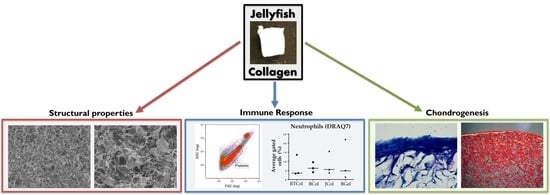
Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity
Abstract
1. Introduction
2. Results
2.1. Structural Characterisation of Jellyfish Collagen Scaffolds
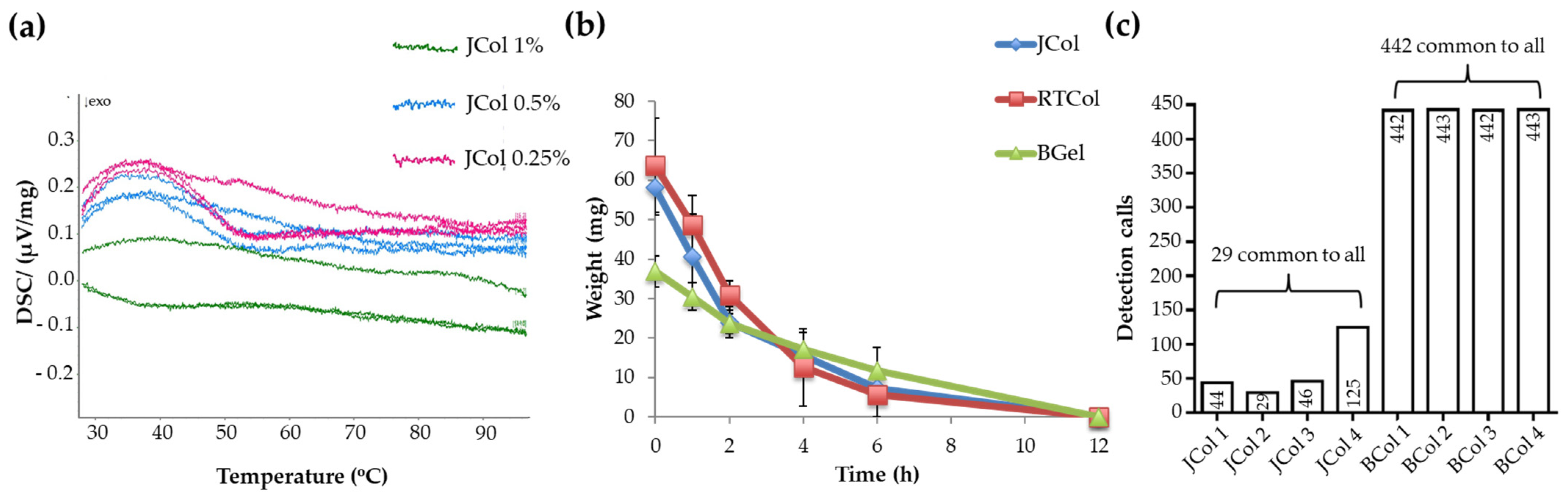
2.2. Stability and Purity of Jellyfish Collagen Scaffolds
2.3. Platelet Activation in Response to Jellyfish Collagen Scaffolds
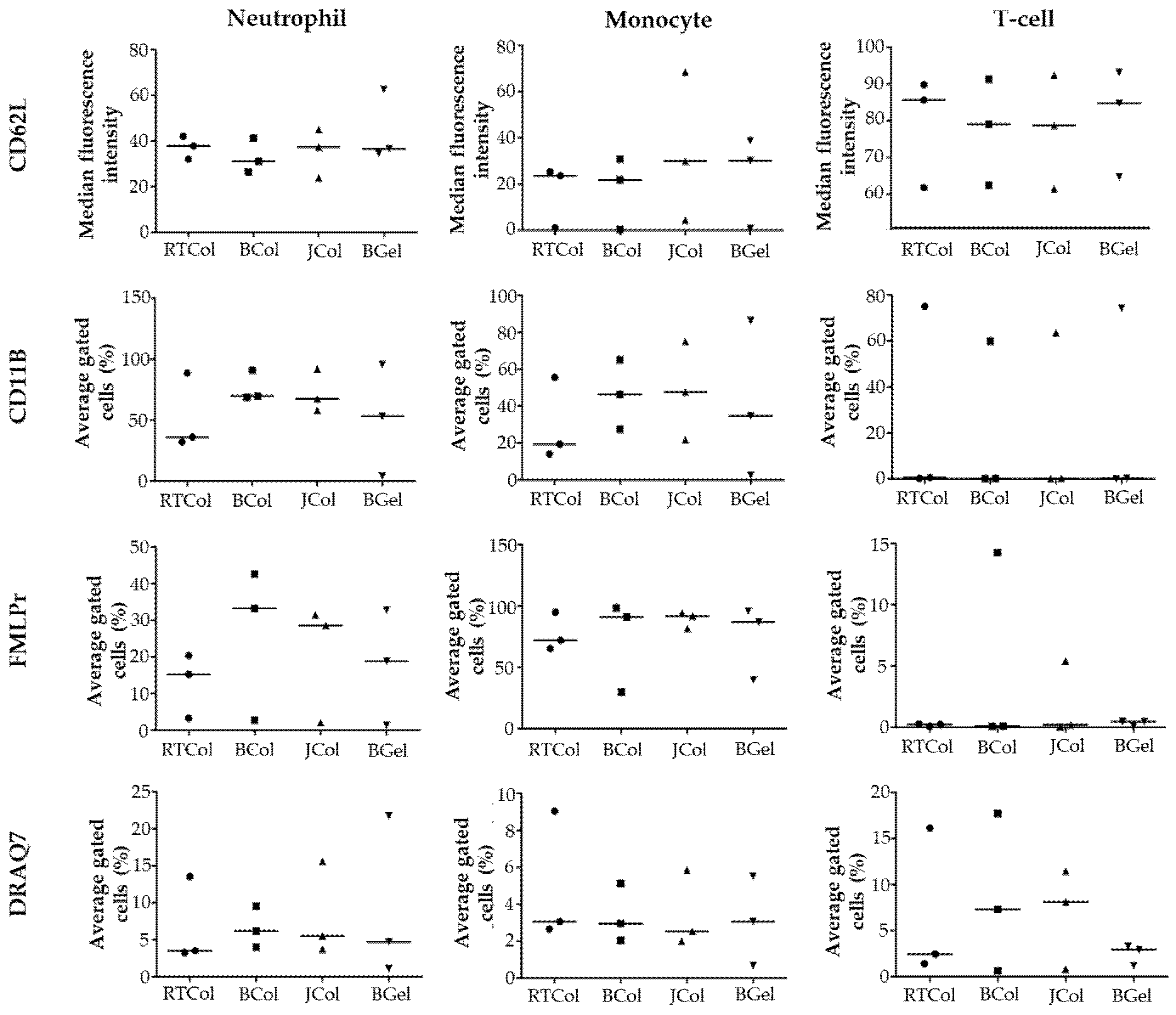
2.4. In Vitro Innate Immune Response to Jellyfish Collagen Scaffolds
2.5. Cytokine Release Profile in the Presence of the Jellyfish Collagen Scaffolds
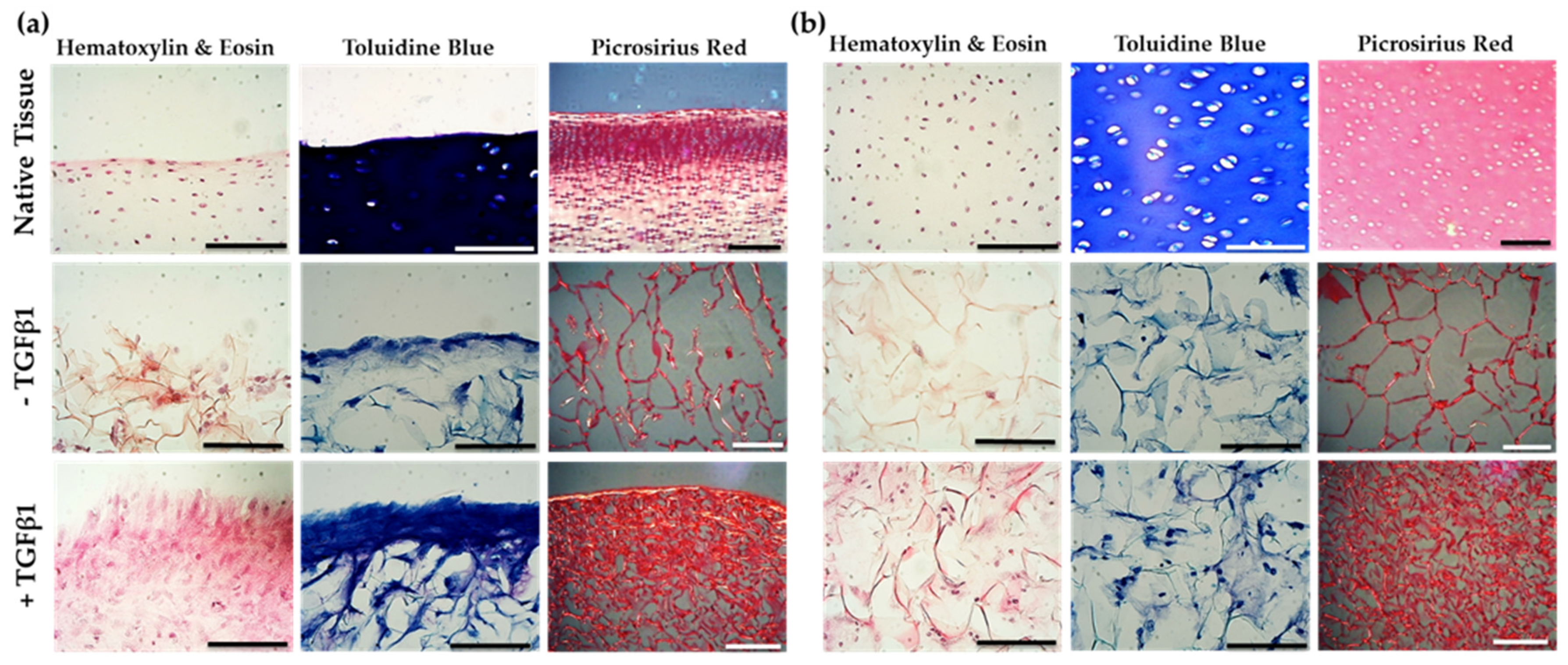
2.6. Jellyfish Sponge Scaffolds Ability to Induce a Chondrogenic Effect
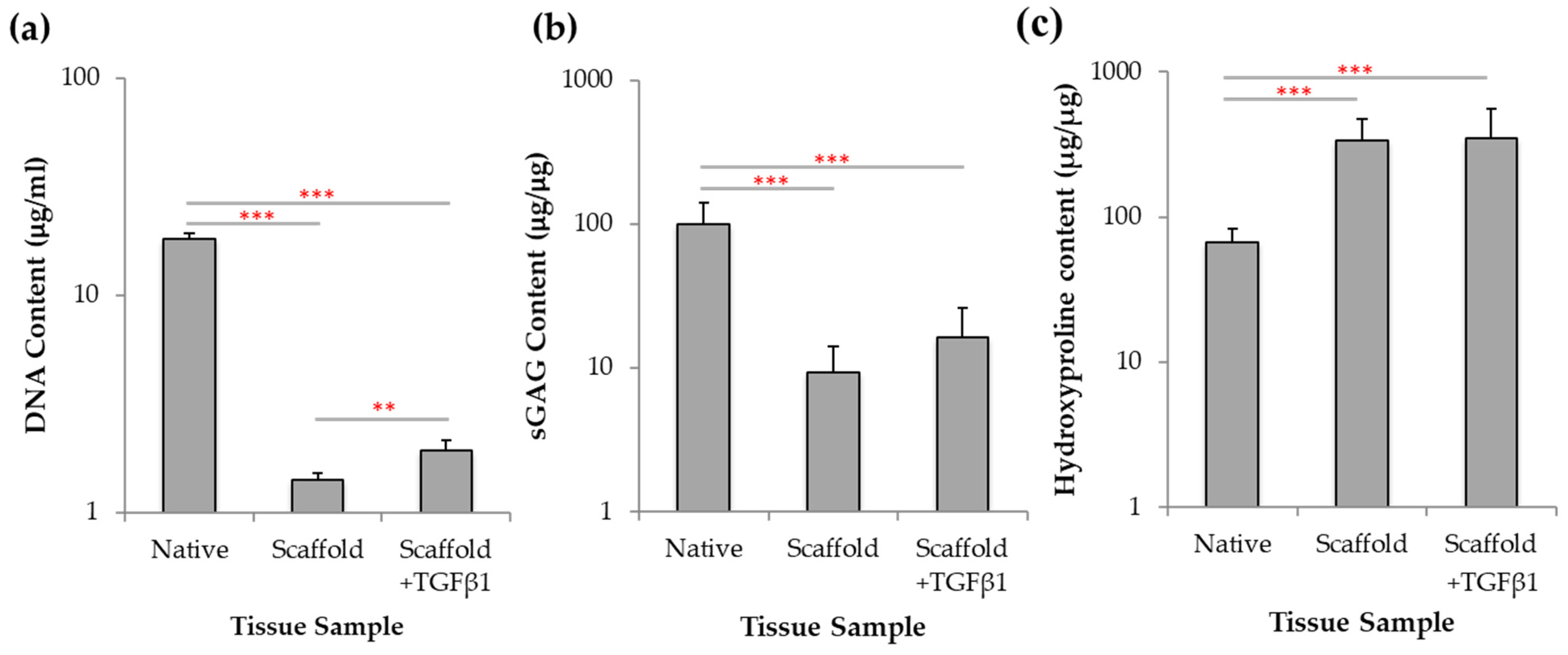
2.7. Biochemical Analysis of Chondrogenesis with Jellyfish Collagen Scaffolds
3. Discussion
4. Materials and Methods
4.1. Jellyfish Collagen
4.2. Collagen Scaffold Fabrication
4.3. Scanning Electron Microscopy Scaffold Characterisation
4.4. Differential Scanning Calorimetry Characterisation
4.5. Collagenase Digest Assay
4.6. Protein Sequencing and Bioinformatics
4.7. miRNA Detection in Collagen
4.8. Whole Blood Collection
4.9. Platelet Activation Assay
4.10. Leukocyte Activation and Death
4.11. Cytokine Release Profile Assay
4.12. Bovine Articular Cartilage Tissue Isolation
4.13. Bovine Chrondroprogenitor Cell Isolation
4.14. Scaffold Cell Seeding
4.15. Histology of Scaffolds and Native Bovine Tissue Seeded with Chrondroprogenitor Cells
4.16. Biochemical Analysis of Chondrogenesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amoako, A.O.; Pujalte, G.G.A. Osteoarthritis in young, active and athletic individuals. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2014, 7, 27–32. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cart. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Blalock, D.; Miller, A.; Tilley, M.; Wang, J. Joint Instability and Osteoarthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- York Health Economics. The Cost of Arthritis: Calculation conducted on behalf of Arthritis Research UK. 2017; Unpublished. [Google Scholar]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D. Collagen of articular cartilage. Arthritis Res. 2002, 4, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef]
- Bark, S.; Piontek, T.; Behrens, P.; Mkalaluh, S.; Varoga, D.; Gille, J. Enhanced microfracture techniques in cartilage knee surgery: Fact or fiction? World J. Orthop. 2014, 5, 444–449. [Google Scholar] [CrossRef]
- Reilingh, M.L.; Lambers, K.T.A.; Dahmen, J.; Opdam, K.T.M.; Kerkhoffs, G.M.M.J. The subchondral bone healing after fixation of an osteochondral talar defect is superior in comparison with microfracture. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Recker, D.; Llgenfritz, J.; Saris, D.B.F.; SUMMIT Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Five-Year Follow-up of a prospective randomized trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Chicatun, F.; Griffanti, G.; McKee, M.D.; Nazhat, S.N. Collagen/chitosan composite scaffolds for bone and cartilage tissue engineering. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Cambridge, UK, 2017; Volume 8, pp. 163–198. [Google Scholar]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Brotz, L.; Cheung, W.W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in large marine ecosystems. Hydrobiologia 2012, 690, 3–20. [Google Scholar] [CrossRef]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef]
- Flaig, I.; Randenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In vivo analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-blum, S.; Lethias, C. Isolation, characterisation and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema Esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Rastian, Z.; Pütz, S.; Wang, Y.J.; Kumar, S.; Fleissner, F.; Weidner, T.; Parekh, S.H. Type I Collagen from Jellyfish Catostylus Mosaicus for Biomaterial Applications. ACS Biomater. Sci. Eng. 2018, 4, 2115–2125. [Google Scholar] [CrossRef]
- Paradiso, F.; Fitzgerald, J.; Yao, S.; Barry, F.; Taraballi, F.; Gonzalez, D.; Conlan, R.S.; Francis, L. Marine collagen substrates for 2D and 3D ovarian cancer cell systems. Front. Bioeng. Biotechnol. 2019, 7, 343. [Google Scholar] [CrossRef]
- Benefits of Next Generation Collagen for Lab Applications—Jellagen. Available online: https://www.jellagen.co.uk/products/cell-culture-reagents/benefits-of-next-generation-collagen-for-lab-applications/ (accessed on 15 December 2019).
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen Scaffolds derived from a marine source and their biocompatibilty. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Pugliano, M.; Vanbellinghen, X.; Schwinté, P.; Benkirane-Jessel, N.; Keller, L. Combined jellyfish collagen type II, human stem cells and TGF-β3 as a therapecutic implant for cartilage repair. J. Stem Cell Res. Ther. 2017, 7, 4. [Google Scholar]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes- different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.-F.; Poon, A.; Basu, A.; Addleman, N.R.; Chen, J.; Phong, A.; Byers, P.H.; Klein, T.E.; Kwok, P.-Y. Natural variation in four human collagen genes across an ethnically diverse population. Genomics 2008, 91, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Pertsemlidis, A.; Fondon, J.W. Having a BLAST with bioinformatics (and avoiding BLASTphemy). Genome Biol. 2001, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar]
- Duan, X.; Sheardown, H. Crosslinking of collagen with dendrimers. J. Biomed. Mater. Res. A 2005, 75A, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comput. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.A.; Noordin, M.M.; Azmi, T.I.; Kala, U.; Loqman, M.Y. The use of rats and mice as animal models in ex vivo bone growth and development studies. Bone Jt. Res. 2016, 5, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Musson, D.S.; Naot, D.; Chhana, A.; Matthews, B.G.; McIntosh, J.D.; Lin, S.T.C.; Choi, A.J.; Callon, K.E.; Dunbar, P.R.; Lesage, S.; et al. In vitro evaulation of a novel non-mulberry silk scaffold for use in tendon regeneration. Tissue Eng. Part A 2015, 21, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Radley, G.; Pieper, I.L.; Thornton, C.A. The effect of ventricular assist device-associated biomaterials on human blood leukocytes. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Jerushalmy, Z.; Englender, T.; Shaklai, M. Phorbol-myristate-acetate-induced platelet aggregation in the presence of inhibitors. Acta Haematol. 1988, 80, 210–215. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Lacy, P.; Stow, J.L. Cytokinne release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Yamashita, A.; Nishikawa, S.; Rancourt, D.E. Identification of five developmental processes during chondrogenic differentiation of embryonic stem cells. PLoS ONE 2010, 5, e10998. [Google Scholar] [CrossRef]
- Watts, A.E.; Ackerman-Yost, J.C.; Nixon, A.J. A comparison of three-dimensional culture systems to evaluate in vitro chondrogenesis of equine bone marrow-derived mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 2275–2283. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials and scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Makris, E.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef]
- van der Kraan, P.M. Differential role of transforming growth factor beta- in an Osteoarthritic of healthy joint. J. Bone Metab. 2018, 25, 65–72. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signalling in cartilage development and maintenance. Birth Defects Res. C Embryo Today 2014, 102, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.C. The wonderful colors of the Hematoxylin-Eosin stain in diagnostic surgical pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Knudson, C.B.; Knudson, W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta 2011, 1812, 1616–1629. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Sharma, A.; Wood, L.D.; Richardson, J.B.; Roberts, S.; Kuiper, N.J. Glycosaminoglycan profiles of repair tissue formed following autologous chondrocyte implantation differ from control cartilage. Arthritis Res. Ther. 2007, 9, R79. [Google Scholar] [CrossRef]
- Lakin, B.A.; Grasso, D.J.; Shah, S.S.; Stewart, R.C.; Bansal, P.N.; Freedman, J.D.; Grinstaff, M.W.; Snyder, B.D. Cationic agent contrast-enhanced computed tomography imaging of cartilage correlates with the compressive modulus and coefficient of friction. Osteoarthr. Cartil. 2013, 21, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.E. Fell-Muir Lecture: From collagen chemistry towards cell thearpy—A personal journey. Int. J. Exp. Pathol. 2007, 88, 203–214. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Xiao, J.B.; Li, Q.; Zhu, Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.; Dong, M.; Corbeil, D.; Gelinsky, M.; Gnther, K.-P.; Fickert, S. Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems. Biotechnol. Prog. 2009, 25, 1146–1152. [Google Scholar] [CrossRef]
- Peters, M.C.; Mooney, D.J. Synthetic extracellular matrices for cell transplantation. In Materials Science Forum; Trans Tech Publications: Baech, Switzerland, 1997; Volume 250, pp. 43–52. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical engineering. Adv. Drug Deliv. Rev. 2012, 54, 3–12. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef]
- McElroy, K.; Mouton, L.; Pasquier, L.D.; Qi, W.; Ebert, D. Characterisation of a large family of polymorphic collagen-like proteins in the endospore-forming bacterium Pasteuria ramosa. Res. Microbiol. 2011, 162, 701–714. [Google Scholar] [CrossRef]
- Thoreson, A.R.; Hiwatari, R.; An, K.-N.; Amadio, P.C.; Zhao, C. The effect of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Suture coating on tendon repair strength and cell viability in a canine model. J. Hand Surg. Am. 2015, 40, 1986–1991. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. The role of the host immune response in tissue engineering and regenerative medicine. In Principles of Tissue Engineering, 4th ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 25, pp. 497–509. [Google Scholar]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bunc, G.; Klobucar, R.; Gradisnik, L. Biomaterials and host versus graft response: A short review. Bosn. J. Basic Med. Sci. 2016, 16, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, Y. Collagen-based forumlations for wound healing applications. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; Volume 2, pp. 135–149. [Google Scholar]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Nishimoto, S.; Goto, Y.; Morishige, H.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S.; Sugahara, T. Mode of action of the immunostimulatory effect of collagen from jellyfish. Biosci. Biotechnol. Biochem. 2008, 72, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Jeuken, R.M.; Roth, A.K.; Peters, R.J.R.W.; Donkelaar, C.C.V.; Thies, J.C.; Rhijn, L.W.V.; Emans, P.J. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Jacobi, M.; Villa, V.; Mmagussen, R.A.; Neyret, P. MACI—A new era? BMC Sports Sci. Med. Rehabil. 2011, 3, 10. [Google Scholar] [CrossRef]
- McCarthy, H.S.; Richardson, J.B.; Parker, J.C.E.; Roberts, S. Evaluating joint morbidity after chondral harvest for autologous chondrocyte implantation (ACI): A study of ACI-treated ankles and hips with a knee chondral harvest. Cartilage 2016, 7, 7–15. [Google Scholar] [CrossRef]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; Bomhard, A.V.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine collagen scaffolds for nasal cartilage repair: Prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef]
- Bornes, T.D.; Jomha, N.M.; Mulet-Sierra, A.; Adesida, A.B. Optimal seeding densities for in vitro chondrogenesis of two- and three- dimensional isolated and expanded bone marrow derived mesenchymal stromal stem cells within a porous collagen scaffold. Tissue Eng. Part C Methods 2016, 22, 208–220. [Google Scholar] [CrossRef]
- Gaut, C.; Sugaya, K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen. Med. 2015, 10, 665–679. [Google Scholar] [CrossRef]
- Minardi, S.; Taraballi, F.; Cabrera, F.J.; Van Eps, J.; Wang, X.; Gazze, S.A.; Fernandez-Mourev, J.S.; Tampieri, A.; Francis, L.; Weiner, B.K.; et al. Biomimetic hydroxyapatite/collagen composite drives bone niche recapitulation in a rabbit orthotopic model. Mater. Today Bio 2019, 2, 100005. [Google Scholar] [CrossRef] [PubMed]
- Harland, B.; Walcott, S.; Sun, S.X. Adhesion dynamics and durotaxis in migrating cells. Phys. Biol. 2011, 8, 015011. [Google Scholar] [CrossRef]
- Juhász, T.; Matta, C.; Somogyi, C.; Katona, E.; Takács, R.; Soha, R.F.; Szabó, I.A.; Cserháti, C.; Sződy, R.; Karácsony, Z.; et al. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell Signal. 2014, 26, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Chow, H.H.; Lai, J.Y.; Liu, H.L.; Tsai, W.B. Dynamic compression modulates chondrocyte proliferation and matrix biosynthesis in chitosan/gelatin scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-B.; Su, D.-H.; Liu, P.; Ma, Y.-Q.; Shao, Z.-Z.; Dong, J. Shape-memory collgaen scaffold for enhanced cartilage regeneration: Native collagen versus denatured collagen. Osteoarthr. Cartil. 2018, 26, 1389–1399. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Z.; Powell, L.C.; Matin, N.; Mearns-Spragg, A.; Thornton, C.A.; Khan, I.M.; Francis, L.W. Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity. Mar. Drugs 2021, 19, 405. https://doi.org/10.3390/md19080405
Ahmed Z, Powell LC, Matin N, Mearns-Spragg A, Thornton CA, Khan IM, Francis LW. Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity. Marine Drugs. 2021; 19(8):405. https://doi.org/10.3390/md19080405
Chicago/Turabian StyleAhmed, Zara, Lydia C. Powell, Navid Matin, Andrew Mearns-Spragg, Catherine A. Thornton, Ilyas M. Khan, and Lewis W. Francis. 2021. "Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity" Marine Drugs 19, no. 8: 405. https://doi.org/10.3390/md19080405
APA StyleAhmed, Z., Powell, L. C., Matin, N., Mearns-Spragg, A., Thornton, C. A., Khan, I. M., & Francis, L. W. (2021). Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity. Marine Drugs, 19(8), 405. https://doi.org/10.3390/md19080405