activity of carraginanes from red algae for infections caused by the herpes simplex virus. Sanit. Vrač (Sanit. Dr.), 2020; 10–20. [Google Scholar] [CrossRef]
- Killington, R.A.; Powell, K.L. Growth, Assay, and Purification of Herpesviruses. In Virology: A Practical Approach; Mahy, B.W., Ed.; IRL Press: Oxford, UK, 1991. [Google Scholar]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The amphibian antimicrobial peptide temporin b inhibits in vitro herpes simplex virus 1 infection. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Wu, Y.; Bi, J.; Wang, S.; Li, F.; Kong, W.; Barbier, J.; Cintrat, J.-C.; Gao, F.; Gillet, D.; et al. Antiviral Effects of ABMA against Herpes Simplex Virus Type 2 In Vitro and In Vivo. Viruses 2018, 10, 119. [Google Scholar] [CrossRef] [Green Version]

| Source of CRG | Sample of CRGs | Structure of Disaccharide Repeating Unit | Molar Ratio AnGal: Gal:SO4−2 | MW, kDa | |
|---|---|---|---|---|---|
| 3-Linked | 4-Linked | ||||
| ΣCRG κ + λ C. armatus | CRG κ CRG λ | G4S G2S | DA D2S,6S | 1:15:1.8 1:18:12.5 | 185.0 |
| T. crinitus | CRG2 κ/β- | G4S/G | DA/DA | 1: 0.75:0.5 | 413.0 |
| A. flabelliformis | CRG3 ι/κ- | G4S/G4S | DA2S/DA | 1:1.8:2.9 | 307.0 |
| CRG3 ι/κ-OS | G4S/G4S | DA2S/DA | 1:1.6:2.0 | 9.1 | |
| CRG Units | E (kcal/mol) | Don | h-Acceptor | Ionic |
|---|---|---|---|---|
| β | −11.8 | – | 5 | – |
| k | −43.4 | 2 | 5 | 5 |
| i | −59.2 | 1 | 4 | 15 |
| λ | −75.9 | 2 | 7 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krylova, N.V.; Kravchenko, A.O.; Iunikhina, O.V.; Pott, A.B.; Likhatskaya, G.N.; Volod’ko, A.V.; Zaporozhets, T.S.; Shchelkanov, M.Y.; Yermak, I.M. Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties. Mar. Drugs 2022, 20, 60. https://doi.org/10.3390/md20010060
Krylova NV, Kravchenko AO, Iunikhina OV, Pott AB, Likhatskaya GN, Volod’ko AV, Zaporozhets TS, Shchelkanov MY, Yermak IM. Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties. Marine Drugs. 2022; 20(1):60. https://doi.org/10.3390/md20010060
Chicago/Turabian StyleKrylova, Natalia V., Anna O. Kravchenko, Olga V. Iunikhina, Anastasia B. Pott, Galina N. Likhatskaya, Aleksandra V. Volod’ko, Tatyana S. Zaporozhets, Mikhail Y. Shchelkanov, and Irina M. Yermak. 2022. "Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties" Marine Drugs 20, no. 1: 60. https://doi.org/10.3390/md20010060
APA StyleKrylova, N. V., Kravchenko, A. O., Iunikhina, O. V., Pott, A. B., Likhatskaya, G. N., Volod’ko, A. V., Zaporozhets, T. S., Shchelkanov, M. Y., & Yermak, I. M. (2022). Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties. Marine Drugs, 20(1), 60. https://doi.org/10.3390/md20010060