A Novel Gelatinase from Marine Flocculibacter collagenilyticus SM1988: Characterization and Potential Application in Collagen Oligopeptide-Rich Hydrolysate Preparation
Abstract
:1. Introduction
2. Results and Discussion
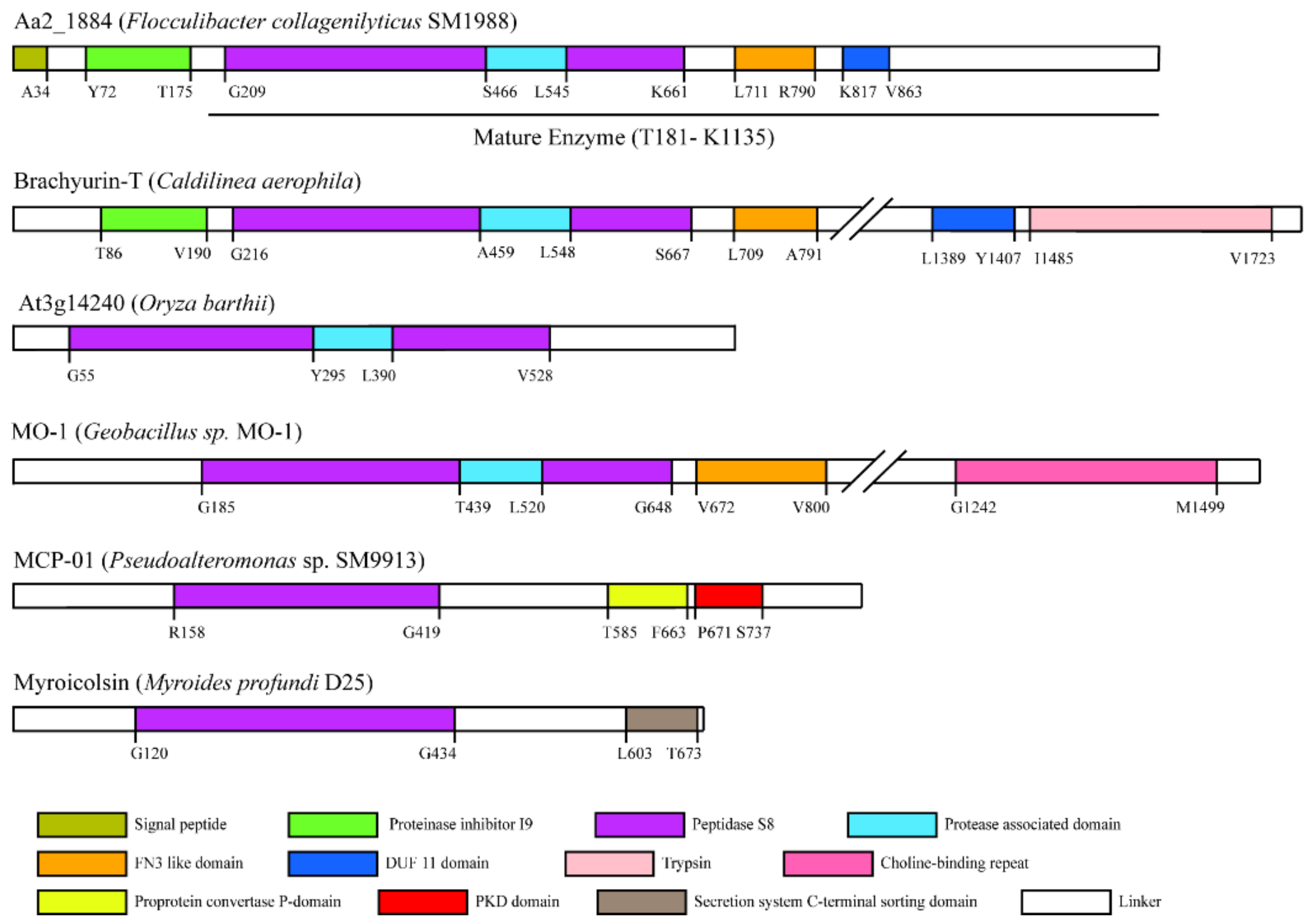
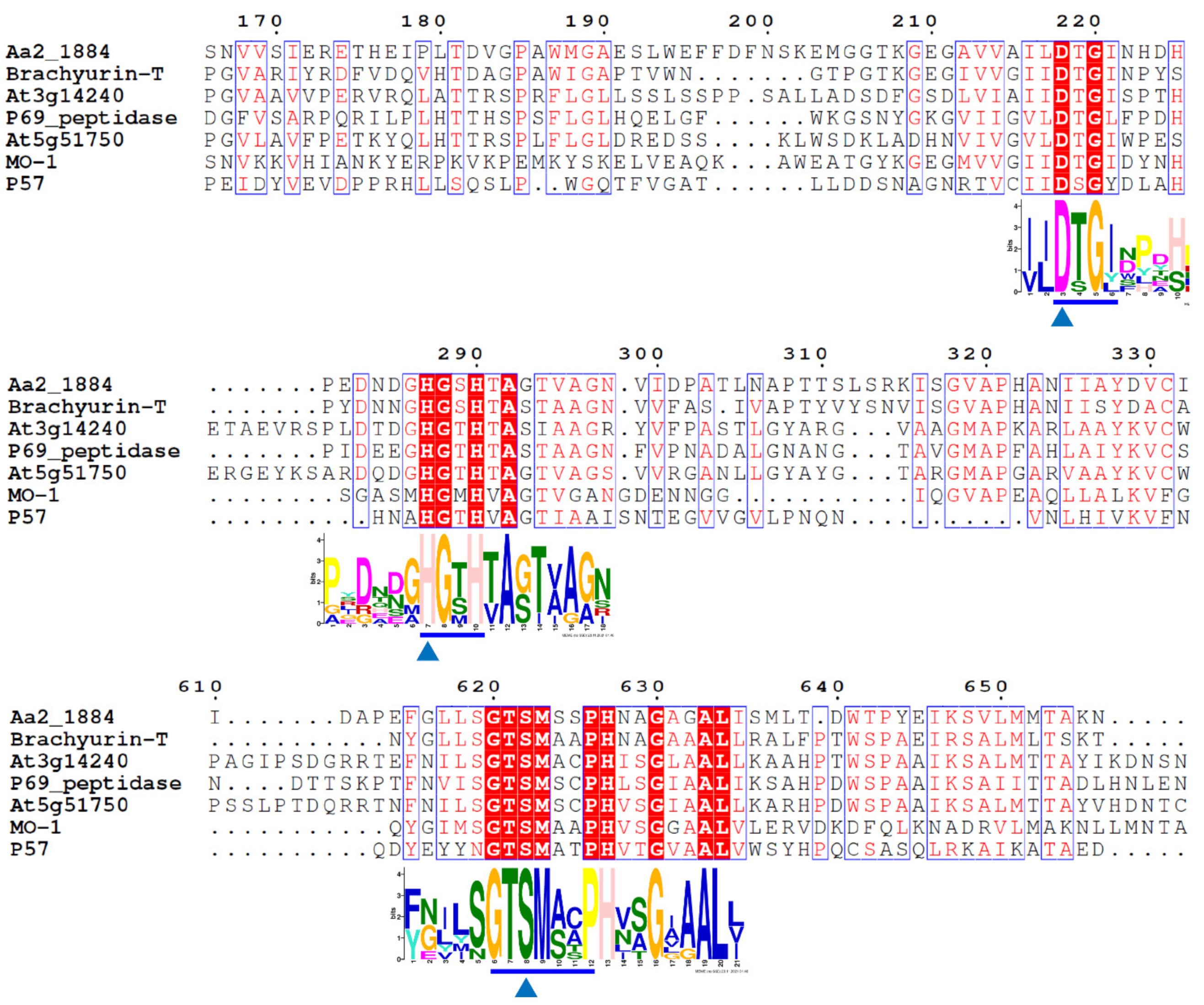
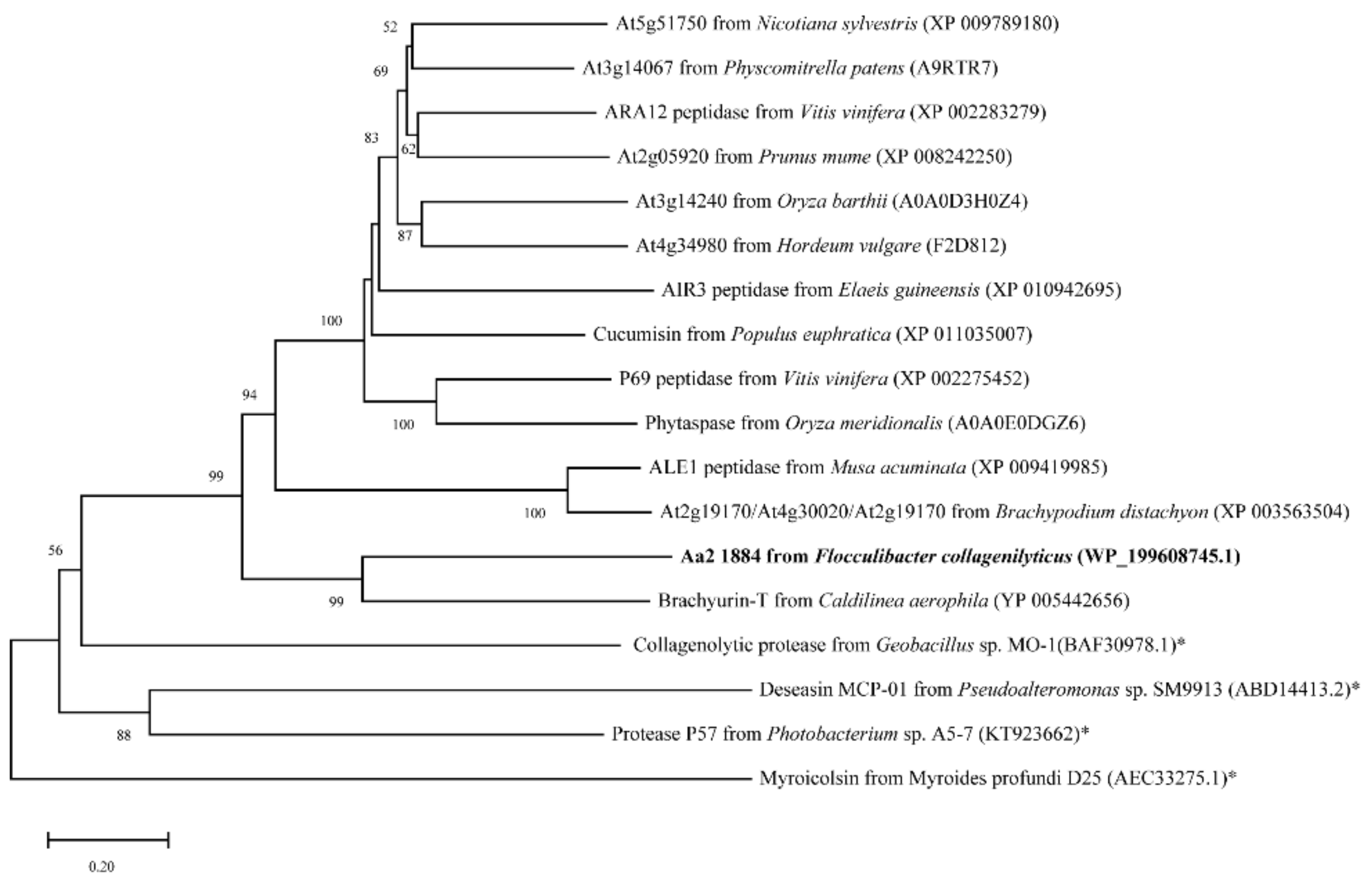
2.1. Aa2_1884 Is a Novel Multimodular Peptidase of the S8 Family
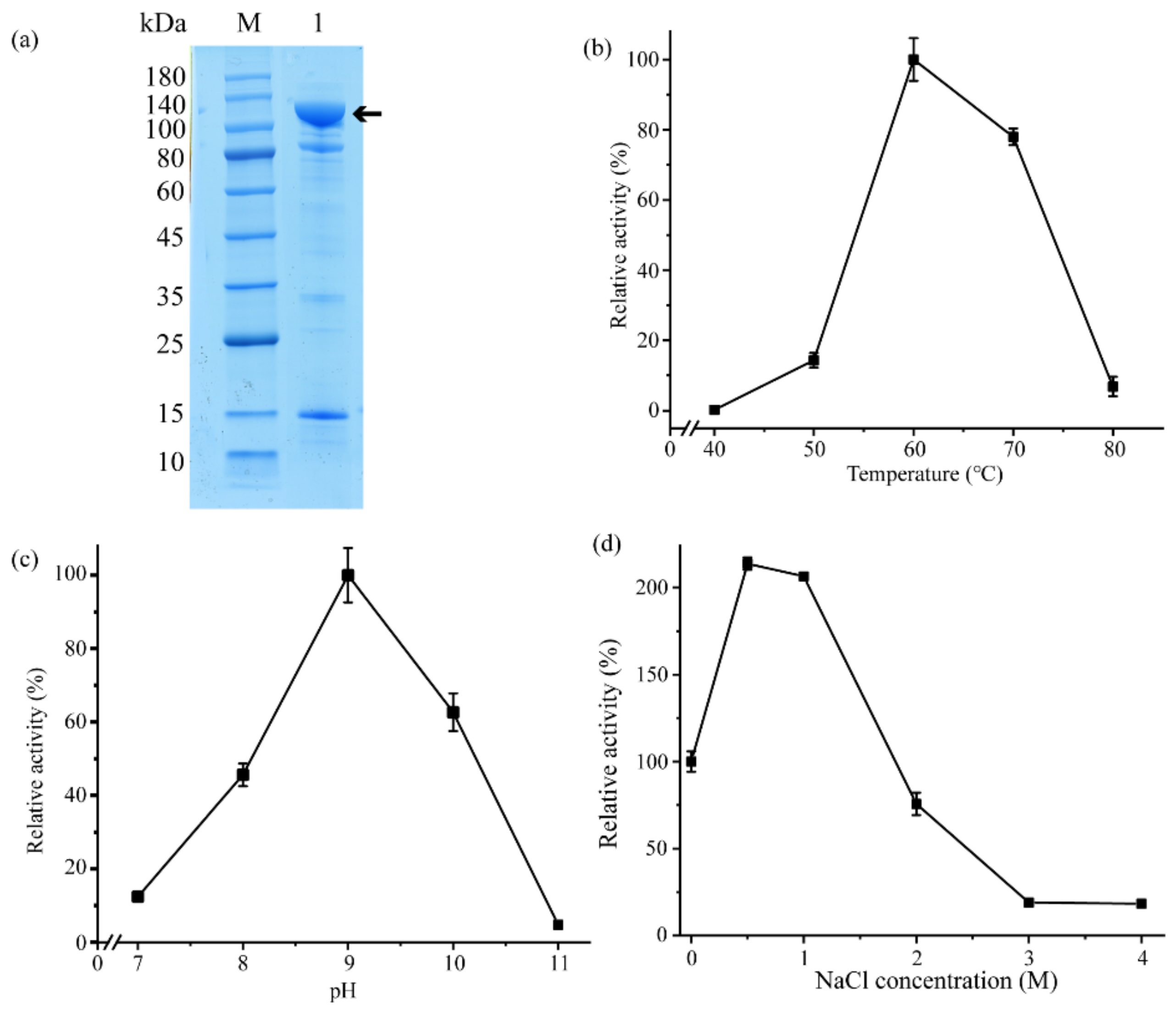
2.2. Aa2_1884 Is a Gelatinase with High Activity toward Denatured Collagens
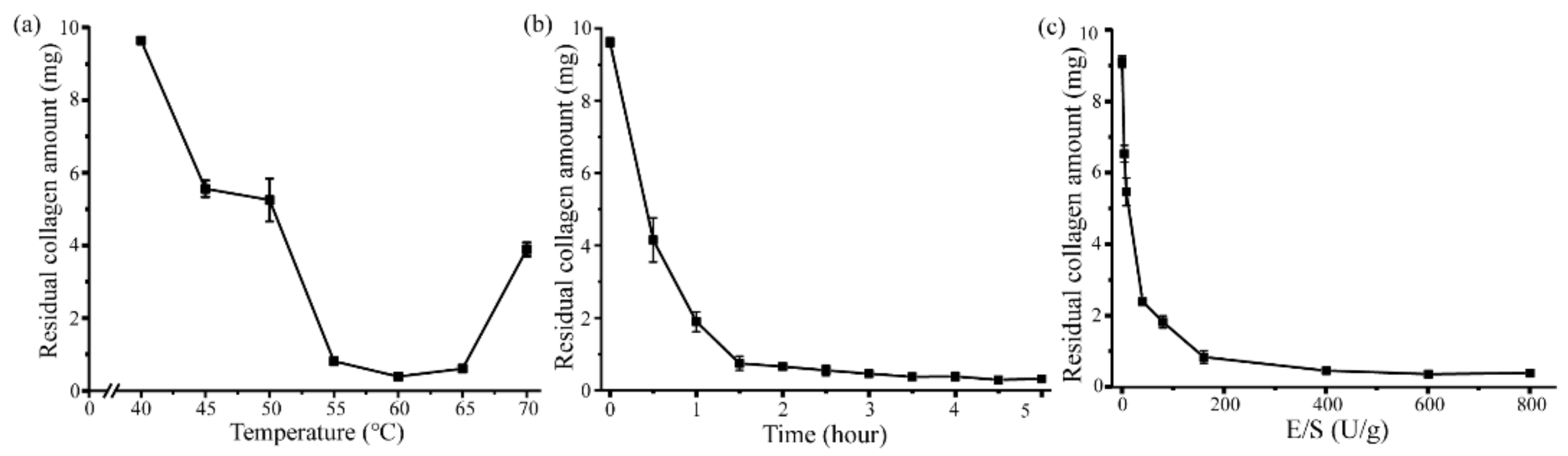
2.3. Aa2_1884 Shows High Hydrolytic Efficiency on Bovine Bone Collagen
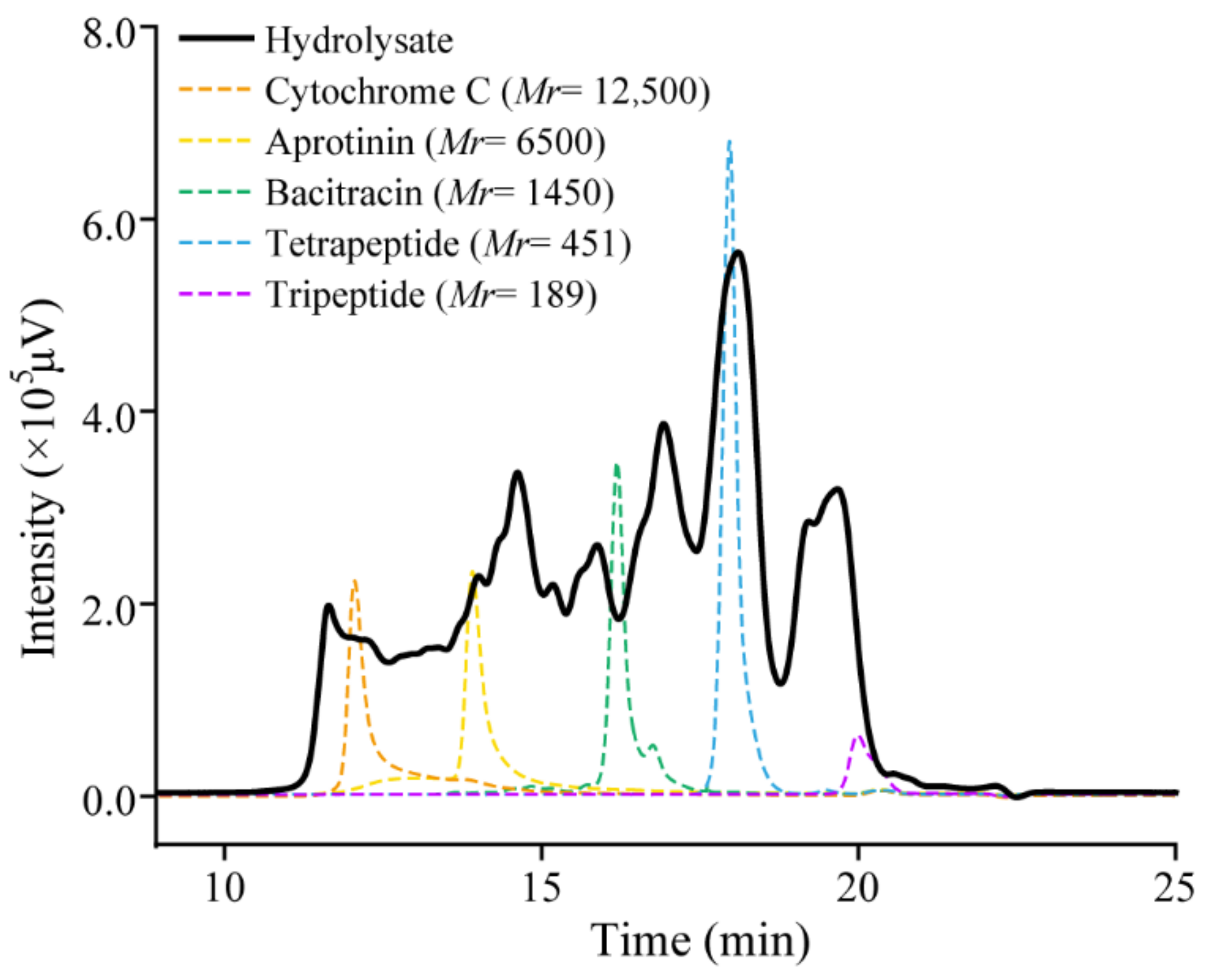
2.4. The Collagen Hydrolysate Prepared with Aa2_1884 Is Rich in Collagen Oligopeptides
2.5. Antioxidant Activity of Bovine Bone Collagen Hydrolysate
3. Materials and Methods
3.1. Materials
3.2. Sequence Analysis
3.3. Protein Expression and Purification
3.4. Enzyme Assay
3.5. Enzyme Characterization
3.6. Optimization of the Enzymatic Hydrolysis Parameters
3.7. Preparation and Evaluation of Collagen Hydrolysate
3.8. Analysis of the Antioxidant Activity of the Collagen Hydrolysate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamyatnin, A.A. Structural-functional diversity of the natural oligopeptides. Prog. Biophys. Mol. Biol. 2018, 133, 1–8. [Google Scholar] [CrossRef]
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactive peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Nasri, M. Marine-derived bioactive peptides as new anticoagulant agents: A review. Curr. Protein Pept. Sci. 2013, 14, 199–204. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Son, A.; Katekaew, S. Purification and characterization of angiotensin converting enzyme-inhibitory derived from crocodile blood hydrolysates. Food Sci. Technol. 2019, 39, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.L.; Peng, M.; Li, J.; Tang, B.L.; Shao, X.; Zhao, F.; Liu, C.; Zhang, X.Y.; Li, P.Y.; Shi, M.; et al. Preparation and functional evaluation of collagen oligopeptide-rich hydrolysate from fish skin with the serine collagenolytic protease from Pseudoalteromonas sp. SM9913. Sci. Rep. 2017, 7, 15716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Salampessy, J.; Phillips, M.; Seneweera, S.; Kailasapathy, K. Release of antimicrobial peptides through bromelain hydrolysis of leatherjacket (Meuchenia sp.) insoluble proteins. Food Chem. 2010, 120, 556–560. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, J.; Guo, Y. Effect of Collagen Peptide, Alone and in Combination with Calcium Citrate, on Bone Loss in Tail-Suspended Rats. Molecules 2020, 25, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, L.; Li, B.; Chen, Y.; Li, L.; Chen, X.; Wang, L.; Lu, F.; Luo, G.; Li, G.; Zhang, Y. Discovery of Anti-Hypertensive Oligopeptides from Adlay Based on In Silico Proteolysis and Virtual Screening. Int. J. Mol. Sci. 2016, 17, 2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Hur, J.; Ham, S.A.; Jo, Y.; Lee, S.; Choi, M.J.; Seo, H.G. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Gollhofer, A.; König, D. Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides. Appl. Physiol. Nutr. Metab. 2017, 42, 588–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral Intake of Collagen Peptide Attenuates Ultraviolet B Irradiation-Induced Skin Dehydration In Vivo by Regulating Hyaluronic Acid Synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Gu, R.-Z.; Li, C.-Y.; Liu, W.-Y.; Yi, W.-X.; Cai, M.-Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wang, B.; Chi, C.-f.; Zhang, Q.-H.; Gong, Y.-d.; Tang, J.-J.; Luo, H.-y.; Ding, G.-f. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Adekoya, O.A.; Sylte, I. The thermolysin family (M4) of enzymes: Therapeutic and biotechnological potential. Chem. Biol. Drug Des. 2009, 73, 7–16. [Google Scholar] [CrossRef]
- Rawlings, N. Introduction: Serine Peptidases and Their Clans. In Handbook of Proteolytic Enzymes, 2nd ed.; Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Okamoto, M.; Yonejima, Y.; Tsujimoto, Y.; Suzuki, Y.; Watanabe, K. A thermostable collagenolytic protease with a very large molecular mass produced by thermophilic Bacillus sp. strain MO-1. Appl. Microbiol. Biotechnol. 2001, 57, 103–108. [Google Scholar] [CrossRef]
- Chen, X.L.; Xie, B.B.; Lu, J.T.; He, H.L.; Zhang, Y. A novel type of subtilase from the psychrotolerant bacterium Pseudoalteromonas sp. SM9913: Catalytic and structural properties of deseasin MCP-01. Microbiology 2007, 153, 2116–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, L.Y.; Su, H.N.; Zhou, M.Y.; Wang, L.; Chen, X.L.; Xie, B.B.; Song, X.Y.; Shi, M.; Qin, Q.L.; Pang, X.; et al. Characterization of a novel subtilisin-like protease myroicolsin from deep sea bacterium Myroides profundi D25 and molecular insight into its collagenolytic mechanism. J. Biol. Chem. 2014, 289, 6041–6053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.J.; Tang, B.L.; Shao, X.; Liu, B.X.; Zheng, X.Y.; Han, X.X.; Li, P.Y.; Zhang, X.Y.; Song, X.Y.; Chen, X.L. Characterization of a New S8 serine Protease from Marine Sedimentary Photobacterium sp. A5-7 and the Function of Its Protease-Associated Domain. Front. Microbiol. 2016, 7, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Hamid, A.; Bakar, J.; Bee, G.H. Nutritional quality of spray dried protein hydrolysate from Black Tilapia (Oreochromis mossambicus). Food Chem. 2002, 78, 69–74. [Google Scholar] [CrossRef]
- Li, J.; Cheng, J.H.; Teng, Z.J.; Sun, Z.Z.; He, X.Y.; Wang, P.; Shi, M.; Song, X.Y.; Chen, X.L.; Zhang, Y.Z.; et al. Taxonomic and Enzymatic Characterization of Flocculibacter collagenilyticus gen. nov., sp. nov., a Novel Gammaproteobacterium With High Collagenase Production. Front. Microbiol. 2021, 12, 621161. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Jordan, F.; Inouye, M. Functional Analysis of the Propeptide of Subtilisin E as an Intramolecular Chaperone for Protein Folding: Refolding And Inhibitory Abilities of Propeptide Mutants (∗). J. Biol. Chem. 1995, 270, 25127–25132. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Minagawa, T.; Miura, K. The propeptide of subtilisin BPN’ as a temporary inhibitor and effect of an amino acid replacement on its inhibitory activity. FEBS Lett. 1997, 411, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Hofmann, K. The protease-associated domain: A homology domain associated with multiple classes of proteases. Trends Biochem. Sci. 2001, 26, 147–148. [Google Scholar] [CrossRef]
- Kagawa, T.F.; O’Connell, M.R.; Mouat, P.; Paoli, M.M.; O’Toole, P.W.; Cooney, J.C. Model for substrate interactions in C5a peptidase from Streptococcus pyogenes: A 1.9 A crystal structure of the active form of ScpA. J. Mol. Biol. 2009, 386, 754–772. [Google Scholar] [CrossRef]
- Morrissey, J.H. Chapter 641—Coagulation Factor VIIa. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2905–2908. [Google Scholar]
- Page, M.J.; Craik, C.S. Chapter 669—Brachyurins. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3049–3052. [Google Scholar]
- Fuller, R.S.; Brake, A.; Thorner, J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc. Natl. Acad. Sci. USA 1989, 86, 1434–1438. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Martin, S.; Lipkind, G.; LaMendola, J.; Steiner, D.F. Regulatory roles of the P domain of the subtilisin-like prohormone convertases. J. Biol. Chem. 1998, 273, 11107–11114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, N.D.; Barrett, A.J. Chapter 559—Introduction: Serine Peptidases and Their Clans. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2491–2523. [Google Scholar]
- Graycar, T.P.; Bott, R.R.; Power, S.D.; Estell, D.A. Chapter 693—Subtilisins. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3148–3155. [Google Scholar]
- Sacco, E.; Elena Regonesi, M.; Vanoni, M. Chapter 711—Archaean Serine Proteases. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3224–3233. [Google Scholar]
- Uesugi, Y.; Arima, J.; Usuki, H.; Iwabuchi, M.; Hatanaka, T. Two bacterial collagenolytic serine proteases have different topological specificities. Biochim. Biophys. Acta 2008, 1784, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, N.; Nakayama, T.; Ashida, M.; Hemmi, H.; Nakao, M.; Minakata, H.; Oyama, H.; Oda, K.; Nishino, T. Collagenolytic serine-carboxyl proteinase from Alicyclobacillus sendaiensis strain NTAP-1: Purification, characterization, gene cloning, and heterologous expression. Appl. Environ. Microbiol. 2003, 69, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.-H.; Zhang, X.-Y.; Wang, Z.; Zhang, X.; Liu, S.-C.; Song, X.-Y.; Zhang, Y.-Z.; Ding, J.-M.; Chen, X.-L.; Xu, F. Potential of Thermolysin-like Protease A69 in Preparation of Bovine Collagen Peptides with Moisture-Retention Ability and Antioxidative Activity. Mar. Drugs 2021, 19, 676. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, A.K.M.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Haq, M.; Chun, B.-S. Characterization of pepsin-solubilised collagen recovered from mackerel (Scomber japonicus) bone and skin using subcritical water hydrolysis. Int. J. Biol. Macromol. 2020, 148, 1290–1297. [Google Scholar] [CrossRef]
- Medina-Medrano, J.R.; Quiñones-Muñoz, T.A.; Arce-Ortíz, A.; Torruco-Uco, J.G.; Hernández-Martínez, R.; Lizardi-Jiménez, M.A.; Varela-Santos, E. Antioxidant Activity of Collagen Extracts Obtained from the Skin and Gills of Oreochromis sp. J. Med. Food 2019, 22, 722–728. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhao, Y.Q.; Wang, Y.M.; Chi, C.F.; Wang, B. Eight Collagen Peptides from Hydrolysate Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation. Mar. Drugs 2019, 17, 224. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Xiong, Y.L.; Jie, C. Andoxidant and emulsifying properties of potato protein hydrolysate in soybean oil-in-water emulsions. Food Chem. 2010, 120, 101–108. [Google Scholar]
- Jahanbani, R.; Ghaffari, S.M.; Salami, M.; Vahdati, K.; Sepehri, H.; Sarvestani, N.N.; Sheibani, N.; Moosavi-Movahedi, A.A. Antioxidant and Anticancer Activities of Walnut (Juglans regia L.) Protein Hydrolysates Using Different Proteases. Plant. Food Hum. Nutr. 2016, 71, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, L.J. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- He, H.; Chen, X.; Sun, C.; Zhang, Y.; Gao, P. Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Bioresour. Technol. 2006, 97, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Ho, T.C.; Ahmed, R.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Biofunctional properties of bacterial collagenolytic protease-extracted collagen hydrolysates obtained using catalysts-assisted subcritical water hydrolysis. J. Ind. Eng. Chem. 2020, 81, 332–339. [Google Scholar] [CrossRef]
- Yamamoto, S.; Deguchi, K.; Onuma, M.; Numata, N.; Sakai, Y. Absorption and Urinary Excretion of Peptides after Collagen Tripeptide Ingestion in Humans. Biol. Pharm. Bull. 2016, 39, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, R.; Haq, M.; Chun, B.-S. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, A.; Hakim, T.R.; Abidin, M.Z.; Fitriyanto, N.A.; Jamhari, J.; Rusman, R.; Erwanto, Y. Angiotensin-converting enzyme inhibitor activity of peptides derived from Kacang goat skin collagen through thermolysin hydrolysis. Vet. World 2021, 14, 161–167. [Google Scholar] [CrossRef]
- Blum, M.; Chang, H.Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Chen, X.L.; Zhao, H.L.; Xie, B.B.; Zhou, B.C.; Zhang, Y.Z. Hydrolysis of insoluble collagen by deseasin MCP-01 from deep-sea Pseudoalteromonas sp. SM9913: Collagenolytic characters, collagen-binding ability of C-terminal polycystic kidney disease domain, and implication for its novel role in deep-sea sedimentary particulate organic nitrogen degradation. J. Biol. Chem. 2008, 283, 36100–36107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- He, H.L.; Chen, X.L.; Li, J.W.; Zhang, Y.Z.; Gao, P.J. Taste improvement of refrigerated meat treated with cold-adapted Protease. Food Chem. 2004, 84, 307–311. [Google Scholar] [CrossRef]
- Chen, X.L.; Xie, B.B.; Bian, F.; Zhao, G.Y.; Zhao, H.L.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Ecological function of myroilysin, a novel bacterial M12 metalloprotease with elastinolytic activity and a synergistic role in collagen hydrolysis, in biodegradation of deep-sea high-molecular-weight organic nitrogen. Appl. Environ. Microbiol. 2009, 75, 1838–1844. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.L.; Zhao, F.; Shi, M.; Zhang, X.Y.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [Green Version]


| Substrate | Enzymatic Activity (U/mL) |
|---|---|
| Bovine bone collagen | 801.15 ± 46.45 |
| Bovine tendon collagen | 761.27 ± 43.41 |
| Gelatin | 834.00 ± 61.39 |
| Casein | 51.75 ± 1.85 |
| Elastin-orcein | – |
| Metal Ion | Relative Activity (%) | Metal Ion | Relative Activity (%) | ||
|---|---|---|---|---|---|
| 2 mM | 4 mM | 2 mM | 4 mM | ||
| Control | 100 | 100 | K+ | 108.62 ± 8.24 | 116.60 ± 4.18 |
| Ca2+ | 263.67 ± 7.06 | 251.90 ± 10.14 | Sn2+ | 97.01 ± 0.12 | 100.32 ± 4.40 |
| Ba2+ | 250.54 ± 11.34 | 276.80 ± 10.14 | Zn2+ | 56.50 ± 2.99 | 40.69 ± 1.33 |
| Sr2+ | 245.91 ± 6.45 | 210.87 ± 29.18 | Fe2+ | 43.73 ± 4.78 | 5.05 ± 2.04 |
| Mg2+ | 217.58 ± 7.25 | 217.25 ± 32.74 | Ni2+ | – b | – |
| Li+ | 111.41 ± 4.40 | 116.60 ± 4.18 | Co2+ | – | – |
| Cu2+ | 100.32 ± 2.96 | 122.83 ± 2.43 | Mn2+ | – | – |
| Amino Acid | Total Amino Acids (g/100 g) | Free Amino Acids (g/100 g) |
|---|---|---|
| Ala | 6.87 ± 0.05 | 0.07 ± 0.02 |
| Arg | 7.16 ± 0.06 | |
| Asp | 3.39 ± 0.03 | 0.10 ± 0.02 |
| Cit | 0.07 ± 0.03 | |
| Cys | 0.56 ± 0.04 | 0.12 ± 0.01 |
| Glu | 4.04 ± 0.03 | |
| Gly | 17.21 ± 0.12 | 0.02 ± 0.01 |
| His | 0.67 ± 0.02 | |
| Ile | 1.54 ± 0.04 | 0.07 ± 0.01 |
| Leu | 2.73 ± 0.06 | 0.04 ± 0.01 |
| Lys | 3.23 ± 0.05 | |
| Met | 0.75 ± 0.06 | 0.10 ± 0.01 |
| Orn | 0.17 ± 0.01 | |
| Phe | 1.83 ± 0.11 | |
| Pro | 10.10 ± 0.08 | |
| Ser | 3.03 ± 0.03 | 0.15 ± 0.04 |
| Thr | 2.47 ± 0.03 | |
| Trp b | – | |
| Tyr | 0.79 ± 0.10 | 0.09 ± 0.07 |
| Val | 2.10 ± 0.02 | 0.14 ± 0.01 |
| Hylys | 1.04 ± 0.05 | 0.19 ± 0.05 |
| Hypro | 8.18 ± 0.11 |
| Molecular-Weight Range (Da) | Hydrolysate (%) |
|---|---|
| >10,000 | 9.68 ± 0.13 |
| 5000–10,000 | 6.25 ± 0.02 |
| 3000–5000 | 12.46 ± 0.11 |
| 1000–3000 | 16.49 ± 0.09 |
| 500–1000 | 15.60 ± 0.07 |
| <500 | 39.50 ± 0.20 |
| Antioxidant Activity | Hydrolysate Concentration (mg/mL) | Enzyme | Method | Collagen Source | Reference |
|---|---|---|---|---|---|
| 32.8% a | 10 | Aa2_1884 (S8) | Enzymolysis | Bovine bone | This study |
| 40.7% a | 30 | A69 (M4) | Enzymolysis | Bovine bone | [43] |
| 341.91% a | 10 | MCP-01 (S8) | Enzymolysis | Fish skin | [7] |
| 50% a | 8.38 | SWH b | Fish bone | [44] | |
| 50% a | 7.58 | SWH | Fish skin | [44] | |
| 50% a | 5.81 | Pepsin | Enzymolysis | Fish skin | [45] |
| 50% a | 1.57 | Pepsin | Enzymolysis and fraction isolation | Fish skin | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cheng, J.-H.; Teng, Z.-J.; Zhang, X.; Chen, X.-L.; Sun, M.-L.; Wang, J.-P.; Zhang, Y.-Z.; Ding, J.-M.; Tian, X.-M.; et al. A Novel Gelatinase from Marine Flocculibacter collagenilyticus SM1988: Characterization and Potential Application in Collagen Oligopeptide-Rich Hydrolysate Preparation. Mar. Drugs 2022, 20, 48. https://doi.org/10.3390/md20010048
Li J, Cheng J-H, Teng Z-J, Zhang X, Chen X-L, Sun M-L, Wang J-P, Zhang Y-Z, Ding J-M, Tian X-M, et al. A Novel Gelatinase from Marine Flocculibacter collagenilyticus SM1988: Characterization and Potential Application in Collagen Oligopeptide-Rich Hydrolysate Preparation. Marine Drugs. 2022; 20(1):48. https://doi.org/10.3390/md20010048
Chicago/Turabian StyleLi, Jian, Jun-Hui Cheng, Zhao-Jie Teng, Xia Zhang, Xiu-Lan Chen, Mei-Ling Sun, Jing-Ping Wang, Yu-Zhong Zhang, Jun-Mei Ding, Xin-Min Tian, and et al. 2022. "A Novel Gelatinase from Marine Flocculibacter collagenilyticus SM1988: Characterization and Potential Application in Collagen Oligopeptide-Rich Hydrolysate Preparation" Marine Drugs 20, no. 1: 48. https://doi.org/10.3390/md20010048
APA StyleLi, J., Cheng, J.-H., Teng, Z.-J., Zhang, X., Chen, X.-L., Sun, M.-L., Wang, J.-P., Zhang, Y.-Z., Ding, J.-M., Tian, X.-M., & Zhang, X.-Y. (2022). A Novel Gelatinase from Marine Flocculibacter collagenilyticus SM1988: Characterization and Potential Application in Collagen Oligopeptide-Rich Hydrolysate Preparation. Marine Drugs, 20(1), 48. https://doi.org/10.3390/md20010048





