Abstract
In our ongoing search for bioactive substances from marine organisms, novel alkaloids have been isolated. Pinnatoxins and pinnamine, potent shellfish poisons, were purified from the Okinawan bivalve Pinna muricata. Pinnatoxins activate Ca2+ channels. Halichlorine was isolated from the marine sponge Halichondria okadai. This compound inhibits the induction of VCAM-1. Drugs that block VCAM-1 may be useful for treating coronary artery diseases, angina, and noncardiovascular inflammatory diseases. Pinnaic acids, which are cPLA2 inhibitors, were also obtained from P. muricata. Interestingly, the structures of pinnaic acids are closely related to that of halichlorine. Norzoanthamine hydrochloride, isolated from the colonial zoanthid Zoanthus sp., suppresses decreases in bone weight and strength in ovariectomized mice, and could be a good candidate for an osteoporotic drug. Ircinamine, purified from the marine sponge Ircinia sp., has a reactive thioester. Aburatubolactams, inhibitors of superoxide anion generation, were isolated from Streptomyces sp. This article covers the bioactive marine alkaloids that have been recently isolated by this research group.
Introduction
Alkaloids are nitrogen-containing compounds that occur naturally not only in plants but also in microorganisms, marine organisms, and animals. Although it is not clear why alkaloids show significant biological activity, they are often useful as drugs or biological probes for physiological studies. As new and more complicated diseases are encountered worldwide, the importance of bioactive alkaloids has increased due to their potential application in chemotherapy. As the application of alkaloids has expanded, the definition of alkaloids has become less restricted.
Results and Discussion
Ca2+ Channel-Activating Shellfish Poisons (Pinnatoxins)
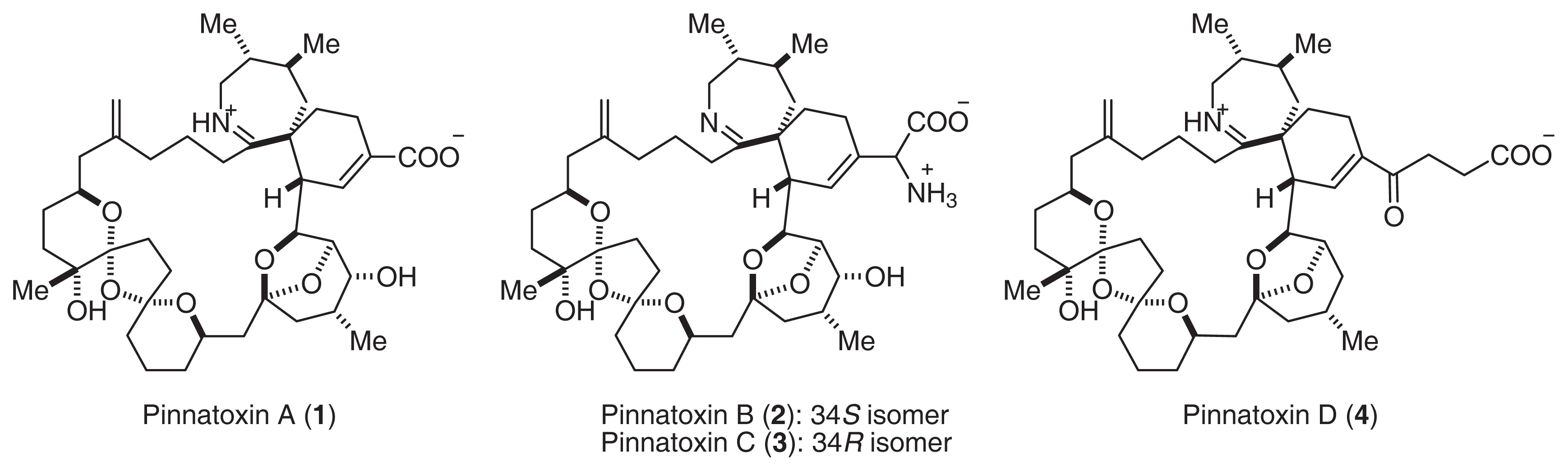
Shellfish of the genus Pinna live mainly in shallow waters of the temperate and tropical zones of the Indian and Pacific Oceans [1]. The adductor muscle of this bivalve is eaten in Japan and China, and food poisoning resulting from its ingestion occurs frequently [2]. Chinese investigators have reported that a toxic extract from P. attenuata, referred to as pinnatoxin, is a Ca2+ channel activator [2]. We isolated pinnatoxin A (1), a mixture of B and C (2, 3), and D (4) from P. muricata (Fig. 1) [3–7].
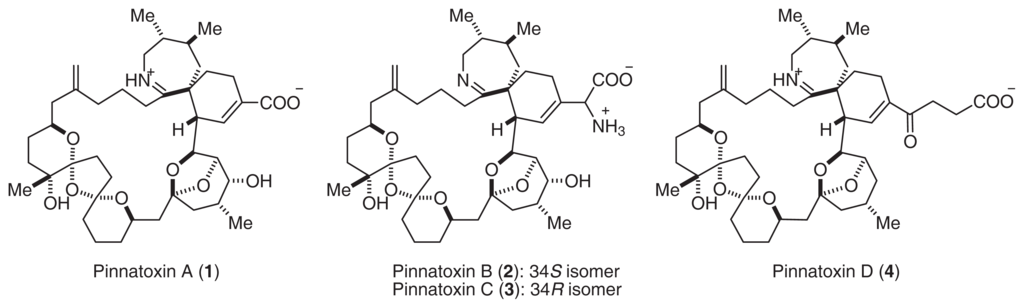
Fig. 1.
Structures of Pinnatoxin.
The structures and stereochemistry of pinnatoxins were clarified by extensive NMR experiments and positive ion ESI MS/MS spectra [3–8]. Pinnatoxins consist of a 20-membered ring, i.e., with 5,6-bicyclo, 6,7-azaspiro, and 6,5,6-triketal moieties in their structure. In particular, pinnaic acids contain a carboxylate anion and an iminium cation or an ammonium cation. Recently, Kishi’s group achieved the total synthesis of 1[9]. This investigation also supported the stereochemistry of 1.
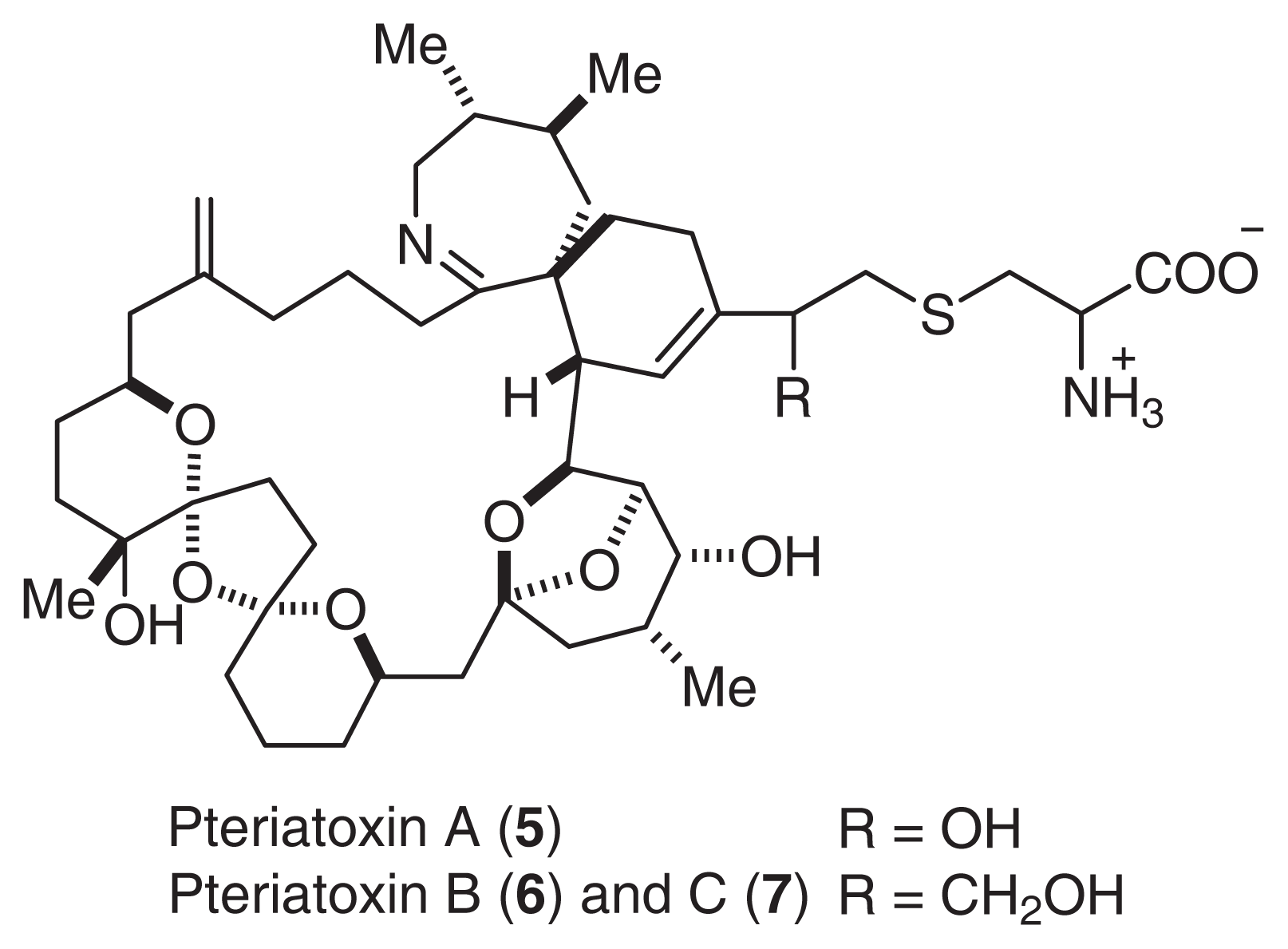
Pinnatoxin A (1) showed potent acute toxicity against mice (LD50 2.7 μg/MU (i.p.)). It was noted that the toxicity of 2, 3 (LD50 0.99 μg/MU) was as potent as that of tetrodotoxin. Although the acute toxicity of 4 (LD50 > 10 μg/MU) was weaker than that of the other pinnatoxins, 4 showed the strongest cytotoxicity against the murine leukemia cell line P388 (IC50 2.5 μg/ml). Pteriatoxins A (5), B, and C (6, 7: a 1:1 mixture) were also isolated from the Okinawan bivalve Petria penguin [10]. Pteriatoxins (5, 6, and 7) showed significant acute toxicity against mice (LD99 100; 8 mg/kg) (Fig. 2).
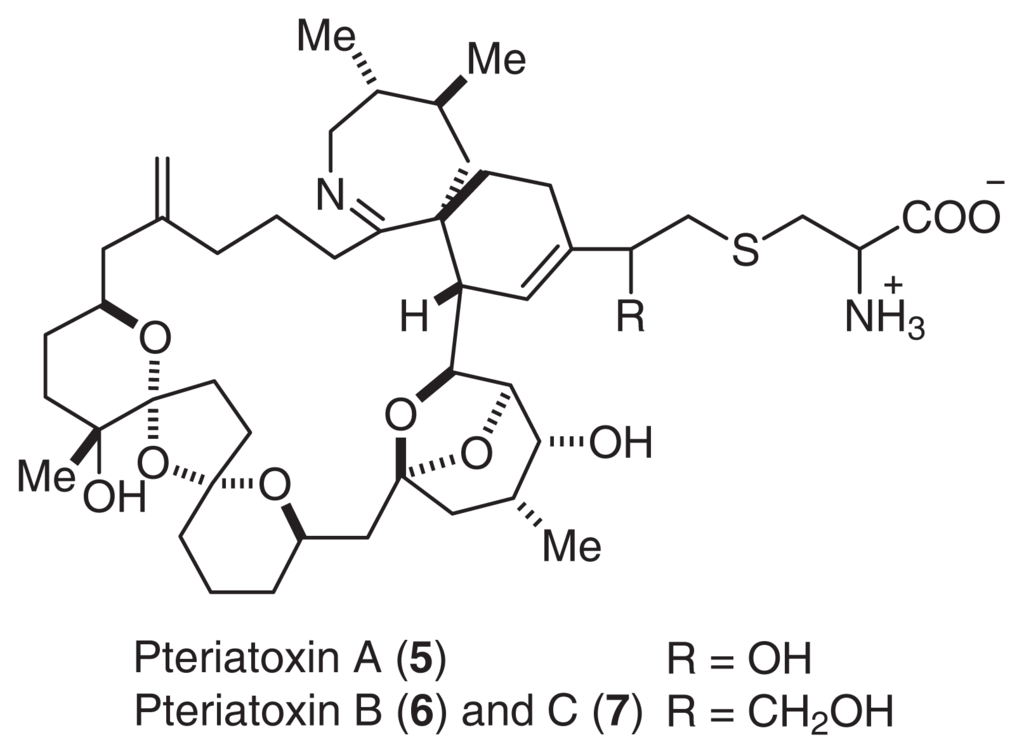
Fig. 2.
Structures of Pteriatoxin.
Extracts from the digestive glands of several Pinna sp., including P. muricata, P. attenuata, P. atropupurea, and the commonly eaten shellfish Atrina pectinata, all produced the same symptoms of poisoning in mice. These data suggest that Pinna shellfish may become toxic as the result of feeding on toxic organisms such as dinoflagellates [10].
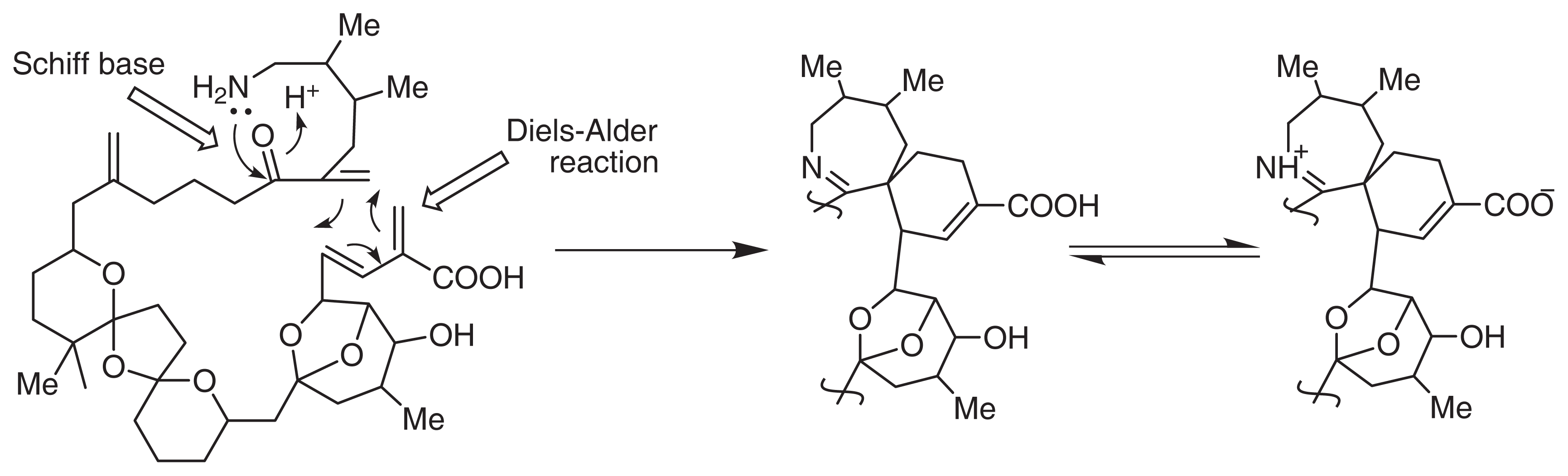
Interestingly, the backbone of pinnatoxins and their analogues could be configured from C1 to C34 in a single carbon chain. Marine organisms usually produce super carbon chain molecules with a terminal amino group, e.g., palytoxin. In this study, we proposed a polyketide biogenetic pathway for pinnatoxins, shown in Fig. 3.
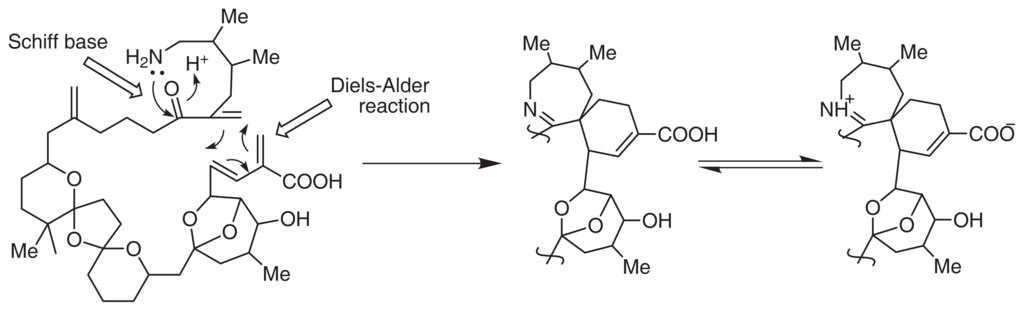
Fig. 3.
Biogenesis of Pinnatoxin
An Inhibitor of VCAM-1 (Vascular Cell Adhesion Molecule-1) Induction (Halichlorine)
A recent study suggested that adhesion molecules may some day be used clinically as anti-inflammatory agents and immunosuppressive agents, provided that the function of the adhesive molecules can be controlled [11].
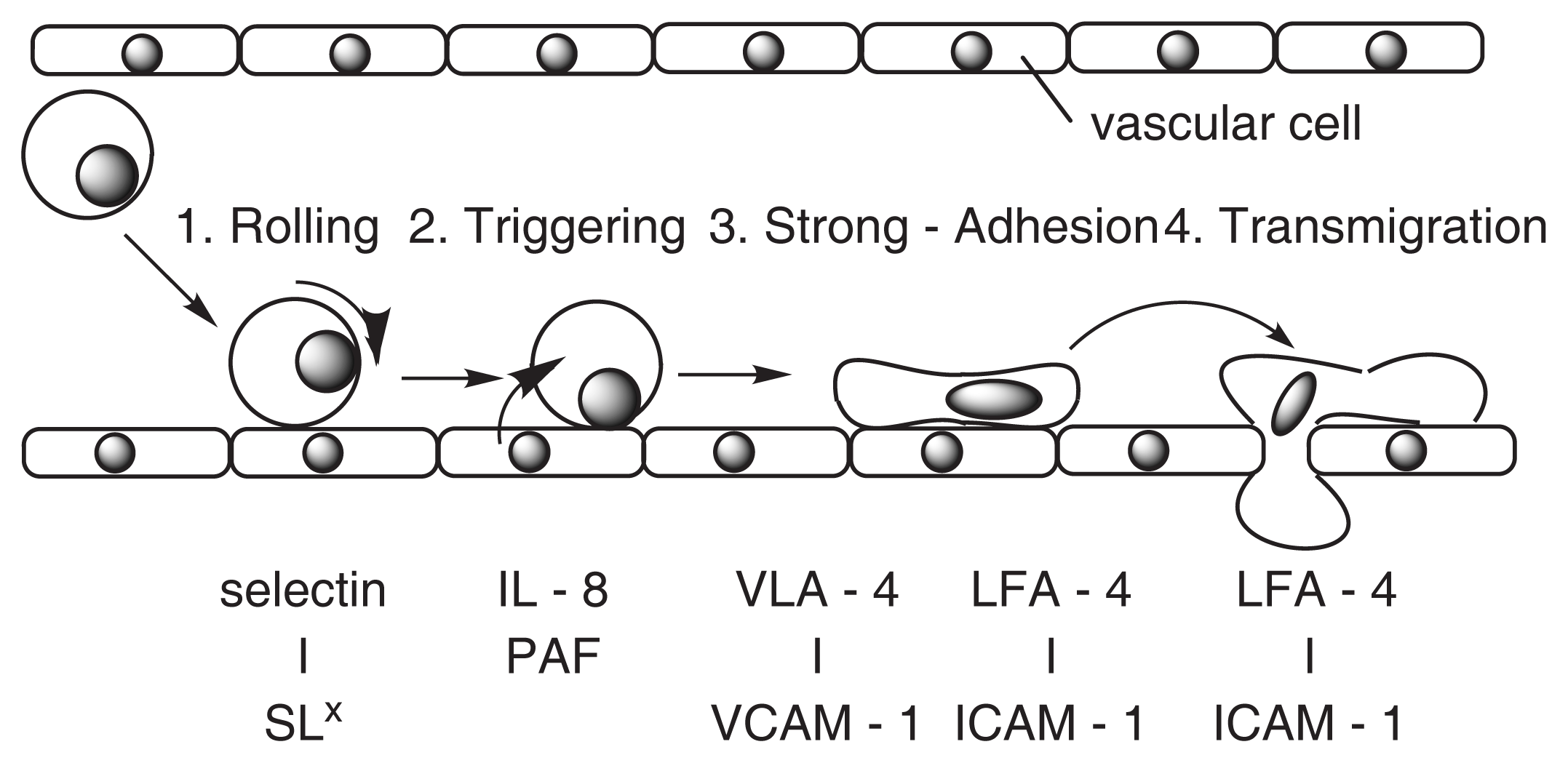
A simple model of multistage adhesion between leukocyte and vascular cells is shown in Fig. 4. VCAM-1 [12] is affected during the phase of Strong Adhesion. Drugs that block the induced expression of VCAM-1 may be useful for treating atherosclerosis, coronary artery diseases, angina, and noncardiovascular inflammatory diseases [12].
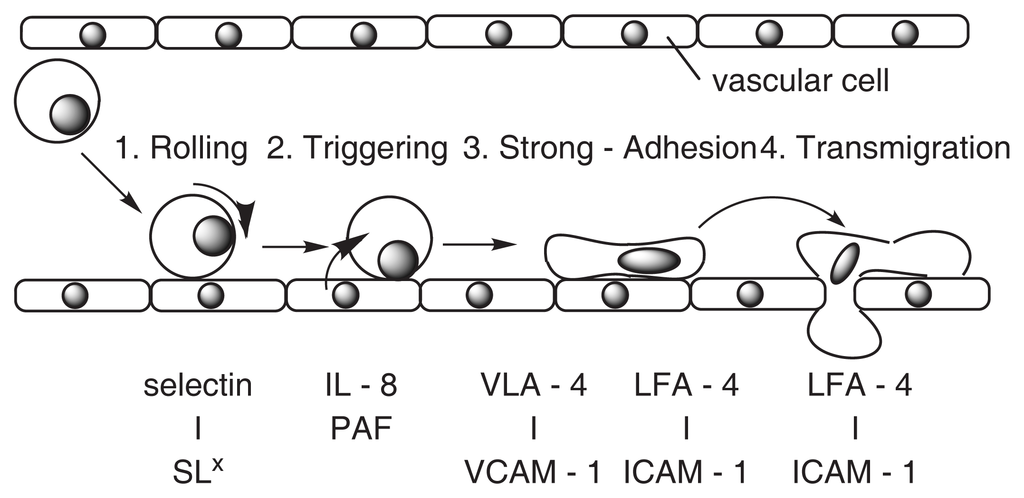
Fig. 4.
A model of adhesion between leukocyte and vascular cells.
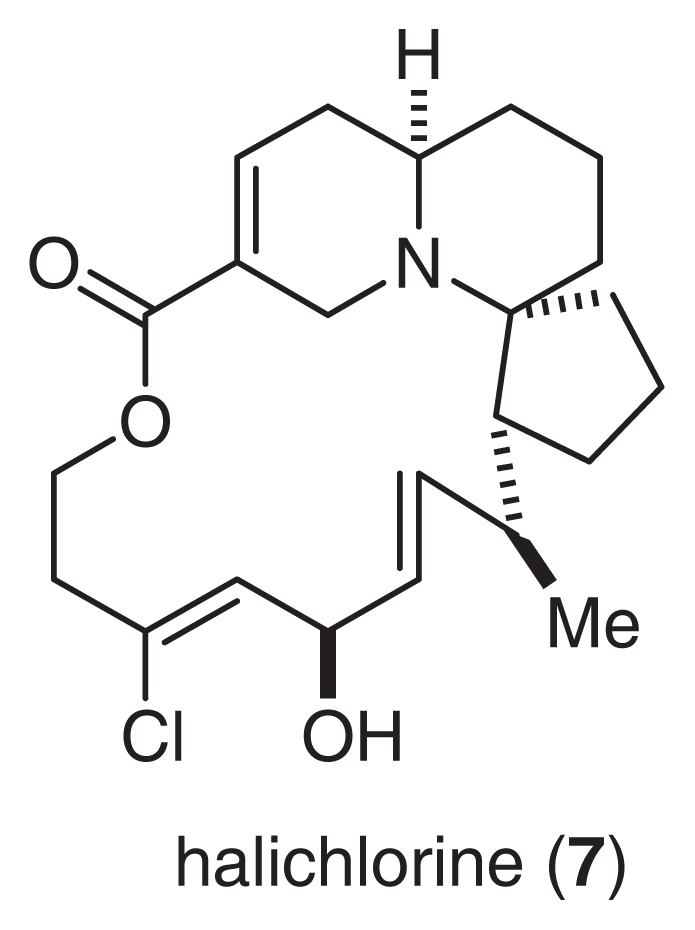
Halichlorine (7) was isolated from the marine sponge H. okadai Kadota [13]. The gross structure of 7 was elucidated by an analysis of MS, IR and extensive 2D NMR spectra, as shown in Fig. 5[14,15]. Halichlorine consists of a sterically hindered 15-membered lactone, an azabicyclo [4.4.0] ring, and a [5.6]-spiro ring moiety. Oxidative degradation of 7, as well as asymmetric synthesis of the degradation product, allowed us to determine the absolute stereochemistry of halichlorine [16]. The first total synthesis by Danishefsky and co-workers [17,18] also supported our conclusions regarding the structure of 7. Halichlorine inhibits the induction of VCAM-1 at IC50 7 μg/ml. Although VCAM-1 and ICAM belong to the same immunoglobulin superfamily, halichlorine does not affect ICAM (IC50 > 100 μg/ml) [10,19]. It is largely unknown why halichlorine affects only VCAM-1. Thus, additional research will be needed to clarify the functions and mechanisms of action of VCAM-1.
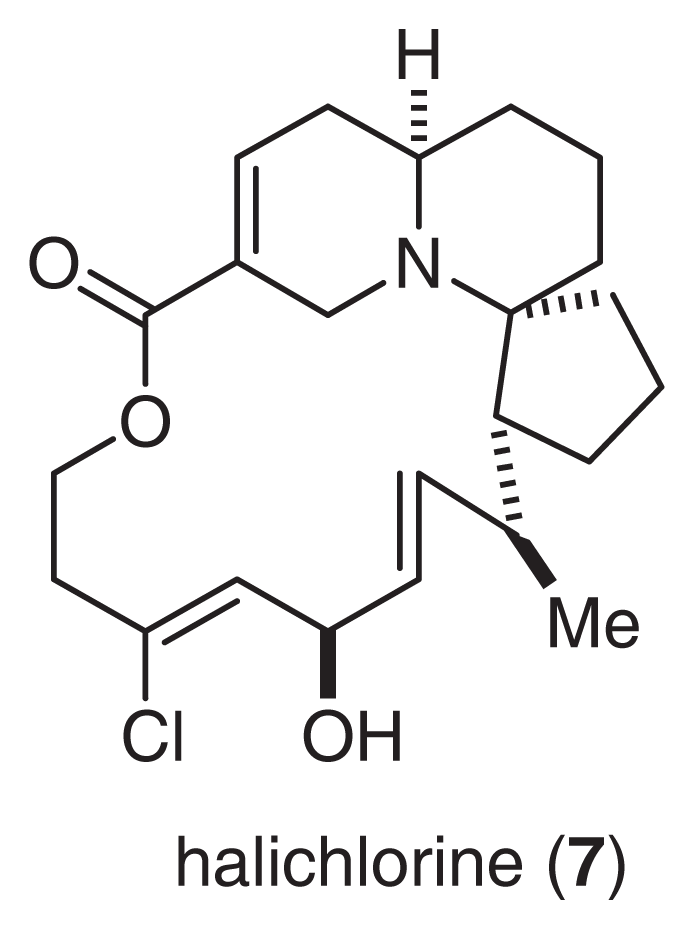
Fig. 5.
Structure of Halichlorine.
cPLA2 Inhibitors (Pinnaic Acids)
Specific inhibitors of phospholipase A2 (PLA2) have been considered as potential drugs for the treatment of inflammation and other disease states, since PLA2 is linked to the initial step in the cascade of enzymatic reactions that lead to the generation of inflammatory mediators [20–22]. Marine natural products such as manoalide [23] and luffariellolide [24] have been reported to be potent PLA2 inhibitors [25,26]. A cytosolic 85-kDa phospholipase (cPLA2) [27,28] exhibits specificity for the release of arachidonic acid from membrane phospholipids [29]. Therefore, compounds that inhibit cPLA2 activity have been targeted as anti-inflammatory agents.
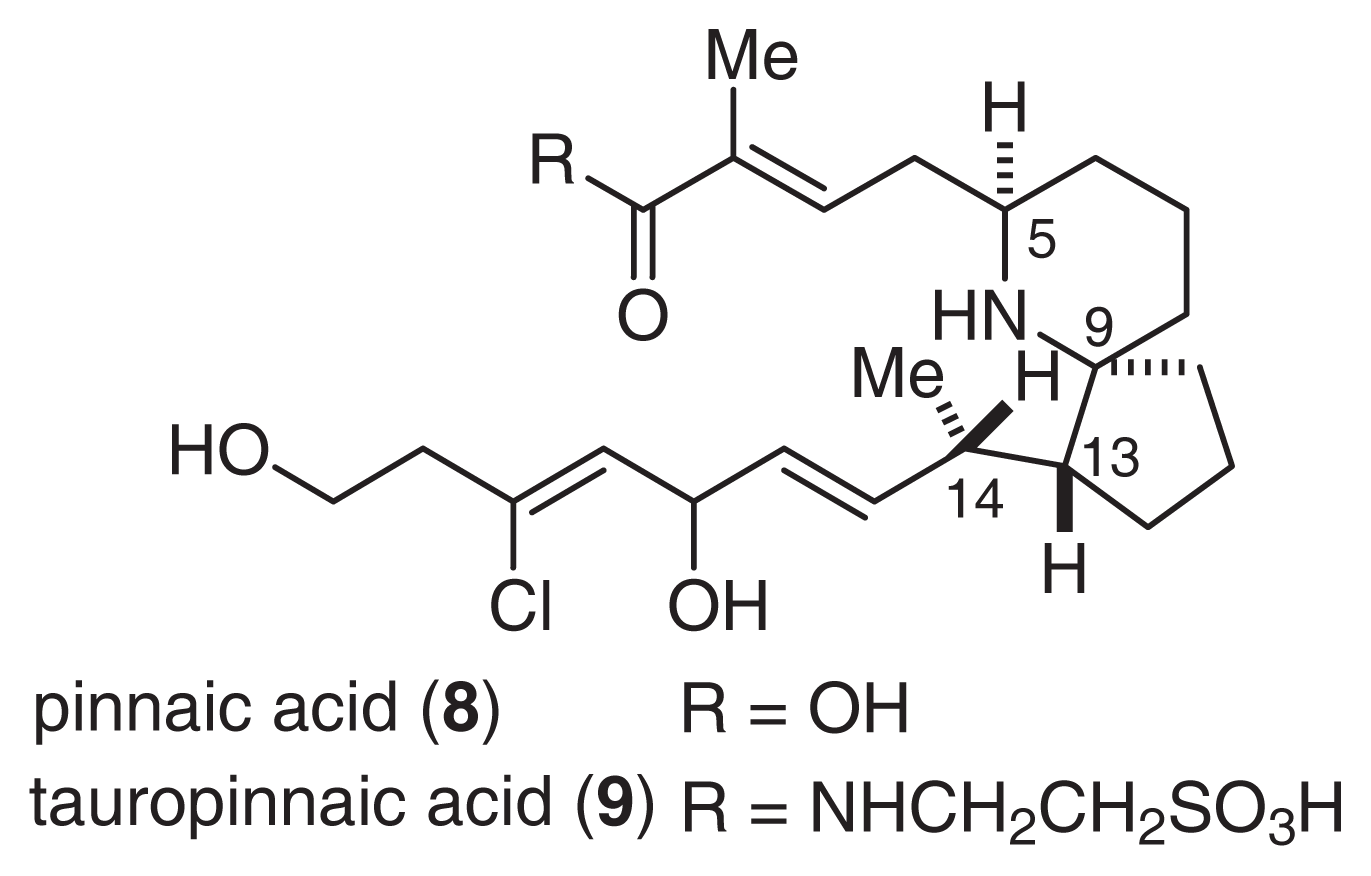
Pinnaic acids (8, 9) were isolated from the viscera of P. muricata (Fig. 6) [30]. The structure of 9 was determined by an analysis of NMR spectral data. Tauropinnaic acid (9) has a 6-azaspiro [4.5]decane unit and a taurine moiety. Furthermore, the gross structure of 8 was elucidated by a detailed comparison of the EI-MS fragment peaks with the corresponding peaks of 9. Synthetic studies of pinnaic acid (vide infra) unambiguously established that the relative stereochemistry of pinnaic acid is similar to that of 7[31–33].
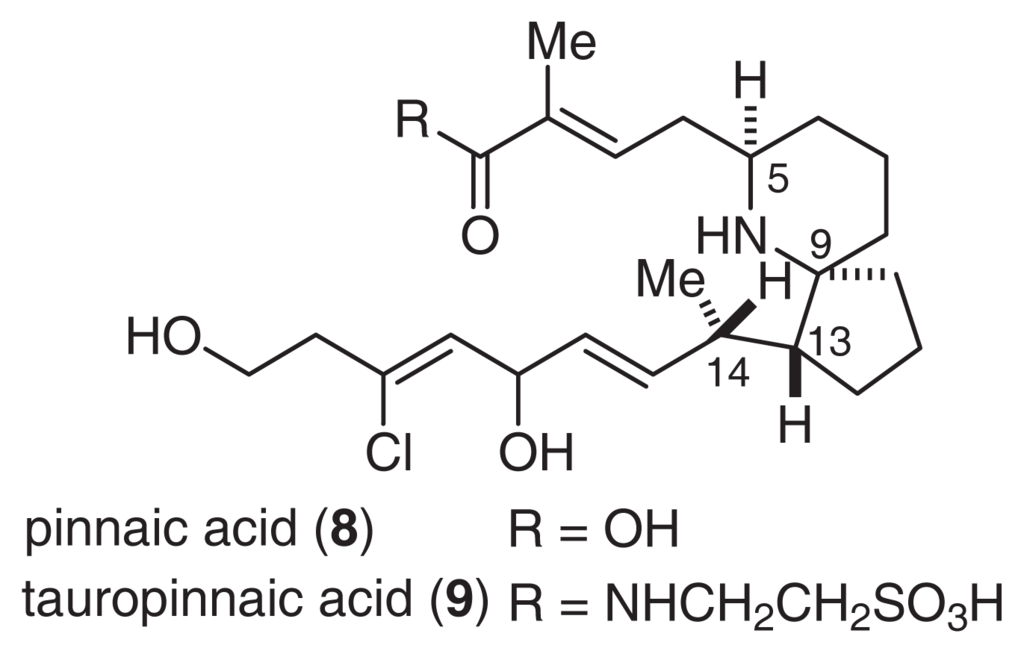
Fig. 6.
Structures of Pinnaic Acids.
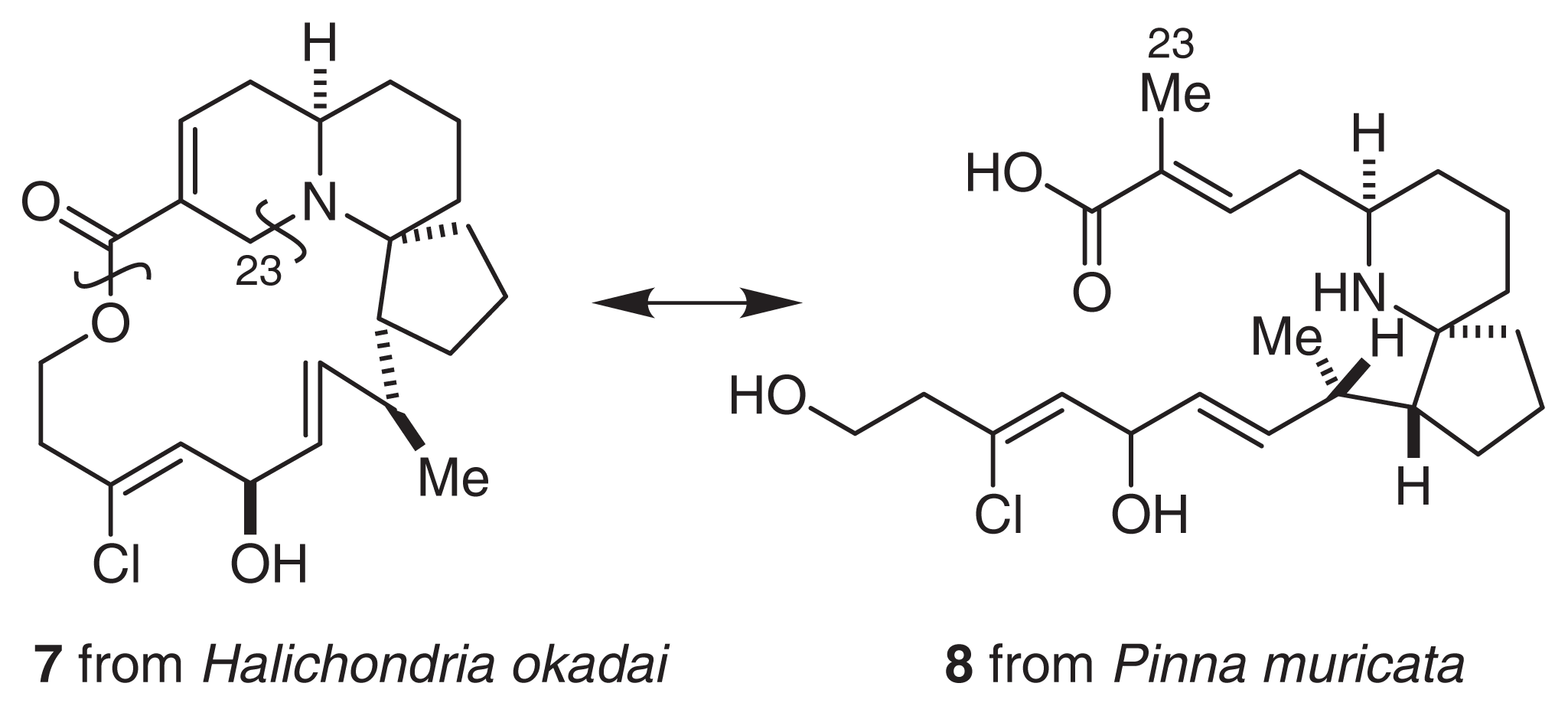
Pinnaic acid (8) and tauropinnaic acid (9) inhibited cPLA2 activity in vitro with IC50 values of 0.2 mM and 0.09 mM, respectively. Although the activity of pinnaic acids was moderate, inhibitors of cPLA2 have not yet been reported. Therefore, it is necessary to clarify the mechanism of action of these inhibitors. As described above, pinnaic acids are closely related to halichlorine. Therefore, each carbon atom has been tentatively numbered according to the supposed biogenetic formation of the N-C23 bond (Fig. 7). It is possible that these bioactive compounds from marine organisms are produced by symbiotic microorganisms.
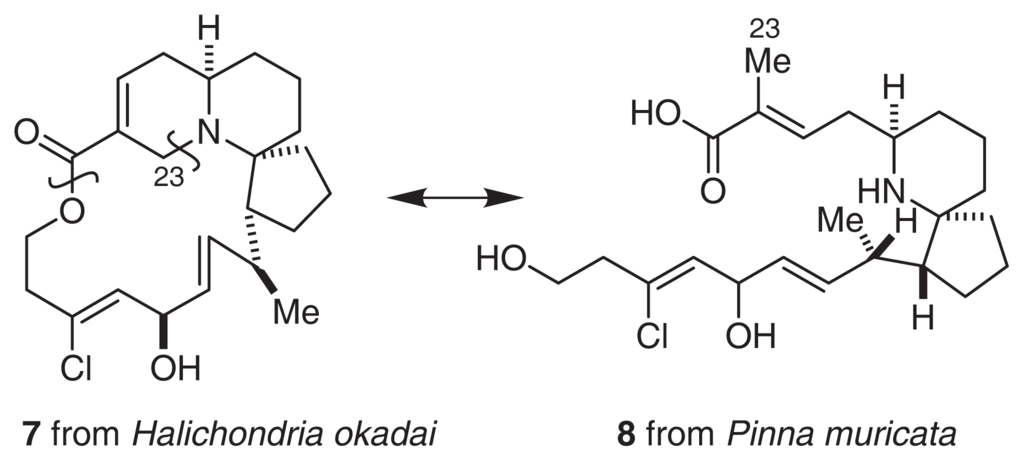
Fig. 7.
Biogenesis of pinnaic acids and halichlorine.
These architecturally novel alkaloids have attracted the attention of synthetic chemists; to date, 14 research groups have published synthetic studies of these alkaloids. The Danishefsky group has achieved the total synthesis of pinnaic acid [31,32] and halichlorine [17,18] in an asymmetric manner. Since pinnaic acid is a zwitterionic molecule, the NMR spectrum is quite sensitive to the measurement conditions. We recently reported a racemic total synthesis of 8 (Scheme 1) [33], and our detailed comparison of the 1H-NMR spectra of both synthetic and natural samples supported Danishefsky’s revision of the configuration at C14.
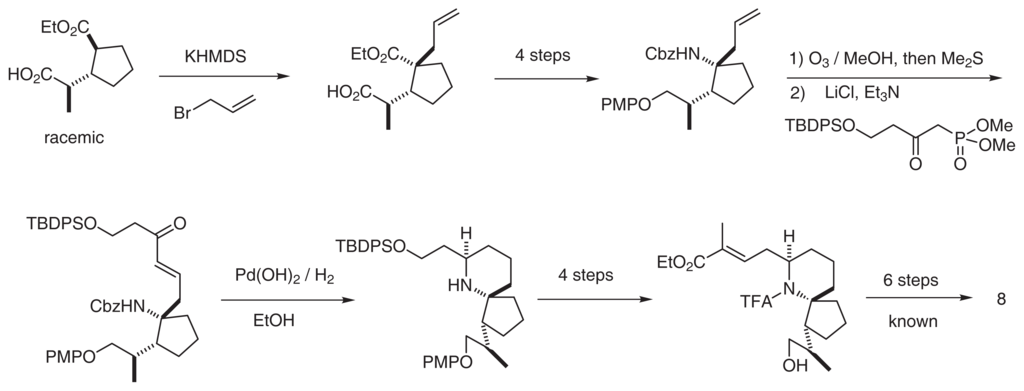
Scheme 1.
Synthesis of (+)-pinnaic acid [33].
A Significant Inhibitor of Osteoporosis (Norzoanthamine)
Osteoporosis is caused by an imbalance between bone resorption and bone formation, which results in bone loss and fractures after mineral flux occurs. The frequency of fracture is significantly increased in patients with osteoporosis, and hip fracture in elderly patients with osteoporosis is a very serious problem because it often limits their quality of life. Therefore, in addition to preventing the loss of bone mass, maintenance of the mechanical strength of bone tissue is a very important point to consider in the development of novel anti-osteoporotic drugs [34].
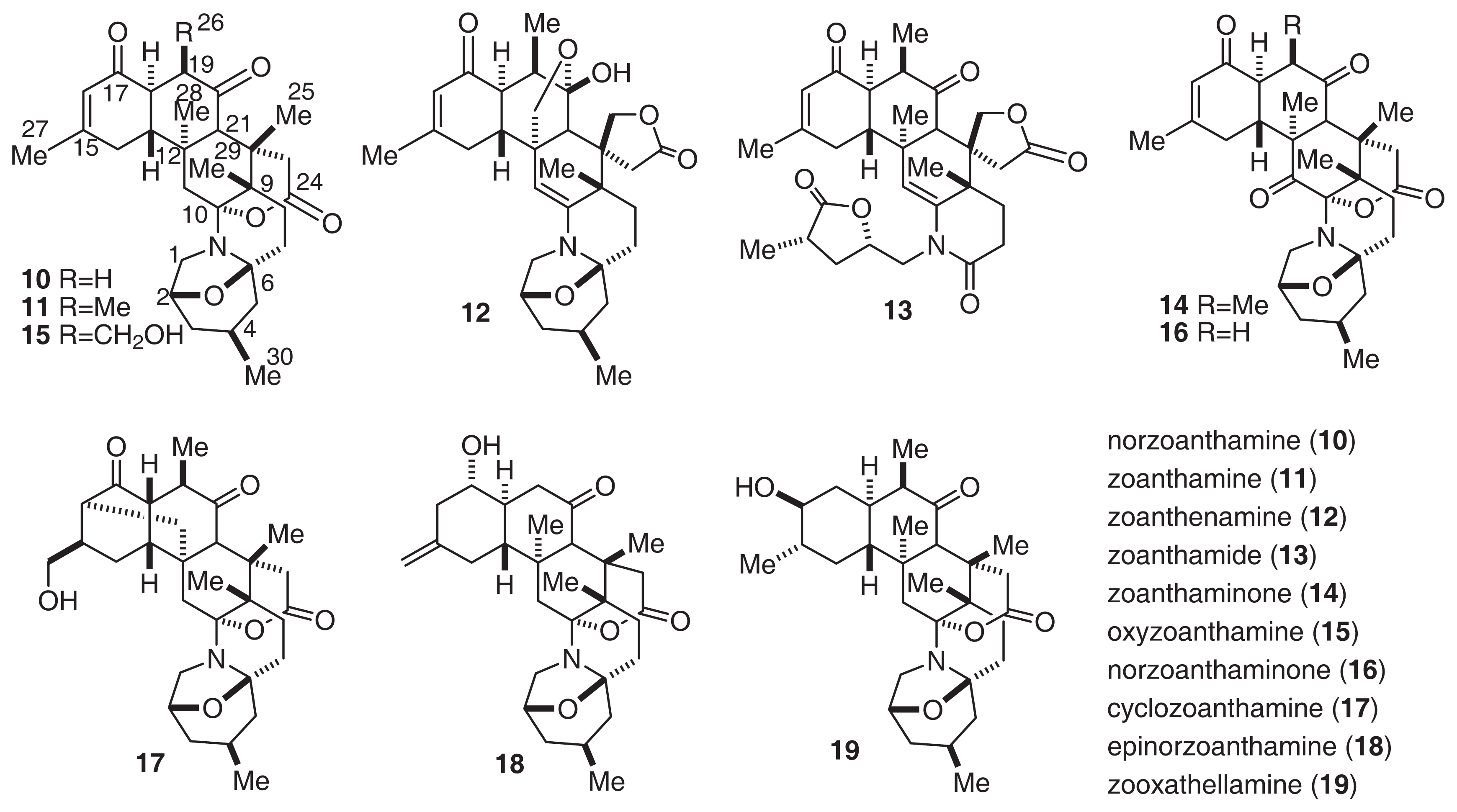
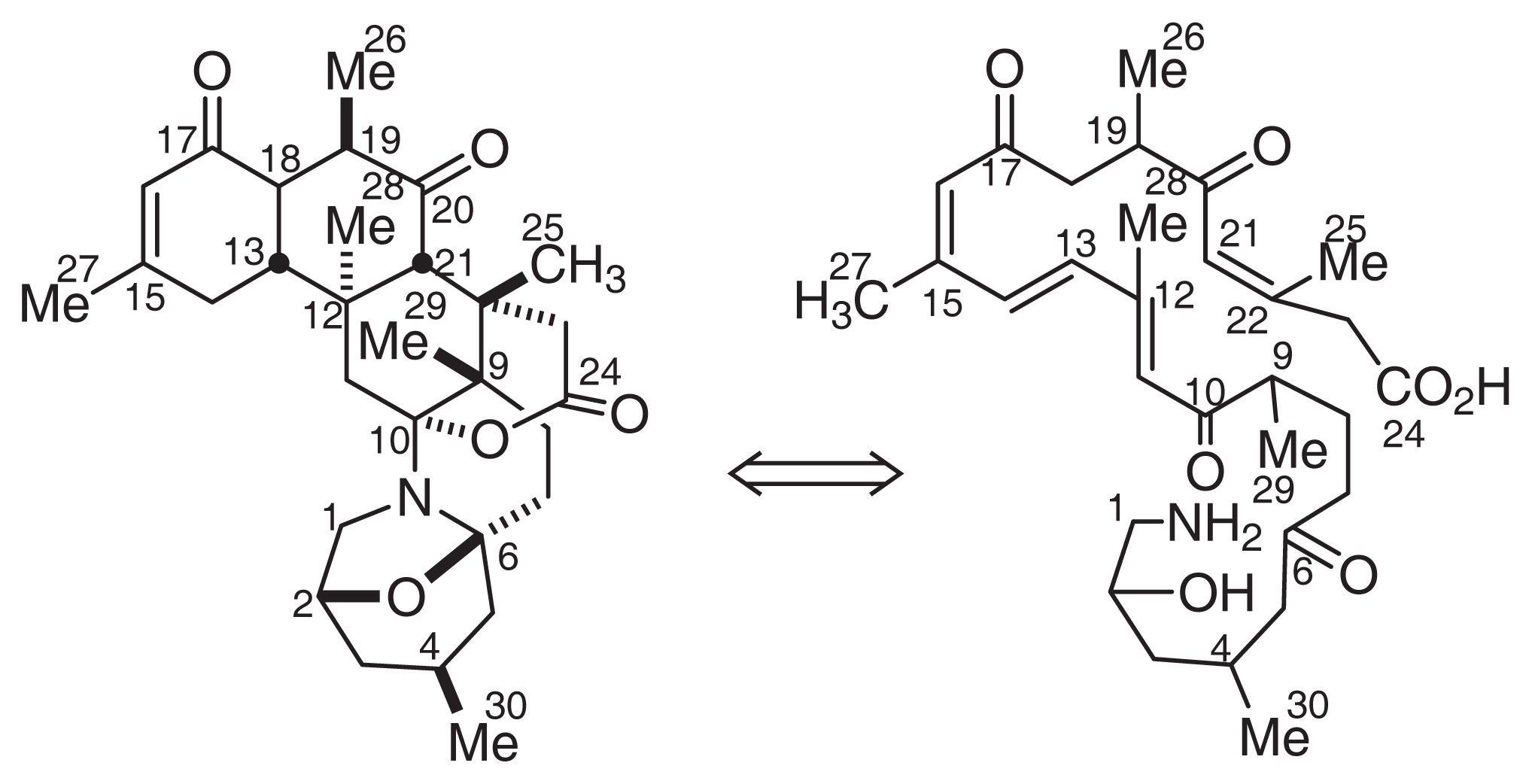
Norzoanthamine (10) [35] and its homologues (11–18) [35–38] were isolated from the genus Zoanthus sp. The relative stereochemistry of norzoanthamines was determined by X-ray analysis. Furthermore, the absolute stereochemistry of norzoanthamine was determined by an advanced version of Mosher’s method, as shown in Fig. 8[39]. IL-6 is known to stimulate osteoclast formation, and the suppression of IL-6 secretion can be effective in the prevention of osteoporosis. Norzoanthamine and norzoanthamine hydrochloride inhibit IL-6 induction at values of 13 and 4.7 μg/ml, respectively [39–41]. Furthermore, norzoanthamine and norzoanthamine hydrochloride, both of which counteract decreases in bone weight and strength in ovariectomized mice, could be good candidates for osteoporotic drugs [34,42].
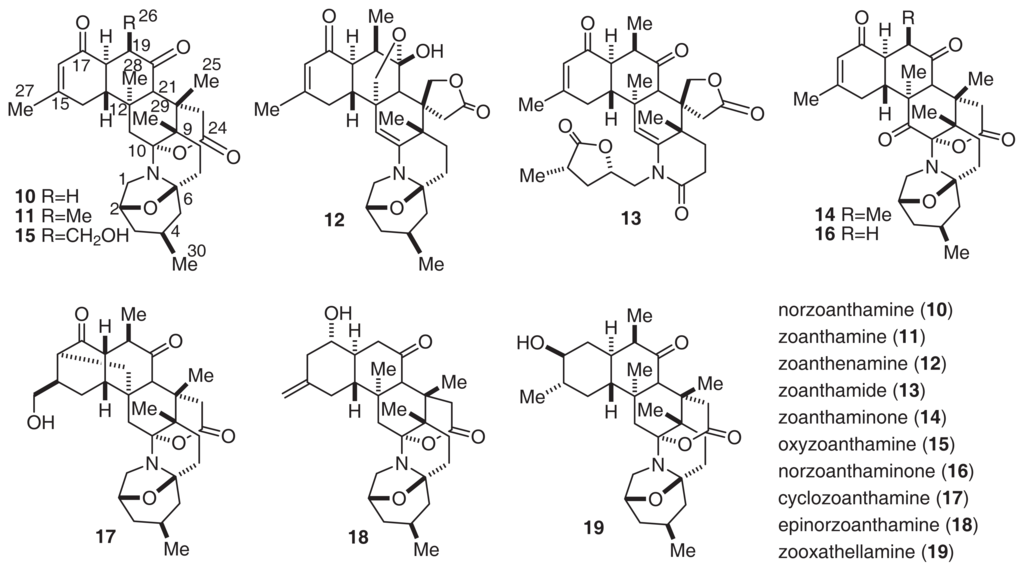
Fig. 8.
Structures of Norzoanthamines.
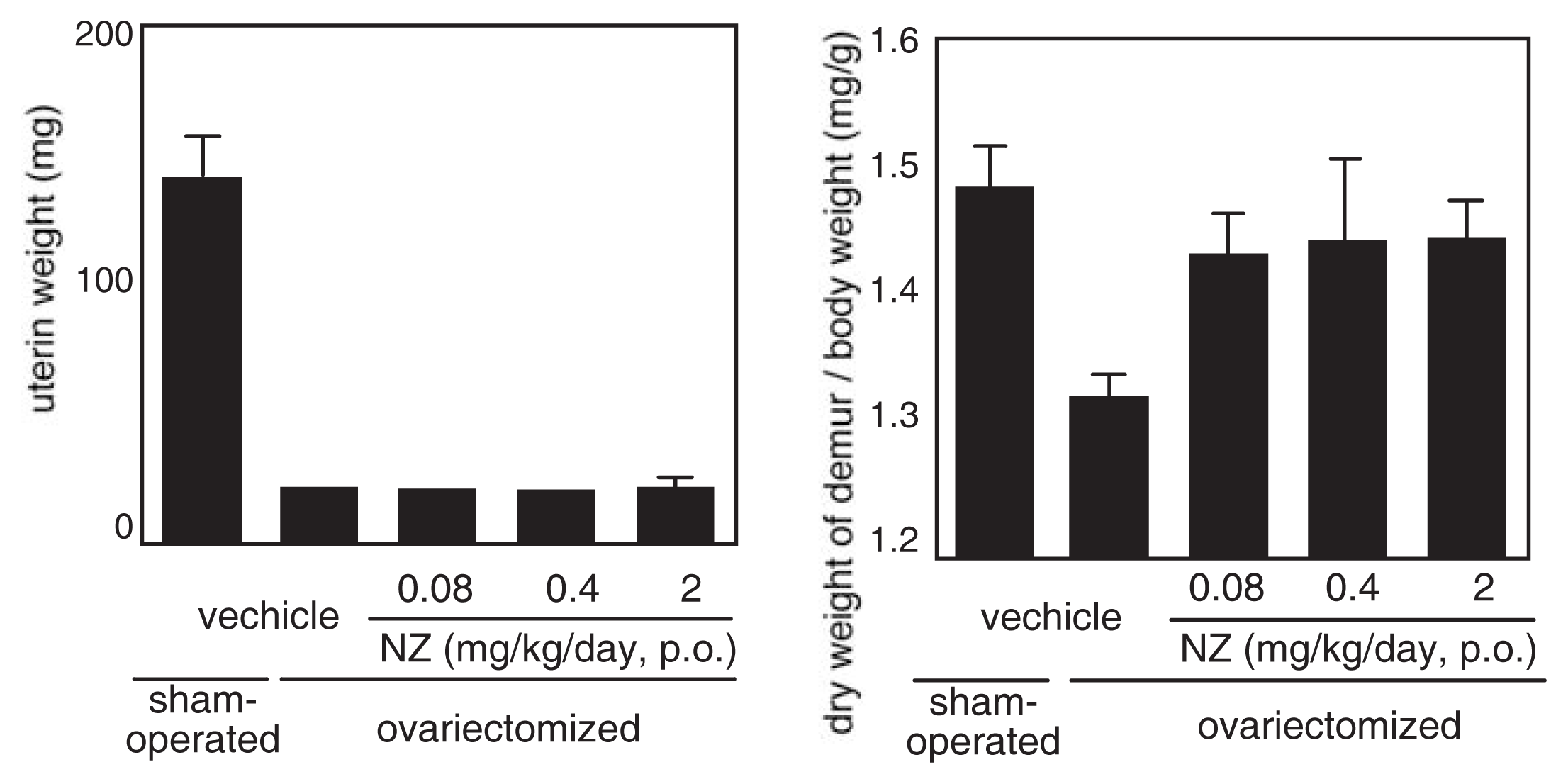
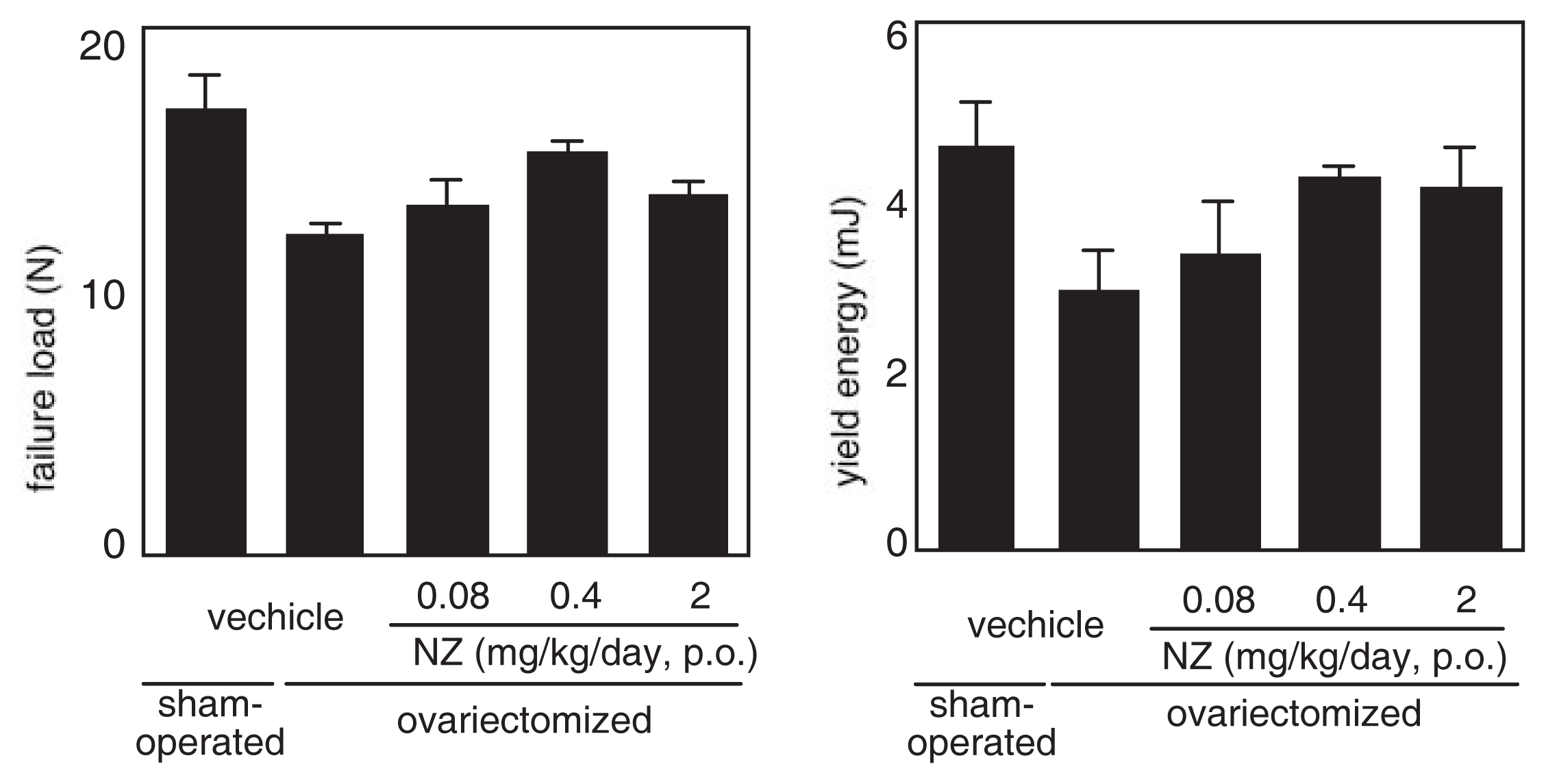
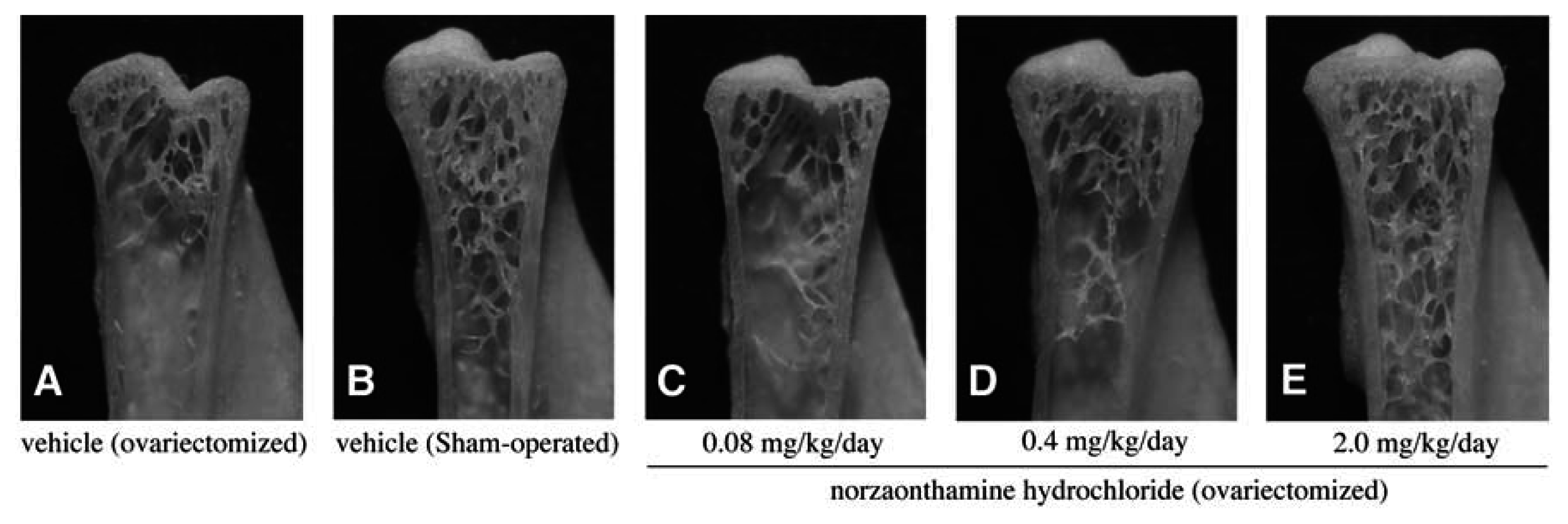
The effect of norzoanthamine hydrochloride on bone weight and strength was tested in ovariectomized mice, an animal model of postmenopausal osteoporosis [39–41,43]. Norzoanthamine hydrochloride (0.08 mg/kg/day, p.o.) significantly suppressed the decrease in femoral weight caused by ovariectomy without an increase in uterine weight (Fig. 9). Such data suggested that the mode of action of norzoanthamine hydrochloride differs from that of estrogen [44]. Furthermore, the failure load and yield energy of the femur were maintained by the administration of norzoanthamine hydrochloride at a dose of 0.4 mg/kg/day (p.o.) (Fig. 10). Finally, the thickness of the cortical bone was measured from a photograph of the ground bone. Ovariectomy caused a decrease in humeralis trabeculae (Fig. 11). Norzoanthamine hydrochloride significantly suppressed this decrease in a dose-dependent manner (Fig. 11C, D, E). In ovariectomized mice treated with norzoanthamine hydrochloride, the primary spongiosa did not significantly increase, and the morphology of the metaphysis remained nearly normal.
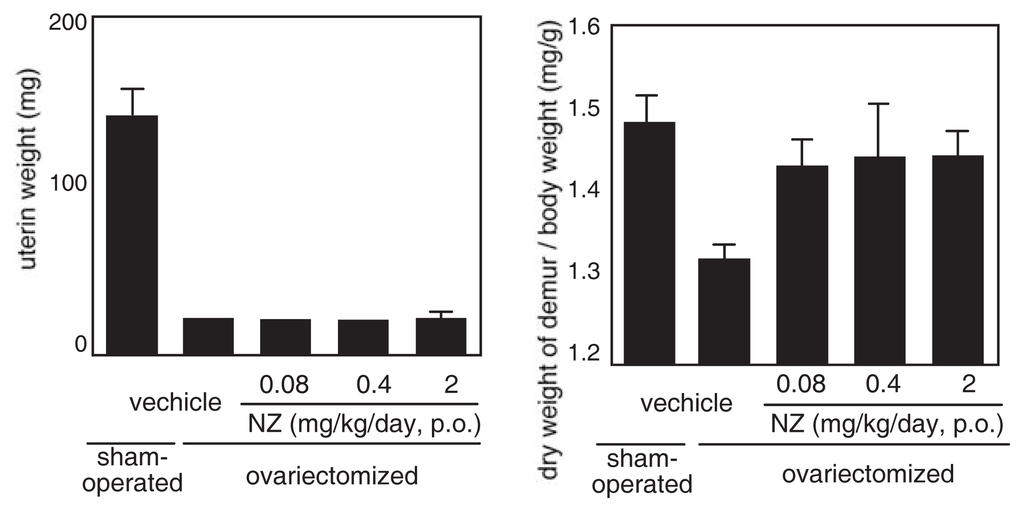
Fig. 9.
Effects of norzoanthamine hydrochloride on uterine and femoral weight.
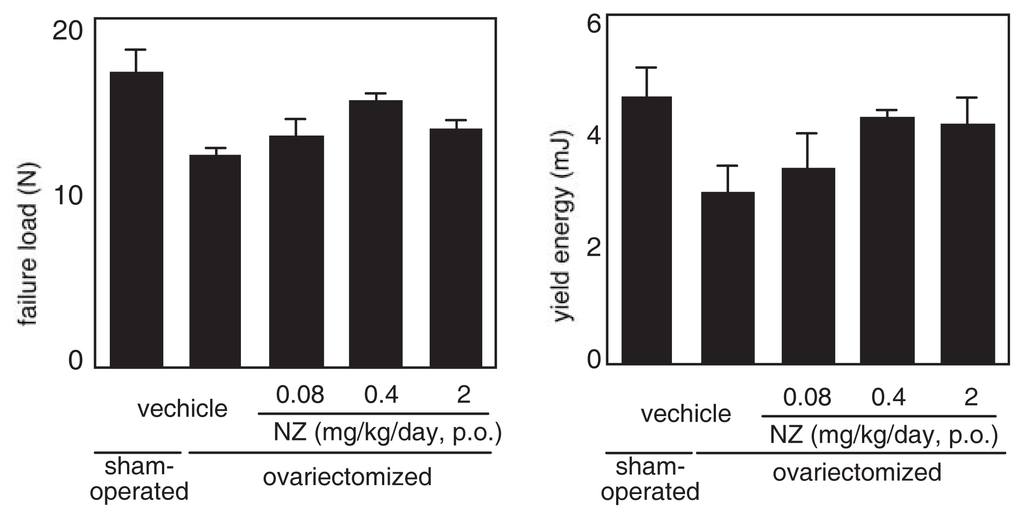
Fig. 10.
Effects of norzoanthamine hydrochloride on failure load and yield energy.
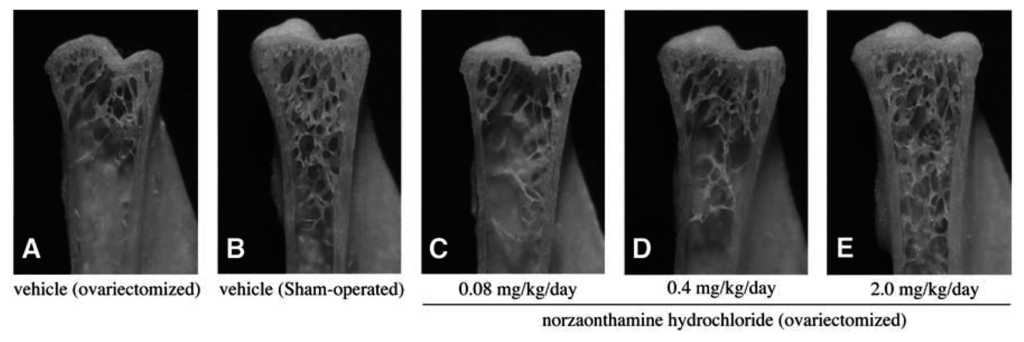
Fig. 11.
Effect of norzoanthamine hydrochloride on humeralis morphology in ovariectomized mice.
Based on their molecular formulas, zoanthamines have been regarded as terpenoids; however, the biogenetic pathway of zoanthamines remains unclear. As described above, marine organisms usually produce super carbon chain molecules with a terminal amino group. Here, we propose a polyketide biogenetic pathway for zoanthamines, as shown in Fig. 12.
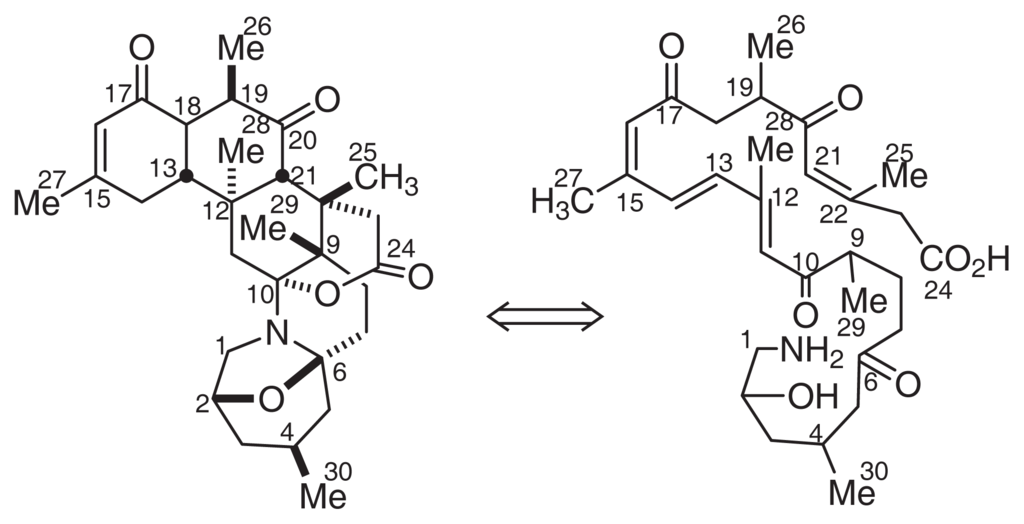
Fig. 12.
Proposed biogenesis of zoanthamines.
Interestingly, Nakamura’s group isolated zooxathellamine (19, Fig. 8) from a symbiotic dinoflagellate Symbiodinium sp. [45]. The absolute stereochemistry of 19 was the same as that of norzoanthamine. The structural similarity of 19 and zoanthid alkaloids suggests that these zoanthamines may have an algal origin. Furthermore, a feeding experiment with a labeled compound suggested the biosynthetic pathway of 19. This pathway was similar to that which we suggested previously.
Other Alkaloids from Marine Organisms (Pinnamine, Ircinamine)
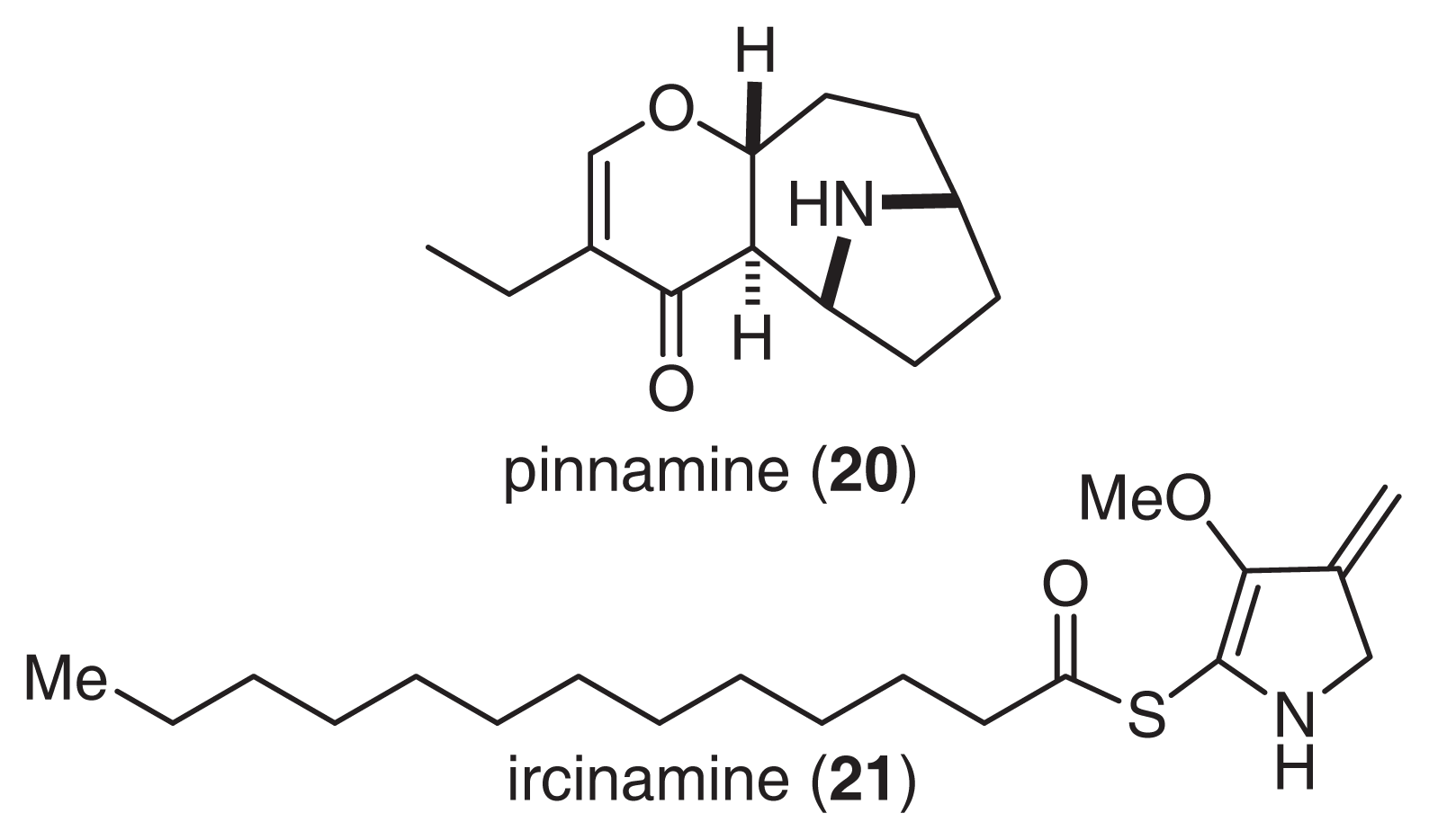
As described above, pinnatoxins, are Ca2+ channel activators that have been isolated from the Okinawan bivalve P. muricata. In a continuation of this work, we isolated pinnamine (20), which produced characteristic toxic symptoms, such as scurrying around [46]. The gross structure of 20 was clarified by a detailed analysis of NMR and CD spectra (Fig. 13) [47–50]. The absolute stereochemistry of 20 was also supported by a synthetic study [51]. Pinnamine exhibited significant acute toxicity against mice, with an LD99 of 0.5 mg/kg.
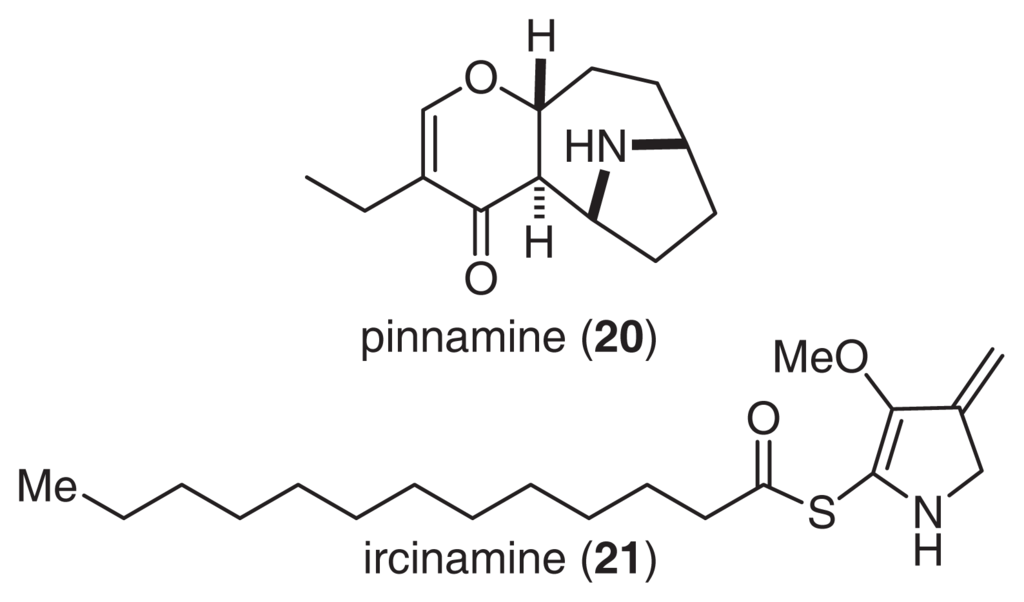
Fig. 13.
Structures of pinnamine and ircinamine.
Ircinamine (21) was isolated from the marine sponge Ircinia sp., and its structure was elucidated by spectroscopic analysis and reductive transformation (Fig. 13) [52]. Ircinamine has a unique structure with an amphibolous pyrroline ring moiety and a reactive thioester unit. Although 21 has only moderate activity toward P388 (LD50 24.6 μg/ml), marked biological activity is expected based on the reactivity of the thioester moiety [53].
These compounds contain unique structural features and may be biosynthesized through unusual biogenetic pathways.
Inhibitors of Superoxide Anion Generation from Marine Microorganisms (Aburatubolactams)
As described above, extremely bioactive, structurally novel compounds have been found in marine organisms. However, their practical use in drugs is considerably limited because of the extraordinarily low amount of physiologically active compounds obtainable from these marine organisms. Therefore, research has necessarily focused on the metabolites of such marine microorganisms, which most likely are the true producers of the active compounds.
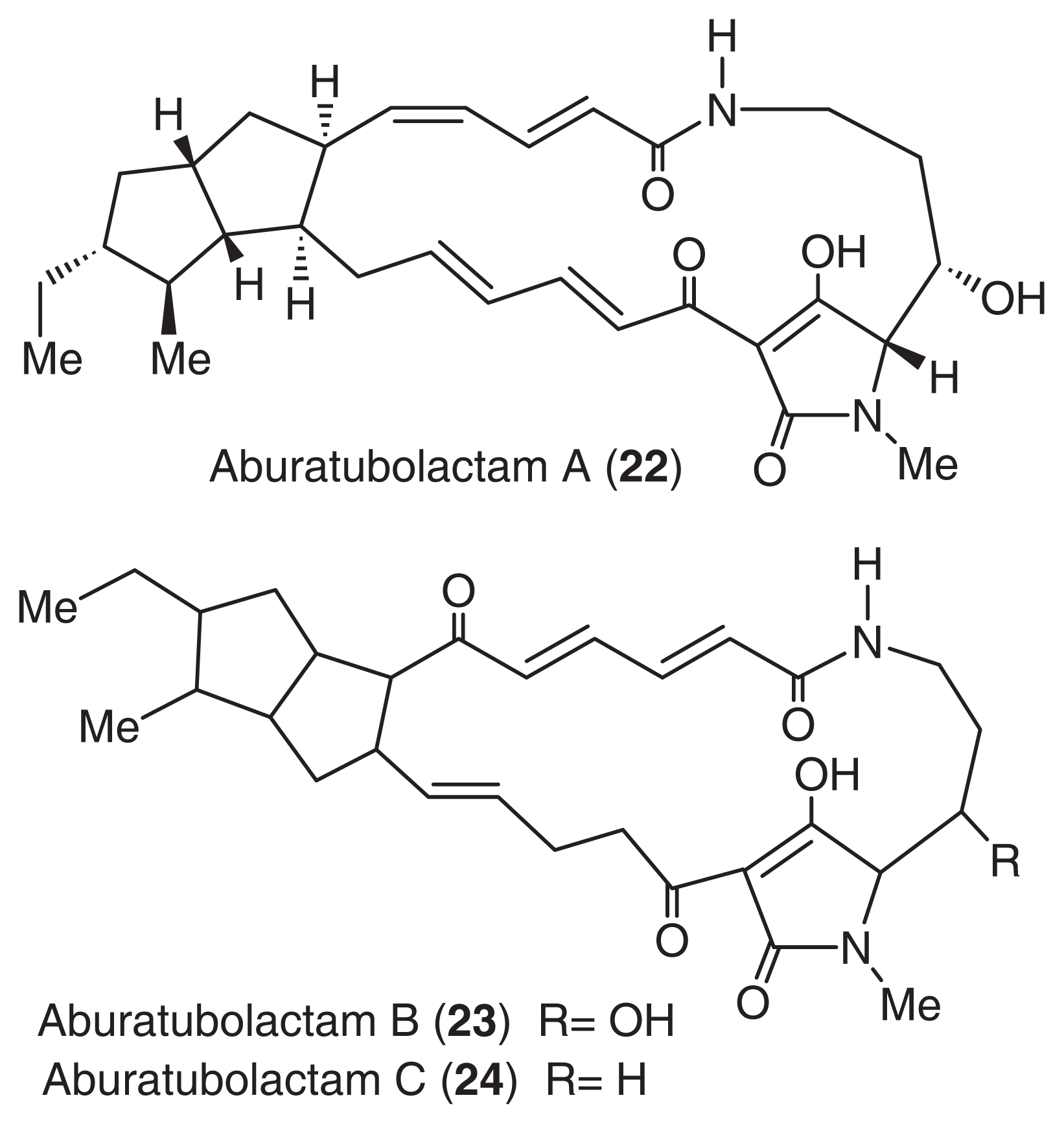
Aburatubolactams (22, 23, 24) [54] were isolated from the cultured broth of a Streptomyces sp., SCRC-A20, in a study that monitored the inhibition of superoxide anion generation [55,56]. Superoxide anions are thought to be closely associated with inflammation, cancer, and aging [57,58]. The structures of these compounds were mainly determined by NMR analysis. Fortunately, a single crystal of aburatubolactam A was obtained from MeOH. The structure of the 20-membered macrocyclic structure is thought to contain diene amide and dienone functionalities. The structures of other aburatubolactams were deduced by a detailed comparison of the NMR spectra with that of aburatubolactam A (Fig. 14).
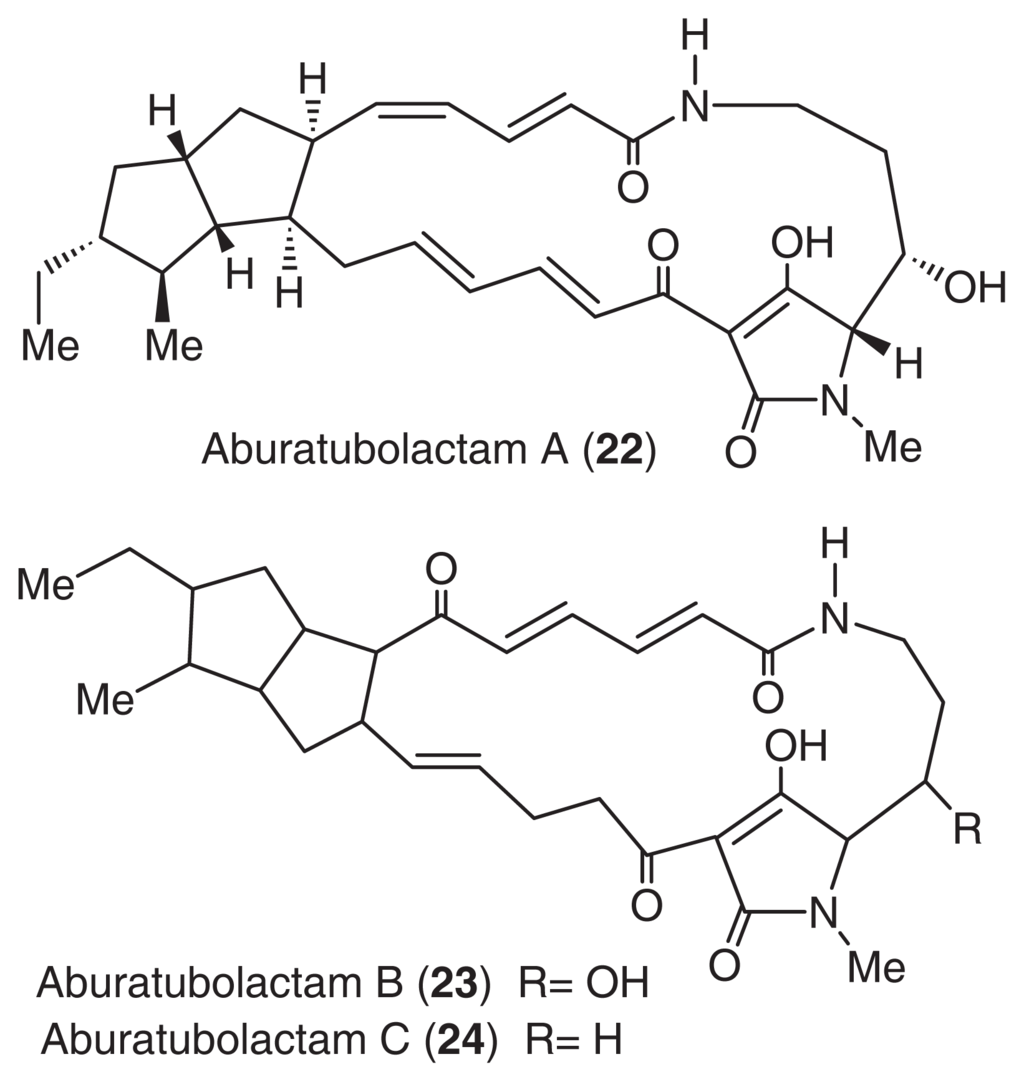
Fig. 14.
Structures of aburatubolactams.
Aburatubolactams (22, 23, 24) inhibited TPA-induced superoxide anion generation by human neutrophils (IC50 26, 6.3, 2.7 μg/ml, respectively). The mechanism of action and the in vivo behavior of aburatubolactams are currently under investigation.
Aburatubolactams, which possess an acyl tetramine structure, are biogenetically related to ikarugamycin [59] from a terrestrial actinomycete, alteramide A [60] from a marine bacterium, and cyrindramine [61] from a marine sponge. These results suggest that microorganisms may be the true producers of most marine metabolites.
Conclusions
As described above, bioactive alkaloids have been isolated from marine organisms. These structures were clarified by spectroscopic analysis and synthetic methods. The biogenesis of these compounds was proposed based on comparisons with their analogs. Additional biological activities of these compounds in vivo are currently under investigation in our laboratories.
Thanks to the development of new analytical instruments and techniques, numerous compounds have been isolated and elucidated from natural resources over the past 30 years. The study of natural resources may lead to the further discovery of novel bioactive compounds.
Acknowledgements
We would like to thank Dr. K. Yamada (Nagoya University) and Dr. K. Yamaguchi (Tokyo University of Fisheries) for testing biological activities. This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We are indebted to Wako Pure Chemical Industries, Ltd.; Banyu Pharmaceutical Co., Ltd.; and the Naito Foundation for their financial support.
References
- Rosewater, J. The family Pinnidae in the Indo-Pacific. Indo-Pacific Mollusca 1961, 1, 53, /501/632.. [Google Scholar]
- Zheng, S. Z.; Huang, F. L.; Chen, S. C.; Tan, X. F.; Zuo, J. B.; Peng, J.; Xie, R. W. The Isolation and Bioactivities of Pinnatoxin. Zhongguo Haiyang Yaowu(Chinese Journal of Marine Drugs). 1990, 9, 33–35. [Google Scholar]
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukuzawa, S.; Zheng, S. Z.; Chen, H. Pinnatoxin A: a Toxic Amphoteric Macrocycle from the Okinawan Bivalve Pinna muricata. J. Am. Chem. Soc 1995, 117, 1155–1156. [Google Scholar]
- Chou, T.; Kamo, O.; Uemura, D. Relative Stereochemistry of Pinnatoxin A, A Potent Shellfish Poison from Pinna muricata. Tetrahedron Lett 1996, 37, 4023–4026. [Google Scholar]
- Chou, T.; Haino, T.; Kuramoto, M.; Uemura, D. Isolation and Structure of Pinnatoxin D, A New Shellfish Poison from the Okinawan Bivalve Pinna muricata. Tetrahedron Lett 1996, 37, 4027–4030. [Google Scholar]
- Takada, N.; Uemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the Most Toxic Components in the Pinnatoxin Series from the Okinawan Bivalve Pinna muricata. Tetrahedron Lett 2001, 42, 3491–3494. [Google Scholar]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a Polyhydroxy-Polyene Antifungal Agent with an Unprecedented Structure, from a Marine Dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc 1991, 113, 9859–9861. [Google Scholar]
- Naoki, H.; Murata, M.; Yasumoto, T. Negative FAB Tandem Mass Spectrometry for Structural Study on Polyether Compounds; Structural Verification of Yessotoxin. Rapid Commun. Mass Sp 1993, 7, 179–182. [Google Scholar]
- McCauley, J. A.; Nakagawa, K.; Lander, P. A.; Mischke, S. G.; Semones, M. A.; Kishi, Y. Total Synthesis of Pinnatoxin A. J. Am. Chem. Soc 1998, 120, 7647–7468. [Google Scholar]
- Takada, N.; Uemura, N.; Suenaga, K.; Uemura, D. Structural Determination of Pteriatoxins A, B and C, Extremely Potent Toxins from the Bivalve Pteria penguin. Tetrahedron Lett 2001, 42, 3495–3497. [Google Scholar]
- Kock, A. E.; Halloran, M. M.; Haskell, C. J.; Sah, M. R.; Polverini, P. J. Angiogenesis Mediated by Soluble forms of E-Selectin and Vascular Cell Adhesion Molecule-1. Nature 1995, 376, 517–519, and references cited therein.. [Google Scholar]
- Osborn, L.; Hession, C.; Tizard, R.; Vassallo, C.; Huhovoskyi, S.; Chi-Rosso, G.; Hobb, R. Direct Expression Cloning of Vascular Cell Adhesion Molecule 1, a Cytokine-Induced Endothelial Protein that Binds to Lymphocytes. Cell 1988, 59, 1203–1211. [Google Scholar]
- Kuramoto, M.; Chou, T.; Yamada, K.; Chiba, T.; Hayashi, Y.; Uemura, D. Halichlorine, an Inhibitor of VCAM-1 Induction from the Marine Sponge Halichondria okadai Kadota. Tetrahedron Lett 1996, 37, 3867–3870. [Google Scholar]
- Nikon, A. A Relationship Between Conformation and Infrared Absorption in 1,2-Halohydrins. J. Am. Chem. Soc 1957, 79, 243–247. [Google Scholar]
- Bohlmann, F. Lupinen-Alkaloide, VIII. Zur Konfigurationsbestimmung von Chinolizidin-Devivaten. Chem. Ber 1958, 91, 2157. [Google Scholar]
- Arimoto, H.; Hayakawa, I.; Kuramoto, M.; Uemura, D. Absolute Stereochemistry of halichlorine; A Potent Inhibitor of VCAM-1 Induction. Tetrahedron. Lett 1998, 39, 861–862. [Google Scholar]
- Trauner, D.; Schwarz, J. B.; Danishefsky, S. J. Total Synthesis of (+)-Halichlorine: An Inhibitor of VCAM-1 Expression. Angew. Chem. Int. Ed 1999, 38, 3542–3545. [Google Scholar]
- Trauner, D.; Danishefsky, S. J. Studies Towards the Total Synthesis of Halichlorine: Asymmetric Synthesis of the Spiroquinolizidine Subunit. Tetrahedron Lett 1999, 40, 6513–6516. [Google Scholar]
- Boschelli, D. H.; Karmer, J. B.; Khatana, S. S.; Sorenson, R. J.; Connor, D. T.; Ferin, M. A.; Wright, C. D.; Lesch, M. E.; Imre, K.; Okonkwo, G. C.; Schrie, D. J.; Conroy, M. C.; Ferguson, E.; Woelle, J.; Saxena, U. Inhibition of E-Selectin-, ICAM-1-, and VCAM-1-Mediated Cell Adhesion by Benzo[b]thiophene-, Benzofuran-, Indole-, and Naphthalene-2- carboxamides: Identification of PD 144795 as An Antiinflammatory Agent. J. Med. Chem 1995, 38, 4597–4614. [Google Scholar]
- Dennis, E. A. The Enzymes; Boyer, P. D., Ed.; Academic Press: New York, 1983; p. 307. [Google Scholar]
- van den Bosch, H. Intracellular Phospholipases A. Biochim. Biophys. Acta 1980, 604, 191–246. [Google Scholar]
- Arita, H.; Nakano, T.; Hanasaki, K. Thromboxane A2: Its Generation and Role in Platelet Activation. Prog. Lipid Res 1989, 28, 273–301. [Google Scholar]
- Scheuer, P. J.; de Silva, E. D. Manoalide, an Antibiotic Sesterterpenoid from the Marine Sponge Luffariella variabilis (polejaeff). Tetrahedron Lett 1980, 21, 1611–1614. [Google Scholar]
- Albizati, K. F.; Holman, T.; Faulkner, D. J.; Glaser, K. B.; Jacobs, R. S. Luffariellolide, an Anti-Infmammatory Sesterterpene from the Marine Sponge Luffariella sp., an Anti-Infmammatory Sesterterpene from the Marine Sponge Luffariella sp. Experientia 1987, 43, 949–950. [Google Scholar]
- Potts, B. C. M.; Faulkner, D. J.; de Carvalho, M. S.; Jacobs, R. S. Chemical Mechanism of Inactivation of Bee Venom Phospholipase A2 by the Marine Natural Products Manoalide, Luffariellolide, and Scalaradial. J. Am. Chem. Soc 1992, 114, 5093–5100. [Google Scholar]
- Potts, B. C. M.; Faulkner, D. J.; Jacobs, R. S. Phospholipase A2 Inhibitors from Marine Organisms. J. Nat. Prod 1992, 55, 1701–17. [Google Scholar]
- Kramer, R.M.; Johansen, B.; Hession, C.; Pepinsky, R.B. Structure and Properties of a Secretable Phospholipase A2 from Human Platelets. Adv. Exp. Med. Biol 1990, 275, 35–53. [Google Scholar]
- Kramer, R. M.; Sharp, J. D. Recent Insights into the Structure, Function and Biology of cPLA2. Agents Actions Suppl 1995, 46, 65–67. [Google Scholar]
- Kim, D. K.; Kudo, I.; Fujimori, Y.; Mizushima, H.; Masuda, M.; Kikuchi, R.; Ikizawa, K; Inoue, K. Detection and Subcellular Localization of Rabbit Platelet Phospholipase A2 which Preferentially Hydrolyzes an Arachidonoyl Residue. J. Biochem 1990, 108, 903–906. [Google Scholar]
- Chou, T.; Haino, T.; Kuramoto, M.; Uemura, D. Pinnaic Acid and Tauropinnaic Acid: Two Novel Fatty Acids Composing a 6-Azaspiro[4.5]decane Unit from the Okinawan Bivalve Pinna muricata. Tetrahedron Lett 1996, 37, 3871–3874. [Google Scholar]
- Carson, M. W.; Kim, G.; Hentemann, M. F.; Trauner, D.; Danishefsky, S. J. Concise Stereoselective Routes to Advanced Intermediates Related to Natural and Unnatural Pinnaic Acid. Angew. Chem. Int. Ed 2001, 40, 4450–4452. [Google Scholar]
- Carson, M. W.; Kim, G.; Danishefsky, D. J. Total Synthesis and Proof of Stereochemistry of Natural and Unnatural Pinnaic Acids: A Remarkable Long-Range Stereochemical Effect in the Reduction of 17-Oxo Precursors of the Pinnaic Acids. Angew. Chem, Int. Ed 2001, 40, 4453–4456. [Google Scholar]
- Hayakawa, I.; Arimoto, H.; Uemura, D. Synthesis of (+)-Pinnaic Acid. Heterocycles 2003, 59, 441–444. [Google Scholar]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C; Bradley, A.; Karsenty, G. Increased Bone Formation in Osteocalcin-Deficient Mice. Nature 1996, 382, 448–451. [Google Scholar]
- Fukuzawa, S.; Hayashi, Y.; Uemura, D.; Nagastu, A.; Yamada, K.; Ijyuin, Y. The Isolation and Structures of Five New Alkaloids, Norzoanthamine, Norzoanthaminone, Cyclozoanthamine, Oxyzoanthamine and Epinorzoanthamine. Heterocycl. Commun 1995, 1, 207–217. [Google Scholar]
- Rao, C. B.; Anjaneyula, A. S. R.; Sarma, N. S.; Venkatateswarlu, Y.; Rosser, R. M.; Faulkner, D. J.; Chen, M. H. M.; Clardy, J. Zoanthamine, A Novel Alkaloid from a Marine Zoanthid. J. Am. Chem. Soc 1984, 106, 7983–7984. [Google Scholar]
- Rao, C. B.; Anjaneyula, A. S. R.; Sarma, N. S.; Venkatateswarlu, Y.; Rosser, R. M.; Faulkner, D. J.; Chen, M. H. M.; Clardy, J. Alkaloids from a Marine Zoanthid. J. Org. Chem 1985, 50, 3757–3760. [Google Scholar]
- Rahman, A. U.; Alvi, K. A.; Abbas, S. A.; Choudhary, M. I.; Clardy, J. Zoanthaminone, a New Alkaloid from a Marine Zoanthid. Tetrahedron Lett 1989, 30, 6825–6828. [Google Scholar]
- Kuramoto, M.; Hayashi, K.; Fujitani, Y.; Yamaguchi, K.; Tsuji, T.; Yamada, K.; Ijyuin, Y.; Uemura, D. Absolute Configuration of Norzoanthamine, a Promising Candidate for an Osteoporotic Drug. Tetrahedron Lett 1997, 38, 5683–5686. [Google Scholar]
- Kuramoto, M.; Hayashi, K.; Yamaguchi, K.; Yada, M.; Tsuji, T.; Uemura, D. Structure-Activity Relationship of Norzoanthamine, Exhibiting Significant Inhibition of Osteoporosis. Bull. Chem. Soc. Jpn 1998, 71, 771–779. [Google Scholar]
- Kuramoto, M.; Yamaguchi, K.; Tsuji, T.; Uemura, D. Drugs from the Sea; Fusetani, N., Ed.; Karger: Basel, 2000; pp. 98–106. [Google Scholar]
- Turner, C. H.; Burr, D. B. Basic Biomechanical Measurements of Bone: a Tutorial. Bone 1993, 14, 595–608. [Google Scholar]
- Yamaguchi, K.; Yada, M.; Tsuji, T.; Kuramoto, M.; Uemura, D. Suppressive Effect of Norzoanthamine Hydrochloride on Experimental Osteoporosis in Ovariectomized Mice. Biol. Pharm. Bull 1999, 22, 920–924. [Google Scholar]
- Kuiper, G.G; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J. A. Cloning of a Novel Receptor Expressed in Rat Prostate and Ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar]
- Nakamura, H.; Kawase, Y.; Maruyama, K.; Murai, A. Studies on Polyketide Metabolites of a Symbiotic Dinoflagellate, Symbidinium sp.: A New C30 Marine Alkaloid, Zooxanthellamine, a Plausible Precursor for Zoanthid Alkaloids. Bull. Chem. Soc. Jpn 1998, 71, 781–787. [Google Scholar]
- Takada, N.; Iwatsuki, M.; Suenaga, K.; Uemura, D. Pinnamine, an Alkaloidal Marine Toxin, Isolated from Pinna muricata. Tetrahedron Lett 2000, 41, 6425–6428. [Google Scholar]
- Djerassi, C.; Records, R.; Bunnenberg, E.; Mislow, K.; Miscowitz, A. Inherently Dissymetric Chromophores. Optical Rotatory Dispersion of α,β-Unsaturated Ketones and Conformational Analysis of Cyclohexenones. J. Am. Chem. Soc 1962, 84, 870–872. [Google Scholar]
- Snatzke, G. Circulardichroismus-VIII: Modifizierung der Octantenregel für, α,β-Ungesättigte Ketone: Theorie. Tetrahedron 1965, 21, 413–419. [Google Scholar]
- Snatzke, G. Circulardichroismus-IX: Modifizierung der Octantenregel für, α,β-Ungesättigte Ketone: Transoid Enone. Tetrahedron 1965, 21, 421–438. [Google Scholar]
- Snatzke, G. Circulardichroismus-X: Modifizierung der Octantenregel für, α,β-Ungesättigte Ketone: Cisoide Enone, Dienone und Arylketone. Tetrahedron 1965, 21, 439–448. [Google Scholar]
- Kigoshi, H.; Hayashi, N.; Uemura, D. Stereoselective Synthesis of Pinnamine, an Alkaloidal Marine Toxin from Pinna muricata. Tetrahedron Lett 2001, 42, 7469–7471. [Google Scholar]
- Kuramoto, M.; Fujita, T.; Ono, N. Ircinamine, a Novel Cytotoxic Alkaloid from Ircinia sp. Chem. Lett 2002, 31, 464–465. [Google Scholar]
- Fenteany, G.; Standaert, R.F.; Lane, W. S.; Choi, S.; Corey, E. J.; Schreiber, S. L. Cloning of a Novel receptor Expressed in Rat Prostate and Ovary. Science 1995, 268, 726–731. [Google Scholar]
- Yamada, K.; Kuramoto, M.; Uemura, D. Aburatubolactams and Zoanthamines, Naturally Occurring Bioactive Alkaloids. Recent Res. Devel. Pure & Applied Chem 1999, 3, 245–254. [Google Scholar]
- Krochak, H. M.; Vienne, K.; Rutherford, L. E.; Wilkenfeld, C.; Finkelstein, M. C.; Weissmann, G. Stimulus Response Coupling in the Human Neutrophil. II. Temporal Analysis of Changes in Cytosolic Calcium and Calcium Efflux. J. Biol. Chem 1984, 259, 4076–4082. [Google Scholar]
- Bae, M. A.; Yamada, K.; Ijyuin, Y.; Tsuji, T.; Yazawa, K.; Tomono, Y.; Uemura, D. Aburatubolactam A, a Novel Inhibitor of Superoxide Anion Generation from a Marine Microorganism. Heterocycl. Commun 1996, 2, 315–318. [Google Scholar]
- Nakano, Y.; Kawaguchi, T.; Sumimoto, J.; Takizawa, T.; Uetsuki, S.; Suenaga, M.; Kido, M. Novel Inhibitors of Superoxide Anion Generation, OPC-15160 and OPC-15161. Taxonomy, Fermentation, Isolation, Physico-Chemical Properties, Biological Characteristics and Structure Determination. J. Antibiot 1991, 44, 52–58. [Google Scholar]
- Badwey, J. A.; Karnovsky, M. L. Active Oxygen Species and the Functions of Phagocytic Leukocytes. Annu. Rev. Biochem 1980, 49, 695–726. [Google Scholar]
- Ito, S.; Hirata, Y. The Structure of Ikarugamycin, an Acyltetramic Acid Antibiotic Possessing a Unique as-Hydrindacene Skeleton. Bull. Chem. Soc. Jpn 1977, 50, 1813–1820. [Google Scholar]
- Shigemori, H.; Bae, M.-A.; Yazawa, K.; Sasaki, T.; Kobayashi, J. Alteramide A, a New Tetracyclic Alkaloid from a Bacterium Alteromonas sp. Associated with the Marine Sponge Halichondria okadai. J. Org. Chem 1992, 57, 4317–4320. [Google Scholar]
- Kanazawa, S.; Fusetani, N.; Matsunaga, S. Cylindramide: Cytotoxic Tetramic Acid Lactam from the Marine Sponge Halichondria cylindrata Tanita & Hoshino. Tetrahedron Lett 1993, 34, 1065–1068. [Google Scholar]
© 2004 by MDPI Reproduction is permitted for noncommercial purposes.