Microalgae as Contributors to Produce Biopolymers
Abstract
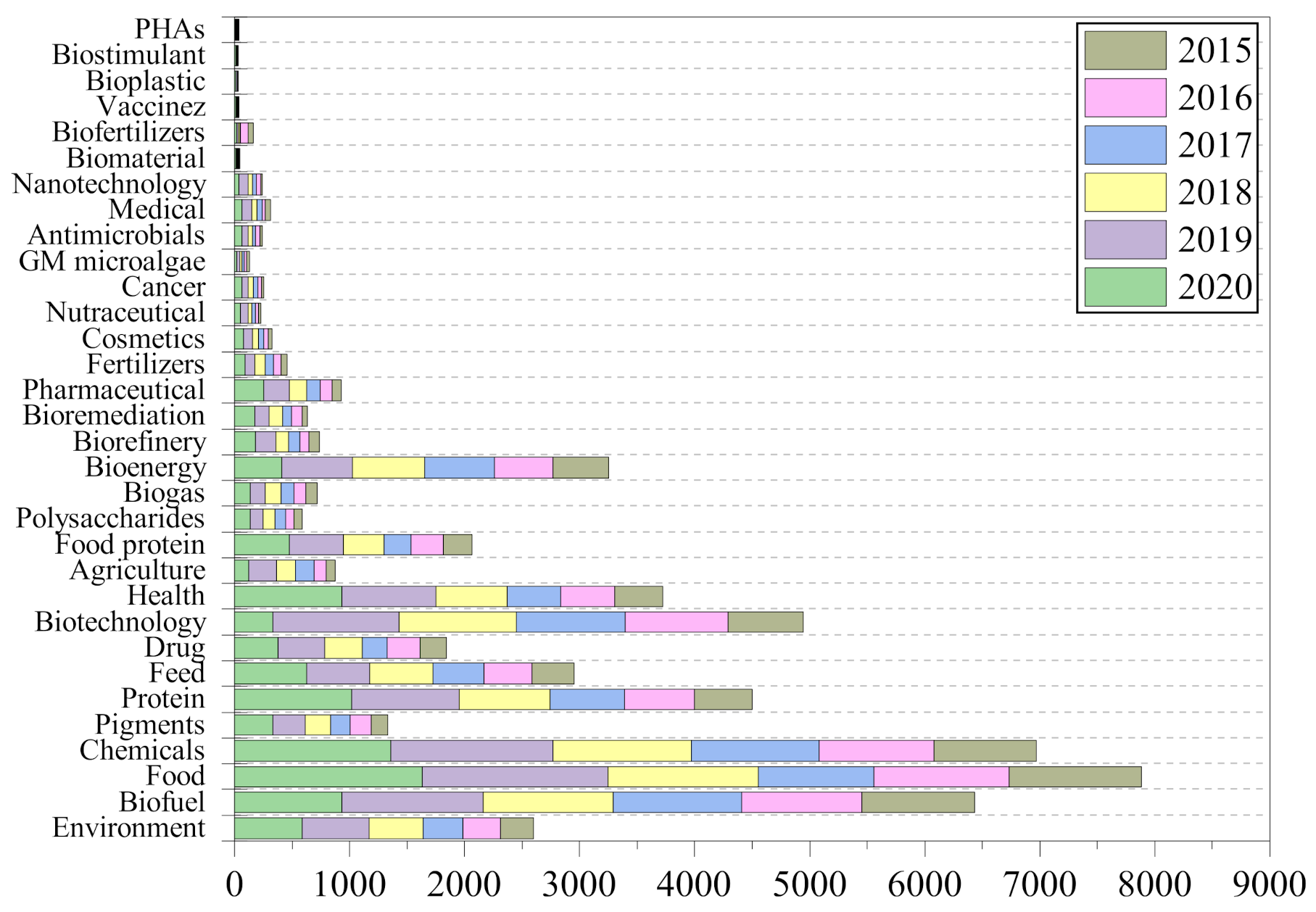
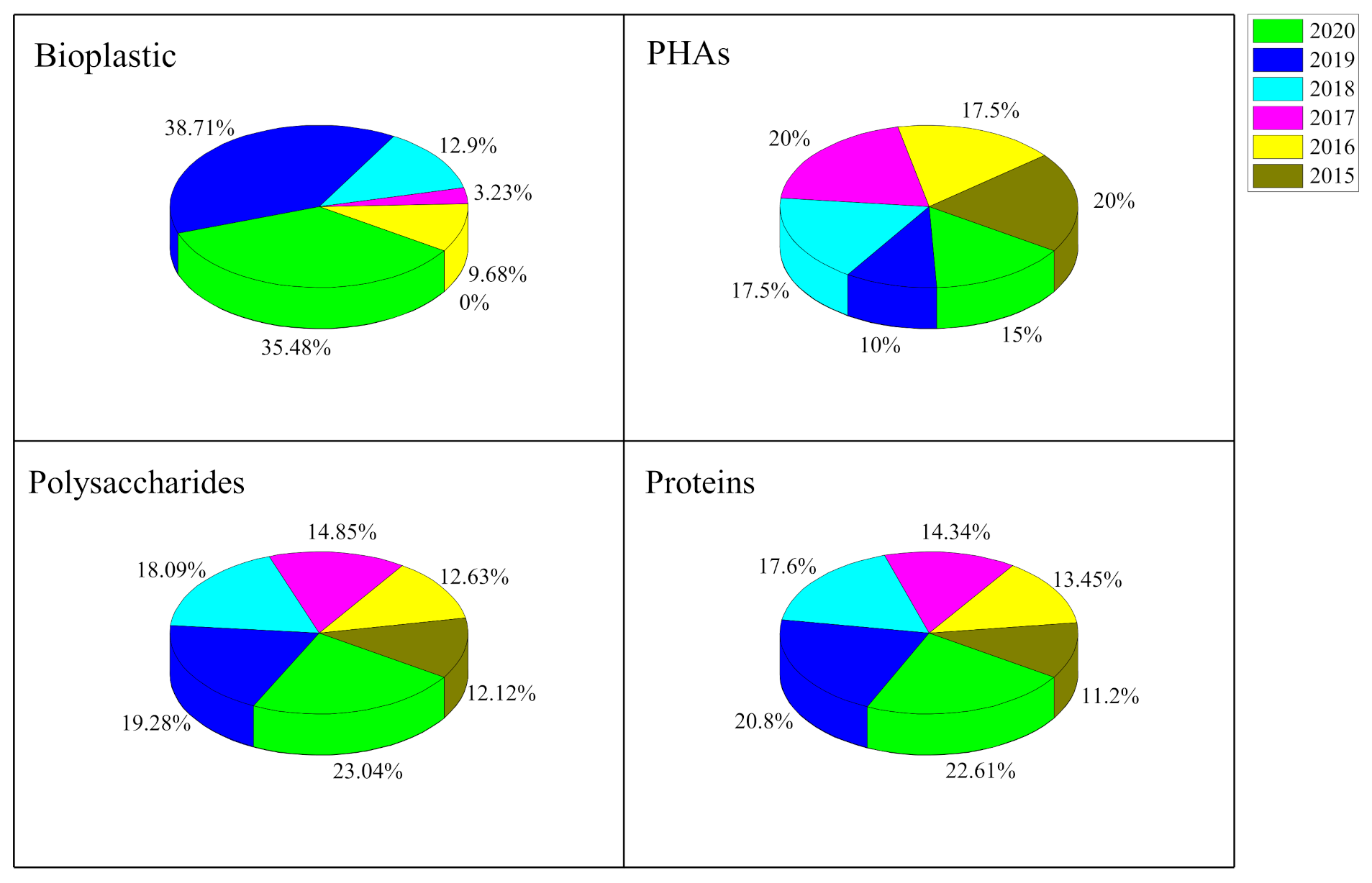
:1. Introduction
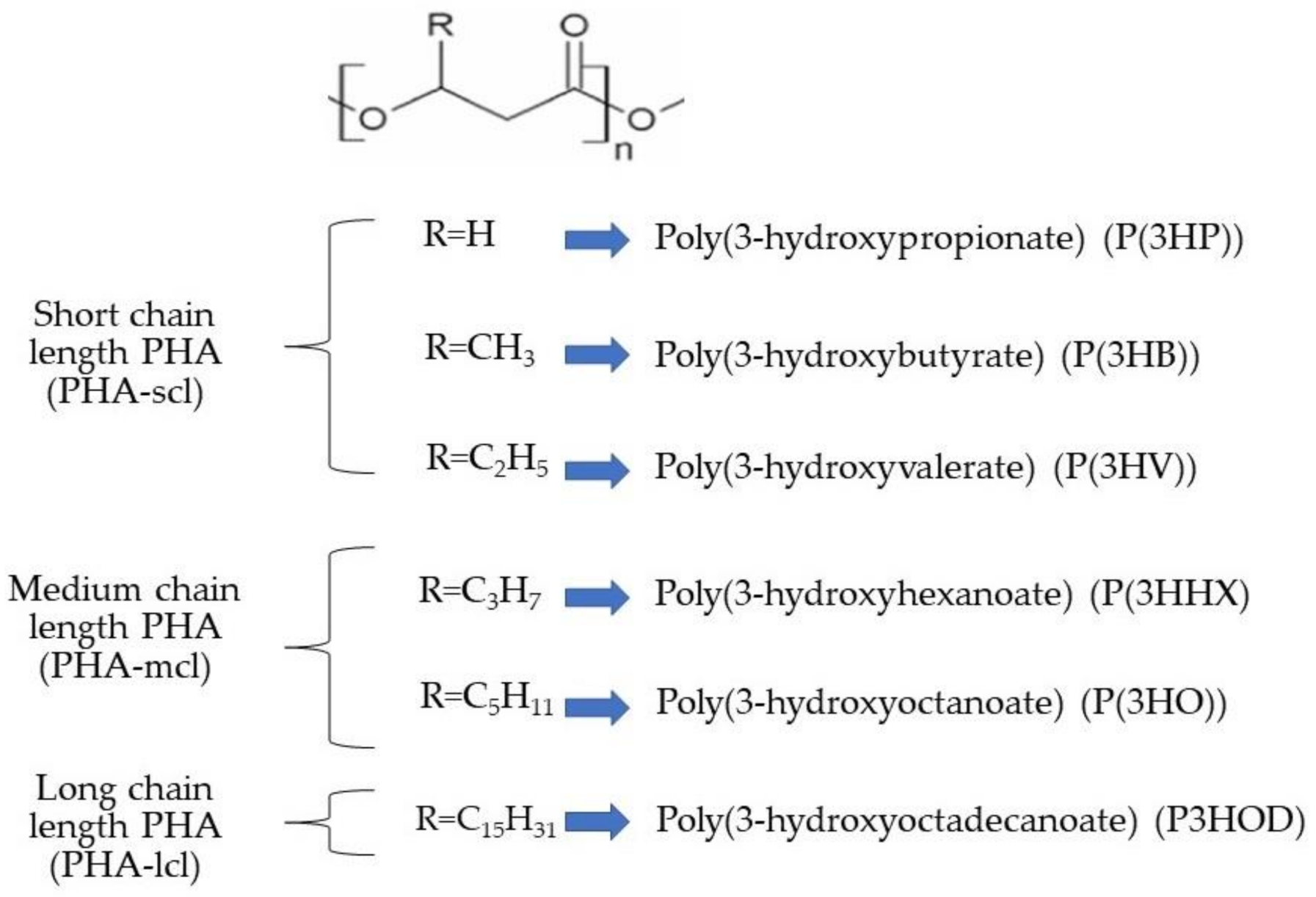
2. Polyhydroxyalkanoates (PHAs) in Microalgae
2.1. PHA-Based Blends and Composites
2.2. Improvement of PHA Accumulation in Algae
3. Microalgal Proteins
3.1. Protein-Based Blends and Composites
3.2. Improvement of Protein Content in Microalgae
4. Microalgal Starch
- (1)
- Indirectly converting starch into the monomers, which are used in the synthesis of polymers such as poly(lactic acid) from lactic acid, polyethylene from ethylene prepared by ethanol dehydration, or even PHAs.
- (2)
- Using starch as a raw material to produce low-molecular-weight hydroxylated compounds. Dextrins and glycolized products are two examples of polymers used in polyurethane formulations.
- (3)
- Using starch as a filler in other plastics or as thermoplastic starch.
4.1. Starch-Based Blends and Composites
4.2. Improvement of Starch Content in Microalgae
4.2.1. Nitrogen, Phosphorous, and Sulfur Limitation
4.2.2. Temperature and Irradiance
4.2.3. Inorganic Carbon
5. Microalgal Cellulose
Cellulose-Based Blends and Composites
6. Conclusions, Future Perspectives, and Personal Reflections
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Ness, D. Sustainable urban infrastructure in China: Towards a Factor 10 improvement in resource productivity through integrated infrastructure systems. Int. J. Sustain. Dev. World Ecol. 2008, 15, 288–301. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Zetterholm, J.; Bryngemark, E.; Ahlström, J.; Söderholm, P.; Harvey, S.; Wetterlund, E. Economic Evaluation of Large-Scale Biorefinery Deployment: A Framework Integrating Dynamic Biomass Market and Techno-Economic Models. Sustainability 2020, 12, 7126. [Google Scholar] [CrossRef]
- Modolo, R.; Benta, A.; Ferreira, V.M.; Machado, L.M. Pulp and Paper Plant Wastes Valorisation in Bituminous Mixes. Waste Manag. 2010, 30, 685–696. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Science of the Total Environment Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muneer, F. Biocomposites from Natural Polymers and Fibers; Faculty of Landscape Architecture, Horticulture and Crop Production Science, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2015; Volume 3, (Report). [Google Scholar]
- Madadi, R.; Tabatabaei, M.; Aghbashlo, M.; Zahed, M.A.; Pourbabaee, A.A. Biodiesel from Microalgae. In Waste to Wealth; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Springer: Singapore, 2018; pp. 277–318. [Google Scholar] [CrossRef]
- Martins, A.; Caetano, N.S.; Mata, T.M. Microalgae for Biodisel Production and Other Applications: A Review. Renewable Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Singh, D.; Saxena, R.; Rani, R.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. High-Value Coproducts from Algae—An Innovational Way to Deal with Advance Algal Industry. In Waste to Wealth; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Springer: Singapore, 2018; pp. 343–363. ISBN 9789811074318. [Google Scholar]
- Rahman, A.; Miller, C.D. Microalgae as a Source of Bioplastics. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–138. ISBN 9780444637840. [Google Scholar]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-Milled Biochar for Galaxolide Removal: Sorption Performance and Governing Mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef]
- da Costa, T.P.; Quinteiro, P.; Tarelho, L.A.C.; Arroja, L.; Dias, A.C. Environmental Assessment of Valorisation Alternatives for Woody Biomass Ash in Construction Materials. Resour. Conserv. Recycl. 2019, 148, 67–79. [Google Scholar] [CrossRef]
- Onen, C.S.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Kaparapu, J. Polyhydroxyalkanoate (PHA) Production by Genetically Engineered Microalgae: A Review. J. New Biol. Rep. 2018, 7, 68–73. [Google Scholar]
- Lambert, S.; Wagner, M. Environmental Performance of Bio-Based and Biodegradable Plastics: The Road Ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Balaji, S.; Gopi, K.; Muthuvelan, B. Review Article: A Review on Production of Poly β hydroxybutyrates from Cyanobacteria for the Production of Bioplastics. ALGAL 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Dai, Z.; Zou, X.; Chen, G. Biomaterials Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) as an Injectable Implant System for Prevention of Post-Surgical Tissue Adhesion. Biomaterials 2009, 30, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Y.; Wu, Q.; Chen, G. Evaluation of Three-Dimensional Scaffolds Prepared from Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for Growth of Allogeneic Chondrocytes for Cartilage Repair in Rabbits. Biomaterials 2008, 29, 2858–2868. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chun Chen, J.; Chen, G. Polyhydroxyalkanoate (PHA) Scaffolds with Good Mechanical Properties and Biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Bayón, B.; Berti, I.R.; Gagneten, A.M.; Castro, G.R. Biopolymers from Wastes to High-Value Products in Biomedicine. In Waste to Wealth. Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 1–44. [Google Scholar]
- Lodi, P.C.; Bueno, B.S.; Vilar, O.M. UV Exposure of Polymeric Geomembranes. In Geosynthetics in Civil and Environmental Engineering; Li, G.X., Chen, Y.M., Tang, X.W., Eds.; Springer: Shanghai, China, 2009; pp. 44–48. [Google Scholar]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. Progress in Polymer Science The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Kovalcik, A.A.; Meixner, K.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Stelzer, F.; Drosg, B.; Zeilinger, W.; Fritz, I.; Fuchs, W.; et al. Characterization of Polyhydroxyalkanoates Produced by Synechocystis salina from Digestate Supernatant. Int. J. Biol. Macromol. 2017, 102, 497–504. [Google Scholar] [CrossRef]
- Roja, K.; Sudhakar, D.R.; Anto, S.; Mathimani, T. Extraction and Characterization of Polyhydroxyalkanoates from Marine Green Alga and Cyanobacteria. Biocatal. Agric. Biotechnol. 2019, 22, 101358. [Google Scholar] [CrossRef]
- Noda, I.; Green, P.R.; Satkowski, M.M.; Schechtman, L.A. Preparation and Properties of a Novel Class of Polyhydroxyalkanoate Copolymers. Biomacromolecules 2005, 6, 580–586. [Google Scholar] [CrossRef]
- Carpine, R.; Olivieri, G.; Hellingwerf, K.J.; Pollio, A.; Marzocchella, A. Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge? Processes 2020, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, Modifications and Applications. Nat. Publ. Gr. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Assis, D.D.J.; Valéria, G.; Gomes, P.; Roberto, D.; Pinho, L.S.; Brandão, L.; Chaves, O.; Druzian, J.I. Simultaneous Biosynthesis of Polyhydroxyalkanoates and Extracellular Polymeric Substance (EPS) from Crude Glycerol from Biodiesel Production by Different Bacterial Strains. Appl. Biochem. Biotechnol. 2016, 180, 1110–1127. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent Advances in Microbial Polyhydroxyalkanoates. Process Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Zhang, C.; Show, P.; Ho, S. Bioresource Technology Progress and Perspective on Algal Plastics–A Critical Review. Bioresour. Technol. 2019, 289, 121700. [Google Scholar] [CrossRef]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering. 2017, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as Source of Polyhydroxyalkanoates (PHAs)—A Review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Microalgal Biorefineries for Industrial Products. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–195. [Google Scholar]
- Abdo, S.M.; Ali, G.H. Analysis of Polyhydroxybutrate and Bioplastic Production from Microalgae. Bull. Natl. Res. Cent. 2019, 5, 3. [Google Scholar] [CrossRef]
- Das, S.K.; Sathish, A.; Stanley, J. Production of Biofuel and Bioplastic from Chlorella Pyrenoidosa. Mater. Today Proc. 2018, 5, 16774–16781. [Google Scholar] [CrossRef]
- Cassuriaga, A.P.A.; Freitas, B.C.B.; Morais, M.G.; Costa, J.A. V Bioresource Technology Innovative Polyhydroxybutyrate Production by Chlorella fusca Grown with Pentoses. Bioresour. Technol. 2018, 265, 456–463. [Google Scholar] [CrossRef]
- Kato, N. Bioresource Technology Reports Production of Crude Bioplastic-Beads with Microalgae: Proof-of-Concept. Bioresour. Technol. Rep. 2019, 6, 81–84. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and Characterization of Poly-β-hydroxybutyrate (PHB) Polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 803–814. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; de Assis, D.J.; Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of Nitrogen on Growth, Biomass Composition, Production, and Properties of Polyhydroxyalkanoates (PHAs) by Microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Bhati, R.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Copolymer Production by the Diazotrophic Cyanobacterium Nostoc muscorum Agardh: Process Optimization and Polymer Characterization. ALGAL 2015, 7, 78–85. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening Doors for a Sustainable Future. NPG Asia Mater. 2016, 8, e265-20. [Google Scholar] [CrossRef]
- Wang, C.; Venditti, R.A.; Zhang, K. Tailor-Made Functional Surfaces based on Cellulose-Derived Materials. Appl. Microbiol. Biotechnol. 2015, 99, 5791–5799. [Google Scholar] [CrossRef]
- Chan, R.T.H.; Garvey, C.J.; Marc, H.; Russell, R.A.; Holden, P.J.; Foster, L.J.R. Manipulation of Polyhydroxybutyrate Properties through Blending with Ethyl-Cellulose for a Composite Biomaterial. Int. J. Polym. Sci. 2011, 2011, 651549. [Google Scholar] [CrossRef] [Green Version]
- Lukasiewicz, B.; Basnett, P.; Nigmatullin, R.; Matharu, R.; Knowles, J.C.; Roy, I. Acta Biomaterialia Binary Polyhydroxyalkanoate Systems for Soft Tissue Engineering. Acta Biomater. 2018, 71, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, T.; Mussone, P.; Bressler, D. Progress in Bio-Based Plastics and Plasticizing Modifications. J. Mater. Chem. A. 2013, 13379–13398. [Google Scholar] [CrossRef] [Green Version]
- Mukheem, A.; Hossain, M.; Shahabuddin, S.; Muthoosamy, K. Bioplastic Polyhydroxyalkanoate (PHA): Recent Advances in Modification and Medical Applications. 2018. Corpus ID: 53071354. Available online: https://europepmc.org/article/ppr/ppr48118 (accessed on 1 July 2021). [CrossRef] [Green Version]
- Bhatt, R.; Shah, D.; Patel, K.C.; Trivedi, U. PHA-Rubber Blends: Synthesis, Characterization and Biodegradation. Bioresour. Technol. 2008, 99, 4615–4620. [Google Scholar] [CrossRef]
- Gerard, T.; Budtova, T. Morphology and Molten-State Rheology of Polylactide and Polyhydroxyalkanoate Blends. Eur. Polym. J. 2012, 48, 1110–1117. [Google Scholar] [CrossRef]
- Loureiro, N.C.; Esteves, J.L.; Viana, J.C.; Ghosh, S.J. Thermoplast. Compos. Mater. 2013, 2, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Mousavioun, P.; George, G.A.; Doherty, W.O.S. Environmental Degradation of Lignin/Poly(hydroxybutyrate) blends. Polym. Degrad. Stab. 2012, 97, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Godbole, S. Preparation and Characterization of Biodegradable Poly-3-hydroxybutyrate–Starch Blend Films. Bioresour. Technol. 2003, 86, 33–37. [Google Scholar] [CrossRef]
- Lai, M.; Botsis, J.; Cugnoni, J. Studies of Hygrothermal Degradation of a Single Fiber Composite: An Iterative Approach with Embedded Optical Sensors and Numerical Analysis. Compos. Part B Eng. 2014, 60, 577–585. [Google Scholar] [CrossRef]
- Lim, J.; Seow, M.; Chong, K.; Teo, E.Y.; Chen, G.; Chan, J.K.Y.; Teoh, S. Biocompatibility Studies and Characterization of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/ Polycaprolactone Blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Sotgiu, M.G.; Vinci, B.; Domenici, C.; Giusti, P. Poly(e-caprolactone) Blends for Tissue Engineering Applications in the Form of Hollow Fibers. J. Biomed. Mater. Res. Part A 2008, 85A, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Z.; Cao, C.; Wang, C.; Fang, Y.; Huang, Y. Self-Sacrificed Template Synthesis of Ribbon-like Hexagonal Boron Nitride Nano-Architectures and their Improvement on Mechanical and Thermal Properties of PHA Polymer. Sci. Rep. 2017, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Lee, W.; Jin, H.; Chin, I.; Kim, M.; Go, J. Toughening of poly(3-hydroxybutyrate) with poly(cis-1,4-isoprene). Eur. Polym. J. 1999, 35, 781–788. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.; Zhao, S.; Huang, Z. Biodegradable polymer blends of poly (3-hydroxybutyrate) and starch acetate. Polym. Int. 1997, 44, 104–110. [Google Scholar] [CrossRef]
- El-Shafee, E.; Saad, G.R.; Fahmy, S.M. Miscibility, crystallization and phase structure of poly(3-hydroxybutyrate)/cellulose acetate butyrate blends. Eur. Polym. J. 2001, 37, 2091–2104. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, G.; Ma, S.; Cai, Z.; Zhang, L. Crystallization behavior, mechanical properties, and environmental biodegradability of poly(?-hydroxybutyrate)/cellulose acetate butyrate blends. J. Appl. Polym. Sci. 2003, 89, 2116–2122. [Google Scholar] [CrossRef]
- Kai, Z.; Ying, D.; Guo-Qiang, C. Effects of surface morphology on the biocompatibility of polyhydroxyalkanoates. Biochem. Eng. J. 2003, 16, 115–123. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, K.; Chen, G. Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials 2002, 23, 1391–1397. [Google Scholar] [CrossRef]
- Zhao, H.; Bian, Y.; Li, Y.; Dong, Q.; Han, C.; Dong, L. Bioresource-based blends of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and stereocomplex polylactide with improved rheological and mechanical properties and enzymatic hydrolysis. J. Mater. Chem. A 2014, 2, 8881. [Google Scholar] [CrossRef]
- Chen, M.G.; Wu, Q. Microbial production and applications of chiral hydroxyalkanoates. Appl. Microbiol. Biotechnol. 2005, 67, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, C.; Fioravanzo, L.; Folin, M.; Marchi, F.; Ercolani, E.; Bianco, A. Electrospun tubular scaffolds: On the effectiveness of blending poly (e-caprolactone) with poly (3-hydroxybutyrate-co-3-hydroxyvalerate). J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 108, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Doi, Y. Enzymatic degradation and morphologies of binary blends of microbial poly(3-hydroxy butyrate) with poly(ε-caprolactone), poly(1,4-butylene adipate and poly(vinyl acetate). Polym. Degrad. Stab. 1992, 36, 241–248. [Google Scholar] [CrossRef]
- Dufresne, A.; Vincendon, M. Poly(3-hydroxybutyrate) and Poly(3-hydroxyoctanoate) Blends: Morphology and Mechanical Behavior. Macromolecules 2000, 33, 2998–3008. [Google Scholar] [CrossRef]
- Sombatmankhong, K.; Suwantong, O.; Waleetorncheepsawat, S.; Supaphol, P. Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2923–2933. [Google Scholar] [CrossRef]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Gnatowski, M.; Kowalczuk, M.; Jedliński, Z. Polymer Blends of Natural Poly(3-hydroxybutyrate- co -3-hydroxyvalerate) and a Synthetic Atactic Poly(3-hydroxybutyrate). Characterization and Biodegradation Studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Xin, L.; Hong-ying, H.; Yu-ping, Z. Bioresource Technology Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol. 2011, 102, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Chu, F.; Lam, P.K.S.; Zeng, R.J. Biosynthesis of high yield fatty acids from Chlorella vulgaris NIES-227 under nitrogen starvation stress during heterotrophic cultivation. Water Res. 2015, 81, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Chu, P.; Cai, P.; Li, W.; Lam, P.K.S.; Zeng, R.J. Bioresource Technology Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency. Bioresour. Technol. 2013, 134, 341–346. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García-galán, M.J.; García, J. Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: Effect of nutrients limitation and photoperiods. N. Biotechnol. 2018, 42, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Sun, B.; She, X.; Zhao, F.; Cao, Y.; Ren, D.; Lu, J. Lipid production and composition of fatty acids in Chlorella vulgaris cultured using different methods: Photoautotrophic, heterotrophic, and pure and mixed conditions. Ann. Microbiol. 2014, 64, 1239–1246. [Google Scholar] [CrossRef]
- Koller, M. Cyanobacterial Polyhydroxyalkanoate Production: Status Quo and Quo Vadis? Curr. Biotechnol. 2016, 4, 464–480. [Google Scholar] [CrossRef]
- Bhati, R.; Samantaray, S.; Sharma, L.; Mallick, N. Poly-β-hydroxybutyrate accumulation in cyanobacteria under photoautotrophy. Biotechnol. J. 2010, 5, 1181–1185. [Google Scholar] [CrossRef]
- Miyake, M.; Erata, M.; Asada, Y. A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J. Ferment. Bioeng. 1996, 82, 512–514. [Google Scholar] [CrossRef]
- Sharma, L.; Mallick, N. Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: Regulation by pH, light–dark cycles, N and P status and carbon sources. Bioresour. Technol. 2005, 96, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Kaewbai-ngam, A.; Incharoensakdi, A.; Monshupanee, T. Bioresource Technology Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 80. Bioresour. Technol. 2016, 212, 342–347. [Google Scholar] [CrossRef]
- Taepucharoen, K.; Tarawat, S.; Puangcharoen, M.; Incharoensakdi, A.; Monshupanee, T. Bioresource Technology Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N 2 -fixing cyanobacterium. Bioresour. Technol. 2017, 239, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bao, T.; Shen, Z.; Wu, Q. Sodium acetate stimulates PHB biosynthesis in synechocystis sp. PCC 6803. Tsinghua Sci. Technol. 2002, 7, 435–438. [Google Scholar]
- Samantaray, S.; Mallick, N. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by the diazotrophic cyanobacterium Aulosira fertilissima CCC 444. J. Appl. Phycol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Koksharova, M.O.A. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 2002, 58, 123–137. [Google Scholar] [CrossRef]
- Carpine, R.; Du, W.; Olivieri, G.; Pollio, A.; Hellingwerf, K.J.; Marzocchella, A.; Branco, F. Genetic engineering of Synechocystis sp. PCC6803 for poly- β-hydroxybutyrate overproduction. Algal Res. 2017, 25, 117–127. [Google Scholar] [CrossRef]
- Wagner, J.; Bransgrove, R.; Beacham, T.A.; Allen, M.J.; Meixner, K.; Drosg, B.; Ting, V.P.; Chuck, C.J. Bioresource Technology Co-production of bio-oil and propylene through the hydrothermal liquefaction of polyhydroxybutyrate producing cyanobacteria. Bioresour. Technol. 2016, 207, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Haase, S.M.; Huchzermeyer, B.; Rath, T. PHB accumulation in Nostoc muscorum under different carbon stress situations. J. Appl. Phycol. 2012, 24, 157–162. [Google Scholar] [CrossRef]
- Mendhulkar, V.D.; Shetye, L.A. Synthesis of Biodegradable Polymer Polyhydroxyalkanoate (PHA) in Cyanobacteria Synechococcus elongates Under Mixotrophic Nitrogen-and Phosphate-Mediated Stress Conditions. Ind. Biotechnol. 2017, 13, 85–93. [Google Scholar] [CrossRef]
- Chakravarty, P.; Mhaisalkar, V.; Chakrabarti, T. Bioresource Technology Study on poly-hydroxyalkanoate (PHA) production in pilot scale continuous mode wastewater treatment system. Bioresour. Technol. 2010, 101, 2896–2899. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Mallick, N. Enhanced poly-?-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007, 44, 194–198. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Sili, C.; Vincenzini, M. Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. J. Gen. Microbiol. 1992, 138, 1623–1628. [Google Scholar] [CrossRef] [Green Version]
- Jau, M.; Yew, S.; Toh, P.S.Y.; Chong, A.S.C.; Chu, W.; Phang, S.; Najimudin, N.; Sudesh, K. Biosynthesis and mobilization of poly(3-hydroxybutyrate) [P(3HB)] by Spirulina platensis. Int. J. Biol. Macromol. 2005, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chaogang, W.; Zhangli, H.; Anping, L.; Baohui, J. BIOSYNTHESIS OF POLY-3-HYDROXYBUTYRATE (PHB) IN THE TRANSGENIC GREEN ALGA CHLAMYDOMONAS REINHARDTII. J. Phycol. 2010, 46, 396–402. [Google Scholar] [CrossRef]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbüchel, A.; Maier, U.G. Microalgae as bioreactors for bioplastic production. Microb. Cell Fact. 2011, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Kovacs, T.; Stefan, P.; Druzhinina, I.; Kroll, P.; Lackner, M.; Herwig, C. Bioresource Technology Increased poly- β -hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: Mutant generation and characterization. Bioresour. Technol. 2018, 266, 34–44. [Google Scholar] [CrossRef]
- Nikel, P.I.; Pettinari, M.J.; Galvagno, M.A.; Me, B.S. Poly (3-Hydroxybutyrate) Synthesis by Recombinant Escherichia coli arcA Mutants in Microaerobiosis. Appl. Environ. Microbiol. 2006, 72, 2614–2620. [Google Scholar] [CrossRef] [Green Version]
- Toh, Y.; Jau, P.S.; Yew, M.H.; Abed, S.P.M.; Sudesh, R.M.K. Comparison of Polyhydroxyalkanoates Biosynthesis, Mobilization and the Effects on Cellular Morphology in Spirulina Platensis and Synechocystis Sp. Uniwg. J. Biosci. 2008, 19, 21–38. [Google Scholar]
- Chen, L.; Yu, Z.; Xu, H.; Wan, K.; Liao, Y.; Ma, X. Microwave-assisted co-pyrolysis of Chlorella vulgaris and wood sawdust using different additives. Bioresour. Technol. 2019, 273, 34–39. [Google Scholar] [CrossRef]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A. Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mandal, A.; Ayton, E.; Hunt, R. Modification of Protein Rich Algal-Biomass to Form Bioplastics and Odor Removal. In Protein Byproducts; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128023914. [Google Scholar]
- Verdugo, M.; Lim, L. Electrospun Protein Concentrate Fibers from Microalgae Residual Biomass Electrospun Protein Concentrate Fibers from Microalgae Residual Biomass. J. Polym. Environ. 2014, 22, 373–383. [Google Scholar] [CrossRef]
- Zeller, M.A.; Hunt, R.; Jones, A.; Sharma, S. Bioplastics and their Thermoplastic Blends from Spirulina and Chlorella Microalgae. J. Appl. Polym. Sci. 2013, 103, 1–13. [Google Scholar] [CrossRef]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Barbarino, E.; Louren, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. LWT-Food Science and Technology Extraction of protein from the macroalga Palmaria palmata. LWT Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Iban, E. Food Chemistry Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Safi, C.; Olivieri, G.; Campos, R.P.; Engelen-Smit, N.; Mulder, W.J.; Van Den, B.L.A.M.; Sijtsma, L. Bioresource Technology Biorefinery of microalgal soluble proteins by sequential processing and membrane filtration. Bioresour. Technol. 2017, 225, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-cubero, R.; Teles, I.; Cabanelas, D.; Sijtsma, L.; Kleinegris, D.M.M.; Barbosa, M.J. Production of exopolysaccharide by Botryococcus braunii CCALA 778 under laboratory simulated Mediterranean climate conditions. Algal Res. 2018, 29, 330–336. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Wang, K. Bio-plastic potential of spirulina microalgae. In Proceedings of the Beijing Institute of Fashion Technology, Beijing, China, May 2014; Available online: https://getd.libs.uga.edu/pdfs/wang_kun_201405_ms.pdf (accessed on 1 July 2021).
- Rocha, D.N.; Martins, M.A.; Soares, J.; Vaz, M.G.M.V.; de Oliveira Leite, M.; Covell, L.; Mendes, L.B.B. Combination of trace elements and salt stress in different cultivation modes improves the lipid productivity of Scenedesmus spp. Bioresour. Technol. 2019, 289, 121644. [Google Scholar] [CrossRef]
- Fazeli Danesh, A.; Ebrahimi, S.; Salehi, A.; Parsa, A. Impact of nutrient starvation on intracellular biochemicals and calorific value of mixed microalgae. Biochem. Eng. J. 2017, 125, 56–64. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Soares, J.; Kriiger Loterio, R.; Rosa, R.M.; Santos, M.O.; Nascimento, A.G.; Santos, N.T.; Williams, T.C.R.; Nunes-Nesi, A.; Arêdes Martins, M. Scenedesmus sp. cultivation using commercial-grade ammonium sources. Ann. Microbiol. 2018, 68, 35–45. [Google Scholar] [CrossRef]
- Batista, A.D.; Rosa, R.M.; Machado, M.; Magalhães, A.S.; Shalaguti, B.A.; Gomes, P.F.; Covell, L.; Vaz, M.G.M.V.; Araújo, W.L.; Nunes-Nesi, A. Increased urea availability promotes adjustments in C/N metabolism and lipid content without impacting growth in Chlamydomonas reinhardtii. Metabolomics 2019, 15, 31. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Madsen, M. Algal proteins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 353–394. [Google Scholar]
- Kose, A.; Oncel, S.S. Properties of microalgal enzymatic protein hydrolysates: Biochemical composition, protein distribution and FTIR characteristics. Biotechnol. Rep. 2015, 6, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Liu, P.; Xia, J.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.; Qiu, G. The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, J.; Wang, Y.; Feng, Y.; Cui, Q.; Lu, Y. Artificial creation of Chlorella pyrenoidosa mutants for economic sustainable food production. Bioresour. Technol. 2018, 268, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S.; Mahamud, A. Nanocellulose Reinforced Starch Polymer Composites: A Review of Preparation, Properties and Application. In Proceedings of the 5th International Conference on Applied Sciences and Engineering Application (ICASEA 2018), Cameron Highlands, Malaysia, 7–8 April 2018. [Google Scholar]
- Li, J.; Luo, X. Starch-based Blends. In Starch-based blends, Composites and Nanocomposites; RSC: London, UK, 2015; pp. 263–325. [Google Scholar]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae-novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Mathiot, C.; Ponge, P.; Gallard, B.; Sassi, J.-F.; Delrue, F.; Le Moigne, N. Microalgae starch-based bioplastics: Screening of ten strains and plasticization of unfractionated microalgae by extrusion. Carbohydr. Polym. 2019, 208, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Ramli, R.N.; Lee, C.K.; Kassim, M.A. Extraction and Characterization of Starch from Microalgae and Comparison with Commercial Corn Starch. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012012. [Google Scholar] [CrossRef]
- Asmawi, N.N.M.; Sapuan, S.M.; Ilyas, R.A. Starch/pla blended composites; a review of preparation and potential applications. In Proceedings of the Seminar Enau Kebangsaan 2019, Bahau, Negeri Sembilan, Malaysia, 1 April 2019. [Google Scholar]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch-Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F. Thermoplastic starch blends: A review of recent works. Polym. Sci. Ser. A 2012, 54, 165–176. [Google Scholar] [CrossRef]
- Martin, O.; Schwach, E.; Averous, L.; Couturier, Y. Properties of Biodegradable Multilayer Films Based on Plasticized Wheat Starch. Starch Stärke 2001, 53, 372. [Google Scholar] [CrossRef]
- Gattin, R.; Copinet, A.; Bertrand, C.; Couturier, Y. Biodegradation study of a starch and poly(lactic acid) co-extruded material in liquid, composting and inert mineral media. Int. Biodeterior. Biodegrad. 2002, 50, 25–31. [Google Scholar] [CrossRef]
- Famá, L.; Rojo, P.G.; Bernal, C.; Goyanes, S. Biodegradable starch based nanocomposites with low water vapor permeability and high storage modulus. Carbohydr. Polym. 2012, 87, 1989–1993. [Google Scholar] [CrossRef]
- Visakh, P.M.; Mathew, A.P.; Thomas, S. Advances in Natural Polymers. In Advanced Structured Materials; Thomas, S., Visakh, P.M., Mathew, A.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 18, ISBN 978–3-642–20939–0. [Google Scholar]
- Averous, L. Properties of thermoplastic blends: Starch–polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Chadehumbe, C. Tensile properties of thermoplastic starch and its blends with polyvinyl butyral and polyamides. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, August 2006. [Google Scholar]
- Dammak, M.; Hadrich, B.; Miladi, R.; Barkallah, M.; Hentati, F.; Hachicha, R.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effects of nutritional conditions on growth and biochemical composition of Tetraselmis sp. Lipids Health Dis. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hing, Y.; Soopna, L.; Chee, P.; Lee, K. Nutritional optimization of Arthrospira platensis for starch and Total carbohydrates production. Biotechnol. Prog. 2019, 35, 1–9. [Google Scholar] [CrossRef]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Wang, Y.; Huang, W.; Xu, J. Enhanced Accumulation of Carbohydrate and Starch in Chlorella zofingiensis Induced by Nitrogen Starvation. Appl. Biochem. Biotechnol. 2014, 174, 2435–2445. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol 2012, 96, 631–645. [Google Scholar] [CrossRef]
- Eduardo, C.; Silva, D.F.; Sforza, E. Enhancing Carbohydrate Productivity in Photosynthetic Microorganism Production: A Comparison Between Cyanobacteria and Microalgae and the Effect of Cultivation Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128179376. [Google Scholar]
- Ji, C.; Yu, X.; Chen, Z.; Xue, S.; Legrand, J.; Zhang, W. Effects of nutrient deprivation on biochemical compositions and photo-hydrogen production of Tetraselmis subcordiformis. Int. J. Hydrog. Energy 2011, 36, 5817–5821. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Anal, A.K. Enhanced lipid and starch productivity of microalga (Chlorococcum sp. TISTR 8583) with nitrogen limitation following effective pretreatments for biofuel production. Biotechnol. Rep. 2019, 21, e00298. [Google Scholar] [CrossRef] [PubMed]
- Barati, B.; Lim, S.Y.G.P.E.; Phang, J.B.S.M. Green algal molecular responses to temperature stress. Acta Physiol. Plant. 2019, 41, 1–19. [Google Scholar] [CrossRef]
- González-fernández, C.; Ballesteros, M. Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, X.; Yen, H.; Ho, S.; Cheng, C. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Hosono, H.; Uemura, I.; Takumi, T.; Nagamune, T.; Yasuda, T.; Kishimoto, M.; Nagashima, H.; Shimomura, N.; Natori, M.; Endo, I. Effect of culture temperature shift on the cellular sugar accumulation of Chlorella vulgaris SO-26. J. Ferment. Bioeng. 1994, 78, 235–240. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Effects of phosphorus concentration and light intensity on the biomass composition of Arthrospira (Spirulina) platensis. World J. Microbiol. Biotechnol. 2012, 28, 2661–2670. [Google Scholar] [CrossRef]
- Samiee-zafarghandi, R.; Karimi-sabet, J.; Ali, M. Increasing microalgal carbohydrate content for hydrothermal gasi fi cation purposes. Renew. Energy 2018, 116, 710–719. [Google Scholar] [CrossRef]
- Ho, S.; Chen, C.; Chang, J. Bioresource Technology Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Lohman, E.J.; Gardner, R.D.; Pedersen, T.; Peyton, B.M.; Cooksey, K.E.; Gerlach, R. Optimized inorganic carbon regime for enhanced growth and lipid accumulation in Chlorella vulgaris. Biotechnol. Biofuels 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tourang, M.; Baghdadi, M.; Torang, A.; Sarkhosh, S. Optimization of carbohydrate productivity of Spirulina microalgae as a potential feedstock for bioethanol production. Int. J. Environ. Sci. Technol. 2019, 16, 1303–1318. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323. [Google Scholar] [CrossRef]
- Mousavi, S.; Najafpour, G.D.; Mohammadi, M. CO2 bio-fixation and biofuel production in an airlift photobioreactor by an isolated strain of microalgae Coelastrum sp. SM under high CO2 concentrations. Environ. Sci. Pollut. Res. 2018, 25, 30139–30150. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Beardall, J.; Bhattacharya, S.; Wangikar, P.P. Isolation and biochemical characterisation of two thermophilic green algal species- Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res. 2018, 30, 28–37. [Google Scholar] [CrossRef]
- de Freitas, B.C.B.; Brächer, E.H.; de Morais, E.G.; Atala, D.I.P.; de Morais, M.G.; Costa, J.A.V. Cultivation of different microalgae with pentose as carbon source and the effects on the carbohydrate content. Environ. Technol. 2019, 40, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Astri Rinanti, R.P. Increasing carbohydrate and lipid productivity in tropical microalgae biomass as a sustainable biofuel feed stock microalgae biomass as a sustainable biofuel feed stock. Energy Procedia 2019, 158, 1215–1222. [Google Scholar] [CrossRef]
- Ho, S.; Ye, X.; Hasunuma, T.; Chang, J.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef]
- Ghag, S.B.; Vavilala, S.L.; D’Souza, J.S. Metabolic Engineering and Genetic Manipulation of Novel Biomass Species for Biofuel Production. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–34. ISBN 9780128179413. [Google Scholar]
- Prakash, M.M.; Selvakumar, R.; Suresh, K.P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Youngblood, J.; Martini, A.; Nairn, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Simas-Rodrigues, C.; Villela, H.D.M.; Martins, A.P.; Marques, L.G.; Colepicolo, P.; Bioquímica, D.D.; Química, I.D.; Paulo, U.D.S.; Prestes, A.L. Microalgae for economic applications: Advantages and perspectives for bioethanol. J. Exp. Bot. 2015, 66, 4097–4108. [Google Scholar] [CrossRef] [Green Version]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Baudelet, P.; Ricochon, G.; Linder, M.; Muniglia, L. Review article A new insight into cell walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Rashidi, B.; Trindade, L.M. Detailed biochemical and morphologic characteristics of the green microalga Neochloris oleoabundans cell wall. Algal Res. 2018, 35, 152–159. [Google Scholar] [CrossRef]
- Mihranyan, A. Cellulose from cladophorales green algae: From environmental problem to high-tech composite materials. J. Appl. Polym. Sci. 2011, 119, 2449–2460. [Google Scholar] [CrossRef]
- Siddhanta, A.K.; Chhatbar, M.U.; Mehta, G.K.; Sanandiya, N.D.; Kumar, S.; Oza, M.D.; Prasad, K.; Meena, R. The cellulose contents of Indian seaweeds. J. Appl. Phycol. 2011, 23, 919–923. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef]
- Sugiyama, J.; Vuong, R.; Chanzy, H. Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 1991, 24, 4168–4175. [Google Scholar] [CrossRef]
- Constante, A.; Pillay, S.; Ning, H.; Vaidya, U.K. Utilization of algae blooms as a source of natural fi bers for biocomposite materials: Study of morphology and mechanical performance of Lyngbya fi bers. ALGAL 2015, 12, 412–420. [Google Scholar] [CrossRef]
- Samiee, S.; Ahmadzadeh, H.; Hosseini, M.; Lyon, S. Algae as a Source of Microcrystalline Cellulose. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 331–350. [Google Scholar]
- Sun, X.; Lu, C.; Liu, Y.; Zhang, W.; Zhang, X. Melt-processed poly (vinyl alcohol) composites filled with microcrystalline cellulose from waste cotton fabrics. Carbohydr. Polym. 2014, 101, 642–649. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.; Mun, S.C.; Chang, Y.K.; Choi, S.Q. A new method to produce cellulose nanofibrils from microalgae and the measurement of their mechanical strength. Carbohydr. Polym. 2018, 180, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and Composition of the Nannochloropsis gaditana Cell Wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassab, Z.; Ben, Y.H.; Hannache, H.; El Achaby, M. Isolation of Cellulose Nanocrystals from Various Lignocellulosic Materials: Physico-chemical characterization and Application in Polymer Composites Development. Mater. Today Proc. 2019, 13, 964–973. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crop. Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Nunes, R.C.R. Rubber nanocomposites with nanocellulose. In Progress in Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 463–494. ISBN 9780081004098. [Google Scholar]
- Nyström, G.; Mihranyan, A.; Razaq, A.; Lindström, T.; Nyholm, L.; Strømme, M. A Nanocellulose Polypyrrole Composite Based on Microfibrillated Cellulose from Wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, C.; Min, S.K.; Jung, S.-M.; Shin, H.S. In vitro evaluation of the effects of electrospun PCL nanofiber mats containing the microalgae Spirulina (Arthrospira) extract on primary astrocytes. Colloids Surf. B Biointerfaces 2012, 90, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Hermosilla, J.; Acevedo, F.; Navia, R. The influences of solvents on the electrospun of whole Scenedesmus almeriensis and poly (ethylene oxide) for the preparation of composite nanofibers. Compos. Commun. 2018, 10, 18–24. [Google Scholar] [CrossRef]
- de Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [Google Scholar] [CrossRef]
- Aguirre, A.-M.; Bassi, A.; Saxena, P. Engineering challenges in biodiesel production from microalgae. Crit. Rev. Biotechnol. 2013, 33, 293–308. [Google Scholar] [CrossRef]
- Jennifer Perr EU Green Deal: Creating a Circular Economy for Plastics. Available online: https://www.openaccessgovernment.org/circular-economy-for-plastics/97160/ (accessed on 1 July 2021).
- European Green Deal Striving to be the first Climate-Neutral Continent. Available online: https://ec.europa.eu/info/strategy/priorities-2019–2024/european-green-deal_en (accessed on 1 July 2021).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- World Economic Forum. World Economic Forum Annual Meeting. Available online: https://www.weforum.org/events/world-economic-forum-annual-meeting-2019 (accessed on 1 July 2021).
- Julia Nightengale. Movement of Plastics Industry Toward Sustainability: Panel Discussion. Available online: https://thesunflower.com/56099/lifestyle/arts-culture/movement-of-plastics-industry-toward-sustainability-panel-discussion/ (accessed on 1 July 2021).
- Matt Migliore. The Plastics Problem–A Roundtable Discussion. Available online: https://fiberjournal.com/the-plastics-problem-a-roundtable-discussion/ (accessed on 1 July 2021).
- Circpack. A new Circular Economy for the Plastic Packaging Sector. Available online: https://circpack.eu/home/ (accessed on 1 July 2021).
- Unilever. Rethinking Plastic Packaging. Available online: https://www.unilever.com/planet-and-society/waste-free-world/rethinking-plastic-packaging/ (accessed on 1 July 2021).
- European Bioplastics. Bioplastics Market Data 2018; Berlin, Germany, 2020. Available online: https://www.european-bioplastics.org/market/ (accessed on 1 July 2021).
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. J. Sustain. Circ. Econ. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
| Polymer Blend (mol·mol−1) | Melting Temperature (°C) | Glass Transition Temperature (°C) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| P(3HB)/starch (70/30) | 165.4 to 167.9 | 165.4 to 167.9 | 4.99 to 19.7 | 578 to 1716 | 3.5 to 9.8 | Coating materials, cardboard for food package | [54] |
| P(3HB)/PIP-g-PVAc (80/20) | 175 | 6 | 14.3 | 711 | 13 | [59] | |
| P(3HB)/starch acetate | 171.0 to 175.9 | 8.6 to 9.9 | - | - | - | - | [60] |
| P(3HB)/ethyl cellulose | 175.3 to 177.0 | 44.6 to 56.1, annealed samples | - | - | - | - | [60] |
| P(3HB)/cellulose acetate butyrate | 178.5 to 189.5 | 6.3 to 12.5 | 13.3 to 29.3 | 592.4 to 2288.3 | 2.2 to 7.3 | - | [61,62] |
| P(3HB)/lignin | 152 to 174 | 7.0 to 43.0 | - | - | - | - | [53] |
| P(3HB)/P(3HHx) | Approximately 152 to 165 | Approximately 0.8 to 5.0 | - | 500 to 1210 | - | Scaffolds for tissue engineering with improved biocompatibility | [63,64] |
| P(3HB-co-4HB)/PLA stereocomplex (SC) | PLA SC: 218 | P3/4HB: −12.5 | 4.2 to 6.6 | 30.8 to 46.7 | 362.7 to 949.0 | Enhanced processability and enzymatic hydrolysis rates | [65] |
| P(3HB-co-3HHx)/PCL |
P(3HB-co-3HHx): 95.4 PCL: 61.1 | - | - | 190.9 to 324.6 | - | Improved cell adhesion and proliferation for musculoskeletal tissue engineering | [56,66] |
| P(3HB-co-3HV)/PCL |
PCL: 57.0 to 57.5 P(3HB-co-3HV): 137 to 152.4 | P(3HB-co-3HV): 1.3 | - | 170 to 1200 | 8.0 to 25 | Hollow fibers and tubular scaffold in tissue engineering | [57,67] |
| P(3HB)/PLC (77/23) | 60 to 168 | −60 to 4 | 21 | 730 | 9 | - | [68] |
| P(3HB)/P(3HO) (75/25) | 172 | −35 | 6.2 | 730 | 30 | - | [69] |
| P(3HB)/P(3HB-co-3HV) (25/75) | 152 to 163 | - | 2 | 150 | 7 | Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends | [70] |
| P(3HB-co-3HV)/a-P(3HB) (50/50) | 133 | 2 | 7 | 240 | 33 | - | [71] |
| Produced Polymer and Operational Conditions | Algae Used | Polymer (%Dry Cell Weight) | Ref. |
|---|---|---|---|
| P(3HB) production using CO2 as carbon source (photosynthetic system) | Synechocystis cf. salina | 7.5 | [87] |
| P(3HB-CO-3HV) production under nitrogen deprivation | Oscillatoria okeni TISTR 8549 | 14.4 | [82] |
| P(3HB-CO-3HV) production under nitrogen deprivation and dark condition | Oscillatoria okeni TISTR 8549 | 42.8 | [82] |
| P(3HB) production under phosphate-starved medium + 1% (w/w) glucose + 1% (w/w) acetate with aeration and CO2 addition | Nostoc muscorum | 21.5 | [88] |
| P(3HB) production using CO2 as carbon source (photosynthetic system) under nitrogen deficiency | Calothrix scytonemicola TISTR 8095 | 25.4 | [81] |
| PHA production using BG11 as culture medium | Synechocystis salina | 5.5–6.6% | [26] |
| PHA production under phosphorus and nitrogen deficiency | Synechococcus elongates | 17.15 | [89] |
| PHA production under phosphorus deficiency | Synechococcus elongates | 7.02 | [89] |
| PHA production using wastewater as culture medium | Microalgae consortium | 43 | [90] |
| PHA production under nitrogen deficiency | Synechococcus subsalsus | 16 | [42] |
| PHA production under nitrogen deficiency | Spirulina sp. LEB-18 | 12 | [42] |
| P(3HB) production using 0.11% acetate and 0.08% propionate at pH 8.1 and an incubation period of 16 days | Nostoc muscorum | 31 | [91] |
| P(3HB) production using 0.2% acetate and 0.4% propionate, incubation period of 14 days at pH 8.5 | Nostoc muscorum | 28.2 | [91] |
| P(3HB) production under phosphorus limitation | Spirulina maxima | 1.2 | [92] |
| P(3HB) production under phosphorus limitation, supplemented with acetate (dark incubation for 7 days) | Nostoc muscorum | 35 | [80] |
| P(3HB-CO-3HV) production under phosphate deficiency conditions | Nostoc muscorum Agardh | 71 | [43] |
| P(3HB-CO-3HV) production under nitrogen deficiency conditions | Nostoc muscorum Agardh | 78 | [43] |
| Production of P(3HB) using CO2/acetate as carbon source | Spirulina plantesis | 10 | [93] |
| Production of P(3HB) under phosphate deficiency with gas-exchange limitation (GEL) conditions and using fructose/acetate as carbon source | Synechocystis sp. PCC6803 | 38 | [91] |
| Production of P(3HB) using 0.2% acetate/dark incubation for 7 days | Nostoc muscorum | 35 | [80] |
| Production of P(3HB) under phosphate limited conditions and permanent illumination | Mixed cyanobacterial culture: Aphanocapsa sp. and cf. Chroococcidiopsis sp. | 838 mgL−1 | [75] |
| Production of P(3HB) under nitrogen-limited conditions, | Synechocystis sp. UNIWG and Synechocystis sp. PCC 6803 | 14 | [15] |
| Production of P(3HB) in genetically engineered systems | Incorporation of phbB and phbC genes from R. eutropha into C. reinhardti | - | [94] |
| Production of PHB in genetically engineered systems | Incorporation of full PHB pathway from R. eutropha H16 into P. tricornutum. | 10.6 | [95] |
| PHB production in genetically engineered systems under nitrogen-limited conditions | Synechocystis sp. (genetically modified with overexpressing pha genes) | 35 | [96] |
| Improvement in PHA production after UV light exposure | Synechocystis sp. PCC6714 | 37 | [97] |
| PHB production under nitrogen deficiency and using acetate as carbon source | Synechococcus sp. PCC7942 | 26 | [98] |
| PHB production under 0.26% citrate, 0.28% acetate, and 5.58 mg L−1 K2HPO4 (incubation period of 5 days) | Aulosira fertilissima CCC 444 | 85 | [41] |
| PHB production under nitrogen deficiency | Spirulina platensis | 10 | [99] |
| PHB production under nitrogen deficiency | Synechocystis sp. UNIWG | 14 | [99] |
| P(3HB-CO-3HV) production under phosphorus deficiency and under 0.5% fructose + 0.4% valerate | Aulosira fertilissima CCC 444 | 77 | [84] |
| P(3HB-CO-3HV) production under nitrogen deficiency with acetate supplementation under dark condition | Oscillatoria okeni TISTR 8549 | 42 | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madadi, R.; Maljaee, H.; Serafim, L.S.; Ventura, S.P.M. Microalgae as Contributors to Produce Biopolymers. Mar. Drugs 2021, 19, 466. https://doi.org/10.3390/md19080466
Madadi R, Maljaee H, Serafim LS, Ventura SPM. Microalgae as Contributors to Produce Biopolymers. Marine Drugs. 2021; 19(8):466. https://doi.org/10.3390/md19080466
Chicago/Turabian StyleMadadi, Rozita, Hamid Maljaee, Luísa S. Serafim, and Sónia P. M. Ventura. 2021. "Microalgae as Contributors to Produce Biopolymers" Marine Drugs 19, no. 8: 466. https://doi.org/10.3390/md19080466
APA StyleMadadi, R., Maljaee, H., Serafim, L. S., & Ventura, S. P. M. (2021). Microalgae as Contributors to Produce Biopolymers. Marine Drugs, 19(8), 466. https://doi.org/10.3390/md19080466








