Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab
Abstract
:1. Introduction
2. Results
2.1. In Vitro Study of the Effects of Fucoidans on the Activation and Proliferation of PBMCs
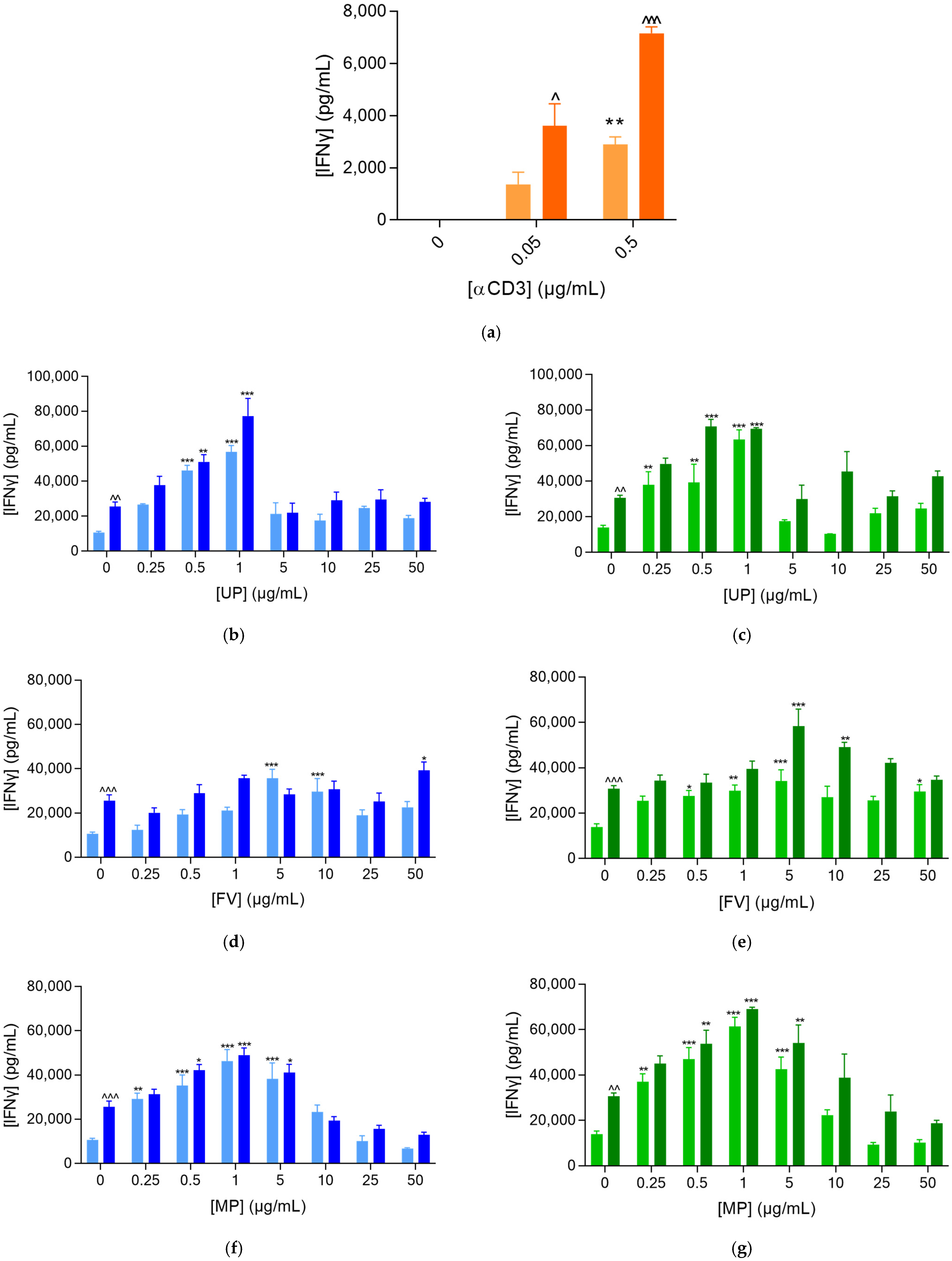
2.1.1. Fucoidans Activated PBMCs in the Presence of αCD3
2.1.2. Fucoidans Optimise PBMC Clusterisation at Lower Concentrations
2.2. Fucoidans Activate PBMCs at Low Concentrations in the Presence of Prostate Cancer Cells
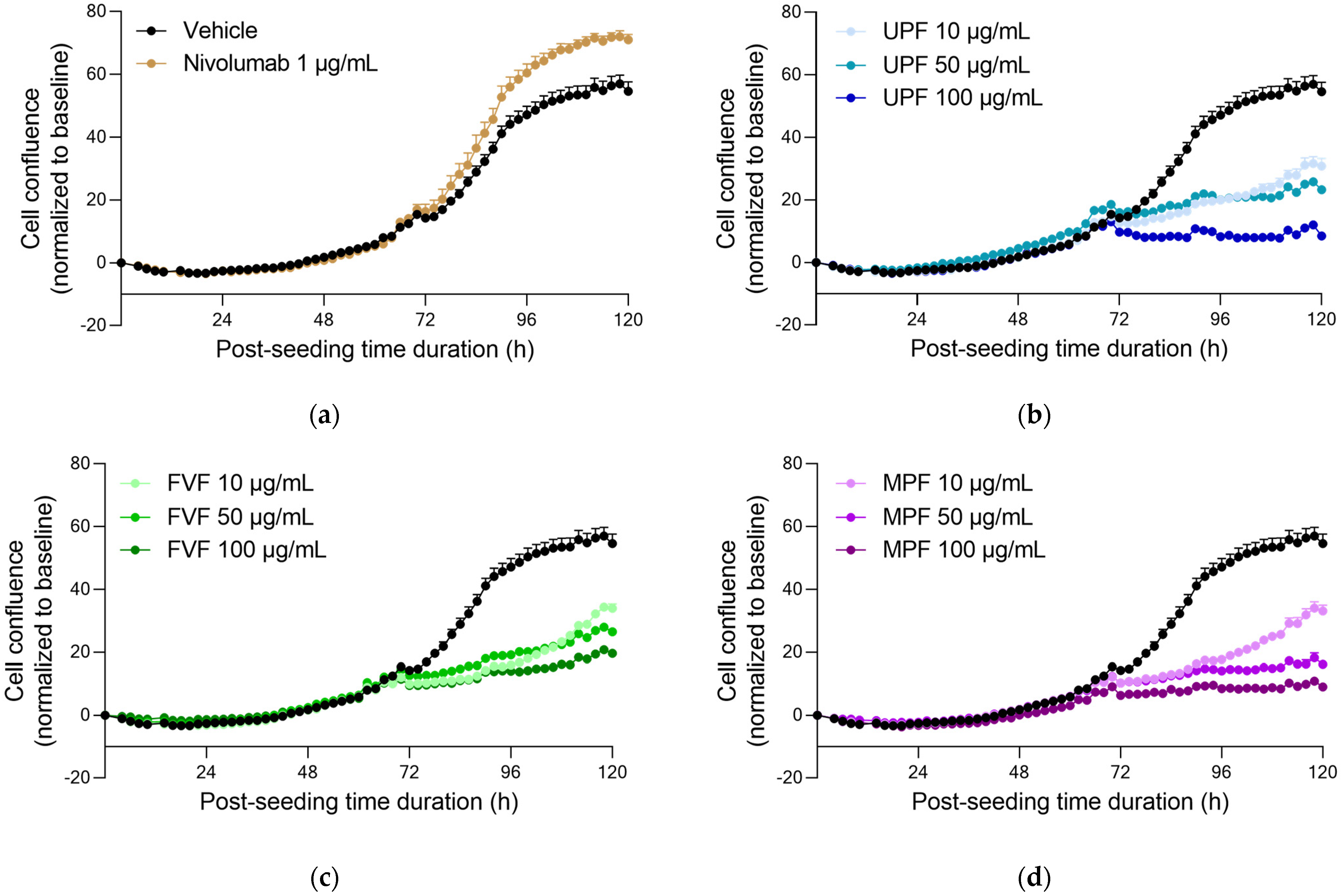
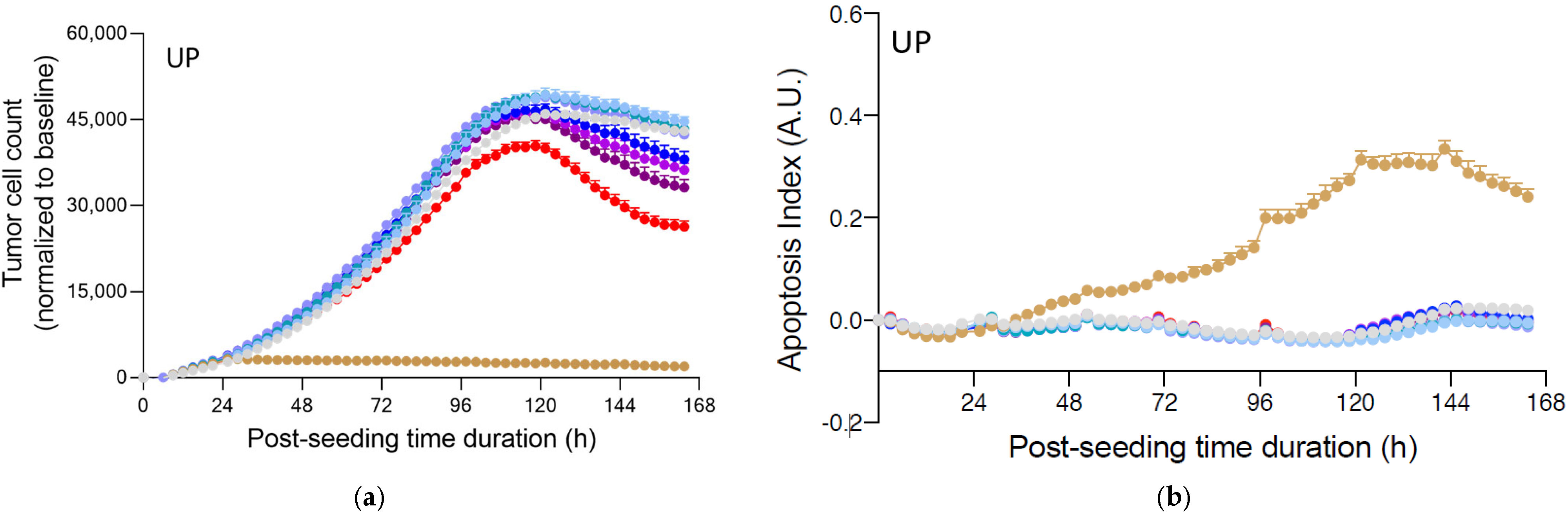
2.3. Fucoidans Alone Decreased PC3 Tumor Cell Proliferation but Did Not Induce Apoptosis in the Absence of PBMCs
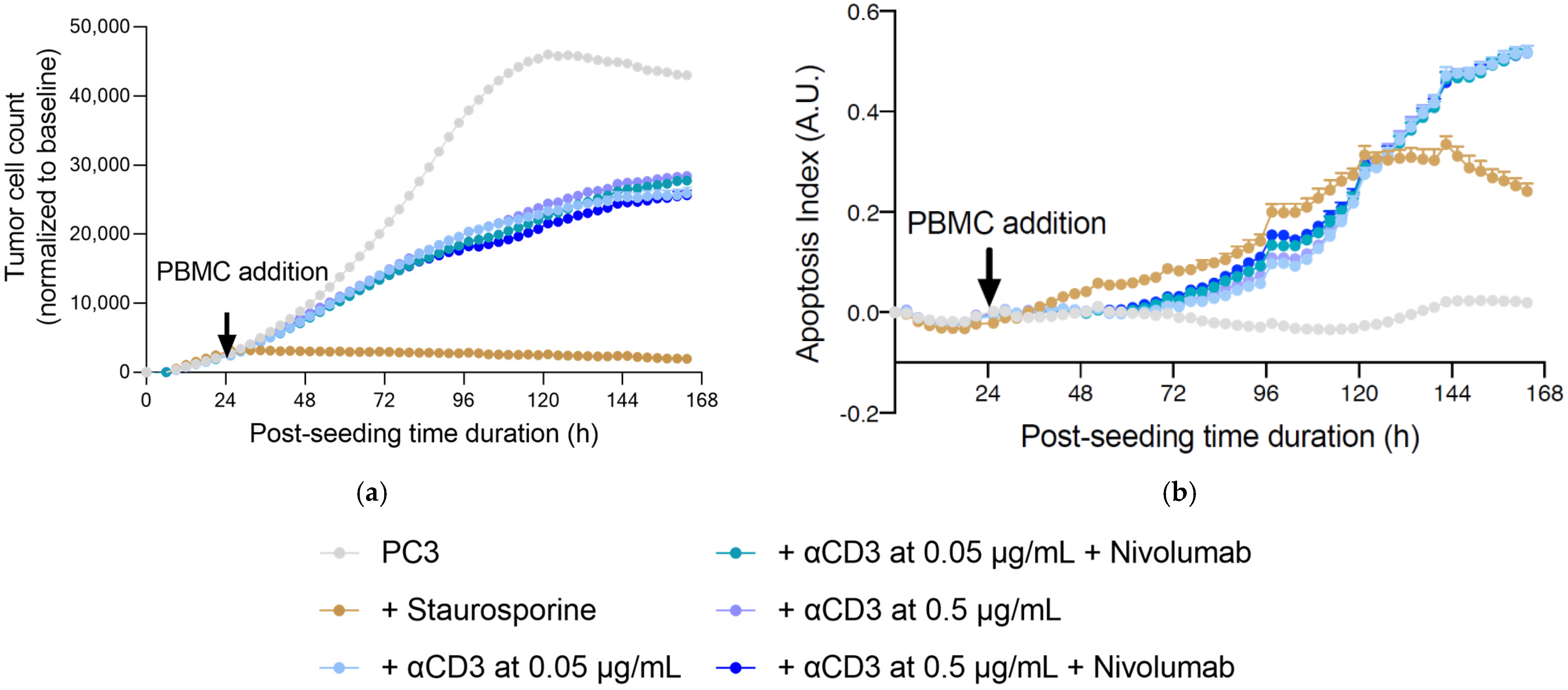
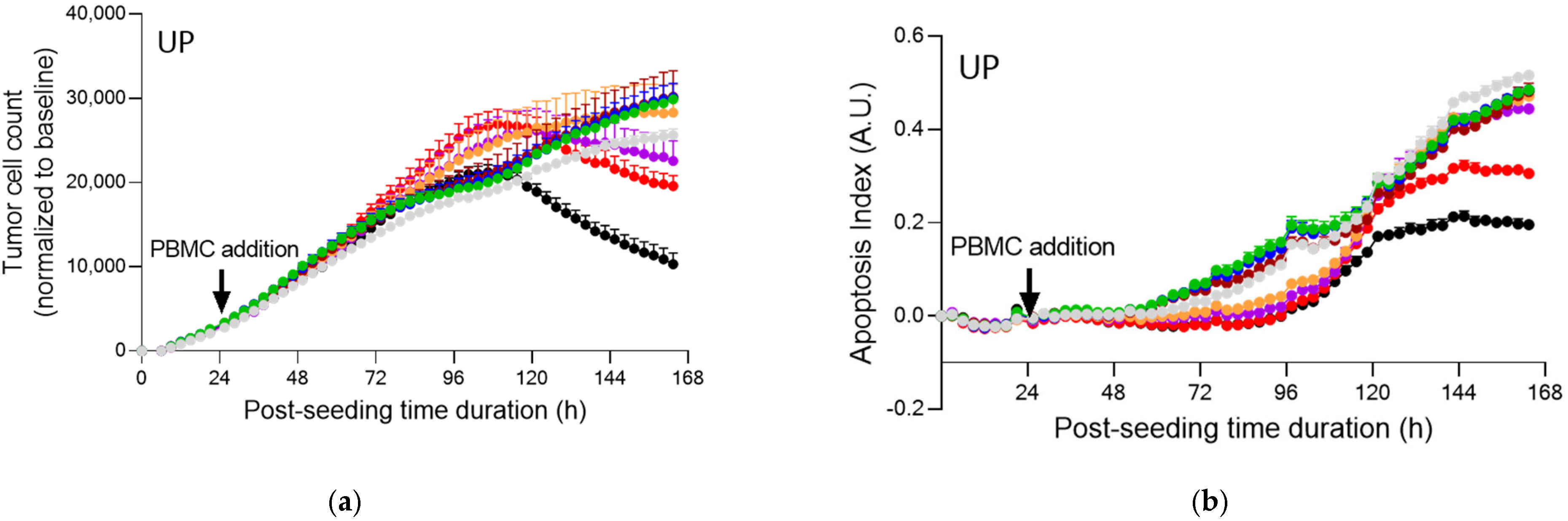
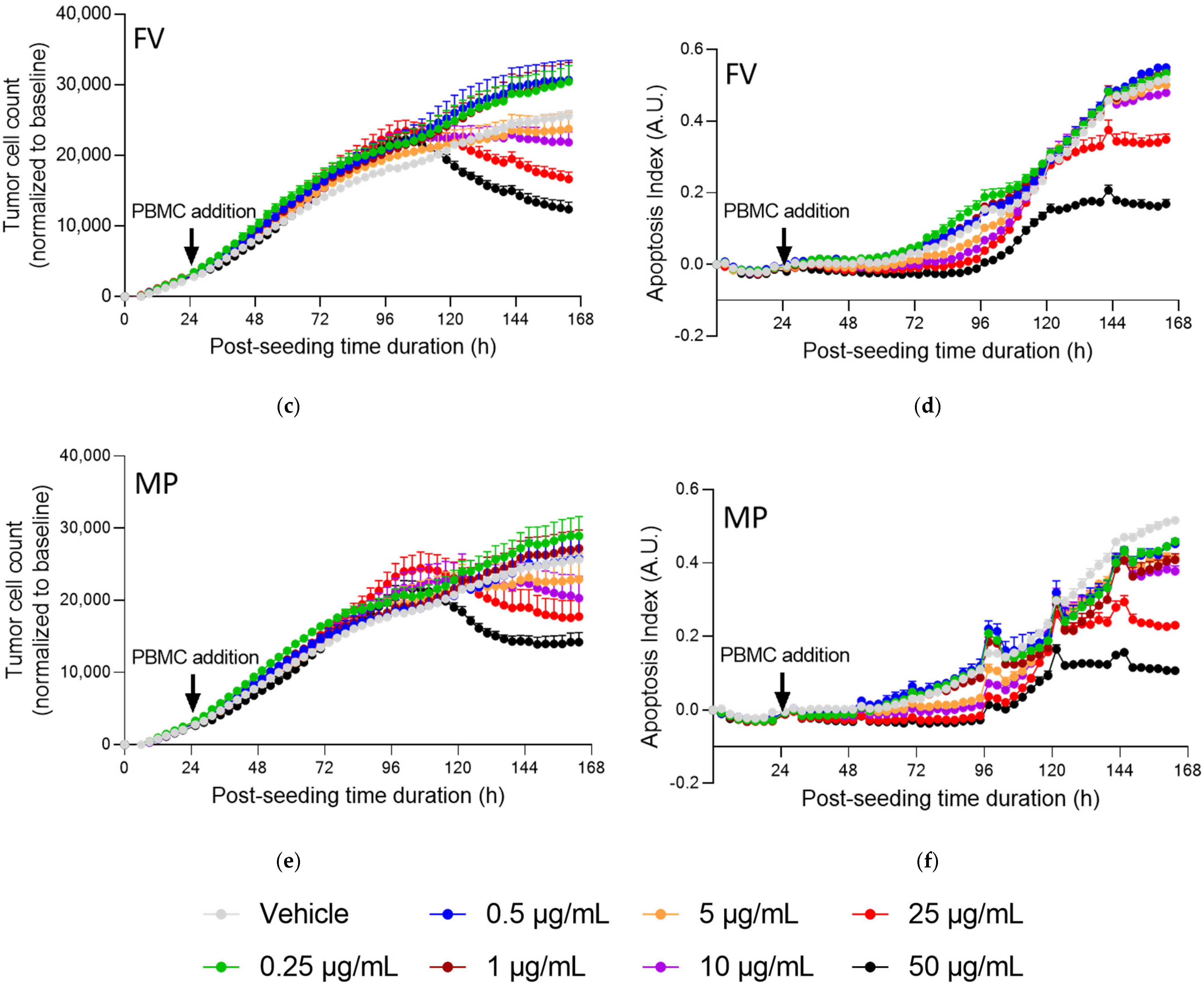
2.4. Fucoidans with Activated PBMCs Stopped Tumor Cell Proliferation but Not through Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Proliferation and Activation of PBMCs
4.3. Data Acquisition and Analysis of PBMCs and IFNγ
4.4. The Immune Cell-Mediated Tumor Killing Activity Assay on PC3 Prostate Tumor Cells
4.5. Data Acquisition and Analysis of PC3 Cells
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, A.; Shamay, Y.; Shah, J.; Brook, S.; Soong, J.; Rajasekhar, V.K.; Humm, J.L.; Healey, J.H.; Powell, S.N.; Baselga, J.; et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nat. Commun. 2017, 8, 14292. [Google Scholar] [CrossRef]
- Hwang, P.A.; Lin, X.Z.; Kuo, K.L.; Hsu, F.Y. Fabrication and Cytotoxicity of Fucoidan-Cisplatin Nanoparticles for Macrophage and Tumor Cells. Materials 2017, 10, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuRoss, A.N.; Landry, M.R.; Thomas, C.R., Jr.; Neufeld, M.J.; Sun, C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021, 500, 208–219. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, W.; Choi, C.; Kim, S.Y.; Son, A.; Kim, H.; Lee, N.; Park, H.C. Fucoidan-Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Co-Targeting Tumor Hypoxia and Angiogenesis. Mar. Drugs 2018, 16, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etman, S.M.; Abdallah, O.Y.; Elnaggar, Y.S.R. Novel fucoidan based bioactive targeted nanoparticles from Undaria Pinnatifida for treatment of pancreatic cancer. Int. J. Biol. Macromol. 2020, 145, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Chiang, C.S.; Hsu, C.H.; Cheng, H.W.; Chen, S.Y. Development and Characterization of a Fucoidan-Based Drug Delivery System by Using Hydrophilic Anticancer Polysaccharides to Simultaneously Deliver Hydrophobic Anticancer Drugs. Biomolecules 2020, 10, 970. [Google Scholar] [CrossRef]
- Oliveira, C.; Goncalves, C.S.; Martins, E.P.; Neves, N.M.; Reis, R.L.; Costa, B.M.; Silva, T.H.; Martins, A. Fucoidan/chitosan nanoparticles functionalized with anti-ErbB-2 target breast cancer cells and impair tumor growth in vivo. Int. J. Pharm. 2021, 600, 120548. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, M.; Snoeck, R.; Pauwels, R.; de Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, T.A.; Smolina, T.P.; Makarenkova, I.D.; Ivanushko, L.A.; Persiyanova, E.V.; Ermakova, S.P.; Silchenko, A.S.; Zaporozhets, T.S.; Besednova, N.N.; Fedyanina, L.N.; et al. Immunoadjuvant Activity of Fucoidans from the Brown Alga Fucus evanescens. Mar. Drugs 2020, 18, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.H.; Chuang, C.C.; Chen, C.C.; Yen, H.J.; Cheng, K.M.; Chen, X.A.; Shyu, H.F.; Lee, C.Y.; Young, J.J.; Kau, J.H. Nanoparticles assembled from fucoidan and trimethylchitosan as anthrax vaccine adjuvant: In vitro and in vivo efficacy in comparison to CpG. Carbohydr. Polym. 2020, 236, 116041. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, Y.; Kim, G.H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Recovers Immunity of Immunosuppressed Mice. J. Microbiol. Biotechnol. 2020, 30, 439–447. [Google Scholar] [CrossRef]
- Zhang, W.; Park, H.B.; Yadav, D.; Hwang, J.; An, E.K.; Eom, H.Y.; Kim, S.J.; Kwak, M.; Lee, P.C.; Jin, J.O. Comparison of human peripheral blood dendritic cell activation by four fucoidans. Int. J. Biol. Macromol. 2021, 174, 477–484. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Lin, N.; Lu, W.; Huang, X.; Weng, J.; Sun, S.; Zhang, C.; Yang, Q.; Zhou, G.; et al. Fucoidan from Fucus vesiculosus attenuates doxorubicin-induced acute cardiotoxicity by regulating JAK2/STAT3-mediated apoptosis and autophagy. Biomed. Pharm. 2020, 130, 110534. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Bin, J.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon nano-onion-mediated dual targeting of P-selectin and P-glycoprotein to overcome cancer drug resistance. Nat. Commun. 2021, 12, 312. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Thomas, D.; Bello, D.M. Adjuvant immunotherapy for melanoma. J. Surg. Oncol. 2021, 123, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Donisi, C.; Puzzoni, M.; Ziranu, P.; Lai, E.; Mariani, S.; Saba, G.; Impera, V.; Dubois, M.; Persano, M.; Migliari, M.; et al. Immune Checkpoint Inhibitors in the Treatment of HCC. Front. Oncol. 2020, 10, 601240. [Google Scholar] [CrossRef] [PubMed]
- Glode, A.E.; May, M.B. Immune checkpoint inhibitors: Significant advancements in non-small cell lung cancer treatment. Am. J. Health-Syst. Pharm. 2021, 78, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Specenier, P. Immunotherapy for head and neck cancer: From recurrent/metastatic disease to (neo)adjuvant treatment in surgically resectable tumors. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 168–177. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Pan, W.; Wang, M.; Lu, Y.; Zhang, J.; Fang, Z.; Zhang, X.; Ji, Y.; Bei, J.X.; et al. Fucoidan-Supplemented Diet Potentiates Immune Checkpoint Blockage by Enhancing Antitumor Immunity. Front. Cell Dev. Biol. 2021, 9, 733246. [Google Scholar] [CrossRef]
- Chiang, C.S.; Lin, Y.J.; Lee, R.; Lai, Y.H.; Cheng, H.W.; Hsieh, C.H.; Shyu, W.C.; Chen, S.Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Yadav, D.; An, E.K.; Kwak, M.; Lee, P.C.; Jin, J.O. Enhancement of Immune Checkpoint Inhibitor-Mediated Anti-Cancer Immunity by Intranasal Treatment of Ecklonia cava Fucoidan against Metastatic Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9125. [Google Scholar] [CrossRef]
- Corban, M.; Ambrose, M.; Pagnon, J.; Stringer, D.; Karpiniec, S.; Park, A.; Eri, R.; Fitton, J.H.; Gueven, N. Pathway Analysis of Fucoidan Activity Using a Yeast Gene Deletion Library Screen. Mar. Drugs 2019, 17, 54. [Google Scholar] [CrossRef] [Green Version]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Sun, B.L. Immunotherapy in treatment of metastatic prostate cancer: An approach to circumvent immunosuppressive tumor microenvironment. Prostate 2021, 81, 1125–1134. [Google Scholar] [CrossRef]
- Kim, T.J.; Koo, K.C. Current Status and Future Perspectives of Checkpoint Inhibitor Immunotherapy for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5484. [Google Scholar] [CrossRef]
- Velho, P.I.; Eisenberger, M.A. There Is Now Compelling Evidence to Further Evaluate Lower Doses of Abiraterone Acetate in Men With Metastatic Prostate Cancer: It Should Be Safer, May Be as Effective and Less Expensive. J. Clin. Oncol. 2018, 36, 3059–3060. [Google Scholar] [CrossRef]
- Nagamine, T.; Kadena, K.; Tomori, M.; Nakajima, K.; Iha, M. Activation of NK cells in male cancer survivors by fucoidan extracted from Cladosiphon okamuranus. Mol. Clin. Oncol. 2020, 12, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Tomori, M.; Nagamine, T.; Miyamoto, T.; Iha, M. Effects of Ingesting Fucoidan Derived from Cladosiphon okamuranus Tokida on Human NK Cells: A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Pilot Study. Mar. Drugs 2021, 19, 340. [Google Scholar] [CrossRef]
- Jin, J.O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Miyata, Y.; Matsuo, T.; Ohba, K.; Mitsunari, K.; Mukae, Y.; Otsubo, A.; Harada, J.; Matsuda, T.; Kondo, T.; Sakai, H. Present Status, Limitations and Future Directions of Treatment Strategies Using Fucoidan-Based Therapies in Bladder Cancer. Cancers 2020, 12, 3776. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef] [PubMed]
- Dithmer, M.; Kirsch, A.M.; Richert, E.; Fuchs, S.; Wang, F.; Schmidt, H.; Coupland, S.E.; Roider, J.; Klettner, A. Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines. Mar. Drugs 2017, 15, 193. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; An, E.K.; Park, H.B.; Hwang, J.; Dhananjay, Y.; Kim, S.J.; Eom, H.Y.; Oda, T.; Kwak, M.; Lee, P.C.; et al. Ecklonia cava fucoidan has potential to stimulate natural killer cells in vivo. Int. J. Biol. Macromol. 2021, 185, 111–121. [Google Scholar] [CrossRef]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar]
- Seya, T.; Takeda, Y.; Takashima, K.; Yoshida, S.; Azuma, M.; Matsumoto, M. Adjuvant immunotherapy for cancer: Both dendritic cell-priming and check-point inhibitor blockade are required for immunotherapy. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Mansoori, S.; Blom, K.; Berglund, M.; Lenhammar, L.; Andersson, C.; Loskog, A.; Fryknas, M.; Nygren, P.; Larsson, R. Mebendazole stimulates CD14+ myeloid cells to enhance T-cell activation and tumour cell killing. Oncotarget 2018, 9, 30805–30813. [Google Scholar] [CrossRef] [Green Version]
- Pozharitskaya, O.; Shikov, A.; Faustova, N.; Obluchinskaya, E.; Kosman, V.; Vuorela, H.; Makarov, V. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Tokita, Y.; Hirayama, M.; Nakajima, K.; Tamaki, K.; Iha, M.; Nagamine, T. Detection of Fucoidan in Urine after Oral Intake of Traditional Japanese Seaweed, Okinawa mozuku (Cladosiphon okamuranus Tokida). J. Nutr. Sci. Vitaminol. 2017, 63, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar. Drugs 2015, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef] [PubMed]



| Fucoidan Extract | Neutral Carbohydrates | Sulfate | Fucoidan | Polyphenol | Molecular Weight (kDa) |
|---|---|---|---|---|---|
| FV | 62.7% | 25.0% | 92.9% | 3.3% | 49.6 |
| UP | 43.5% | 25.9% | 86.0% | <2% | 46.8 |
| MP | 47.0% | 25.7% | 80.0% | <2% | 66.0 |
| Fucose | Xylose | Galactose | Arabinose | Rhamnose | |
|---|---|---|---|---|---|
| FV | 46% | 7% | 4% | 1% | 0% |
| UP | 21% | 1% | 18% | 1% | 0% |
| MP | 31% | 1% | 7% | 1% | 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, A.Y.; Nafia, I.; Stringer, D.N.; Karpiniec, S.S.; Fitton, J.H. Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab. Mar. Drugs 2022, 20, 12. https://doi.org/10.3390/md20010012
Park AY, Nafia I, Stringer DN, Karpiniec SS, Fitton JH. Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab. Marine Drugs. 2022; 20(1):12. https://doi.org/10.3390/md20010012
Chicago/Turabian StylePark, Ah Young, Imane Nafia, Damien N. Stringer, Samuel S. Karpiniec, and J. Helen Fitton. 2022. "Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab" Marine Drugs 20, no. 1: 12. https://doi.org/10.3390/md20010012
APA StylePark, A. Y., Nafia, I., Stringer, D. N., Karpiniec, S. S., & Fitton, J. H. (2022). Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab. Marine Drugs, 20(1), 12. https://doi.org/10.3390/md20010012






