Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins
Abstract
1. Introduction
2. Results and Discussion
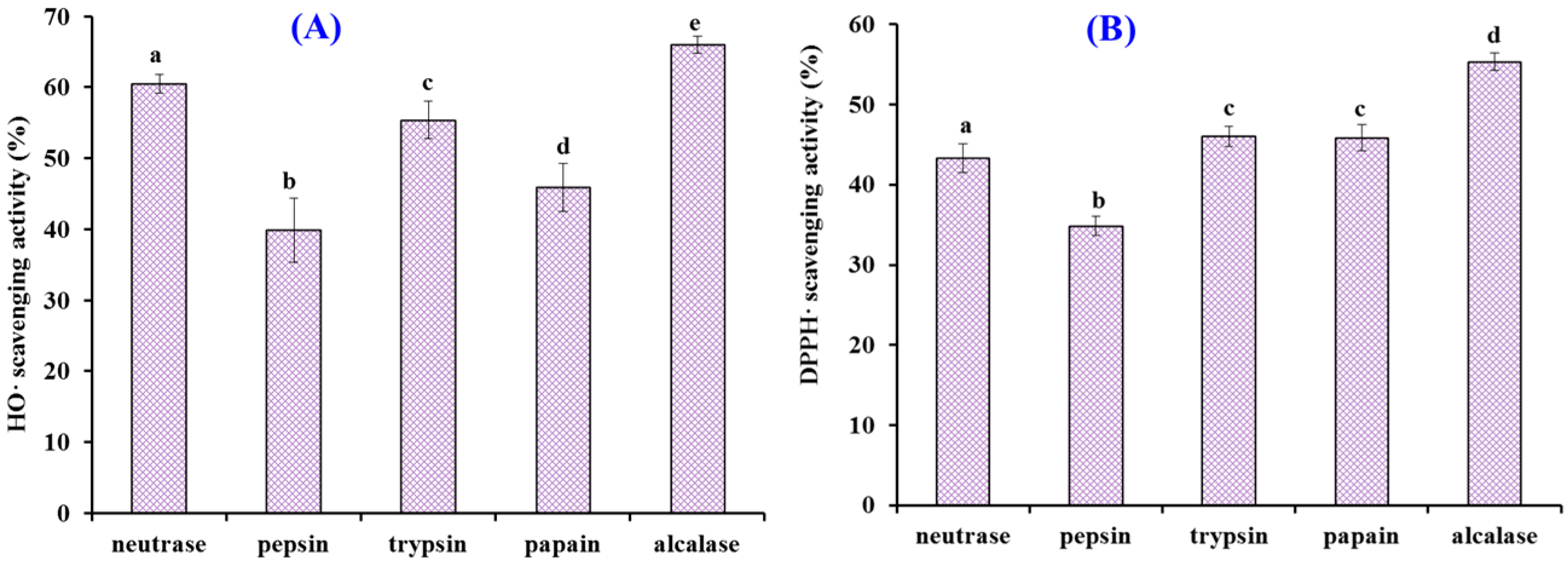
2.1. Preparation of Protein Hydrolysates of Antarctic Krill and Their Radical Scavenging Activities
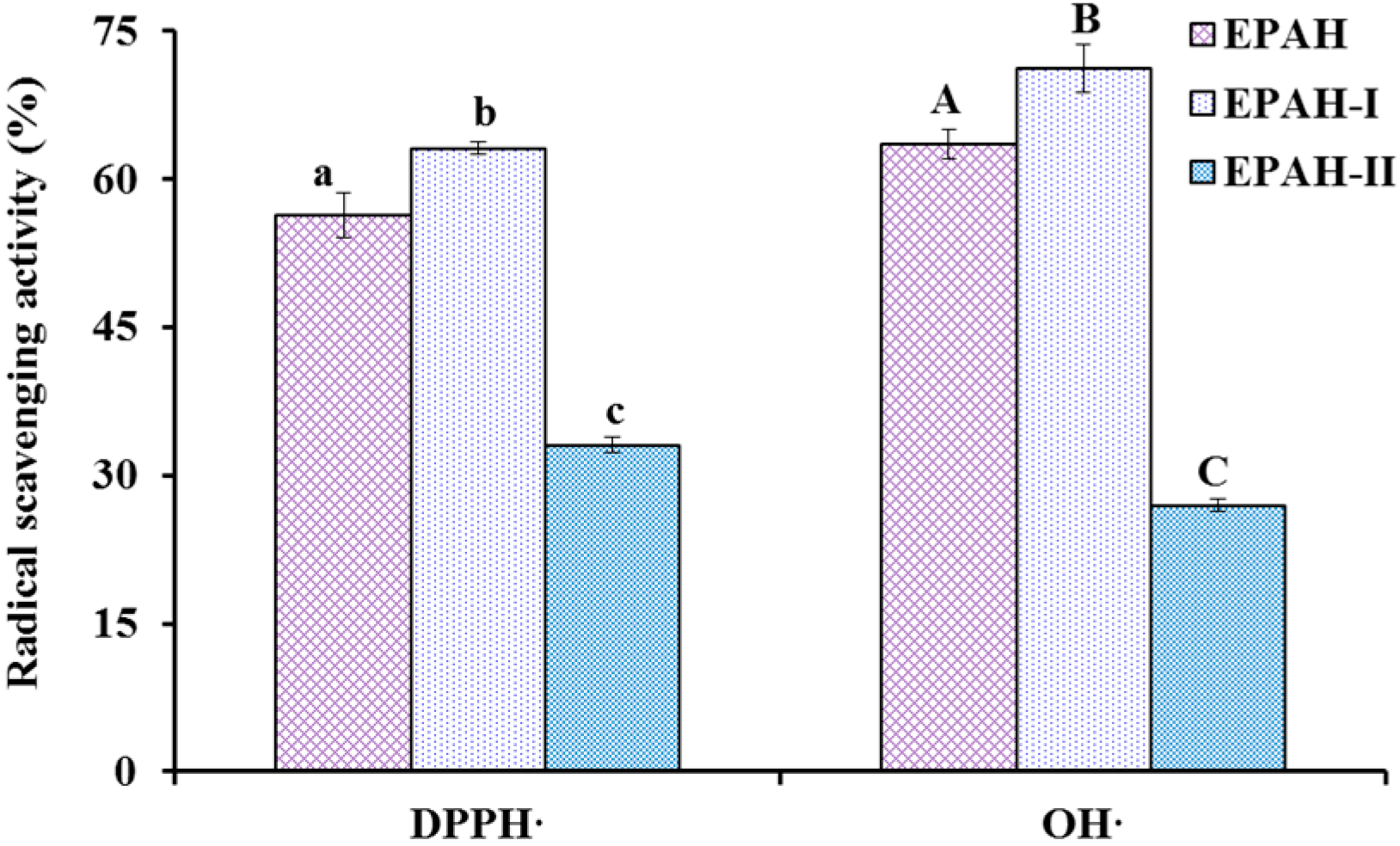
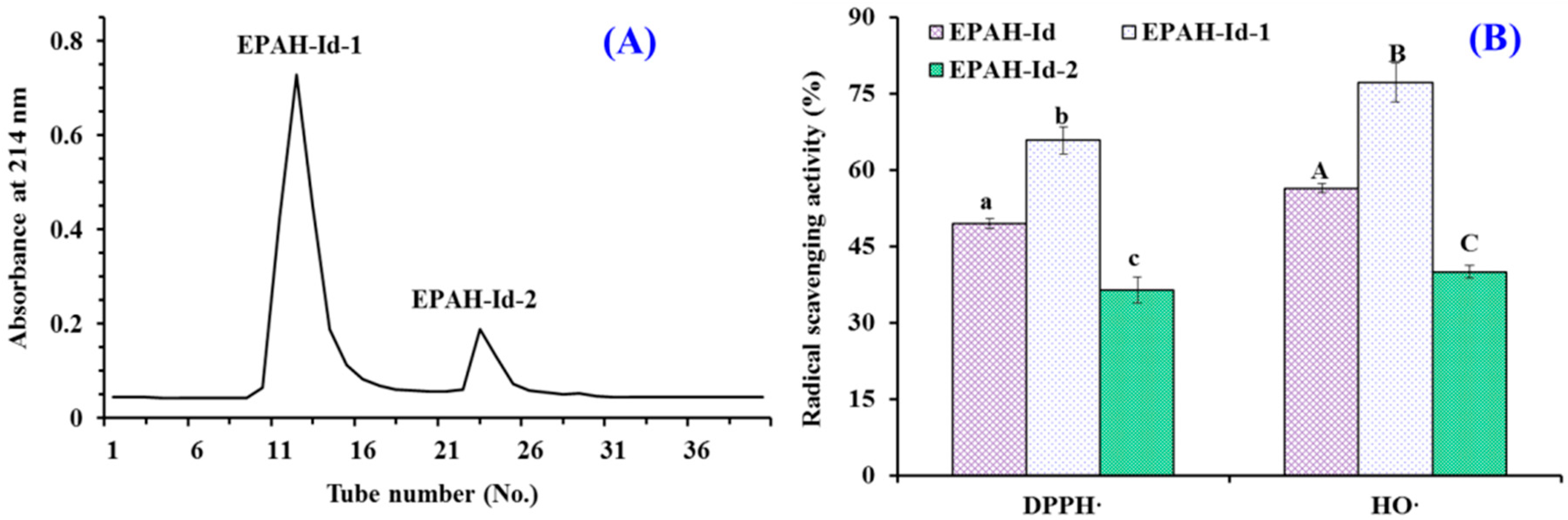
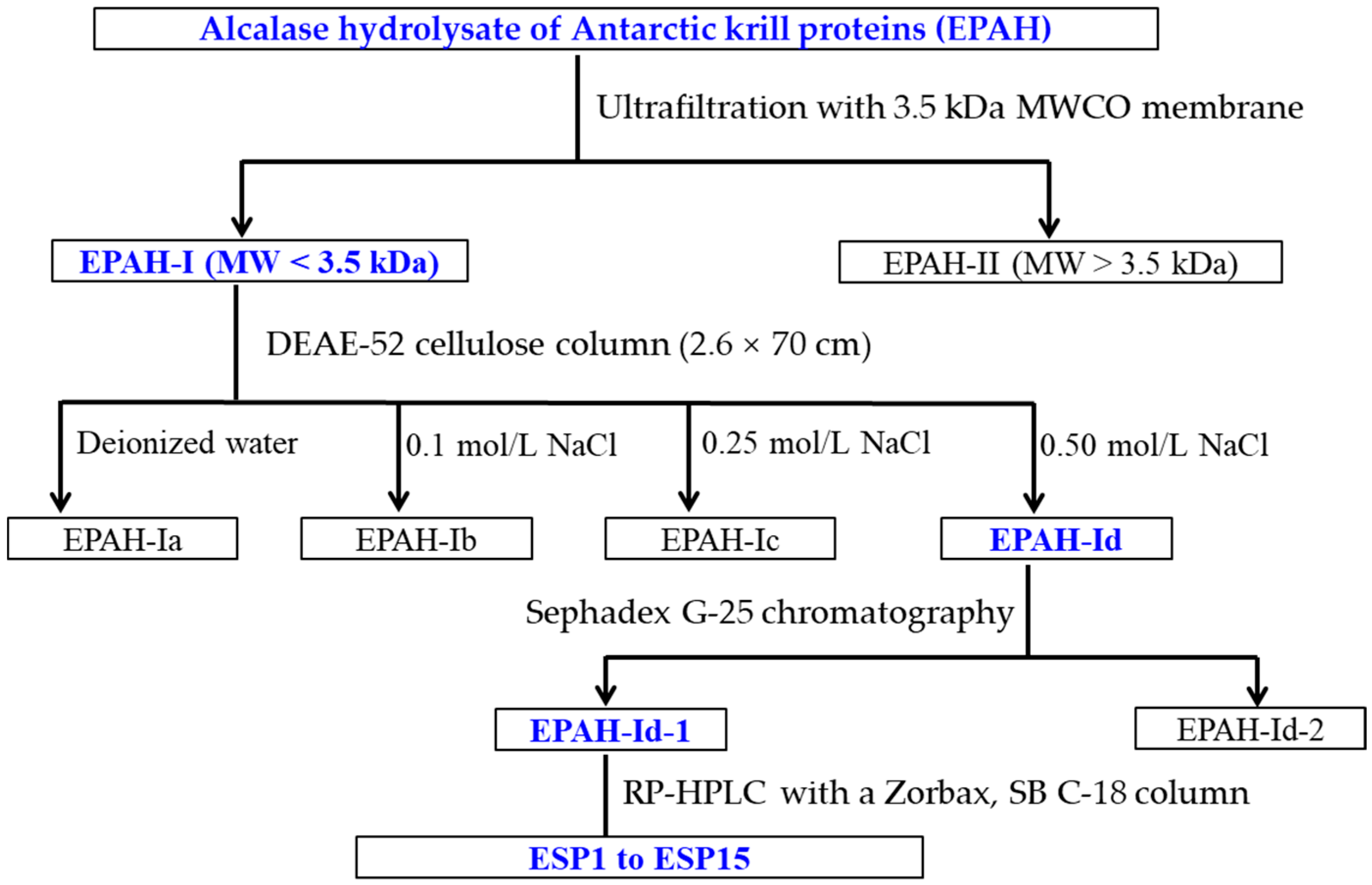
2.2. Preparation of Antioxidant Peptides from EPAH
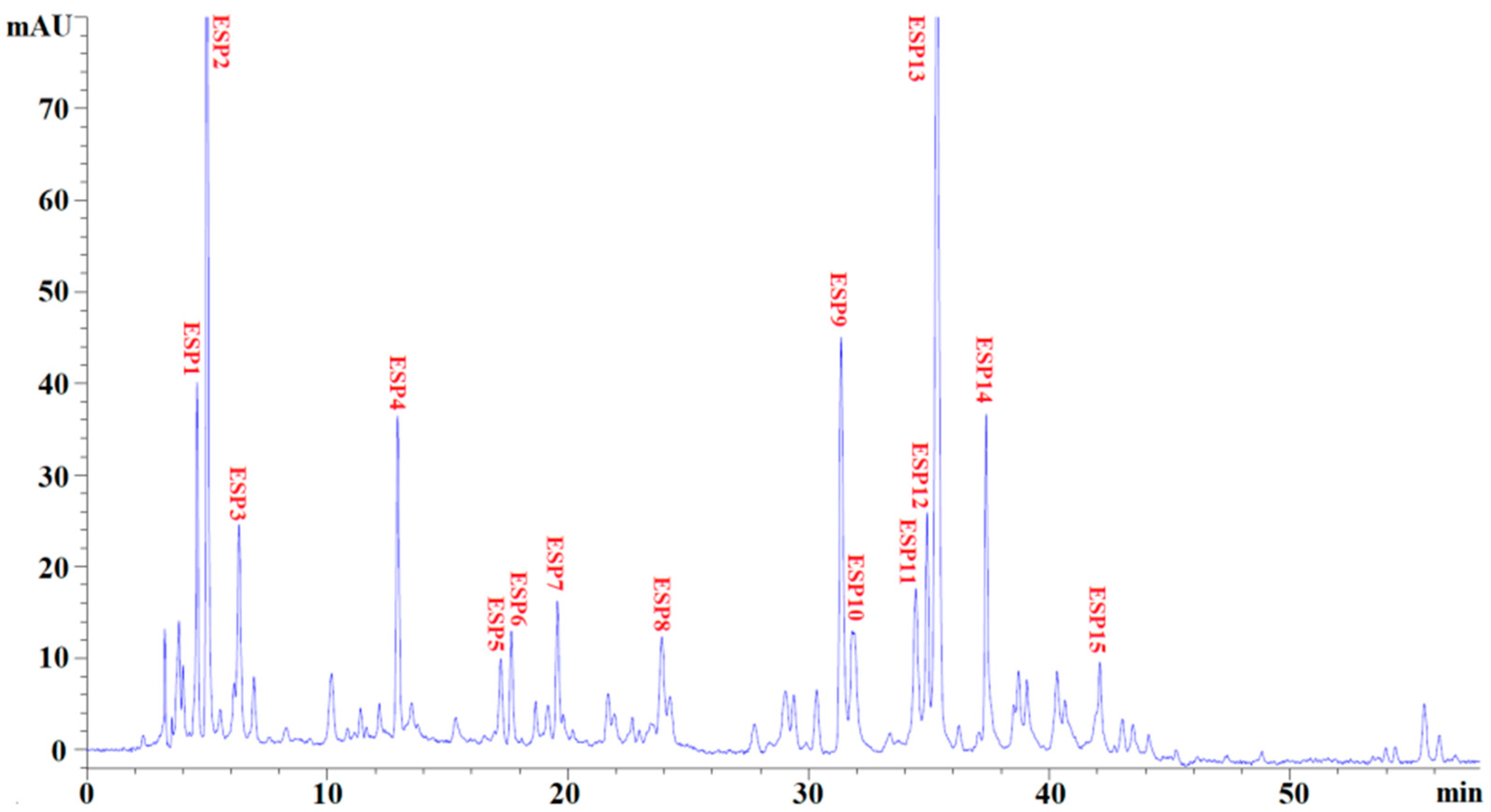
2.3. Identification of Antioxidant Peptides (ESP1 to ESP15) from EPAH-Id-1
2.4. Antioxidant Activity of Antioxidant Peptides (ESP1 to ESP15)
2.4.1. Radical Scavenging Activity of Antioxidant Peptides (ESP1 to ESP15)
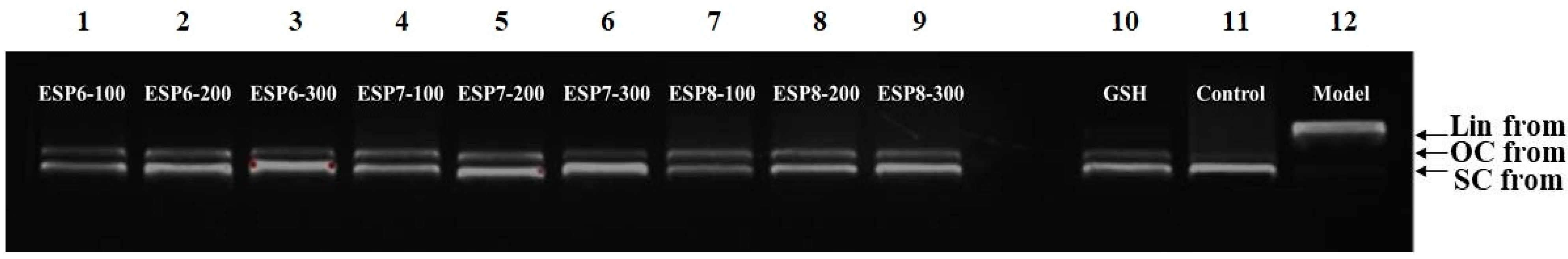
2.4.2. Protective Activity of ESP6, ESP7, and ESP8 against H2O2-Damaged Plasmid DNA
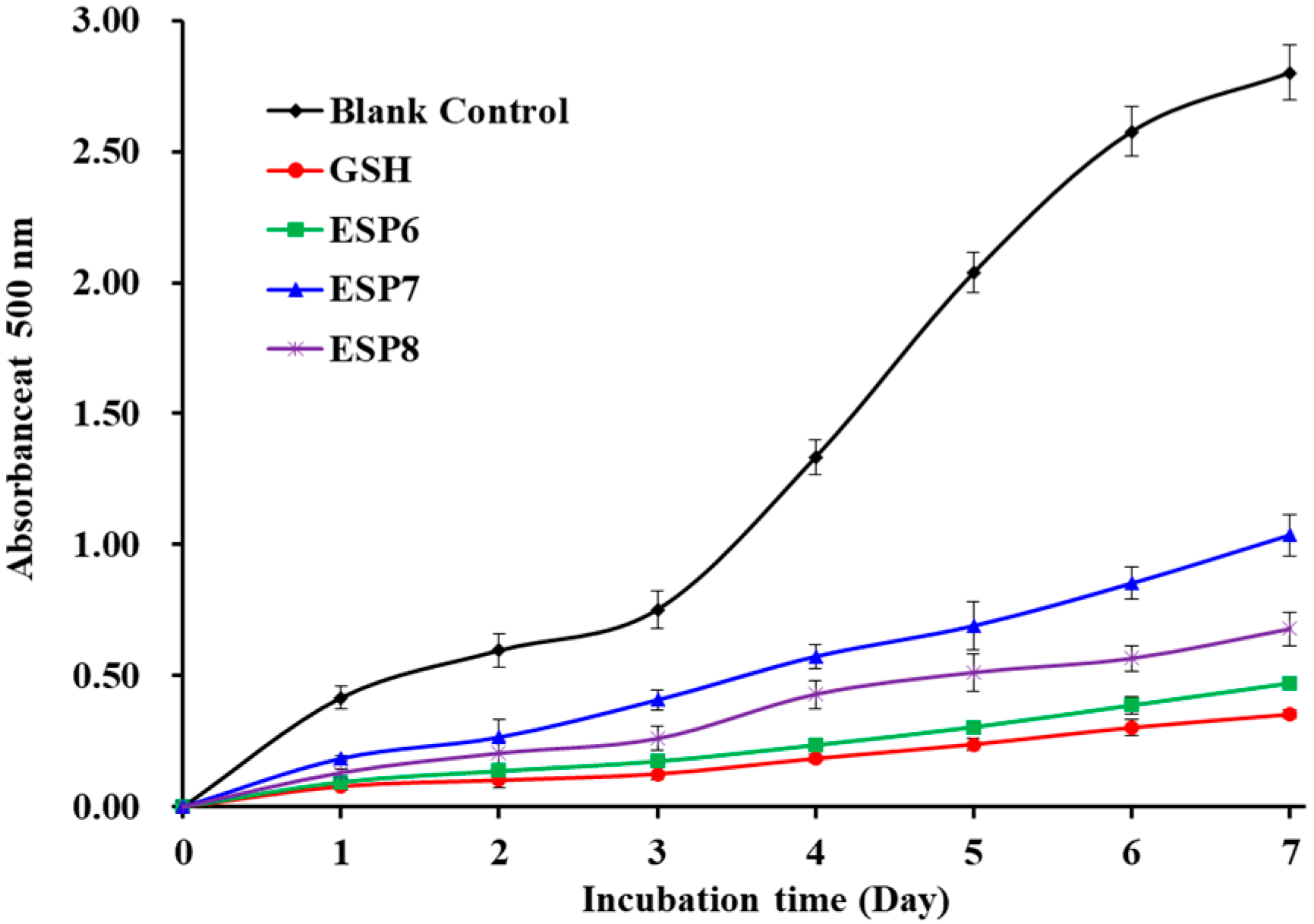
2.4.3. Lipid Peroxidation Inhibition Assay of ESP6, ESP7, and ESP8
2.4.4. Reducing Power of ESP6, ESP7, and ESP8
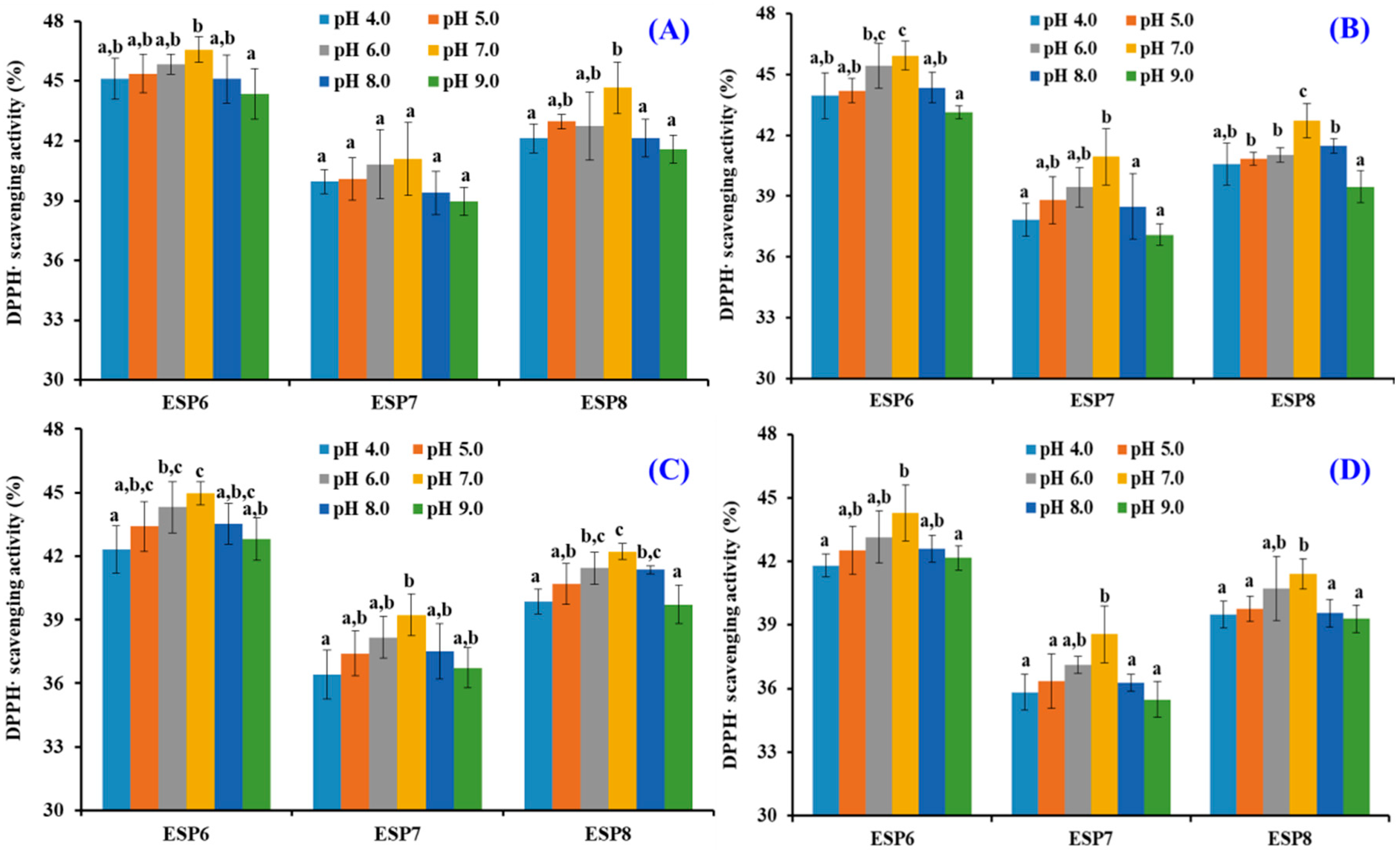
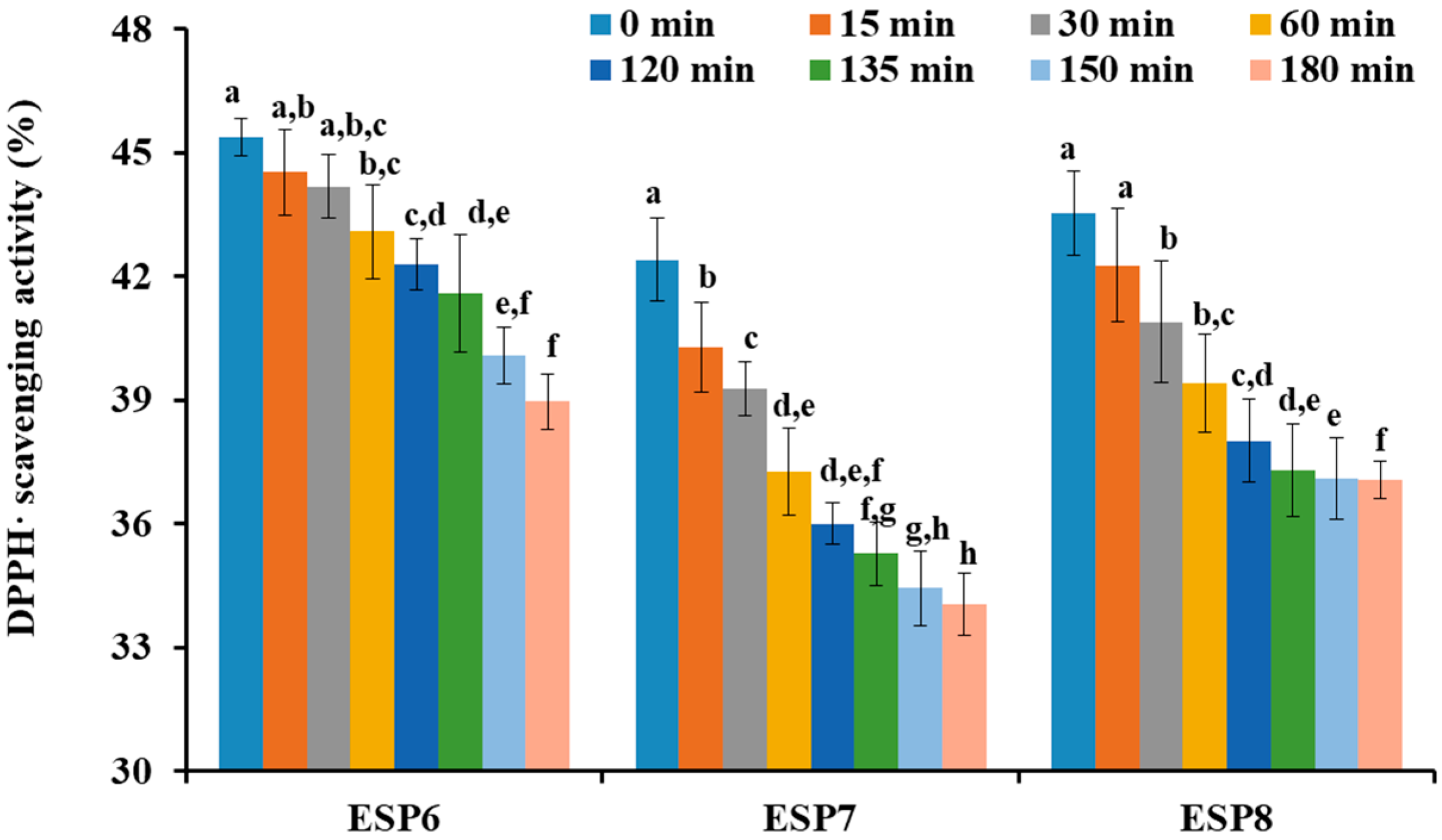
2.5. Effects of pH, Thermal, and Simulated GI Digestion Treatments on the Stability of ESP6, ESP7, and ESP8
3. Materials and Methods
3.1. Materials
3.2. Preparation of Protein Hydrolysate of Antarctic Krill (EPAH)
3.3. Preparation of Antioxidant Peptides from Alcalase Hydrolysate (EPAH) of Antarctic Krill Proteins
3.4. Identification of Antioxidant Peptides (ESP1 to ESP15) from EPAH-Id-1
3.5. Antioxidant Activities of Antioxidant Peptides (ESP1 to ESP15)
3.6. Stability Characteristics of ESP6, ESP7, and ESP8 against the Treatments of Heat, pH, and Simulated Gastrointestinal (GI) Digestion
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nicol, S.; Foster, J.; Kawaguchi, S. The fishery for Antarctic krill—Recent developments. Fish Fish 2011, 13, 30–40. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef]
- Chu, F.; Wang, D.; Liu, T.; Han, H.; Yu, Y.; Yang, Q. An optimized cocktail of chitinolytic enzymes to produce N, N′-diacetylchitobiose and N-acetyl-d-glucosamine from defatted krill by-products. Int. J. Biol. Macromol. 2019, 133, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Zhao, Y.Q.; Li, X.R.; Wang, B. High Fischer ratio oligopeptides determination from Antartic krill: Preparation, peptides profiles, and in vitro antioxidant activity. J. Food Biochem. 2019, 43, e12827. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A.; Miyahara, H.; Suzuki, K.I.; Sato, S. Isolation and identification of antihypertensive peptides from antarctic krill tail meat hydrolysate. J. Food Sci. 2009, 74, H116–H120. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, C.; Ji, H. Purification, identification and molecular mechanism of two dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from Antarctic krill (Euphausia superba) protein hydrolysate. J. Chromatogr. B 2017, 1064, 56–61. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.B.; Hong, J.T. Antarctic krill oil diet protects against lipopolysaccharide-induced oxidative stress, neuroinflammation and cognitive impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Dai, Y.; Han, L.; Zhu, Y.; Xue, C.; Wang, P.; Wang, J. Peptides from Antarctic Krill (Euphausia superba) Improve Osteoarthritis via Inhibiting HIF-2α-Mediated Death Receptor Apoptosis and Metabolism Regulation in Osteoarthritic Mice. J. Agric. Food Chem. 2019, 67, 3125–3133. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; Miao, J.; Leng, K. Chitin from Antarctic krill shell: Eco-preparation, detection, and characterization. Int. J. Biol. Macromol. 2020, 164, 4125–4137. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, X.; Zhou, Y.; Zhou, T.; Deng, S.; Zheng, B.; Zheng, P. Protective effects of Antarctic krill oil in dextran sulfate sodium-induced ulcerative colitis mice. J. Funct. Foods 2021, 79, 104394. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Chai, T.T.; Law, Y.C.; Wong, F.C.; Kim, S.K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs. 2017, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Identification and characterization of novel antioxidant peptides from mackerel (Scomber japonicus) muscle protein hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef]
- Wang, Y.M.; Pan, X.; He, Y.; Chi, C.F.; Wang, B. Hypolipidemic activities of two pentapeptides (VIAPW and IRWWW) from miiuy croaker (Miichthys miiuy) muscle on lipid accumulation in HepG2 cells through regulation of AMPK pathway. Appl. Sci. 2020, 10, 817. [Google Scholar] [CrossRef]
- Manikkam, V.; Vasiljevic, T.; Donkor, O.N.; Mathai, M.L. A review of potential marine-derived hypotensive and anti-obesity peptides. Crit. Rev. Food Sci. Nutr. 2016, 56, 92–112. [Google Scholar] [CrossRef]
- Tu, M.; Liu, H.; Cheng, S.; Mao, F.; Chen, H.; Fan, F.; Lu, W.; Du, M. Identification and characterization of a novel casein anticoagulant peptide derived from in vivo digestion. Food Funct. 2019, 10, 2552–2559. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Chi, C.F.; Wang, B. Anticancer activity of a hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in HeLa cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef]
- Sila, A.; Nedjar-Arroume, N.; Hedhili, K.; Chataigné, G.; Balti, R.; Nasri, M.; Dhulster, P.; Bougatef, A. Antibacterial peptides from barbel muscle protein hydrolysates: Activity against some pathogenic bacteria. LWT 2014, 55, 183–188. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from yellowfin tuna (Thunnus albacares) Skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Deng, S.G.; Ma, J.Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfish (Lophius litulon) muscle: Purification, identification, and cytoprotective function on HepG2 cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef]
- Sierra, L.; Fan, H.; Zapata, J.; Wu, J. Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT 2021, 146, 111631. [Google Scholar] [CrossRef]
- Cai, S.Y.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Cytoprotective effect of antioxidant pentapeptides from the protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy) against H2O2-mediated human umbilical vein endothelial cell (HUVEC) injury. Int. J. Mol. Sci. 2019, 20, 5425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, G.X.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Identification and active evaluation of antioxidant peptides from protein hydrolysates of skipjack tuna (Katsuwonus pelamis) head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Preparation and characterization of gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) bone stimulated by in vitro gastrointestinal digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef]
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Jim Wang, Z.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 127094. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Sadiq, F.A.; Wang, S.; Caiqin, L.; Jianchang, J. Involvement of Nrf2 and Keap1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent peptides from soft-shelled turtle. Process. Biochem. 2020, 92, 174–181. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Han, L.; Mao, X.; Wang, K.; Li, Y.; Zhao, M.; Wang, J.; Xue, C. Phosphorylated peptides from Antarctic krill (Euphausia superba) ameliorated osteoporosis by activation of osteogenesis-related MAPKs and PI3K/AKT/GSK-3β pathways in dexamethasone-treated mice. J. Funct. Foods 2018, 47, 447–456. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, D.; Li, B. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Zhang, L.; Tao, J.; Chi, C.F.; Wang, B. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Two novel bioactive peptides from Antarctic krill with dual angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. J. Food Sci. 2017, 82, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zhao, Y.; Yu, Z.; Tian, Y.; Wang, Y.; Wang, S.; Wang, J.; Xue, C. Phosphorylated peptides from Antarctic krill (Euphausia superba) prevent estrogen deficiency induced osteoporosis by inhibiting bone resorption in ovariectomized rats. J. Agric. Food Chem. 2015, 63, 9550–9557. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and Characterization of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Ren, X.J.; Deng, S.G.; Wu, C.W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef]
- Bougatef, A.; Balti, R.; Haddar, A.; Jellouli, K.; Souissi, N.; Nasri, M. Antioxidant and functional properties of protein hydrolysates of bluefin tuna (Thunnus thynnus) heads as influenced by the extent of enzymatic hydrolysis. Biotechnol. Bioproc. Eng. 2012, 17, 841–852. [Google Scholar] [CrossRef]
- Zhao, W.H.; Luo, Q.B.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Foods 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Chi, C.; Hu, F.; Li, Z.; Wang, B.; Luo, H. Influence of different hydrolysis processes by trypsin on the physicochemical, antioxidant, and functional properties of collagen hydrolysates from Sphyrna lewini, Dasyatis akjei, and Raja porosa. J. Aquat. Food Prod. Technol. 2016, 25, 616–632. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Luo, H.; Gong, Y.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Chang, S.K.; Ismail, A.; Yanagita, T.; Esa, N.M.; Baharuldin, M.T.H. Antioxidant peptides purified and identified from the oil palm (Elaeis guineensis Jacq.) kernel protein hydrolysate. J. Funct. Foods 2015, 14, 63–75. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionizationmass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; Luo, H.Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Wang, Y.M.; Li, L.; Chi, C.F.; Wang, B. Four antioxidant peptides from protein hydrolysate of red stingray (Dasyatis akajei) cartilages: Isolation, identification, and in vitro activity evaluation. Mar. Drugs 2019, 17, 263. [Google Scholar] [CrossRef]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zeng, L.; Yang, Z.S.; Huang, F.F.; Ding, G.F.; Wang, B. Anti-fatigue effect by peptide fraction from protein hydrolysate of croceine croaker (Pseudosciaena crocea) swim bladder through inhibiting the oxidative reactions including DNA damage. Mar. Drugs 2016, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Zhang, L.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Gulçin, I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, S.; Zhou, A.; Miao, J.; Liu, J.; Benjakul, S. A novel antioxidant peptide purified from defatted round scad (Decapterus maruadsi) protein hydrolysate extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103907. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and antifreezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Ong, M.G.-L.; Pang, M.J.; Wong, S.J.; The, L.K.; Chai, T.T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef]
- Orsini Delgado, M.C.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Añón, M.C.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M.; Zhou, G. Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem. 2020, 319, 126534. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhang, W.G.; Zhou, G.H.; Xu, X.L.; Kang, Z.L.; Yin, Y. Isolation and identification of antioxidant peptides from Jinhua ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]



| Retention Time (min) | Amino Acid Sequence | Theoretical MW/Determined MW (Da) | |
|---|---|---|---|
| ESP1 | 4.46 | NQM | 391.44/391.50 |
| ESP2 | 4.92 | WFPM | 579.71/580.03 |
| ESP3 | 6.21 | QNPT | 458.47/458.50 |
| ESP4 | 13.01 | YMNF | 573.66/573.50 |
| ESP5 | 17.19 | SGPA | 330.34/330.15 |
| ESP6 | 17.78 | SLPY | 478.54/478.80 |
| ESP7 | 19.65 | QYPPMQY | 926.05/926.00 |
| ESP8 | 23.84 | EYEA | 510.49/510.60 |
| ESP9 | 31.41 | NWDDMRIVAV | 1218.38/1218.40 |
| ESP10 | 31.93 | WDDMERLVMI | 1307.54/1307.40 |
| ESP11 | 34.46 | NWDDMEPSF | 1140.18/1140.30 |
| ESP12 | 34.97 | NGPDPRPSQQ | 1094.14/1094.21 |
| ESP13 | 35.34 | AFLWA | 649.74/650.10 |
| ESP14 | 37.38 | NVPDM | 574.65/574.60 |
| ESP15 | 42.17 | TFPIYDPQ | 1143.24/1143.30 |
| Amino Acid Sequence | EC50 (mg/mL) | |||
|---|---|---|---|---|
| HO· | DPPH· | · | ||
| ESP1 | NQM | 1.425 ± 0.067 a | 1.695 ± 0.033 a,e,i | 1.796 ± 0.029 a |
| ESP2 | WFPM | 1.751 ± 0.075 b | 5.364 ± 0.337 b | 2.746 ± 0.302 b |
| ESP3 | QNPT | 1.931 ± 0.031 c,h | 7.193 ± 0.460 c | 2.933 ± 0.075 c |
| ESP4 | YMNF | 1.443 ± 0.066 a,j | 1.672 ± 0.044 a,e,i | 1.136 ± 0.063 d |
| ESP5 | SGPA | 2.362 ± 0.021 d | 4.135 ± 0.192 d | 1.863 ± 0.104 a,e |
| ESP6 | SLPY | 0.826 ± 0.027 e | 1.181 ± 0.036 e | 0.789 ± 0.079 f |
| ESP7 | QYPPMQY | 1.022 ± 0.058 f | 1.547 ± 0.150 a,e | 0.913 ± 0.007 f |
| ESP8 | EYEA | 0.946 ± 0.011 e,f | 1.372 ± 0.274 a,e | 0.793 ± 0.056 f |
| ESP9 | NWDDMRIVAV | 2.612 ± 0.013 g | 6.192 ± 0.192 f | 3.756 ± 0.025 g |
| ESP10 | WDDMERLVMI | 1.953 ± 0.042 h | 4.719 ± 0.163 g | 1.996 ± 0.011 e |
| ESP11 | NWDDMEPSF | 2.598 ± 0.036 g | 3.029 ± 0.077 h | 1.862 ± 0.094 a,e |
| ESP12 | NGPDPRPSQQ | 2.742 ± 0.105 i | 7.054 ± 0.460 c | 2.031 ± 0.011 e |
| ESP13 | AFLWA | 1.527 ± 0.080 j,k | 2.029 ± 0.092 i | 1.162 ± 0.036 d |
| ESP14 | NVPDM | 1.839 ± 0.032 b,c | 4.876 ± 0.145 g | 2.916 ± 0.153 c |
| ESP15 | TFPIYDPQ | 1.549 ± 0.072 k | 2.135 ± 0.106 i | 1.252 ± 0.051 d |
| Positive control | GSH | 0.492 ± 0.063 l | 0.073 ± 0.021 j | 0.250 ± 0.023 h |
| Reduced Proportion of DPPH· Scavenging Activity (%) | ||||||
|---|---|---|---|---|---|---|
| pH 4.0 | pH 5.0 | pH 6.0 | pH 7.0 | pH 8.0 | pH 9.0 | |
| ESP6 | 5.76 ± 0.06 | 5.06 ± 0.55 | 4.42 ± 0.73 | 3.29 ± 0.77 | 4.99 ± 0.58 | 5.41 ± 0.95 |
| ESP7 | 6.95 ± 0.65 | 6.44 ± 0.79 | 5.65 ± 1.33 | 4.22 ± 1.34 | 6.49 ± 1.21 | 7.29 ± 0.99 |
| ESP8 | 6.99 ± 6.72 | 6.72 ± 0.87 | 5.74 ± 1.36 | 5.05 ± 0.89 | 6.92 ± 0.99 | 7.19 ± 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-Y.; Zhao, G.-X.; Suo, S.-K.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Mar. Drugs 2021, 19, 347. https://doi.org/10.3390/md19060347
Zhang S-Y, Zhao G-X, Suo S-K, Wang Y-M, Chi C-F, Wang B. Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Marine Drugs. 2021; 19(6):347. https://doi.org/10.3390/md19060347
Chicago/Turabian StyleZhang, Shuang-Yi, Guo-Xu Zhao, Shi-Kun Suo, Yu-Mei Wang, Chang-Feng Chi, and Bin Wang. 2021. "Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins" Marine Drugs 19, no. 6: 347. https://doi.org/10.3390/md19060347
APA StyleZhang, S.-Y., Zhao, G.-X., Suo, S.-K., Wang, Y.-M., Chi, C.-F., & Wang, B. (2021). Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Marine Drugs, 19(6), 347. https://doi.org/10.3390/md19060347








