Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool
Abstract
1. Introduction
2. Cyclic Peptides in Drug Development
2.1. Peptides as Drugs
2.2. Membrane Permeability of Peptides
2.3. Advantages of Cyclic Peptides and Peptidomimetics on Cell Permeability
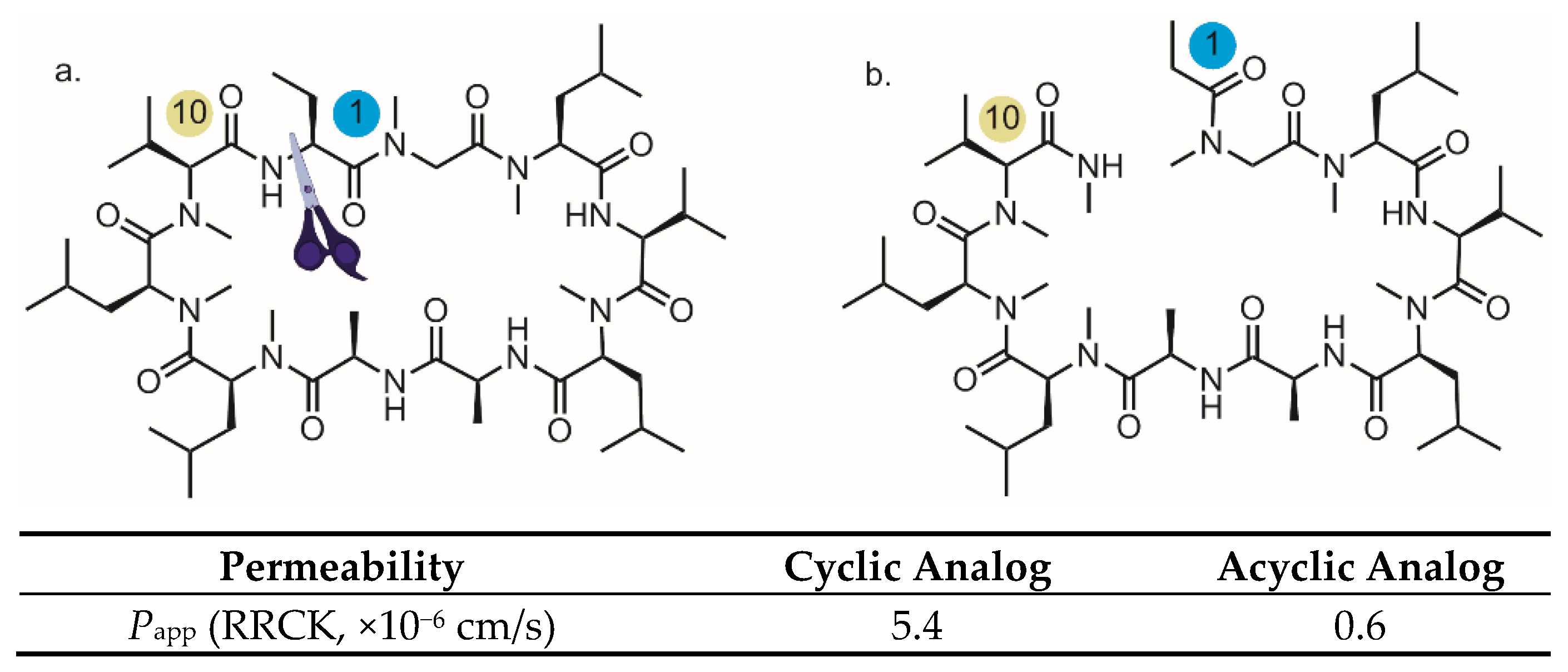
3. Backbone N-Methylation: Pivotal Roles to Improve the Permeability for Cyclic Peptides and Peptidomimetics
3.1. Chemical Synthesis of N-Methylated Cyclic Peptides
3.1.1. Preparation of N-Methyl Amino Acids (NMAAs) as Building Blocks for Solution-Phase Synthesis of Peptides
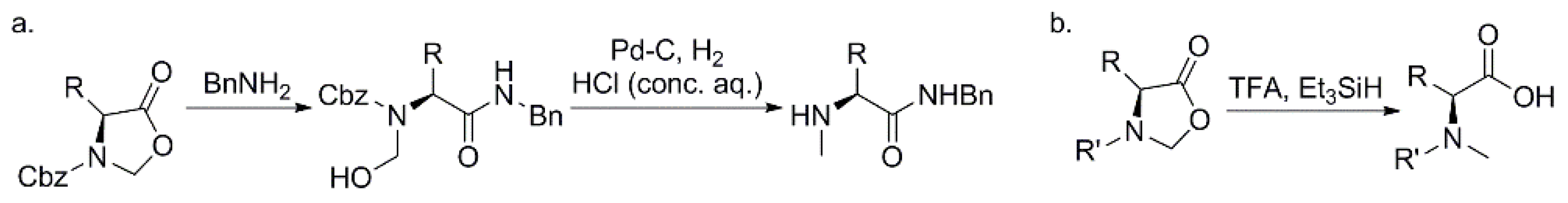
3.1.2. Regio-Specific N-Methylation for Solid-Phase Synthesis of Peptides
3.2. Backbone N-Methylation in the Discovery of Permeable Cyclic Peptide/Peptidomimetic
3.2.1. Studies on Methylated Analogs of Sanguinamide A
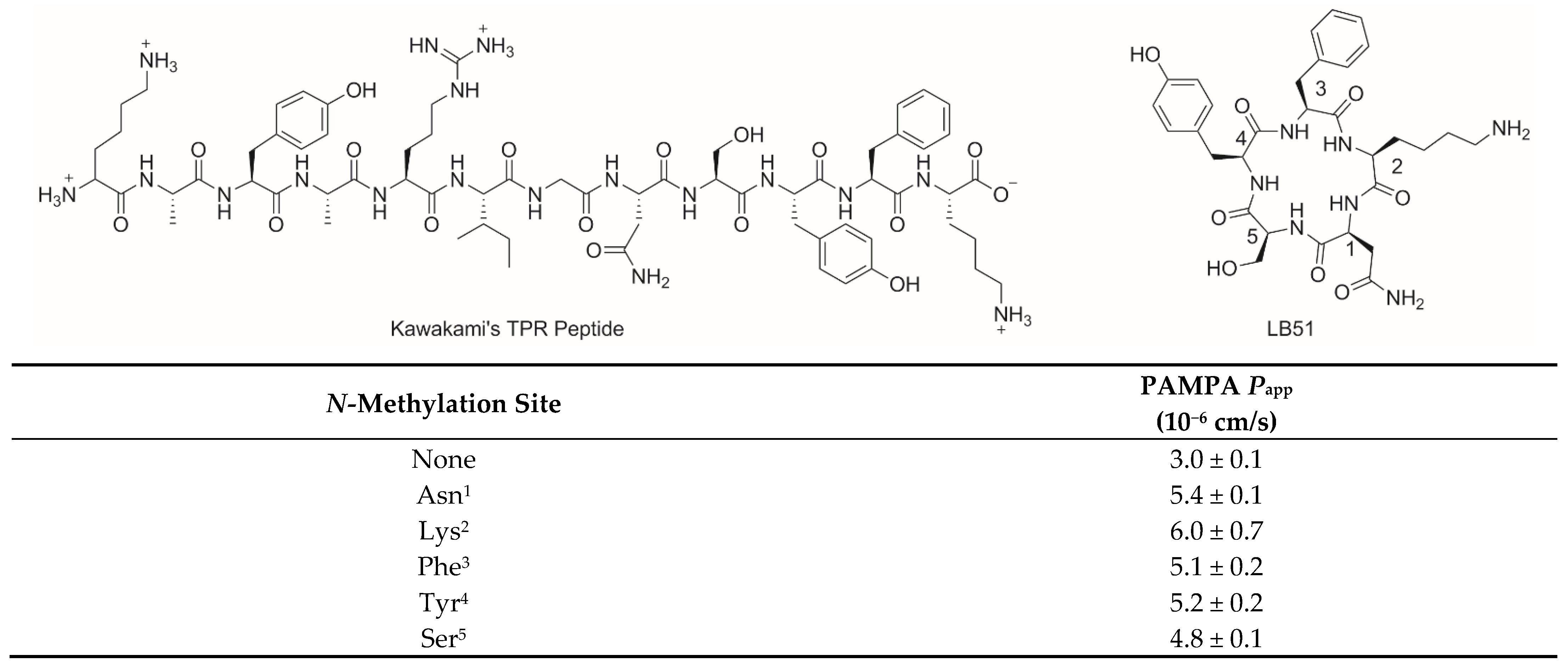
3.2.2. PAMPA Permeability of N-Methylated LB51 Analogs
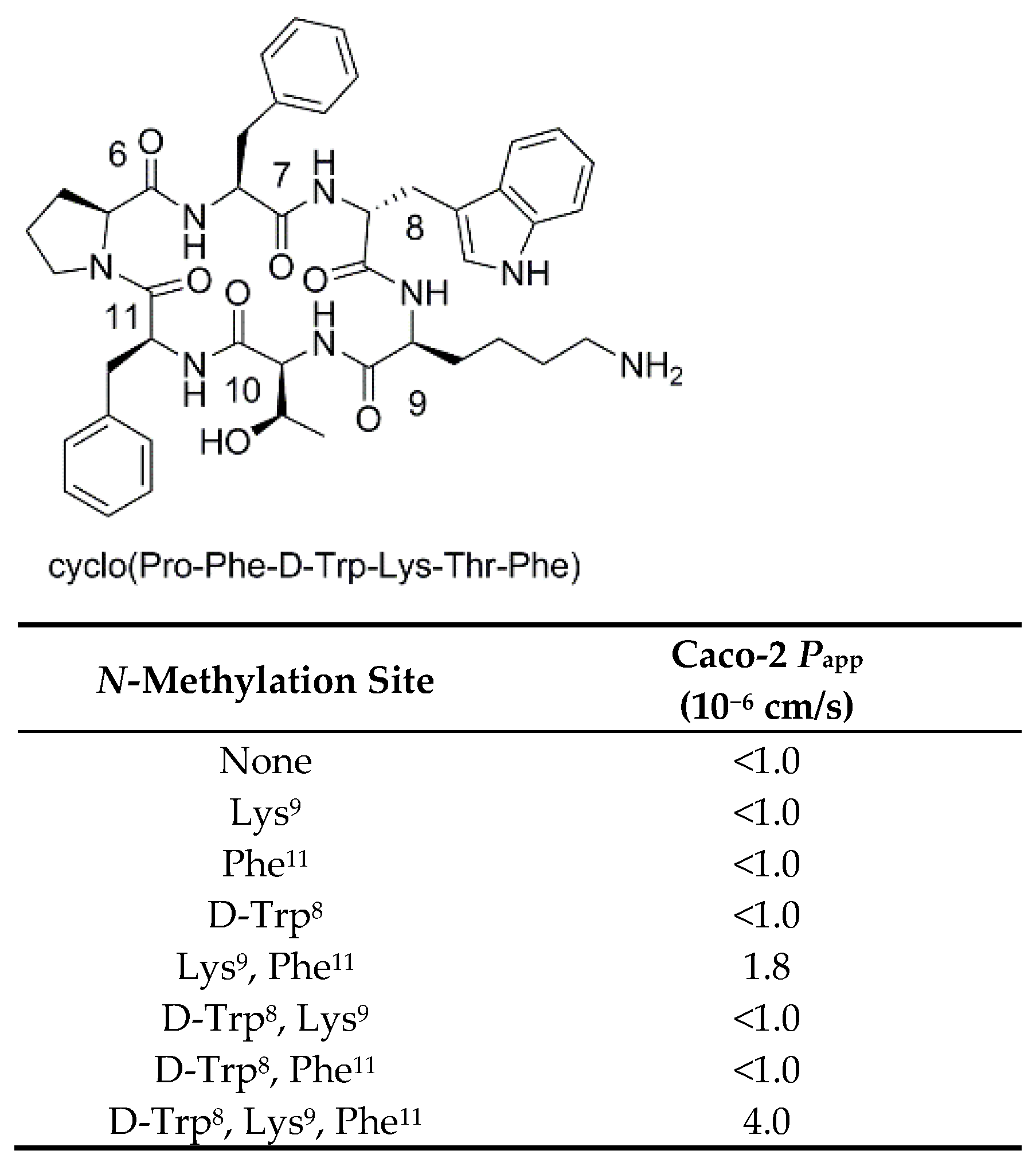
3.2.3. Studies on N-Methylated Analogs of Cyclo(-Pro-Phe-D-Trp-Lys-Thr-Phe-)
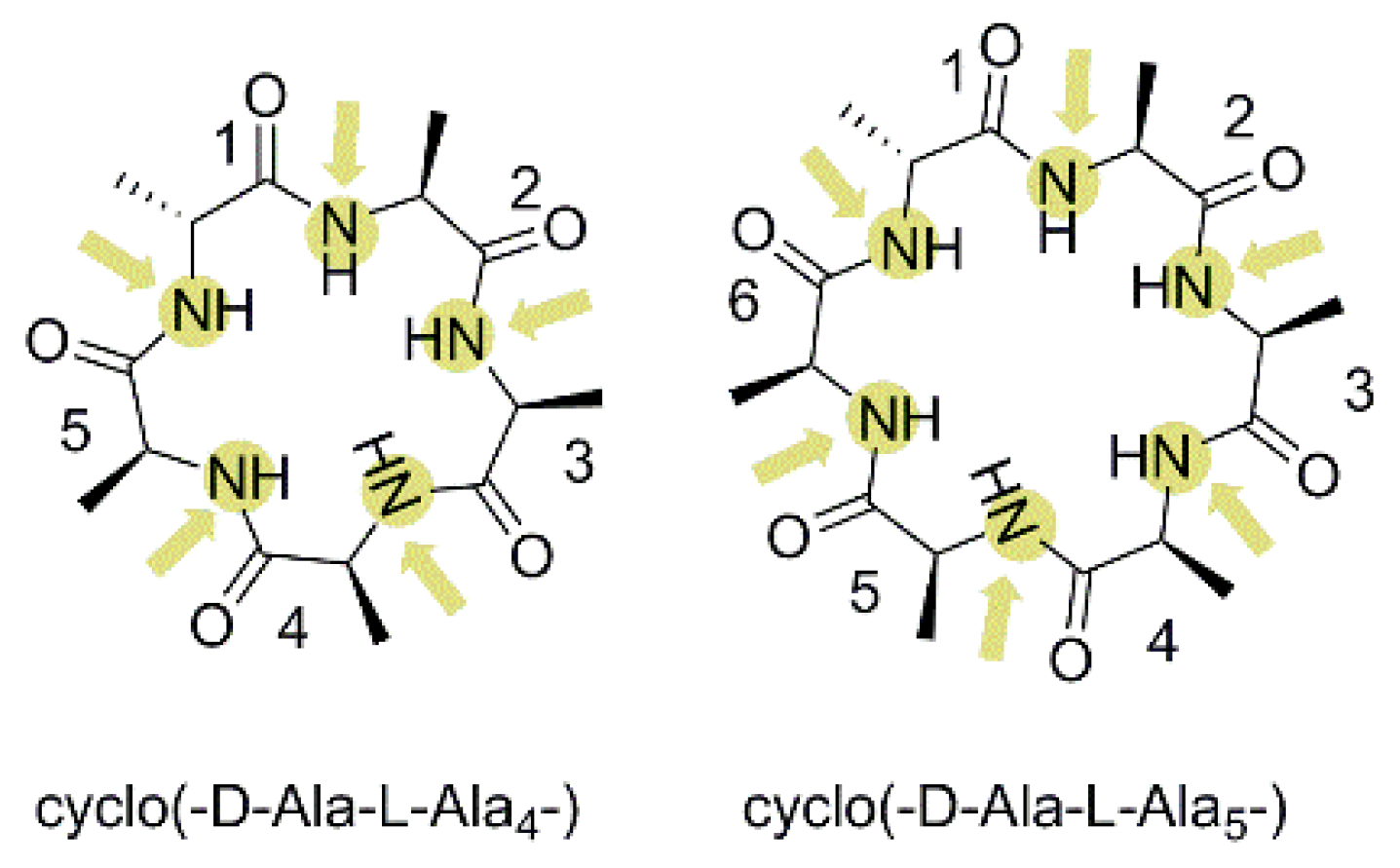
3.2.4. Membrane Permeability of N-Methylated Poly Alanine Cyclic Pentapeptide/hexapeptide
3.2.5. Studies on N-Methylated Analogs of Cyclo(-Leu-Leu-Leu-Leu-Pro-Tyr-)
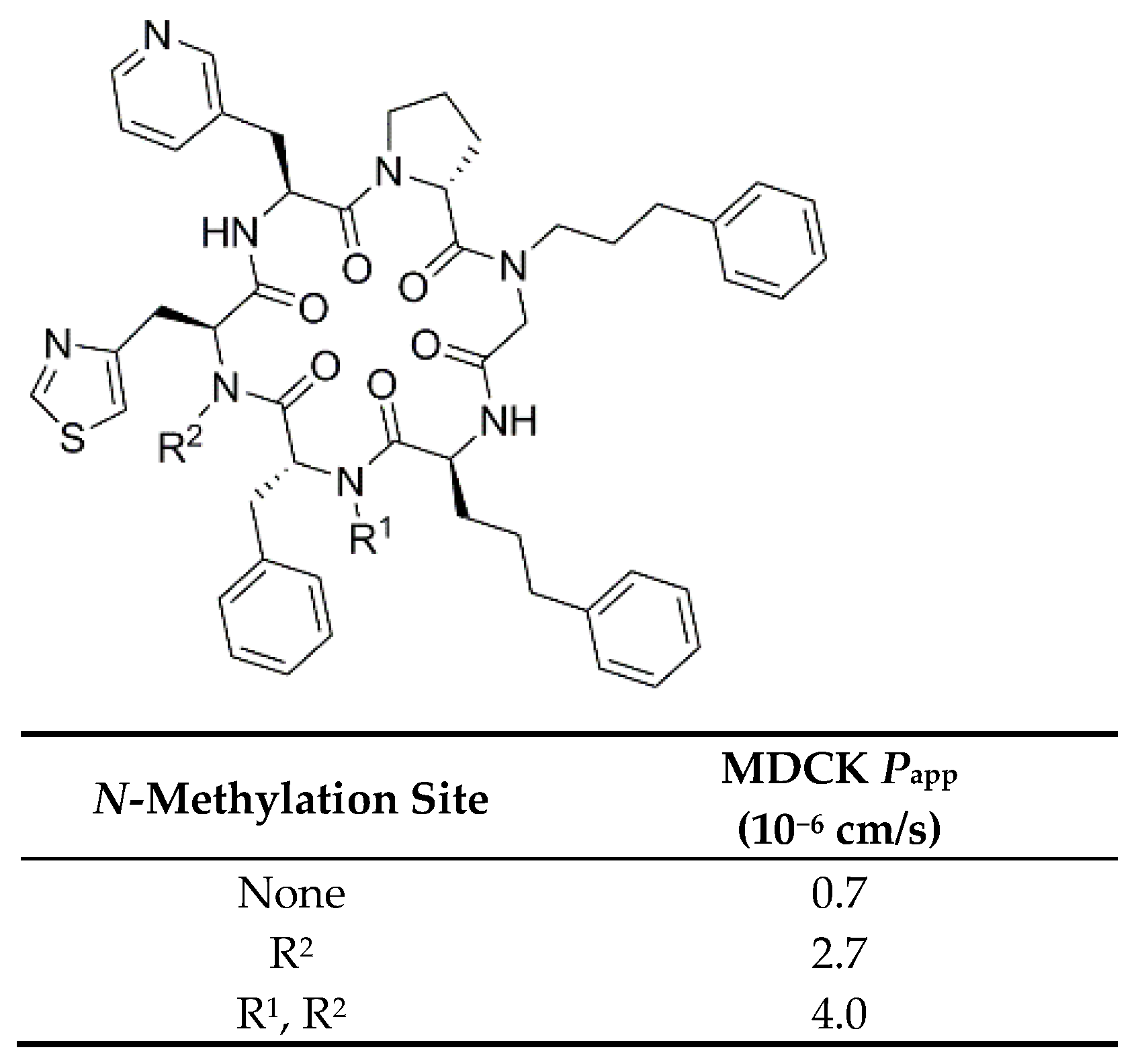
3.2.6. Backbone N-Methylation on Modulators for Chemokine Receptor CXCR7
3.2.7. Influence of N-Methylation on Permeability of Semipeptide Macrocycles
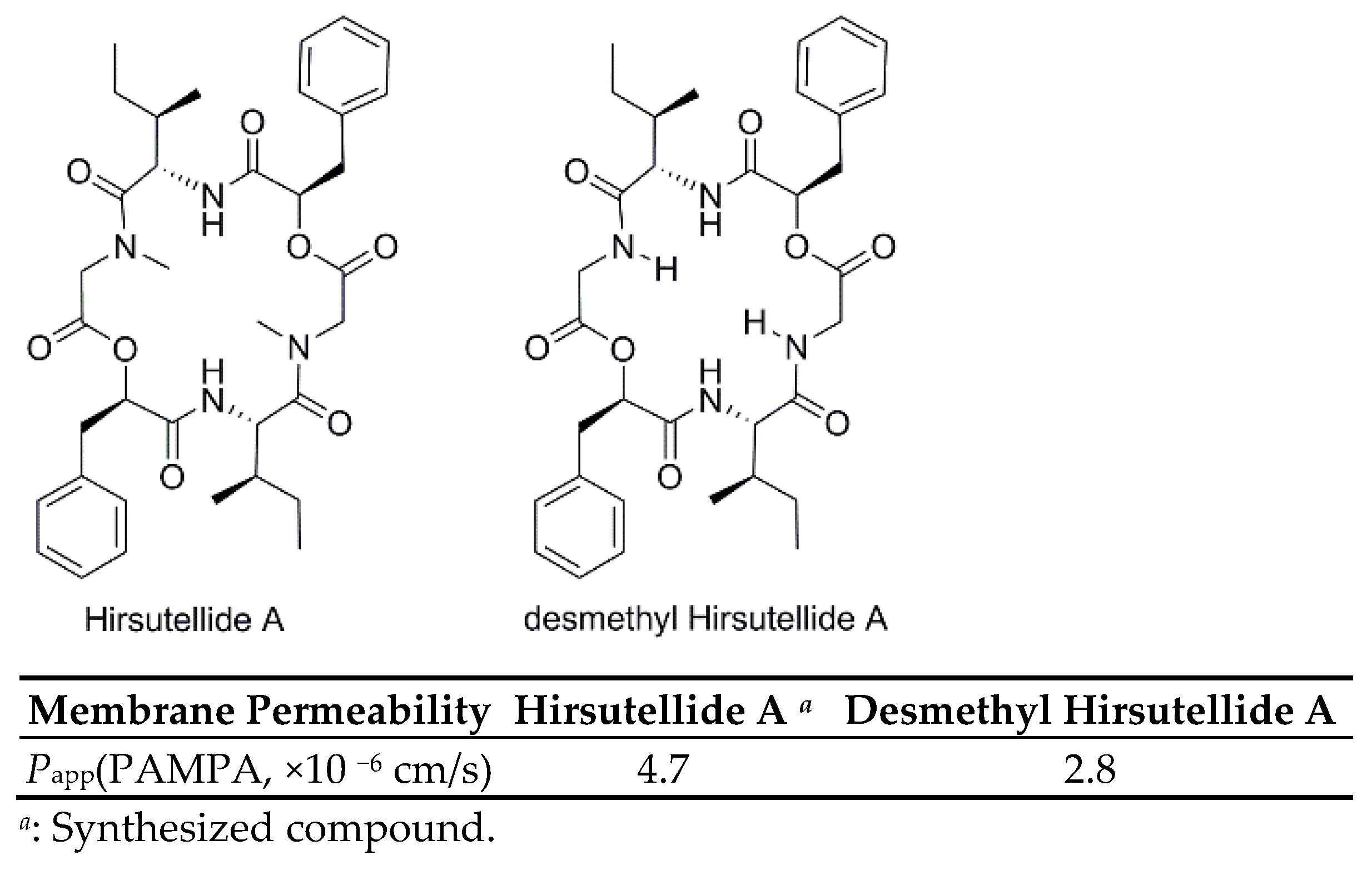
3.2.8. Membrane Permeability of Hirsutellide A and Its Desmethyl Analog
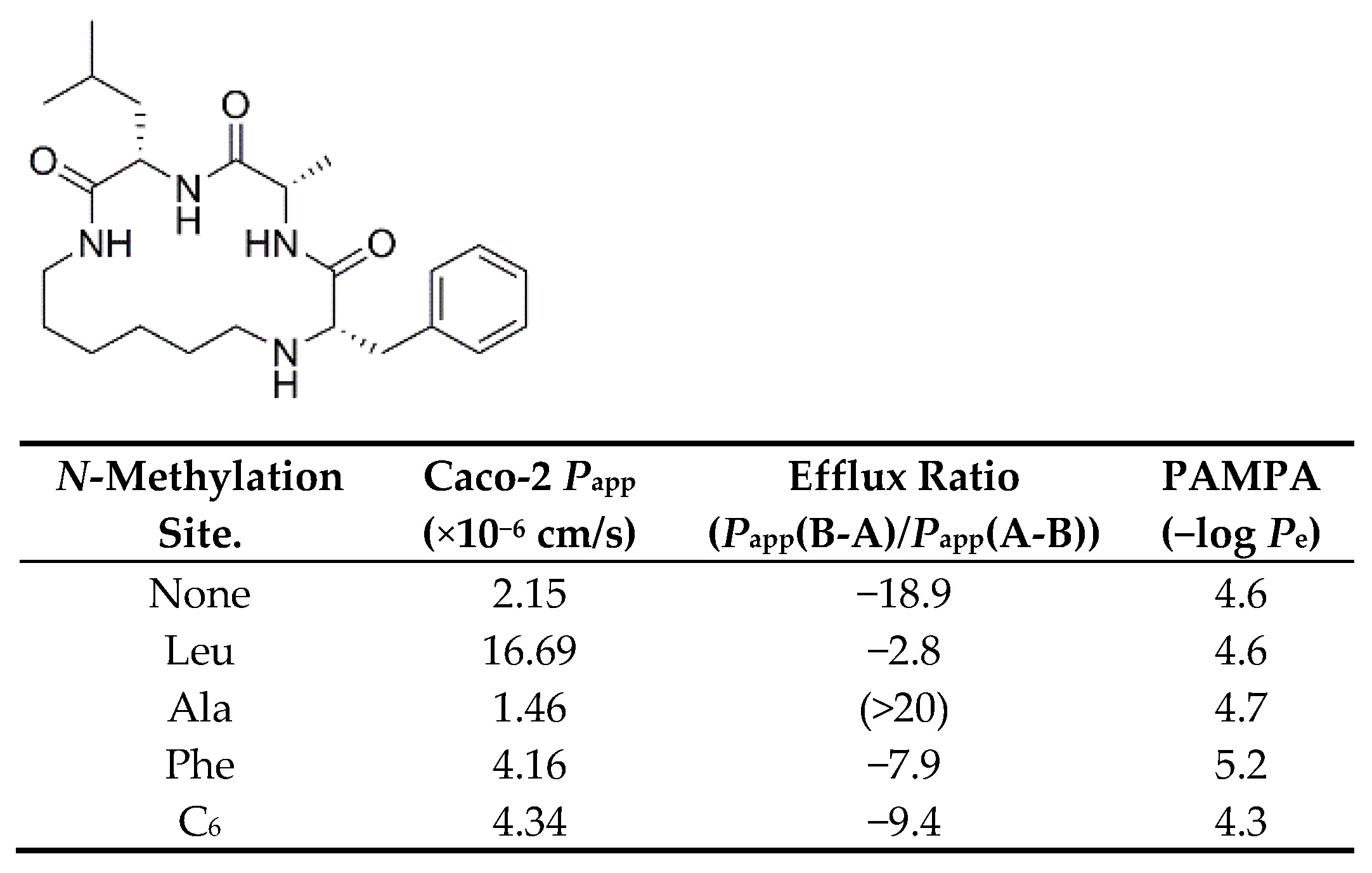
3.2.9. Influence of Backbone N-Methylation on Permeability of Lariat Peptides
3.2.10. Backbone N-Methylation of Hexa-, Hepta- and Octo-Thioether-Containing Cyclic Peptides
4. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wells, J.A.; McClendon, C.L. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 2007, 450, 1001–1009. [Google Scholar] [CrossRef]
- Buchwald, P. Small-molecule protein–protein interaction inhibitors: Therapeutic potential in light of molecular size, chemical space, and ligand binding efficiency considerations. IUBMB Life 2010, 62, 724–731. [Google Scholar] [CrossRef]
- Qian, Z.; Dougherty, P.G.; Pei, D. Targeting intracellular protein-protein interactions with cell-permeable cyclic peptides. Curr. Opin. Chem. Biol. 2017, 38, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Gestwicki, J.E. Inhibitors of protein–protein interactions (PPIs): An analysis of scaffold choices and buried surface area. Curr. Opin. Chem. Biol. 2018, 44, 75–86. [Google Scholar] [CrossRef]
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810. [Google Scholar] [CrossRef]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A comprehensive review on current advances in peptide drug development and design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Albericio, F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Kenworthy, M.N.; Mukherjee, S.; Kopach, M.E.; Wegner, K.; Gallou, F.; Smith, A.G.; Roschangar, F. Sustainability challenges in peptide synthesis and purification: From R&D to production. J. Org. Chem. 2019, 84, 4615–4628. [Google Scholar]
- Reese, H.R.; Shanahan, C.C.; Proulx, C.; Menegatti, S. Peptide science: A “rule model” for new generations of peptidomimetics. Acta Biomater. 2020, 102, 35–74. [Google Scholar] [CrossRef]
- Cheng, F.; Zhao, J.; Wang, Y.; Lu, W.; Liu, Z.; Zhou, Y.; Martin, W.R.; Wang, R.; Huang, J.; Hao, T.; et al. Comprehensive characterization of protein–protein interactions perturbed by disease mutations. Nat. Genet. 2021, 53, 342–353. [Google Scholar] [CrossRef]
- Jwad, R.; Weissberger, D.; Hunter, L. Strategies for fine-tuning the conformations of cyclic peptides. Chem. Rev. 2020, 120, 9743–9789. [Google Scholar] [CrossRef]
- Viarengo-Baker, L.A.; Brown, L.E.; Rzepiela, A.A.; Whitty, A. Defining and navigating macrocycle chemical space. Chem. Sci. 2021, 12, 4309–4328. [Google Scholar] [CrossRef]
- Matsson, P.; Doak, B.C.; Over, B.; Kihlberg, J. Cell permeability beyond the rule of 5. Adv. Drug Deliv. Rev. 2016, 101, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Goetz, G.H.; Philippe, L.; Shapiro, M.J. EPSA: A novel supercritical fluid chromatography technique enabling the design of permeable cyclic peptides. ACS Med. Chem. Lett. 2014, 5, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.N.; Hill, T.A.; Fairlie, D.P. Connecting hydrophobic surfaces in cyclic peptides increases membrane permeability. Angew. Chem. Int. Ed. 2021, 60, 8385–8390. [Google Scholar] [CrossRef]
- Farley, K.A.; Che, Y.; Navarro-Vázquez, A.; Limberakis, C.; Anderson, D.; Yan, J.; Shapiro, M.; Shanmugasundaram, V.; Gil, R.R. Cyclic peptide design guided by residual dipolar couplings, j-couplings, and intramolecular hydrogen bond analysis. J. Org. Chem. 2019, 84, 4803–4813. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, A.; Schwochert, J.; Pye, C.R.; Asano, D.; Edmondson, Q.D.; Turmon, A.C.; Klein, V.G.; Ono, S.; Okada, O.; Lokey, R.S. Drug-Like Properties in Macrocycles above MW 1000: Backbone Rigidity versus Side-Chain Lipophilicity. Angew. Chem. Int. Ed. 2020, 59, 21571–21577. [Google Scholar] [CrossRef]
- Liras, S.; McClure, K.F. Permeability of Cyclic Peptide Macrocycles and Cyclotides and Their Potential as Therapeutics. ACS Med. Chem. Lett. 2019, 10, 1026–1032. [Google Scholar] [CrossRef]
- Stähelin, H.F. The history of cyclosporin A (Sandimmune®) revisited: Another point of view. Experientia 1996, 52, 5–13. [Google Scholar] [CrossRef]
- Graeb, C.; Arbogast, H.; Guba, M.; Jauch, K.W.; Land, W. Cyclosporine: 20 years of experience at the university of munich. Transplant. Proc. 2004, 36, S125–S129. [Google Scholar] [CrossRef] [PubMed]
- Loosli, H.-R.; Kessler, H.; Oschkinat, H.; Weber, H.-P.; Petcher, T.J.; Widmer, A. Peptide conformations. Part 31. The conformation of cyclosporin a in the crystal and in solution. Helv. Chim. Acta 1985, 68, 682–704. [Google Scholar] [CrossRef]
- Wang, C.K.; Swedberg, J.E.; Harvey, P.J.; Kaas, Q.; Craik, D.J. Conformational flexibility is a determinant of permeability for cyclosporin. J. Phys. Chem. B 2018, 122, 2261–2276. [Google Scholar] [CrossRef]
- Witek, J.; Keller, B.G.; Blatter, M.; Meissner, A.; Wagner, T.; Riniker, S. Kinetic models of cyclosporin a in polar and apolar environments reveal multiple congruent conformational states. J. Chem. Inf. Model. 2016, 56, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, G.M.; Gangwar, S.; Okumu, F.W.; Siahaan, T.J.; Stella, V.J.; Borchardt, R.T. Esterase-sensitive cyclic prodrugs of peptides: Evaluation of an acyloxyalkoxy promoiety in a model hexapeptide. Pharm. Res. 1996, 13, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, S.; Pauletti, G.M.; Siahaan, T.J.; Stella, V.J.; Borchardt, R.T. Synthesis of a novel esterase-sensitive cyclic prodrug of a hexapeptide using an (acyloxy)alkoxy promoiety. J. Org. Chem. 1997, 62, 1356–1362. [Google Scholar] [CrossRef]
- Okumu, F.W.; Pauletti, G.M.; Vander Velde, D.G.; Siahaan, T.J.; Borchardt, R.T. Effect of restricted conformational flexibility on the permeation of model hexapeptides across Caco-2 cell monolayers. Pharm. Res. 1997, 14, 169–175. [Google Scholar] [CrossRef]
- Borchardt, R.T. Optimizing oral absorption of peptides using prodrug strategies. J. Control. Release 1999, 62, 231–238. [Google Scholar] [CrossRef]
- Gudmundsson, O.; Jois, S.; Velde, D.V.; Siahaan, T.; Borchardt, R.; Wang, B. The effect of conformation on the membrane permeation of coumarinic acid-and phenylpropionic acid-based cyclic prodrugs of opioid peptides. J. Pept. Res. 1999, 53, 383–392. [Google Scholar] [CrossRef]
- Hill, T.A.; Lohman, R.J.; Hoang, H.N.; Nielsen, D.S.; Scully, C.C.; Kok, W.M.; Liu, L.; Lucke, A.J.; Stoermer, M.J.; Schroeder, C.I.; et al. Cyclic Penta- and Hexaleucine Peptides without N-Methylation Are Orally Absorbed. ACS Med. Chem. Lett. 2014, 5, 1148–1151. [Google Scholar] [CrossRef]
- Pauletti, G.M.; Gangwar, S.; Wang, B.; Borchardt, R.T. Esterase-sensitive cyclic prodrugs of peptides: Evaluation of a phenylpropionic acid promoiety in a model hexapeptide. Pharm. Res. 1997, 14, 11–17. [Google Scholar] [CrossRef]
- Wang, B.; Wang, W.; Zhang, H.; Shan, D.; Nimkar, K.; Gudmundsson, O.; Gangwar, S.; Siahaan, T.; Borchardt, R. Synthesis and evaluation of the physicochemical properties of esterase-sensitive cyclic prodrugs of opioid peptides using coumarinic acid and phenylpropionic acid linkers. J. Pept. Res. 1999, 53, 370–382. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhu, L.J.; Xi, T.K.; Zhu, H.Y.; Chen, X.X.; Wu, M.; Sun, C.; Xu, C.; Fang, G.M.; Meng, X. Delivery of cell membrane impermeable peptides into living cells by using head-to-tail cyclized mitochondria-penetrating peptides. Org. Biomol. Chem. 2019, 17, 9693–9697. [Google Scholar] [CrossRef]
- Price, D.A.; Eng, H.; Farley, K.A.; Goetz, G.H.; Huang, Y.; Jiao, Z.; Kalgutkar, A.S.; Kablaoui, N.M.; Khunte, B.; Liras, S.; et al. Comparative pharmacokinetic profile of cyclosporine (CsA) with a decapeptide and a linear analogue. Org. Biomol. Chem. 2017, 15, 2501–2506. [Google Scholar] [CrossRef]
- Masuda, Y.; Tanaka, R.; Ganesan, A.; Doi, T. Systematic Analysis of the Relationship among 3D Structure, Bioactivity, and Membrane Permeability of PF1171F, a Cyclic Hexapeptide with Paralyzing Effects on Silkworms. J. Org. Chem. 2017, 82, 11447–11463. [Google Scholar] [CrossRef]
- Ovadia, O.; Linde, Y.; Haskell-Luevano, C.; Dirain, M.L.; Sheynis, T.; Jelinek, R.; Gilon, C.; Hoffman, A. The effect of backbone cyclization on PK/PD properties of bioactive peptide-peptoid hybrids: The melanocortin agonist paradigm. Bioorg. Med. Chem. 2010, 18, 580–589. [Google Scholar] [CrossRef]
- Lättig-Tünnemann, G.; Prinz, M.; Hoffmann, D.; Behlke, J.; Palm-Apergi, C.; Morano, I.; Herce, H.D.; Cardoso, M.C. Backbone rigidity and static presentation of guanidinium groups increases cellular uptake of arginine-rich cell-penetrating peptides. Nat. Commun. 2011, 2, 453. [Google Scholar] [CrossRef]
- Buckton, L.K.; Rahimi, M.N.; McAlpine, S.R. Cyclic peptides as drugs for intracellular targets: The next frontier in peptide therapeutic development. Chem. Eur. J. 2021, 27, 1487–1513. [Google Scholar] [CrossRef]
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-methylation of peptides and proteins: An important element for modulating biological functions. Angew. Chem. Int. Ed. 2013, 52, 254–269. [Google Scholar] [CrossRef]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-methylation of peptides: A new perspective in medicinal chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Rader, A.F.B.; Reichart, F.; Weinmuller, M.; Kessler, H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg. Med. Chem. 2018, 26, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Aurelio, L.; Hughes, A.B. Synthesis of N-alkyl amino acids. In Amino Acids, Peptides and Proteins in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 245–289. [Google Scholar]
- Sharma, A.; Kumar, A.; Abdel Monaim, S.A.H.; Jad, Y.E.; El-Faham, A.; de la Torre, B.G.; Albericio, F. N-methylation in amino acids and peptides: Scope and limitations. Biopolymers 2018, 109, e23110. [Google Scholar] [CrossRef]
- Effenberger, F.; Burkard, U.; Willfahrt, J. Aminosäuren, 4. Enantioselektive Synthese N-substituierter α-Aminocarbonsäuren aus α-Hydroxycarbonsäuren. Liebigs Ann. Der Chem. 1986, 1986, 314–333. [Google Scholar] [CrossRef]
- Yang, L.; Chiu, K. Solid phase synthesis of Fmoc N-methyl amino acids: Application of the Fukuyama amine synthesis. Tetrahedron Lett. 1997, 38, 7307–7310. [Google Scholar] [CrossRef]
- Karamanos, N.; Stavropoulos, G.; Napoli, A.; Aksnesc, D.W.; Francis, G.W.; Sindona, G. Redox-alkylation of tosyl protected amino acid and peptide ester. Acta Chem. Scand. 1994, 994, 324–333. [Google Scholar]
- Abdel-Magid, A.F. Reduction of CN to CH–NH by Metal Hydrides. In Comprehensive Organic Synthesis II; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 85–150. [Google Scholar]
- Ben-Ishai, D. Reaction of acylamino acids with paraformaldehyde. J. Am. Chem. Soc. 1957, 79, 5736–5738. [Google Scholar] [CrossRef]
- Auerbach, J.; Zamore, M.; Weinreb, S.M. N-Methylation of amides, lactams, and ureas. J. Org. Chem. 1976, 41, 725–726. [Google Scholar] [CrossRef]
- Freidinger, R.M.; Hinkle, J.S.; Perlow, D.S. Synthesis of 9-fluorenylmethyloxycarbonyl-protected N-alkyl amino acids by reduction of oxazolidinones. J. Org. Chem. 1983, 48, 77–81. [Google Scholar] [CrossRef]
- Aurelio, L.; Box, J.S.; Brownlee, R.T.C.; Hughes, A.B.; Sleebs, M.M. An efficient synthesis of n-methyl amino acids by way of intermediate 5-oxazolidinones. J. Org. Chem. 2003, 68, 2652–2667. [Google Scholar] [CrossRef]
- Aurelio, L.; Brownlee, R.T.C.; Hughes, A.B. A novel synthesis of N-methyl asparagine, arginine, histidine, and tryptophan. Org. Lett. 2002, 4, 3767–3769. [Google Scholar] [CrossRef]
- Reddy, G.V.; Rao, G.V.; Iyengar, D.S. A practical approach for the optically pure N-Methyl-α-amino acids. Tetrahedron Lett. 1998, 39, 1985–1986. [Google Scholar] [CrossRef]
- Fukuyama, T.; Jow, C.-K.; Cheung, M. 2- and 4-Nitrobenzenesulfonamides: Exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett. 1995, 36, 6373–6374. [Google Scholar] [CrossRef]
- Miller, S.C.; Scanlan, T.S. Site-selective N-methylation of peptides on solid support. J. Am. Chem. Soc. 1997, 119, 2301–2302. [Google Scholar] [CrossRef]
- Miller, S.C.; Scanlan, T.S. oNBS−SPPS: A new method for solid-phase peptide synthesis. J. Am. Chem. Soc. 1998, 120, 2690–2691. [Google Scholar] [CrossRef]
- Biron, E.; Chatterjee, J.; Kessler, H. Optimized selective N-methylation of peptides on solid support. J. Pept. Sci. 2006, 12, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.A.; Hauksson, N.E.; Gipe, J.H.; Lokey, R.S. Selective, on-resin N-methylation of peptide N-trifluoroacetamides. Org. Lett. 2013, 15, 5012–5015. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Hoang, H.N.; Lohman, R.J.; Hill, T.A.; Lucke, A.J.; Craik, D.J.; Edmonds, D.J.; Griffith, D.A.; Rotter, C.J.; Ruggeri, R.B.; et al. Improving on nature: Making a cyclic heptapeptide orally bioavailable. Angew. Chem. Int. Ed. 2014, 53, 12059–12063. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.S.; Hoang, H.N.; Lohman, R.J.; Diness, F.; Fairlie, D.P. Total synthesis, structure, and oral absorption of a thiazole cyclic peptide, sanguinamide A. Org. Lett. 2012, 14, 5720–5723. [Google Scholar] [CrossRef] [PubMed]
- Bockus, A.T.; Schwochert, J.A.; Pye, C.R.; Townsend, C.E.; Sok, V.; Bednarek, M.A.; Lokey, R.S. Going Out on a Limb: Delineating The Effects of beta-Branching, N-Methylation, and Side Chain Size on the Passive Permeability, Solubility, and Flexibility of Sanguinamide A Analogues. J. Med. Chem. 2015, 58, 7409–7418. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.; Scheufler, C.; Von Der Mulbe, F.; Fleckenstein, B.; Herrmann, C.; Jung, G.; Moarefi, I.; Hartl, F.U. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 x Hop x Hsp90 complexes. J. Biol. Chem. 2002, 277, 19265–19275. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, C.; Brinker, A.; Bourenkov, G.; Pegoraro, S.; Moroder, L.; Bartunik, H.; Hartl, F.U.; Moarefi, I. Structure of TPR domain–peptide complexes. Cell 2000, 101, 199–210. [Google Scholar] [CrossRef]
- Schmid, A.B.; Lagleder, S.; Grawert, M.A.; Rohl, A.; Hagn, F.; Wandinger, S.K.; Cox, M.B.; Demmer, O.; Richter, K.; Groll, M.; et al. The architecture of functional modules in the Hsp90 co-chaperone Sti1/Hop. EMBO J. 2012, 31, 1506–1517. [Google Scholar] [CrossRef]
- Horibe, T.; Kohno, M.; Haramoto, M.; Ohara, K.; Kawakami, K. Designed hybrid TPR peptide targeting Hsp90 as a novel anticancer agent. J. Transl. Med. 2011, 9, 8. [Google Scholar] [CrossRef]
- Buckton, L.K.; Wahyudi, H.; McAlpine, S.R. The first report of direct inhibitors that target the C-terminal MEEVD region on heat shock protein 90. Chem. Commun. 2016, 52, 501–504. [Google Scholar] [CrossRef]
- MacRaild, C.A.; Seow, J.; Das, S.C.; Norton, R.S. Disordered epitopes as peptide vaccines. Pept. Sci. 2018, 110, e24067. [Google Scholar] [CrossRef]
- Biron, E.; Chatterjee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H.A.; Jelinek, R.; Gilon, C.; Hoffman, A.; et al. Improving oral bioavailability of peptides by multiple N-methylation: Somatostatin analogues. Angew. Chem. Int. Ed. 2008, 47, 2595–2599. [Google Scholar] [CrossRef]
- Chatterjee, J.; Mierke, D.; Kessler, H. N-Methylated cyclic pentaalanine peptides as template structures. J. Am. Chem. Soc. 2006, 128, 15164–15172. [Google Scholar] [CrossRef]
- Ovadia, O.; Greenberg, S.; Chatterjee, J.; Laufer, B.; Opperer, F.; Kessler, H.; Gilon, C.; Hoffman, A. The effect of multiple N-methylation on intestinal permeability of cyclic hexapeptides. Mol. Pharm. 2011, 8, 479–487. [Google Scholar] [CrossRef]
- Beck, J.G.; Chatterjee, J.; Laufer, B.; Kiran, M.U.; Frank, A.O.; Neubauer, S.; Ovadia, O.; Greenberg, S.; Gilon, C.; Hoffman, A.; et al. Intestinal Permeability of Cyclic Peptides: Common Key Backbone Motifs Identified. J. Am. Chem. Soc. 2012, 134, 12125–12133. [Google Scholar] [CrossRef] [PubMed]
- Marelli, U.K.; Bezencon, J.; Puig, E.; Ernst, B.; Kessler, H. Enantiomeric cyclic peptides with different Caco-2 permeability suggest carrier-mediated transport. Chem. Eur. J. 2015, 21, 8023–8027. [Google Scholar] [CrossRef]
- White, T.R.; Renzelman, C.M.; Rand, A.C.; Rezai, T.; McEwen, C.M.; Gelev, V.M.; Turner, R.A.; Linington, R.G.; Leung, S.S.; Kalgutkar, A.S.; et al. On-resin N-methylation of cyclic peptides for discovery of orally bioavailable scaffolds. Nat. Chem. Biol. 2011, 7, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Beaumont, K.; Jones, R.; Kalgutkar, A.S.; Zhang, L.; Atkinson, K.; Bai, G.; Brown, J.A.; Eng, H.; Goetz, G.H.; et al. Discovery of potent and orally bioavailable macrocyclic peptide-peptoid hybrid CXCR7 modulators. J. Med. Chem. 2017, 60, 9653–9663. [Google Scholar] [CrossRef]
- Le Roux, A.; Blaise, E.; Boudreault, P.L.; Comeau, C.; Doucet, A.; Giarrusso, M.; Collin, M.P.; Neubauer, T.; Kolling, F.; Goller, A.H.; et al. Structure-permeability relationship of semipeptidic macrocycles-understanding and optimizing passive permeability and efflux ratio. J. Med. Chem. 2020, 63, 6774–6783. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Duan, X.; Meng, Y.; Jiang, L.; Li, M.; Zhao, G.; Li, Y. Total synthesis of Hirsutellide A. Tetrahedron Lett. 2005, 46, 4377–4379. [Google Scholar] [CrossRef]
- Vongvanich, N.; Kittakoop, P.; Isaka, M.; Trakulnaleamsai, S.; Vimuttipong, S.; Tanticharoen, M.; Thebtaranonth, Y. Hirsutellide A, a new antimycobacterial cyclohexadepsipeptide from the entomopathogenic fungus hirsutella kobayasii. J. Nat. Prod. 2002, 65, 1346–1348. [Google Scholar] [CrossRef]
- Sahile, H.A.; Martínez-Martínez, M.S.; Dillenberger, M.; Becker, K.; Imming, P. Synthesis and Evaluation of Antimycobacterial and Antiplasmodial Activities of Hirsutellide A and Its Analogues. ACS Omega 2020, 5, 14451–14460. [Google Scholar] [CrossRef]
- Kelly, C.N.; Townsend, C.E.; Jain, A.N.; Naylor, M.R.; Pye, C.R.; Schwochert, J.; Lokey, R.S. Geometrically Diverse Lariat Peptide Scaffolds Reveal an Untapped Chemical Space of High Membrane Permeability. J. Am. Chem. Soc. 2021, 143, 705–714. [Google Scholar] [CrossRef]
- Golosov, A.A.; Flyer, A.N.; Amin, J.; Babu, C.; Gampe, C.; Li, J.; Liu, E.; Nakajima, K.; Nettleton, D.; Patel, T.J.; et al. Design of thioether cyclic peptide scaffolds with passive permeability and oral exposure. J. Med. Chem. 2021, 64, 2622–2633. [Google Scholar] [CrossRef]
- Feldwisch, J.; Tolmachev, V. Engineering of affibody molecules for therapy and diagnostics. In Therapeutic Proteins: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; Volume 899, pp. 103–126. [Google Scholar]
- Frejd, F.Y.; Kim, K.-T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Karlström, A.E.; Frejd, F.Y.; Nygren, P.-Å.; Löfblom, J. Affibody molecules in biotechnological and medical applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Ingram, J.R.; Schmidt, F.I.; Ploegh, H.L. Exploiting nanobodies’ singular traits. Annu. Rev. Immunol. 2018, 36, 695–715. [Google Scholar] [CrossRef]
- Beghein, E.; Gettemans, J. Nanobody technology: A versatile toolkit for microscopic imaging, protein-protein interaction analysis, and protein function exploration. Front. Immunol. 2017, 8, 771. [Google Scholar] [CrossRef]
- Širochmanová, I.; Čomor, Ľ.; Káňová, E.; Jiménez-Munguía, I.; Tkáčová, Z.; Bhide, M. Permeability of the blood-brain barrier and transport of nanobodies across the blood-brain barrier. Folia Vet. 2018, 62, 59–66. [Google Scholar] [CrossRef][Green Version]
- Herce, H.D.; Schumacher, D.; Schneider, A.F.L.; Ludwig, A.K.; Mann, F.A.; Fillies, M.; Kasper, M.-A.; Reinke, S.; Krause, E.; Leonhardt, H.; et al. Cell-permeable nanobodies for targeted immunolabelling and antigen manipulation in living cells. Nat. Chem. 2017, 9, 762–771. [Google Scholar] [CrossRef]
- Schumacher, D.; Helma, J.; Schneider, A.F.L.; Leonhardt, H.; Hackenberger, C.P.R. Nanobodies: Chemical functionalization strategies and intracellular applications. Angew. Chem. Int. Ed. 2018, 57, 2314–2333. [Google Scholar] [CrossRef]
- Bruce, V.J.; Lopez-Islas, M.; McNaughton, B.R. Resurfaced cell-penetrating nanobodies: A potentially general scaffold for intracellularly targeted protein discovery. Protein Sci. 2016, 25, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Mix, K.A.; Lomax, J.E.; Raines, R.T. Cytosolic delivery of proteins by bioreversible esterification. J. Am. Chem. Soc. 2017, 139, 14396–14398. [Google Scholar] [CrossRef]

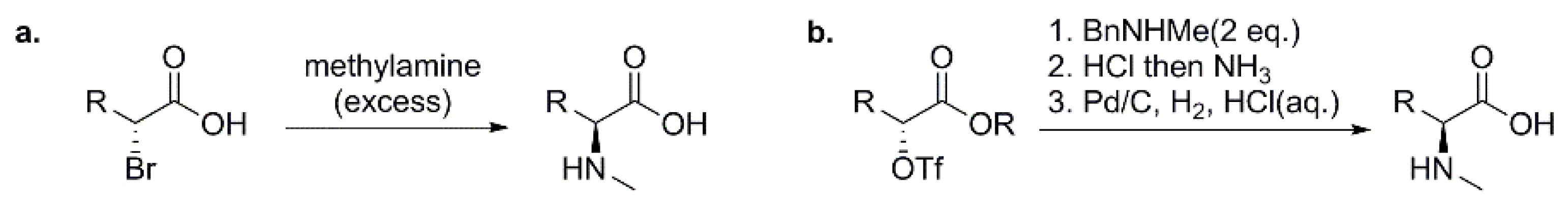







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, W.; Xu, Z. Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool. Mar. Drugs 2021, 19, 311. https://doi.org/10.3390/md19060311
Li Y, Li W, Xu Z. Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool. Marine Drugs. 2021; 19(6):311. https://doi.org/10.3390/md19060311
Chicago/Turabian StyleLi, Yang, Wang Li, and Zhengshuang Xu. 2021. "Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool" Marine Drugs 19, no. 6: 311. https://doi.org/10.3390/md19060311
APA StyleLi, Y., Li, W., & Xu, Z. (2021). Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool. Marine Drugs, 19(6), 311. https://doi.org/10.3390/md19060311




